Abstract
rsTagRFP the first monomeric red fluorescent protein with reversibly photoswitchable absorbance spectra. The switching is realized by irradiation of rsTagRFP with blue (440 nm) and yellow (567 nm) light, turning the protein fluorescence ON and OFF, respectively. It is perhaps the most useful probe in this color class that has yet been reported. Because of the photoswitchable absorbance, rsTagRFP can be used as an acceptor in photochromic Förster resonance energy transfer (pcFRET). Yellow fluorescent proteins YPet and mVenus have been demonstrated to be excellent pcFRET donors for the rsTagRFP acceptor in its fusion constructs. Analysis of X-ray structures has shown that photoswitching of rsTagRFP is accompanied by cis-trans isomerization and protonation/deprotonation of the chromophore, with the deprotonated cis- and protonated trans- isomers corresponding to its ON and OFF states. Unlike in other photoswitchable fluorescent proteins, both conformers of rsTagRFP chromophore are essentially coplanar. Two other peculiarities of the rsTagRFP chromophore are an essentially hydrophobic environment of its p-hydroxyphenyl site and the absence of direct hydrogen bonding between this moiety and the protein scaffold. The influence of the immediate environment on rsTagRFP chromophore was probed by site-directed mutagenesis. Residues Glu145 and His197 were found to participate in protonation/deprotonation of the chromophore accompanying the photoswitching of rsTagRFP fluorescence, whereas the residues Met160 and Leu174 were shown to spatially restrict chromophore isomerization, favoring its radiative decay.
Keywords: KFP, Dronpa, TagRFP, PAmCherry, FRET
Reversibly switchable fluorescent proteins (rsFPs) are the new, recently developed class of fluorescent proteins (FPs), that unlike conventional FPs, demonstrating permanent fluorescence, can be reversibly switched between the non-fluorescent (OFF) and fluorescent (ON) forms by irradiation with light of an appropriate wavelength.1–3 Monomeric rsFPs have several advantages over conventional FPs in monitoring intracellular dynamics. First, rsFPs are applied to study reversible processes such as a transport of the tagged proteins between different compartments in a single cell.4 Second, rsFPs are used in several emerging super-resolution microscopy techniques that enable imaging of single protein molecules tagged with rsFPs at low light intensities.5–9 Third, rsFPs substantially improve signal contrast from the tagged proteins obtained by comparing images of a cell over several photoswitching cycles in so-called optical lock-in detection (OLID) techniques.10
According to the changes in their fluorescence accompanying photoswitching, monomeric rsFPs could be divided in two groups: the first one changes quantum yield of fluorescence, while the second one changes its absorbance spectrum. The major advantage of monomeric rsFPs of the second kind is a unique possibility to use them as acceptors in the novel technique, called a photochromic Förster resonance energy transfer (pcFRET). In pcFRET, fluorescence of a donor can be modulated by reversible changes in the absorbance of the photochromic acceptor. Thus, the donor fluorescence can be measured in the “presence” (a large overlap between the donor emission and the acceptor absorbance spectra) and in the “absence” (a small or no overlap) of the acceptor. The advantage of pcFRET over other FRET approaches is an accurate and reproducible FRET quantification for the same FRET pair within the same cell without the need for corrections based on reference images acquired from control cells. As the photoswitching of rsFPs can be accomplished relatively fast, pcFRET could provide a sub-second resolution important for characterization of protein-protein interactions, once those are brief or occur in rapidly moving cell compartments.
Until recently, monomeric rsFPs with reversible change of the absorbance spectra were limited to two type of cyan-green probes, such as mTFP0.711 and Dronpa variants.4, 12–14 However, cyan-green rsFPs have limited use in pcFRET because of the very few suitable FP donors available. On the other hand, red rsFPs with modulated changes in absorbance could be used with numerous green, yellow, orange, and large Stokes shift FPs as the pcFRET donors.
rsTagRFP protein is the first and currently the only red monomeric rsFP with reversible change of the absorbance spectra.15 rsTagRFP is turned ON and OFF by illumination with 440 and 567 nm light, respectively. Fluorescence of rsTagRFP in the ON state is seen as the emission band with peak at 585 nm; the transition to fluorescent state is accompanied by disappearance of 440 and appearance of 567 nm absorbance bands (see Fig. S1a in the Supporting Information). Remarkably, ON-to-OFF and OFF-to-NO transitions of rsTagRFP are not equivalent in terms of the consumed light energy. It takes about 1,000-fold more energy to switch the fluorescent chromophore OFF than to turn the OFF-chromophore into fluorescent state.15 rsTagRFP was generated from the permanently red fluorescent protein TagRFP (λexmax/λemmax 555/585 nm)16 and presents a variant with the following seven mutations: Phe80Trp / Ile121Leu / Asn143Ala / Ser158Gly / Phe174Leu / Lys182Asn / Tyr194Phe. A recently reported crystal structure of TagRFP17 demonstrated that its chromophore forms direct hydrogen bonds with residues Asn143 and Ser158, stabilizing its trans- conformation. As few as two mutations in TagRFP, Asn143Ala and Ser158Gly, were reported to be essential to yield a variant with reversible photoswitchability.15
Other red rsFPs developed so far are asFP59518 and its mutant asFP595/A143G (KFP)19 as well as rsCherry and rsCherryRev (see Table S1 in the Supporting Information).8 asFP595 and KFP are obligate tetramers, preventing their use for protein tagging. rsCherry and rsCherryRev are monomeric but have been reported to exhibit low brightness and a complex switching behavior.20 However, unlike rsTagRFP, these red rsFPs change quantum yield rather than the absorbance spectrum upon photoswitching. Among red rsFPs, crystal structures have been reported for only asFP595 and its mutants.21, 22
Our comparison of rsTagRFP with rsCherryRev, which is brighter than rsCherry and requires ON and OFF light of wavelengths similar to that for rsTagRFP, showed that, even after ten photoswitching cycles, rsCherryRev was 10-fold less bright than rsTagRFP (Fig. 1a). Moreover, the photoswitching contrast of rsTagRFP was 15-fold at the first cycle and almost did not change over the cycles, whereas the contrast of rsCherryRev increased over the cycles, reaching 3-fold after the 10-th cycle only. These results suggested that rsTagRFP should outperform rsCherryRev in fluorescence applications that require reversible photoswitching.
Fig. 1.
Photoswitching behavior of rsTagRFP in pcFRET. (a) Changes of fluorescence intensity of rsTagRFP and rsCherryRev proteins during the photoswitching cycles. (b) Changes of fluorescence intensity of YPet donor and FRET signal in YPet-rsTagRFP fusion construct. (c) Comparison of the changes of FRET signal in mEGFP-rsTagRFP, YPet-rsTagRFP, mVenus-rsTagRFP, EYFP-rsTagRFP and Citrine-rsTagRFP fusion constructs. The magnitude of the changes in fluorescence signal for each donor corresponds to the FRET efficiency in the respective fusion construct. All fluorescence signals were normalized to the maximum rsTagRFP fluorescence intensity in ON state after the first photoswitching cycle.
We also tested several common monomeric FPs, such as mEGFP (Clontech), YPet,23 mVenus,24 EYFP (Clontech), and Citrine,25 for being pcFRET donors for rsTagRFP in its fusion constructs. The green and yellow FPs have been chosen because of substantial overlap between their emission spectra and the absorbance spectrum of rsTagRFP in the ON state, and because of a minimal effect of blue light required for their excitation on the rsTagRFP photoswitching. When the rsTagRFP acceptor in the YPet-rsTagRFP fusion construct was switched ON there was FRET from the YPet donor (Fig. 1b). When rsTagRFP was subsequently switched OFF, the overlap between the donor emission and rsTagRFP absorbance disappeared, resulting in a substantial decrease of the FRET. There were reversible 28% changes in the pcFRET signal over multiple rsTagRFP photoswitching cycles (Fig. 1b). Similar ~28% changes of pcFRET signal were observed with the mVenus donor, whereas for EYFP, Citrine and mEGFP the changes were 19%, 19%, and 10%, respectively (Fig. 1c). The smaller modulation of the pcFRET signal in the case of mEGFP was possibly caused by smaller overlap of the mEGFP and rsTagRFP spectra. The smaller changes of FRET signal for EYFP and Citrine likely resulted from suboptimal orientation of the excited state dipoles of the donor and acceptor chromophores. At least in this setup, EYFP, which has been recently used in pcFRET with rsTagRFP to study protein-protein interactions,17 is not the best donor for rsTagRFP, and YPet and mVenus donors substantially outperform it.
To determine the structural basis of the rsTagRFP photoswitching, we have crystallized rsTagRFP and determined its structures in the ON and OFF states. Fluorescence of rsTagRFP in crystals was turned ON and OFF by illumination with 405 and 532 nm light, respectively. Upon irradiation, the crystals were immediately flash frozen in 100 K nitrogen stream to trap the desired chromophore state. The overall quality of the diffraction data was high as indicated by data collection statistics (see Table S2 in the Supporting Information). The electron density of rsTagRFP in the OFF state corresponds to the chromophore adopting exclusively trans-conformation, whereas in the ON state it indicates the presence of a mixture of cis- and trans-isomers (see Fig. S2 in the Supporting Information).
Subunits B and D of rsTagRFP in the ON state contain 1:1 mixture of cis- and trans-chromophores, whereas subunits A and C contain 60% trans- and 40% cis- isomers of the chromophore, respectively. Examination of the crystal packing revealed that, unlike in solution, monomeric rsTagRFP forms in the crystal tetramers in which the pairs of subunits AC and BD make different crystal contacts (see Fig. S3 in the Supporting Information). The loops formed by residues 34–40 and 68–80 participate in the crystal contacts in subunits A and C, whereas in subunits B and D they are exposed to solvent channel. The difference in the cis-trans isomerization rates in rsTagRFP subunits imply that photoisomerization of the chromophore is accompanied by expansion/contraction of the β-barrel. A similar breathing of β-barrel accompanying photoswitching was earlier reported for Dronpa,26 thus one could suggest that certain flexibility of the protein scaffold is required for efficient cis-trans photoisomerization of the chromophore in rsFPs.
The nearest environment of p-hydroxyphenyl ring of both the ON and OFF chromophores has an essentially hydrophobic character originating from the Asn143Ala, Ser158Gly, and Phe174Leu mutations (Fig. 2a,b). The phenolate moiety of the rsTagRFP chromophore, in contrast to other rsFP and parent TagRFP, does not form any direct hydrogen bonds with the protein scaffold. However, in both the cis- and trans-conformations, it forms hydrogen bonds with water molecules occupying the space available after replacement of bulkier Asn143, Ser158, and Phe174 with smaller Ala143, Gly158, and Leu174.
Fig. 2.
The nearest chromophore environment of rsTagRFP in its ON (a,d) and OFF (b,e) states, and of TagRFP (c,f). The environment of the p-hydroxyphenyl ring in both the ON (a) and OFF (b) states of rsTagRFP chromophore has a clearly hydrophobic character resulting in the absence of direct hydrogen bonds between the chromophore phenolate and the protein scaffold. In contrast, in parental TagRFP the phenolate group of the chromophore is stabilized in its trans-conformation by two hydrogen bonds with Asn143 and Ser158 (c). The environment of the imidazolinone ring of rsTagRFP in both ON (d) and OFF (e) states is equivalent to that of parental TagRFP (f). The ring is stabilized by five hydrogen bonds with Arg67, Trp90, Arg92, Gln106, and Glu215 and is involved in numerous van der Waals contacts with surrounding residues.
Four other substitutions made in TagRFP in order to obtain rsTagRFP were Phe80Trp, Ile121Leu, Lys182Asn, and Tyr194Phe; they are somewhat distant from the chromophore and merely serve to improve folding, brightness, and photoswitchability of rsTagRFP (see Fig. S4 in the Supporting Information). The environment of the imidazolinone ring of rsTagRFP is similar to that found in progenitor TagRFP. The ring forms five hydrogen bonds with Arg67, Trp90, Arg92, Gln106, and Glu215 and participates in numerous van der Waals contacts with the nearby residues (Fig. 2d,e).
To probe the mechanism of proton transfer around the p-hydroxyphenyl ring of rsTagRFP we generated two mutants, Glu145Gln and His197Arg, yielding the variants lacking one of the ionogenic groups. As demonstrated by the absorbance spectra recorded from these variants in the ON and OFF states, the absence of either of these ionogenic residues near the p-hydroxyphenyl group of the chromophore results in the OFF state mutants (see Fig. S1b,c in the Supporting Information). To clarify the role of the steric restrictions imposed by Met160 and Leu174 on the ON/OFF transition of the chromophore, we generated two other mutants, Met160Val and Leu174Val. Met160 was substituted with a smaller Val and yielded a mutant with a 14-fold decreased quantum yield (see Table S3 in the Supporting Information). Leu174Val mutant has shown an accelerated ON/OFF transition of the chromophore: the corresponding half-time is two-times shorter than for the original rsTagRFP (see Table S3 in the Supporting Information), implying that the Leu174Val substitution facilitates rotation of the p-hydroxyphenyl ring of the chromophore around the Cα2-Cβ2 bond.
The structures of rsTagRFP in ON and OFF states have shown three notable features associated with its chromophore. The first was facilitated rotation around the Cα2-Cβ2 bond, directly associated with cis-trans isomerization and ON/OFF fluorescence switching. Such freedom of rotation has been enabled by substitution of Asn143, Ser158, and Phe174 with less bulky Ala, Gly, and Leu, respectively, and resulted in enlargement of the cavity around the p-hydroxyphenyl site of the chromophore. Similar to Padron0.9,27 asFP595,21 and KFP,1 cis-trans isomerization of rsTagRFP chromophore leaves its nearest environment intact. From our structural data we can conclude that cis-trans isomerization is accompanied by a slight “breathing” of the β-barrel - a phenomenon earlier reported for Dronpa.26 On the other hand, for rsFPs with tightly packed chromophore environment - mTFP0.7,11 Dronpa,28 and IrisFP,29 - photoisomerization causes concerted rearrangement of amino acid residues close to the chromophore. It is noteworthy that, in Dronpa, switching requires high-energy UV or blue light, respectively, whereas for asFP595 and rsTagRFP, for which no residue rearrangements have been observed, green and blue light is sufficient.28
The second feature is the lack of direct stabilizing hydrogen bonds between the phenolic ring of the chromophore and the protein matrix caused by Asn143Ala and Ser158Gly substitutions. To our knowledge, a complete absence of direct hydrogen bonding with the protein scaffold has not been seen before for any other reversibly photoswitchable FPs. The absence of such H-bonding, is, at least partly, responsible for the easiness of cis-trans isomerization of its chromophore and is seen as an increase of pKa from 3.8 for parent TagRFP to 6.6 for rsTagRFP.
The third feature involves essential coplanarity of both the cis- and trans-forms of the chromophore, with the angles between its five- and six- membered rings of less than 10°. Low deviation from planarity is a key feature for the fluorescent cis- conformation and was reported for most rsFPs and FPs, whereas planar trans- conformation is quite unusual. For the majority of rsFPs, trans-chromophores have a highly distorted planarity with the angles between the five-and six-membered rings ranging from 20° to 45° (see Fig. S5 in the Supporting Information).
In spite of being planar, the trans- conformation of rsTagRFP chromophore corresponds to its non-fluorescent (OFF) state. The respective absorbance spectra show the appearance of 438 nm band (see Fig. S1a in the Supporting Information), indicating transition of the chromophore into the blue form. This kind of spectra change could be caused by distortion of the conjugation system of the chromophore and by its protonation.15 Photoswitching of the previously reported red rsFP is indeed accompanied by both cis-trans isomerization and protonation/deprotonation of the chromophore.8,21,30,31 As it was shown by Subach et al., variation of pH affects the fluorescence and the absorbance spectra of rsTagRFP similar to 445/25 nm light: basic pH results in increase of the ON fraction of the chromophore, whereas at pH < 5, most of it exists in the OFF state.15 The same pH-dependence is presumably accountable for the incomplete OFF-to-ON conversion of rsTagRFP in the examined crystals, as they were obtained in acidic crystallization media (pH 5.5; see Materials and Methods section in the Supporting Information). The results of site-directed mutagenesis point out that His197 and Glu145 are crucial for chromophore deprotonation (hence, fluorescence), as their replacement with residues incapable of proton transfer interrupts the proton wire responsible for chromophore deprotonation.
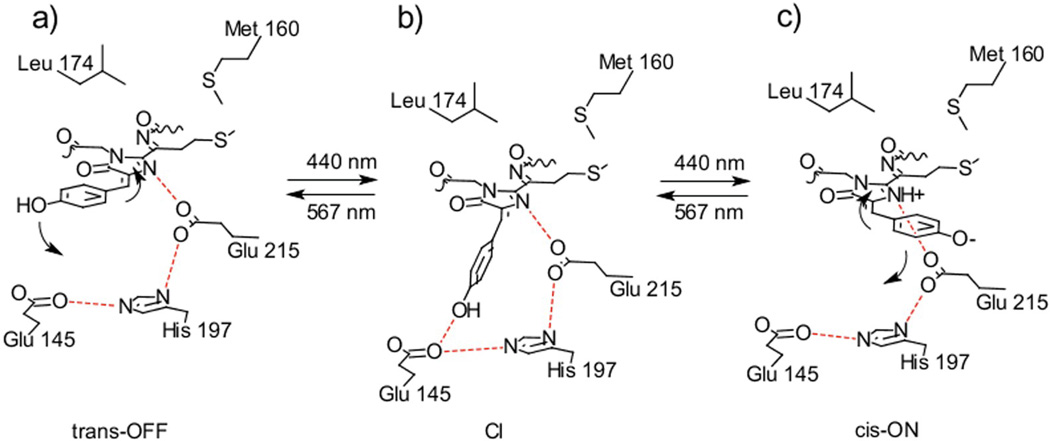
The exact mechanism of chromophore protonation/deprotonation remains unclear as X-ray data account only for static interactions of the chromophore. In their detailed theoretical study, Schaefer et al. remarked that for reversible ON-OFF photoswitching, the fluorescent chromophore anion should be able to undergo protonation.30 This requires a proton donor in close proximity to the phenolate oxygen. Studying photoswitching mechanism of asFP595, Schaefer et al. demonstrated that cis-trans isomerization of the chromophore occurs as a complex hula-hoop motion.31 Because molecular dynamics (MD) simulations and X-ray data provide only structural information, we cannot distinguish whether the observed protonation/deprotonation and cis-trans isomerization occur simultaneously or sequentially.28 In analogy with multiconfigural ab initio (CASSCF) calculations and QM/MM excited state MD simulations with explicit surface hopping for asFP59531, we suggest that, in rsTagRFP, the trans-to-cis transition starts when the p-hydroxyphenyl ring of the chromophore tilts towards Glu145 and His197, while Cβ2-H-group moves away from these residues (Scheme 1a). The chromophore then reaches the conical intersection (CI) point, characterized by the strongest distortion of chromophore geometry (Scheme 1b). Protonated phenolic group of the CI-chromophore forms a hydrogen bond with Glu145 and undergoes deprotonation. The proton is then shuttled to the imidazolinone ring of the chromophore via His197 and Glu215 residues forming a fluorescent zwitterionic species. CI-chromophore then finalizes the hula-hoop motion to adopt cis-conformation (Scheme 1c). In the course of cis-to-trans conversion, the proton wire provides a reverse transition of zwitterionic species to a neutral one. Apparently, transitions between zwitterionic and neutral forms of the chromophore are not equally favored: as one could see from the transition times and the source power, ON-to-OFF transition needs about 1,000-fold more energy than the opposite one.15
Scheme 1.
A proposed mechanism of rsTagRFP photoswitching. (a) Protonated trans-chromophore. (b) Conical intersection (CI) point of the OFF-to-ON / ON-to-OFF transition. (c) Deprotonated cis- chromophore. Upon excitation with blue light neutral trans- chromophore (a) undertakes complex hula-hoop motion in which its p-hydroxyphenyl moiety approaches Glu145 forming with the latter a hydrogen bond (b). At this moment the proton of the phenolate oxygen of the chromophore relocates to the nitrogen atom of its imidazolinone ring via the residues Glu145, His197 and Glu215 resulting in zwitterionic chromophore species. The chromophore then completes its hula-hoop motion to accommodate cis- conformation (c). Excitation with yellow light result in an opposite motion of the chromophore accompanied by a reverse transfer of the proton from its imidazolinone to p-hydroxyphenyl group via Glu215, His197 and Glu145 proton wire.
While cis-trans-isomerization and the protonation/deprotonation pathway describe the mechanism of rsTagRFP photoswitching, they do not explain the difference in the rates of ON-to-OFF and OFF-to-ON transitions. The second pair of the generated mutants, Met160Val and Leu174Val, deals with the dynamics of the chromophore cis-trans isomerization, probing the steric restrictions of the movements of isomerizing chromophore. Replacement of Met160 by a smaller Val resulted in a weakly fluorescent variant (see Table S3 in the Supporting Information). The absorbance spectrum of Met160Val shows two bands, characteristic to ON (553 nm) and OFF (430 nm) states of the chromophore; their relative intensities correspond to a mixture of 2/3 OFF and 1/3 ON rsTagRFP (see Fig. S1e in the Supporting Information). Apparently, unrestricted by a rigid support of Met160 side chain, cis- (ON) chromophore absorbs 567 nm light and undergoes fast isomerization, turning it OFF. The remainder 1/3 of ON chromophores, having greater freedom, may undergo nonradiative pathway of de-excitation. A similar influence of Met160 replacement was observed by Stiel et al. in Dronpa, when they found that replacement of Met159 (in their sequence) with a smaller Thr, Ser, or Ala accelerates the photoswitching to the dark state more than 1,000-fold, decreasing the fluorescence quantum yield.13 Leu174Val mutant, on the other hand, yields a variant that favors cis- (ON) form of the chromophore and increases 2-fold both the ON-to-OFF and OFF-to-ON transition rates (see Table S3 and Fig. S1d in the Supporting Information). Similar to Met160Val, the chromophore of the Leu174Val mutant has a larger surrounding cavity and hence has greater freedom of motion, favoring its nonradiative de-excitation. In the parent TagRFP position 174 is occupied by bulkier Phe, which upon two substitutions, Asn143Ala and Ser158Gly, yielded a photoswitchable rsFP variant, indicating that Leu in position 174 is not crucial for photoswitching properties but merely improves them by stabilizing the chromophore in cis-(ON) conformation.
Summary
In conclusion, in the present paper we have demonstrated the advantages of rsTagRFP comparing to rsCherryRev. Additionally, we have found the donors that are substantially better for pcFRET than EYFP: YPet and mVenus. We have further determined the crystal structures of rsTagRFP in its ON and OFF states, which have revealed that the chromophore of rsTagRFP has a large surrounding cavity and lacks stabilizing direct hydrogen bonding between its tyrosine and the protein scaffold. The chromophore is capable of adopting both the cis- and trans-conformations, stabilized by hydrogen bonding with internal water molecules. Photoswitching of rsTagRFP is enabled by two groups of amino acid residues residing in the immediate surroundings of the chromophore. The first group includes Glu145, His197, and Glu215 and provides protonation/deprotonation of the chromophore. The second group, consisting of Met160 and Leu174, restricts the hula-hoop motion of the chromophore p-hydroxyphenyl ring, creating a barrier that slows down cis-trans isomerization and makes it possible to distinguish and separate the chromophore ON and OFF states.
Supplementary Material
Acknowledgements
We thank K.D. Piatkevich for discussions. We acknowledge the use of beamline 22-BM of the Southeast Regional Collaborative Access Team (SER-CAT), located at the Advanced Photon Source, Argonne National Laboratory. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. This project was supported in part by Federal funds from the National Cancer Institute, National Institutes of Health (NIH) contract No. HHSN261200800001E, the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by the NIH grants GM073913 and CA164468 to V.V.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson JN, Remington SJ. The kindling fluorescent protein: a transient photoswitchable marker. Physiology (Bethesda) 2006;21:162–170. doi: 10.1152/physiol.00056.2005. [DOI] [PubMed] [Google Scholar]
- 2.Piatkevich KD, Verkhusha VV. Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission. Curr. Opin. Chem. Biol. 2010;14:23–29. doi: 10.1016/j.cbpa.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B, Piatkevich KD, Lionnet T, Singer RH, Verkhusha VV. Modern fluorescent proteins and imaging technologies to study gene expression, nuclear localization, and dynamics. Curr. Opin. Cell Biol. 2011;23:310–317. doi: 10.1016/j.ceb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann M, Eggeling C, Jakobs S, Hell SW. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA. 2005;102:17565–17569. doi: 10.1073/pnas.0506010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedecker P, Hotta J, Flors C, Sliwa M, Uji-i H, Roeffaers MB, Ando R, Mizuno H, Miyawaki A, Hofkens J. Subdiffraction imaging through the selective donut-mode depletion of thermally stable photoswitchable fluorophores: numerical analysis and application to the fluorescent protein Dronpa. J. Am. Chem. Soc. 2007;129:16132–16141. doi: 10.1021/ja076128z. [DOI] [PubMed] [Google Scholar]
- 7.Flors C, Hotta J, Uji-i H, Dedecker P, Ando R, Mizuno H, Miyawaki A, Hofkens J. A stroboscopic approach for fast photoactivation-localization microscopy with Dronpa mutants. J. Am. Chem. Soc. 2007;129:13970–13977. doi: 10.1021/ja074704l. [DOI] [PubMed] [Google Scholar]
- 8.Stiel AC, Andresen M, Bock H, Hilbert M, Schilde J, Schonle A, Eggeling C, Egner A, Hell SW, Jakobs S. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys. J. 2008;95:2989–2997. doi: 10.1529/biophysj.108.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler C, Schonle A, Von Middendorff C, Bock H, Eggeling C, Egner A, Hell SW. Resolution of ë/10 in fluorescence microscopy using fast single molecule photo-switching. Appl. Phys. A. 2007;88:223–226. [Google Scholar]
- 10.Yan Y, Marriott ME, Petchprayoon C, Marriott G. Optical switch probes and optical lock-in detection (OLID) imaging microscopy: high-contrast fluorescence imaging within living systems. Biochem. J. 2011;433:411–422. doi: 10.1042/BJ20100992. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JN, Ai HW, Campbell RE, Remington SJ. Structural basis for reversible photobleaching of a green fluorescent protein homologue. Proc. Natl. Acad. Sci. USA. 2007;104:6672–6677. doi: 10.1073/pnas.0700059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando R, Flors C, Mizuno H, Hofkens J, Miyawaki A. Highlighted generation of fluorescence signals using simultaneous two-color irradiation on Dronpa mutants. Biophys. J. 2007;92:L97–L99. doi: 10.1529/biophysj.107.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiel AC, Trowitzsch S, Weber G, Andresen M, Eggeling C, Hell SW, Jakobs S, Wahl MC. 1.8 A bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants. Biochem. J. 2007;402:35–42. doi: 10.1042/BJ20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andresen M, Stiel AC, Folling J, Wenzel D, Schonle A, Egner A, Eggeling C, Hell SW, Jakobs S. Photoswitchable fluorescent proteins enable monochromatic multilabel imaging and dual color fluorescence nanoscopy. Nat. Biotechnol. 2008;26:1035–1040. doi: 10.1038/nbt.1493. [DOI] [PubMed] [Google Scholar]
- 15.Subach FV, Zhang L, Gadella TW, Gurskaya NG, Lukyanov KA, Verkhusha VV. Red fluorescent protein with reversibly photoswitchable absorbance for photochromic FRET. Chem. Biol. 2010;17:745–755. doi: 10.1016/j.chembiol.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merzlyak EM, Goedhart J, Shcherbo D, Bulina ME, Shcheglov AS, Fradkov AF, Gaintzeva A, Lukyanov KA, Lukyanov S, Gadella TW, Chudakov DM. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- 17.Subach OM, Malashkevich VN, Zencheck WD, Morozova KS, Piatkevich KD, Almo SC, Verkhusha VV. Structural characterization of acylimine-containing blue and red chromophores in mTagBFP and TagRFP fluorescent proteins. Chem. Biol. 2010;17:333–341. doi: 10.1016/j.chembiol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukyanov KA, Fradkov AF, Gurskaya NG, Matz MV, Labas YA, Savitsky AP, Markelov ML, Zaraisky AG, Zhao X, Fang Y, Tan W, Lukyanov SA. Natural animal coloration can Be determined by a nonfluorescent green fluorescent protein homolog. J. Biol. Chem. 2000;275:25879–25882. doi: 10.1074/jbc.C000338200. [DOI] [PubMed] [Google Scholar]
- 19.Chudakov DM, Belousov VV, Zaraisky AG, Novoselov VV, Staroverov DB, Zorov DB, Lukyanov S, Lukyanov KA. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 2003;21:191–194. doi: 10.1038/nbt778. [DOI] [PubMed] [Google Scholar]
- 20.Subach FV, Patterson GH, Manley S, Gillette JM, Lippincott-Schwartz J, Verkhusha VV. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat. Methods. 2009;6:153–159. doi: 10.1038/nmeth.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andresen M, Wahl MC, Stiel AC, Grater F, Schafer LV, Trowitzsch S, Weber G, Eggeling C, Grubmuller H, Hell SW, Jakobs S. Structure and mechanism of the reversible photoswitch of a fluorescent protein. Proc. Natl. Acad. Sci. USA. 2005;102:13070–13074. doi: 10.1073/pnas.0502772102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quillin ML, Anstrom DM, Shu X, O'Leary S, Kallio K, Chudakov DM, Remington SJ. Kindling fluorescent protein from Anemonia sulcata: dark-state structure at 1.38 A resolution. Biochemistry. 2005;44:5774–5787. doi: 10.1021/bi047644u. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- 24.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 25.Heikal AA, Hess ST, Baird GS, Tsien RY, Webb WW. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: coral red (dsRed) and yellow (Citrine) Proc. Natl. Acad. Sci. USA. 2000;97:11996–12001. doi: 10.1073/pnas.97.22.11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno H, Mal TK, Walchli M, Kikuchi A, Fukano T, Ando R, Jeyakanthan J, Taka J, Shiro Y, Ikura M, Miyawaki A. Light-dependent regulation of structural flexibility in a photochromic fluorescent protein. Proc. Natl. Acad. Sci. USA. 2008;105:9227–9232. doi: 10.1073/pnas.0709599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brakemann T, Weber G, Andresen M, Groenhof G, Stiel AC, Trowitzsch S, Eggeling C, Grubmuller H, Hell SW, Wahl MC, Jakobs S. Molecular basis of the light-driven switching of the photochromic fluorescent protein Padron. J. Biol. Chem. 2010;285:14603–14609. doi: 10.1074/jbc.M109.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andresen M, Stiel AC, Trowitzsch S, Weber G, Eggeling C, Wahl MC, Hell SW, Jakobs S. Structural basis for reversible photoswitching in Dronpa. Proc. Natl. Acad. Sci. USA. 2007;104:13005–13009. doi: 10.1073/pnas.0700629104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam V, Lelimousin M, Boehme S, Desfonds G, Nienhaus K, Field MJ, Wiedenmann J, McSweeney S, Nienhaus GU, Bourgeois D. Structural characterization of IrisFP, an optical highlighter undergoing multiple photo-induced transformations. Proc. Natl. Acad. Sci. USA. 2008;105:18343–18348. doi: 10.1073/pnas.0805949105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer LV, Groenhof G, Klingen AR, Ullmann GM, Boggio-Pasqua M, Robb MA, Grubmuller H. Photoswitching of the fluorescent protein asFP595: mechanism, proton pathways, and absorption spectra. Angew. Chem. Int. Ed. Engl. 2007;46:530–536. doi: 10.1002/anie.200602315. [DOI] [PubMed] [Google Scholar]
- 31.Schafer LV, Groenhof G, Boggio-Pasqua M, Robb MA, Grubmuller H. Chromophore protonation state controls photoswitching of the fluoroprotein asFP595. PLoS Comput. Biol. 2008;4:e1000034. doi: 10.1371/journal.pcbi.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.