Abstract
Two main classes of models address the earliest steps of left-right patterning: those postulating that asymmetry is initiated via cilia-driven fluid flow in a multicellular tissue at gastrulation, and those postulating that asymmetry is amplified from intrinsic chirality of individual cells at very early embryonic stages. A recent study revealed that cultured human cells have consistent left-right (LR) biases that are dependent on apical-basal polarity machinery. The ability of single cells to set up asymmetry suggests that cellular chirality could be converted to embryonic laterality by cilia-independent polarity mechanisms in cell fields. To examine the link between cellular polarity and LR patterning in a vertebrate model organism, we probed the roles of apical-basal and planar polarity proteins in the orientation of the LR axis in Xenopus. Molecular loss-of-function targeting these polarity pathways specifically randomizes organ situs independently of contribution to the ciliated organ. Alterations in cell polarity also disrupt tight junction integrity, localization of the LR signaling molecule serotonin, the normally left-sided expression of Xnr-1, and the LR instruction occurring between native and ectopic organizers. We propose that well-conserved polarity complexes are required for LR asymmetry and that cell polarity signals establish the flow of laterality information across the early blastoderm independently of later ciliary functions.
Keywords: left-right asymmetry, Vangl2, Par6, conjoined twins, heterotaxia, ion flux
Introduction
During development, embryos acquire specific patterns in three orthogonal directions, which ultimately align with the dorsal-ventral, anterior-posterior, and left-right (LR) axes. While considerable insight has been generated into the molecular mechanisms of patterning along the anterior-posterior and dorsal-ventral axes, much remains to be understood about how the LR axis is consistently oriented with respect to the other two (Aw and Levin, 2008, 2009; Levin, 2005, 2006; Speder et al., 2007; Tabin, 2005; Vandenberg and Levin, 2010b). Externally, the vertebrate body-plan possesses bilateral symmetry, but this is coupled with a strikingly conserved LR asymmetry of the internal organs including the morphogenesis and placement of the heart, liver, gall bladder, stomach and lungs. Abnormalities in laterality form a class of human birth defects that can affect the health of individuals (Casey, 1998; Casey and Hackett, 2000; Kosaki and Casey, 1998; Peeters and Devriendt, 2006). Individuals with heterotaxia, a condition in which the placement of each organ is determined independently, often have serious medical problems because of the failed connections of major blood vessels. In contrast, individuals with situs inversus, the complete mirror inversion of all body organs, are typically healthy.
Two major classes of models have been proposed to explain how consistent asymmetries of the LR axis originate. In the first type of model (Basu and Brueckner, 2008; Tabin and Vogan, 2003), the LR axis is not oriented until late gastrula / early neurula stages when cells isolated in a small pocket of the embryo [termed the node (mouse), gastrocoel roof plate (GRP; Xenopus), or Kupffer’s Vesicle (zebrafish)] develop primary cilia that beat in a clockwise manner [reviewed in (Basu and Brueckner, 2008)]. The tilting of the cilia, together with this stereotypical motion, is proposed to set up asymmetric fluid flows that are transduced into a cascade of asymmetric gene expression by direct movement of a morphogen (Tanaka et al., 2005) or activation of Ca++ signaling in sensory cilia (McGrath et al., 2003). This is proposed to ultimately lead to asymmetric gene expression and biased organ placement [reviewed in (Tabin and Vogan, 2003)]. In the second class of models, a chiral cytoskeletal organizing element (an intracellular structure such as the centriole) becomes oriented with respect to the other two orthogonal axes within the first few cell cleavages, generating a cytoskeleton able to consistently guide the asymmetric intracellular localization of key maternal components by motor proteins (Aw et al., 2008; Danilchik et al., 2006; Qiu et al., 2005). Such asymmetric distributions of ion channels and pumps give rise to a voltage differential consistently biased along the early LR midline (Adams et al., 2006; Levin et al., 2002), which subsequently drives the establishment a physiological gradient of pre-nervous serotonin (5HT) (Fukumoto et al., 2005a; Fukumoto et al., 2005b), ultimately controlling the expression of asymmetric genes by an HDAC-dependent intracellular receptor (Carneiro et al., 2011).
The ciliary model predicts that primary organizers induced later in development should have normal asymmetry since cilia generated at nodes possess their own fixed chirality determined by their molecular structure. In contrast, the cytoplasmic model suggests that the early cleavage events (allowing alignment of intracellular transport events with major embryonic axes) are indispensible for normal asymmetry. We recently showed that organizers whose induction is delayed by as little as 4 cell divisions give rise to embryos with randomized asymmetry, except when they are induced in a blastoderm containing a primary organizer that was present at the first cleavage (Vandenberg and Levin, 2010a). The inability of organizers to orient asymmetry without instruction from an organizer that had participated in the early-cleavage events led us to propose a ‘big brother effect’ – LR instruction between two organizers embedded in the same blastoderm. We had speculated that Planar Cell Polarity (PCP) is a plausible mechanism for the coordination of organizers’ orientation within a cell sheet.
PCP has been more broadly proposed as a mechanism that could be used to orient the LR axis across a wide variety of embryonic architectures (Aw and Levin, 2009; Vandenberg and Levin, 2009). Several recent studies demonstrated a role for PCP in LR asymmetry (Antic et al., 2010; Borovina et al., 2010; Ferrante et al., 2009; Maisonneuve et al., 2009; May-Simera et al., 2010; Oteiza et al., 2010; Ross et al., 2005; Song et al., 2010; Zhang and Levin, 2009). This has often been interpreted as being due to the actions of PCP in positioning cilia at the node or GRP (Hashimoto et al., 2010; Maisonneuve et al., 2009; Okada et al., 2005). However, inhibition of the PCP protein Vangl2 randomizes asymmetry in chick embryos (Zhang and Levin, 2009), which do not use cilia to establish asymmetry (Gros et al., 2009; Manner, 2001). Likewise, in Xenopus, aspects of PCP are already consistently asymmetric at a stage well before ciliary flow appears (Ohkawara and Niehrs, 2011).
PCP has been shown to affect tissue organization and polarity (Chung et al., 2009; Reynolds et al., 2010). Importantly, overexpression of PCP proteins can lead to inappropriate accumulation of other members of the pathway, producing comparable phenotypes to those induced when PCP proteins are down-regulated (Amonlirdviman et al., 2005). Most previous studies of PCP and LR asymmetry focused on the protein Vangl2. However, other members of the PCP pathway, including disheveled, diversin, and RSG1, a PCP effector gene, have yet to be examined for a role in LR asymmetry (Gray et al., 2009; Schwarz-Romond et al., 2002).
The first step in establishing PCP is to position planar polarity proteins at the apical surface of epithelial cells (Tree et al., 2002). The Par6/aPKC complex is one apical-basal polarity (ABP) complex that has been shown to play this role, and both Par6 and aPKC have been implicated in asymmetric cell divisions in multiple animals and in embryo-wide asymmetries in C. elegans (Burgess, 2008; Guo and Kemphues, 1996; Petronczki and Knoblich, 2001). Previous studies indicate that ABP proteins such as the FERM domain protein Lulu, which is apically localized and required for actin reorganization, are required for orientation of the LR axis in the mouse (Lee et al., 2010). Further, a recent study discovered that single neutrophils in culture display a consistent LR asymmetry (Xu et al., 2007). HL60 cells preferentially extend their pseudopodia to the left of a line connecting the nucleus and centrosome, demonstrating that consistent asymmetries do not require fluid flow in a multicellular node or ciliary motion. The HL60 cells’ biases were shown to be dependent on Par6 and aPKC, which is consistent with the requirement of orienting the LR axis relative to the other two axes. Similar data on intrinsic chirality were recently shown for a wide range of other cell types (Wan et al., 2011).
Thus, it becomes important to determine what roles PCP may have in LR-relevant events that do not involve cilia. Interestingly, no studies linking PCP to LR asymmetry have investigated what happens to the LR axis when PCP is altered in cells that do not contribute to the ciliated node or GRP (Vandenberg and Levin, 2010b). Hence, we probed the role of PCP in early, cilia-independent events in normal singleton embryos as well as during twin-twin LR instruction. Here, we report a role for ABP and PCP proteins including Par6, Vangl2, aPKC, and others in the orientation of the LR axis in Xenopus. Disruption of ABP and PCP by overexpression or inhibition of proteins in these pathways resulted in heterotaxia. Importantly, these effects were observed even when altered expression was limited to cells that do not contribute to the GRP. Disrupted ABP or PCP results in early alterations of the normal localization of 5HT and tight junctional integrity, both known to be crucial for LR asymmetry. Moreover, we observed that both ABP and PCP were required for the LR instruction of a late organizer by an early one in a conjoined twin model. Taken together, the data suggest multiple roles for PCP in laterality; the spatial and temporal aspects of these mechanisms highlight novel, cilia-independent functions during the crucial early events underlying LR patterning.
Results
Alterations in apical-basal and planar cell polarities induce heterotaxia
Previous studies of single cells suggest that ABP proteins could be involved in fundamental, well-conserved mechanisms of establishing LR asymmetry. To determine whether these same proteins are involved in the orientation of the LR axis in vertebrates, we expressed dominant negative (DN) forms of Par6 and aPKC, two well-characterized proteins necessary for the establishment of ABP, in Xenopus embryos. All mRNAs were titered to levels low enough to enable normal development and dissociate subtle patterning roles from housekeeping functions. Injection of either DNPar6 or DNaPKC mRNAs at 1 cell specifically induced heterotaxia – an independent randomization of individual organ situs observed at stage 45 (Figure 1, Table 2). This occurred in the absence of generalized malformations or alterations in the dorso-anterior index. To verify that these constructs were affecting ABP, we examined the expression of an apical marker, cytokeratin, and the basolateral marker β1-integrin. In embryos injected with DNPar6, we observed an expansion of basolateral surface markers and a loss of apical surface markers (Supplemental Figure 1), as well as defects in the pigmented apical surface (data not shown), similar to what was seen previously with expression of DNaPKC (Chalmers et al., 2005). We conclude that normal LR patterning requires Par6 and aPKC, and disruption of these ABP proteins alters not just apical-basal domains but also LR asymmetry.
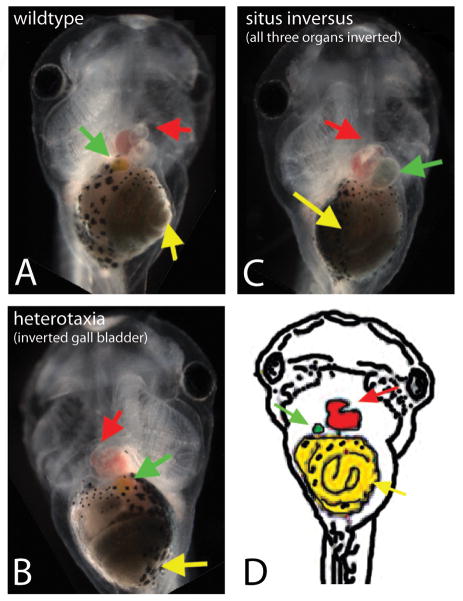
Figure 1. Alterations in apical-basal polarity affect organ situs, including the loss of concordance of the gall bladder with other organs.
(A–C): Organ position in Xenopus embryos at stage 45. A) Wildtype organ situs in an untreated embryo, with the stomach (yellow arrow) coiling to the embryo’s left, the gall bladder (green arrow) on the embryo’s right, and the heart apex (red arrow) located on the embryo’s left. B) Heterotaxic organ situs in an embryo injected with DNaPKC. In a small percentage of embryos, only the position of the gall bladder is affected. C) Situs inversus observed in an embryo injected with DNPar6, marked by the inverted position of all three organs. D) Schematic showing the wildtype position of three asymmetric organs, the heart (red), stomach (yellow), and gall bladder (green).
Table 2.
Heterotaxia is induced when ABP (blue) or PCP (yellow) is disrupted
| Construct/Treatment | % heterotaxia | X2, p-value | % toxicity |
|---|---|---|---|
| Untreated | 2–4% (n=173–248) | 8–15% | |
| DNPar6 | 25% (n=207) | 66.5, p<0.001 | 28% |
| DNaPKC | 27% (n=269) | 48.7, p<0.001 | 21% |
| Const active CDC42 | 12% (n=52) | 5.4, p<0.05 | 80% |
| DN CDC42 | 33% (n=82) | 59.1, p<0.001 | 84% |
| Vangl2 MO | 15% (n=298) | 25.7, p<0.001 | 26% |
| RSG1 | 19% (n=183) | 15.6, p<0.001 | 22% |
| Diversin | 12% (n=194) | 17.7, p<0.001 | 47% |
| Disheveled | 21% (n=48) | 26.5, p<0.001 | 80% |
To determine whether the effects we observed were due to changes in the expression of Par6 and aPKC specifically, or were fundamentally due to alterations in ABP, we examined the effect of several additional mRNA constructs on the orientation of the LR axis. Expression of other ABP-disrupting reagents, including a wildtype and DN CDC42, also induced significant levels of heterotaxia (Table 2). Unsurprisingly, some of these reagents induced measurable toxicity; only embryos with completely normal dorso-anterior indices were included for analysis. We conclude that establishment of left-right asymmetry is dependent on intact apical-basal polarity.
Previous studies using Vangl2 morpholinos (MO) have shown that down-regulation of the PCP protein Vangl2 can disrupt orientation of the LR axis (Antic et al., 2010; Song et al., 2010; Zhang and Levin, 2009). We confirmed this observation, finding that injection of a Vangl2 MO at the 1 cell stage induced heterotaxia in 15% of embryos (Table 2). To determine whether these effects were due to specific actions of Vangl2, or if they were indicative of a more general role of PCP in the orientation of the LR axis, we overexpressed diversin, disheveled, and Rem/Rab-Similar GTPase 1 (RSG1). We observed that altered expression of diversin, disheveled, or RSG1 induced heterotaxia at similar frequencies as those observed with expression of Vangl2 MO (Table 2).
Alterations of ABP or PCP in cells that do not contribute to the ciliated node (GRP) induce heterotaxia
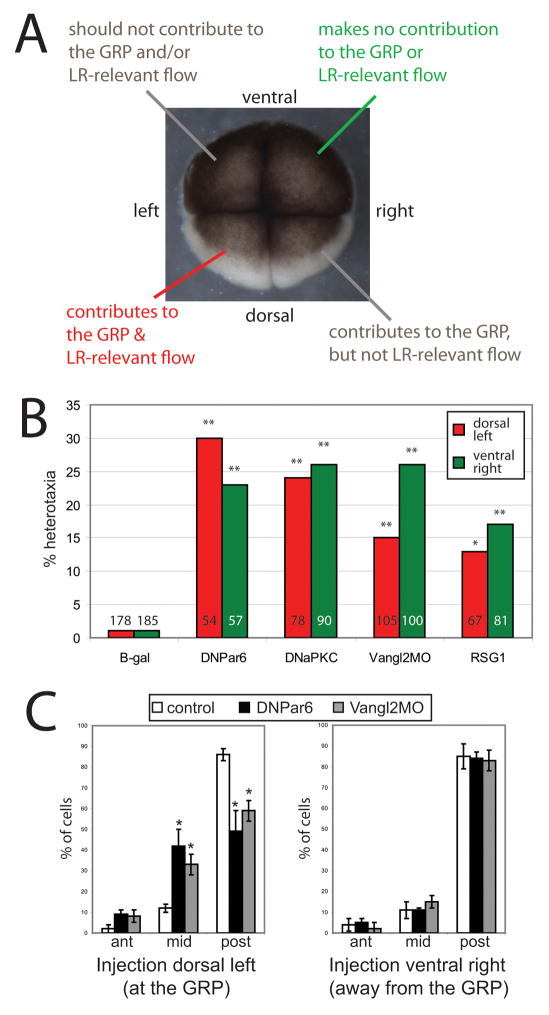
Elegant reverse fate-mapping experiments indicate that only the dorsal blastomeres’ descendants contribute to the GRP (Blum et al., 2009) and only those cells on the left side of the GRP are required to generate asymmetry-relevant flows (Vick et al., 2009). Therefore, the 4-cell embryo contains one blastomere giving rise to daughter cells that contribute to flow at the GRP (left dorsal), one blastomere with daughter cells that do not contribute to the GRP and are not required for LR relevant flow (right ventral), and two other blastomeres with daughter cells that may make minor contributions to flow at the GRP, depending on the accuracy of the first two cleavage furrows (left ventral and right dorsal; see Figure 2A & Supplemental Figure 2).
Figure 2. Altering apical-basal or planar cell polarity in cells that do not contribute to the GRP randomizes asymmetry.
A) Fate mapping experiments of the 4-cell embryo have shown that right ventral cells do not contribute to flow at the GRP, and left dorsal cells contribute to the GRP and are required for LR-relevant flow (Blum et al., 2009; Schweickert et al., 2007; Vick et al., 2009). B) Five constructs were injected into single blastomeres of the 4-cell embryo and then scored at stage 45 for laterality defects. Red bars indicate heterotaxia rates when injected into the dorsal left cells, which are required for cilia-induced flow at the GRP. Green bars indicate heterotaxia rates when injected into the ventral right cells, which do not contribute to flow at the GRP. For all constructs and each blastomere position, at least 50 embryos were examined for organ situs. * p<0.01, ** p<0.001 relative to controls. Numbers on graph indicate total sample sizes that were scored for LR phenotypes. See Supplemental Figure 2 for incidence of heterotaxia associated with injections targeted to each one of the four blastomeres. C) Cilia positioning assays were performed as described in (Antic et al., 2010). Similar to the results reported there, we observed that disruption of PCP signaling via injection of Vangl2MO in dorsal blastomeres significantly alters cilia position, with fewer cells demonstrating posterior localization of cilia. Disruption of ABP signaling via expression of DNPar6 in dorsal cells has similar effects. However, alteration of ABP or PCP signaling in cells that do not contribute to the GRP had no effect on cilia position in cells at the GRP. On both graphs, the X-axis indicates position of the cilia (ant = anterior third of the cell, mid = middle, post = posterior third of the cell.) * p<0.01 relative to controls, ANOVA with Bonferroni post-hoc analysis. Each group (controls, DNPar6, Vangl2MO) at each position (at the GRP, away from the GRP) included at least 100 individual cells from at least 4 different embryos.
To definitively test whether ABP and PCP pathways are required for LR patterning because of their roles in orienting ciliary motion with respect to two axes, we examined the ability of ABP- and PCP-disrupting reagents to randomize when targeted to cells that either do or do not contribute to nodal flow. Single blastomeres of 4-cell stage embryos (oriented both by blastomere size and pigmentation patterns) were injected with the mRNA or morpholino of interest, together with a β-gal lineage tracer to allow subsequent verification of the targeting of injections (Supplemental Figure 2). Expression of DNPar6, DNaPKC, RSG1, or Vangl2MO in right ventral cells induced heterotaxia at rates similar to those obtained when GRP-relevant blastomeres were targeted (Figure 2B).
In the GRP, motile cilia are typically localized to the posterior portion of the cell; the positioning of the cilia is required to establish leftward flow, and cilia remaining at the center of the cell cannot perform this task (Schweickert et al., 2007). Previous studies using a cilia positioning assay have shown that inhibition of Vangl2 in the GRP prevents the localization of cilia to the posterior of the cell (Antic et al., 2010). We used the same assay to determine whether disruption of ABP or PCP signaling in cells that do not contribute to the GRP alters the position of cilia at the GRP. We observed a decrease in the number of cilia positioned at the posterior third of cells when DNPar6 or Vangl2MO were targeted to the GRP (Figure 2C). Crucially however, injection of either of these reagents into ventral blastomeres, which do not contribute to the GRP, had no effect on cilia position at the GRP. From these data, we conclude that the orientation of the LR axis requires broadly intact ABP and PCP with no special status for these processes in cilia-dependent events at the GRP.
ABP and PCP proteins function upstream of Xnr-1
To determine where in the known LR pathway ABP and PCP proteins are playing a role, we examined whether disruption of either of these pathways alters asymmetric gene expression. Xnr-1 is normally expressed only on the left side of the embryo at approximately stage 20 (Sampath et al., 1997). Expression of DNPar6, DNaPKC, RSG1 and Vangl2MO all disrupted the typical asymmetric expression of Xnr-1 (Figure 3). These results indicate that ABP and PCP pathways feed into the well-conserved transcriptional cascades regulating organ situs (Levin and Mercola, 1998a; Yost, 2001), and are functioning upstream of known asymmetric gene expression in the LR pathway.
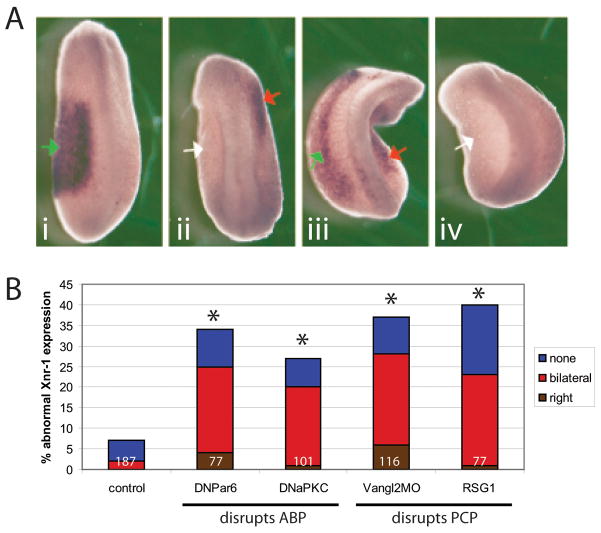
Figure 3. Asymmetric gene expression is affected when apical-basal or planar cell polarity is disrupted.
(A): Xnr-1 localization in embryos injected with DNPar6. In all images, green arrows indicate correct left-sided expression, red arrows indicate ectopic expression, and white arrows indicate absent expression. A.i) Normal left-sided expression of Xnr-1, as observed in the majority of untreated embryos. A.ii) Right-sided expression. A.iii) Bilateral expression. A.iv) Absent Xnr-1 expression. Similar localization patterns were observed for other reagents that disrupt ABP and PCP signaling (not shown). B) Graph of inappropriate Xnr-1 expression observed in untreated and injected embryos. All groups are significantly different from controls (*p<0.001). Numbers on graph indicate sample sizes.
Expression of DNPar6 or Vangl2MO disrupts asymmetric protein localization in early cleavage stages
We next asked how early the polarity pathways play a role in asymmetry, as it has been suggested that PCP is a mechanism used very early to amplify cellular chirality information onto multicellular fields in the blastoderm (Aw and Levin, 2009). In order to determine whether alterations in ABP or PCP have an effect during the first few cleavage stages, we examined the early localization of disheveled-2 (dsh2) in embryos injected with DNPar6 or Vangl2MO. Dsh2 is a key component of the Wnt signaling pathway, and is involved in both canonical and non-canonical (PCP) signaling (Wharton, 2003). In untreated embryos, we found that localization of dsh2 protein had two apparent asymmetries: first, in embryos cut along the animal-vegetal axis, there was a bias observed with significant enrichment in the animal pole (Figure 4A.i). Second, when examining cross-sections collected from regions in the animal pole, we observed that the majority of embryos displayed an asymmetric expression whereby the right ventral blastomere had a stronger signal than the other three (Figure 4A.ii). In embryos expressing DNPar6, sections cut along the animal-vegetal axis still revealed a bias toward the animal pole, although in approximately one-third of embryos, there was a slight shift downward, so that the strongest signal was no longer observed at the apical-most surface of the embryo (Figure 4A.iii). Additionally, in cross-sections collected from the animal pole, we found that expression of Vangl2MO altered the asymmetric distribution among blastomeres such that two, three, or all four blastomeres were now found to have strong dsh2 expression (Figure 4A.iv). We conclude that the effects of our polarity-disrupting reagents are already manifest within the embryo by the second cleavage, and that the normal localization of at least some proteins (e.g., Dsh2) at this stage is downstream of polarity establishment.
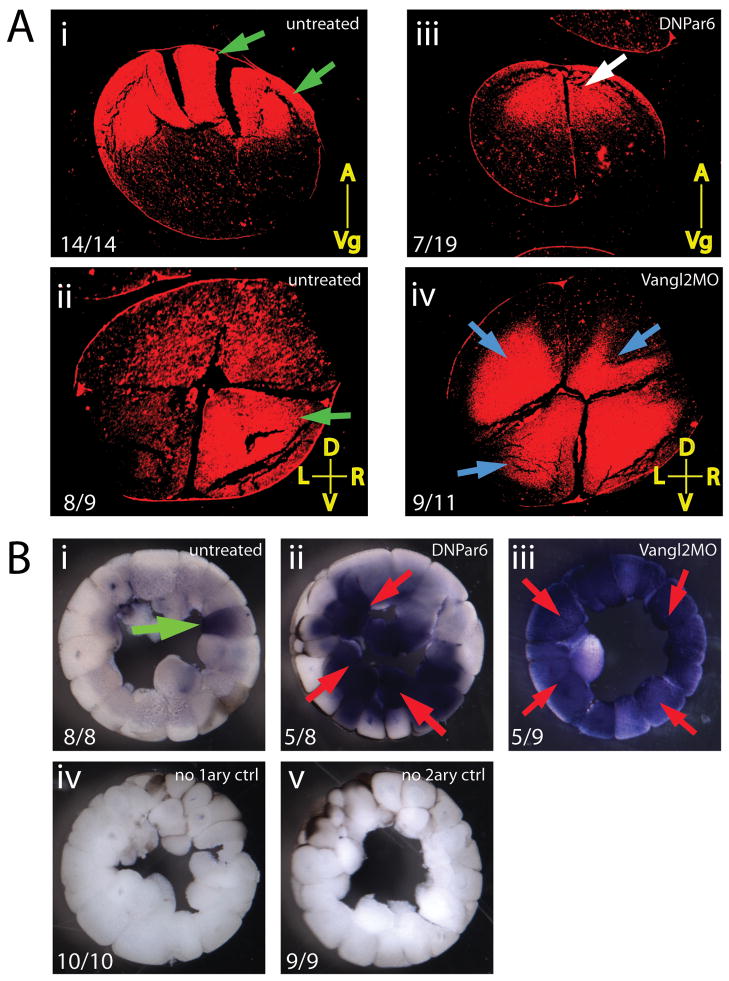
Figure 4. Asymmetric protein localization in cleavage stage embryos is affected by DNPar6 and Vangl2MO.
A) Localization of dsh2 protein in 4-cell embryos. A.i) Animal-vegetal sections through uninjected embryos show strong localization of disheveled-2 at the animal-most position (green arrows) in 100% of embryos. A.ii) In 89% of controls, dsh2 was localized strongly to the right ventral blastomere (green arrow). A.iii) In embryos injected with DNPar6, 37% show a shifted localization of dsh2 protein away from the extremes of the animal pole (white arrow). A.iv) Injection of Vangl2MO disrupted the asymmetric localization of dsh2 in 89% of embryos, with abnormal localization in two, three, or all four blastomeres (blue arrows). Numbers on panels indicate the number of embryos observed with the phenotype over the total number of embryos examined. Yellow lines indicate orientation of the sections (A=animal, Vg=vegetal, D=dorsal, V=ventral, L=left, R=right). B) Expression of 5HT in stage 5/6 embryos. Green arrows indicate appropriate expression, red arrows signal inappropriate 5HT localization. B.i) 5HT was localized to a single blastomere in uninjected embryos. B.ii) Injection of DNPar6 caused a mislocalization of 5HT throughout the embryo. B.iii) Similarly, embryos expressing Vangl2MO showed 5HT localized to most blastomeres. B.iv) Embryos treated for immunohistochemistry without primary antibody or B.v) without secondary antibody show no unspecific staining. Numbers on panels indicate the number of embryos observed with the phenotype over the total number of embryos examined.
Intact ABP and PCP are required for correct localization of pre-nervous serotonin
How might ABP and PCP contribute to early events controlling LR patterning? To link these aspects of cell polarity to known steps in LR patterning, we examined the effect of DNPar6 and Vangl2MO on two mechanisms known to be important at early stages. The first is the localization of serotonin (5HT) at stage 5/6. It has previously been shown that the movement of maternal 5HT from an initially homogenous distribution to a gradient enriched in a small group of right-ventral blastomeres at the ~32 cell stage is required for normal LR patterning (Adams et al., 2006; Carneiro et al., 2011; Esser et al., 2006; Fukumoto et al., 2005b). In control embryos, 5HT signal becomes localized to a single blastomere (Figure 4B.i). In contrast, in embryos where ABP or PCP was targeted, the 5HT signal was disrupted, maintaining distribution throughout the embryo and failing to localize (Figure 4B.ii–iii). These results indicate that both ABP and PCP are required to establish the normal early redistribution of the crucial LR patterning molecule serotonin.
Disruption of ABP and PCP affects tight junction integrity
A second mechanism functioning early in LR patterning is tight-junctional integrity. The Par6 complex and other polarity proteins are required to establish and maintain tight junctions (TJs) in epithelial sheets (Broman et al., 2006; Hurd et al., 2003; Joberty et al., 2000) and their integrity is required for orientation of the LR axis in several species including Xenopus and chick (Brizuela et al., 2001; Simard et al., 2006; Vanhoven et al., 2006). To determine whether alterations in ABP and PCP affect tight junction integrity in the early cleavage stage Xenopus embryo, we performed a biotin-labeling assay on embryos at stage 6. In control embryos, the biotin signal typically penetrated approximately 1 cell depth or less (Figure 5A). In contrast, about 50% of the embryos injected with either DNPar6 or Vangl2MO showed penetrance of the TJ probe further into the embryo, often several cell layers deep (Figure 5B,C). We conclude that the ABP and PCP pathways are required to establish or preserve tight junction integrity, an important downstream step of LR patterning.
Figure 5. Tight junction integrity is disrupted by expression of either DNPar6 or Vangl2MO.
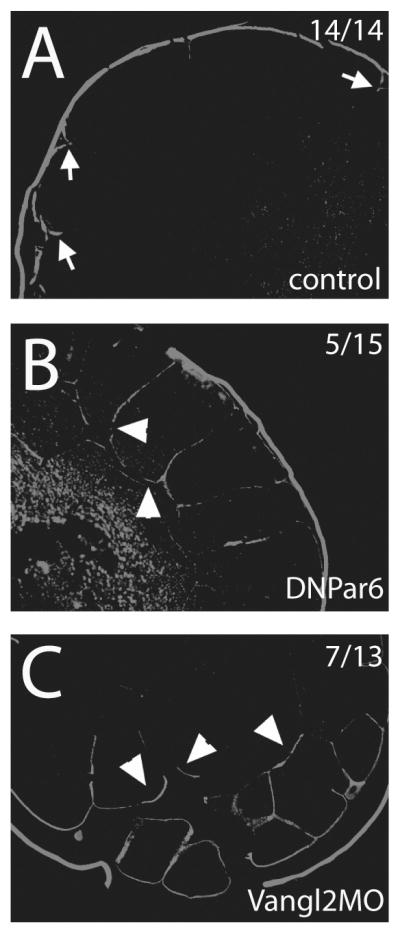
Using a biotin permeability assay, embryos were examined for tight junction integrity. A) Tight junctions were maintained in control embryos, determined because very little biotin was able to penetrate into the embryos. Arrows indicate areas where biotin signal penetrates the embryo; this signal was never observed more than 1 cell depth. B) In embryos injected with DNPar6 or C) Vangl2MO, the biotin signal was observed several cell layers deep in the embryo (arrowheads), indicating that tight junction integrity was affected. Similar results were obtained with embryos cultured in Mg2+/Ca2+-free medium, which is known to break tight junctions (data not shown). This could be interpreted as showing that tight junctions never formed properly in these embryos, or alternatively that tight junctions were disrupted. Numbers on panels indicate the number of embryos observed with the phenotype over the total number of embryos examined.
Par6 and Vangl2 are required for the ‘big brother’ effect in conjoined twins
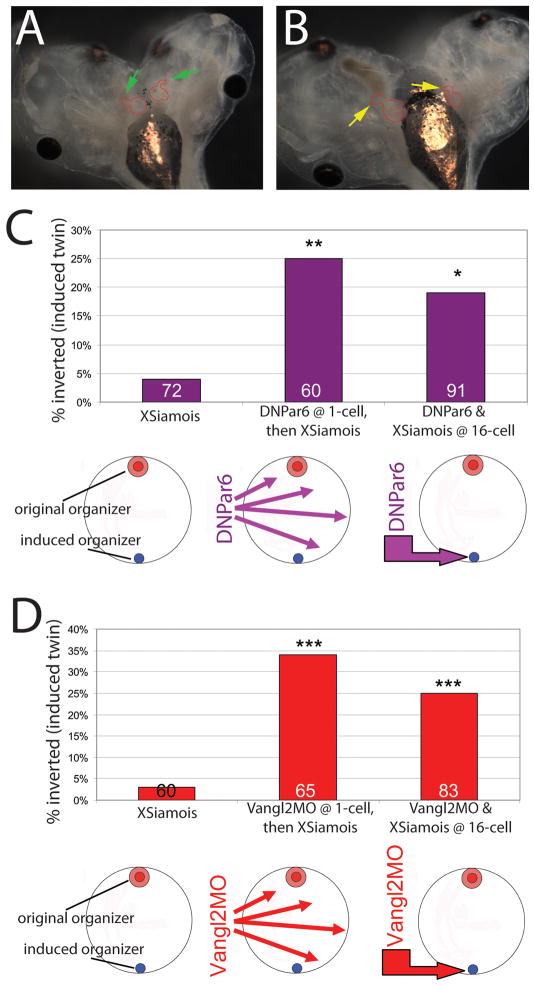
Having observed that PCP and ABP are required for LR patterning in normal embryos, we next examined the role of polarity proteins in the fascinating process of LR instruction of one primary organizer by another in the context of conjoined twins. We hypothesized that all cells of the developing embryo are oriented in a kind of planar polarity, and that an induced (secondary) organizer uses this PCP information to align its LR axis with respect to the blastoderm. To test this hypothesis, we injected 1-cell embryos with either DNPar6 or Vangl2MO to disrupt ABP or PCP, and then induced secondary organizers at mid-blastula transition by injecting the transcription factor XSiamois at the 16-cell stage. While embryos with intact ABP and PCP gave rise to conjoined twins with normal LR asymmetry, we found that pre-injection of either polarity-disrupting construct resulted in randomized heart situs in the induced twin (Figure 6). We conclude that the instruction of the secondary twin by the primary organizer requires ABP and PCP.
Figure 6. Alterations in ABP or PCP randomize heart situs in conjoined twins, regardless of whether polarity is disrupted in one or both twins.
A) Normal heart looping in both twins (green arrows) after injection with XSiamois only. B) Inverted heart looping in both twins (yellow arrows) when XSiamois was paired with DNPar6. C) XSiamois was injected into a single left ventral vegetal blastomere at the 16-cell stage, inducing a secondary organizer (blue circle) directly across from the primary organizer (red circles). A subset of these embryos was injected with DNPar6 at the 1-cell stage, allowing this construct to be expressed in most cells, and then injected with XSiamois at 16-cells. A second subset of embryos was co-injected with both DNPar6 and XSiamois into a single left ventral vegetal blastomere at the 16-cell stage. Inverted heart loops were observed in the induced twins when DNPar6 was injected, regardless of where this construct was introduced. Numbers indicate sample sizes examined, *p=0.01, **p<0.01. D) The same groups were examined as in (C), but this time examining the effects of Vangl2MO. Again, inverted heart loops were observed in the induced twins expressing Vangl2MO, regardless of whether it was injected at 1-cell or co-injected at 16 cell with XSiamois. Numbers indicate sample sizes examined, ***p<0.001.
To gain further insight into the mechanistic role of polarity in this process, we next asked whether the ‘big brother effect’ would occur if polarity were altered in only a small number of cells: is planar polarity necessary for the primary organizer to be able to send the orienting signal, for the secondary organizer to receive the signal, or both? To address this question, we co-injected DNPar6 or Vangl2MO with XSiamois at the 16-cell stage, thus targeting the polarity disruption exclusively to the cells belonging to the ectopic organizer (Supplemental Figure 3). We found that expression of either construct solely at the location of the induced organizer randomized heart situs in the induced twin (Figure 6). These results suggest that intact PCP in the primary organizer is not sufficient for the instructive orientation – the secondary axis must also have normal PCP if it is to receive correct LR patterning information and orient itself within the blastoderm.
Discussion
Here, we have shown that ABP and PCP signaling pathways are required for the proper orientation of the LR axis in Xenopus. Furthermore, our results indicate that these pathways act independent of cilia, a result that is consistent with studies of PCP in chick and other data that indicate the LR axis can be oriented in the absence of cilia [reviewed in (Oviedo and Levin, 2007; Speder et al., 2007; Vandenberg and Levin, 2010b)]. Finally, we have demonstrated that ABP and PCP signaling is required for patterning of the LR axis in induced twins, likely because these mechanisms are needed for communication of orientation cues between the original and the secondary axis.
ABP proteins: a link between asymmetries in single cells and vertebrate embryos
Previous studies show that the Par6/aPKC complex plays important roles in oocyte maturation, maintenance of cell morphology, the asymmetric distribution of proteins to the apical and basolateral surfaces, and the regulation of asymmetric cell divisions in drosophila, C. elegans, Xenopus, and mammals (Burgess, 2008; Chalmers et al., 2005; Etienne-Manneville and Hall, 2003; Hutterer et al., 2004). Additionally, single cells in culture utilize the Par6/aPKC complex to establish and/or maintain LR-like asymmetries, as LR biases were randomized when ABP was disrupted (Xu et al., 2007). Here, we show that ABP machinery is required for orientation of the LR axis in a vertebrate species. Our results closely match the effects of mutant Par6 and aPKC in single cells in culture (Xu et al., 2007), supporting the hypothesis that LR asymmetry is an ancient phenomenon and that the mechanisms utilized to orient the LR axis are highly conserved (Levin and Palmer, 2007; Vandenberg and Levin, 2010a). Importantly, while we have focused on the effects of Par6 and aPKC, other ABP proteins, including CDC42, can also randomize LR asymmetry (Table 2). Collectively, these results suggest that the effects we observed on LR asymmetry are not specific to the Par6/aPKC complex, but a more general effect of altered ABP signaling.
Functional data implicate PCP in the orientation of the LR axis
Downstream of ABP are a group of signaling proteins involved in PCP, which establish polarity perpendicular to the apical-basal axis (McCaffrey and Macara, 2009; Tree et al., 2002). In the Xenopus oocyte, disruption of ABP via depletion of aPKC mRNA alters the localization of Vangl2, a PCP protein (Cha et al., 2011), indicating that an intimate relationship between these signaling proteins exists in the early embryo. A large number of PCP proteins have been well-characterized in biological processes including hair and bristle patterning, drosophila wing shape, ommatidial positioning and rotation in the fly eye, and vertebrate gastrulation [reviewed in (Simons and Mlodzik, 2008)]. The non-canonical Wnt signaling pathway, including molecules such as frizzled, disheveled, non-classical cadherins, and downstream factors such as strabismus (also called van Gogh or Vangl) and prickle, are responsible for establishing PCP in epithelial sheets (Axelrod and McNeill, 2002).
Several studies have linked PCP signaling to the orientation of the LR axis (Antic et al., 2010; Borovina et al., 2010; Ferrante et al., 2009; Maisonneuve et al., 2009; May-Simera et al., 2010; Oteiza et al., 2010; Ross et al., 2005; Song et al., 2010; Zhang and Levin, 2009). Most of these examined the role of Vangl in LR patterning of chick, fish, mouse, and frog embryos using a variety of molecular tools. Our experiments extended these findings by examining the effects of manipulating several PCP proteins on the LR axis, particularly probing a role for PCP in regions of the embryo outside of the ciliated GRP. Most previous studies examining a link between PCP and the LR axis have focused on the effects of Vangl on ciliary placement [reviewed in (Santos and Reiter, 2010)]. Studies in other organ systems indicate that PCP may play a role in the docking of basal bodies to the apical membrane, a process that is important for cilia growth and motility (Lee et al., 2011). However, the requirement for Vangl2 in normal LR patterning in the chick (Zhang and Levin, 2009), which orients the LR axis in a cilia-independent manner (Gros et al., 2009; Manner, 2001), and our data showing that PCP in regions well outside of the cilia-bearing cells of the GRP is still required for normal LR asymmetry, both suggest that PCP has additional roles in laterality that do not involve cilia.
Antic and colleagues disrupted Vangl2 specifically in the cells that contribute to the GRP (Antic et al., 2010), and the genetic mutants utilized in previous studies lack Vangl in all cells of the embryo (Borovina et al., 2010; Song et al., 2010). These experiments did not test cilia/node-independent functions of PCP by asking whether alterations in PCP outside of the node/GRP could affect orientation of the LR axis. This is important, because many of the “ciliary” proteins implicated in LR patterning have other non-ciliary roles including intracellular ones [reviewed in (Armakolas et al., 2010; Levin, 2003; Levin and Palmer, 2007)]. To address this issue, we specifically examined the effect of Vangl2 morpholinos, and other constructs that inhibit PCP and ABP signaling, in cells that do not contribute to the GRP. We found that altered PCP or ABP signaling was effective in randomizing the LR axis, whether it occurred in cells that contributed to the GRP or not (Figure 2B). This result suggests that, similar to the results in chick (Zhang and Levin, 2009), PCP has a cilia-independent role in LR asymmetry. We observed normal GRP cilia when PCP or ABP signaling was disrupted in cells that do not contribute to this structure (Figure 2C) – a result not compatible with hypotheses about impairment of GRP ciliary orientation by PCP defects in distant cells. This is consistent with data reported by Antic et al. (2010), where cilia were only disrupted in GRP cells within clones expressing the Vangl2MO; cilia localization in uninjected cells – near the injected cells, and still within the GRP – was unaffected, confirming a local model of cilia polarization by PCP.
Our results indicate that even species that do appear to use cilia at some point in the LR pathway, such as Xenopus (Vick et al., 2009), still use PCP for other aspects of LR orientation that are distinct from the roles cilia and fluid flow play in establishing LR asymmetry. The ability of PCP-disrupting reagents to specifically randomize the LR axis when not contributing to the cells needed for GRP flow strongly suggests that ciliated cells have no privileged requirement for PCP in LR patterning; these results are instead more compatible with the need for consistent PCP throughout the blastoderm, disruption of which in any region is sufficient to impact global coordination of LR polarity within the embryo.
Polarity proteins maintain TJ integrity, a requirement for LR axis orientation
As early as the 64-cell stage, the Xenopus embryo has characteristics of an epithelium, including the expression of tight junctions, desmosomes and apical-basal domains (Muller and Hausen, 1995). Maintaining the integrity of the epithelium is required for the proper orientation of the LR axis, likely because when tight junctions are disrupted, the ability of the epithelium to establish electrophysiological gradients is diminished (Aw et al., 2010). Polarity proteins are required to establish and maintain tight junctions in epithelial sheets (Hurd et al., 2003; Joberty et al., 2000). Our results indicate that alterations in either ABP or PCP affect tight junctions and epithelial integrity in cleavage stage embryos (Figure 5), suggesting that these pathways could disrupt LR asymmetry also via this second mechanism (Figure 7A).
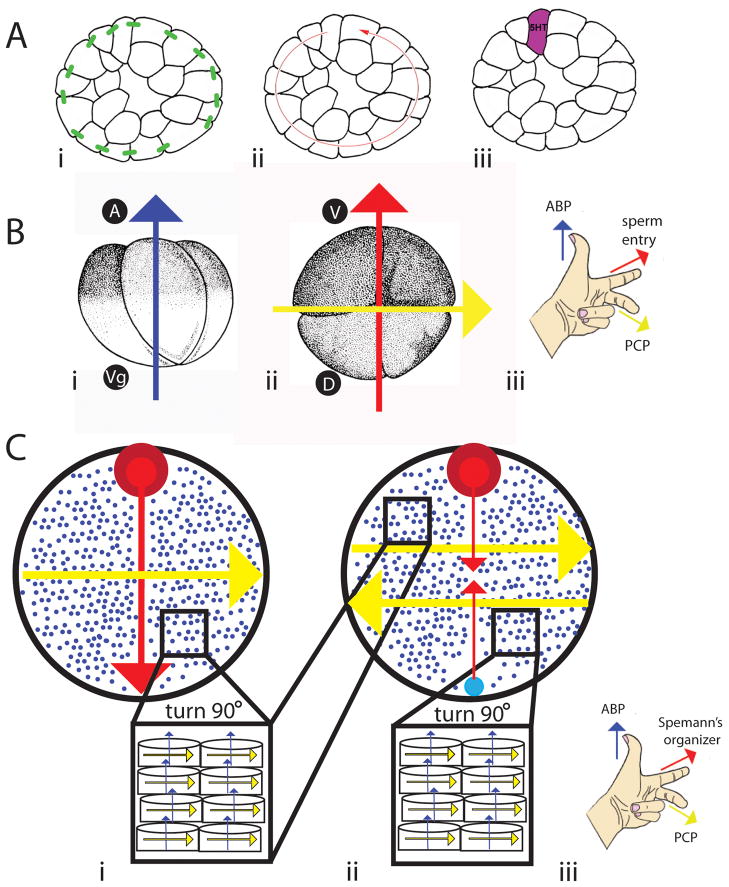
Figure 7. Schematic model for the role of polarity proteins in the orientation of the LR axis.
A) ABP proteins are needed to maintain epithelial integrity via tight junctions (green bars, panel i). This allows physiological gradients, including biophysical ones, to be established (red arrow, panel ii). Ultimately, this – plus a chiral cytoskeleton described in (B), allows for the asymmetric localization of 5HT (purple, panel iii). Thus, ABP is at the center of a well-studied mechanism required for the generation of asymmetry in the early cleavage stage embryo. B) Consistent, biased orientation of 4-cell embryos can be achieved with ABP proteins, which generate an apical-basal axis along the animal (A) and vegetal (Vg) axis (blue arrow, panel i), the sperm entry point, which generates the dorsal (D) ventral (V) axis (red arrow, panel ii), and PCP proteins, which establish LR differences (yellow arrow, panel ii). Together (iii), these three signals work to distinguish all three axes. C) In embryos that establish their axes when thousands of cells are present, one axis is set by ABP proteins (blue arrows and small blue dots marking the tips of arrows coming out of the plane of the page). The organizer (red circle) marks the dorsal-ventral axis (red arrow), and PCP proteins orient the LR axis perpendicular to the other two (yellow arrow, panel i). When a secondary organizer (large blue circle, panel ii) is introduced across from the original one, the dorsal-ventral axis is shifted 180 degrees, which also causes a shift in PCP proteins (yellow arrows, panel ii). Together (iii), ABP proteins, signals from Spemann’s organizer (like XSiamois), and PCP proteins work to distinguish all three axes.
The ultimate consequence for disrupting ABP or PCP pathways is the mislocalization of the LR signaling molecule, 5HT (Figure 4B). In normal embryos, 5HT becomes strongly concentrated in a single right ventral blastomere at stage 5/6 (Adams et al., 2006; Fukumoto et al., 2005b), and in this position is thought to repress the expression of laterality-related genes, leading to their appearance solely on the left side (Carneiro et al., 2011; Levin, 2006; Levin et al., 2006). The establishment of a chiral cytoskeleton and ion flux pathways are both upstream of asymmetric 5HT localization (Adams et al., 2006), therefore ABP and PCP pathways could be disrupting LR asymmetry via either or both mechanism.
ABP and PCP are necessary for intracellular protein localization and LR events at the multi-cellular level
The early cleavage stage embryo has a chiral cytoskeleton, resulting in biased protein distribution among the blastomeres (Aw et al., 2008), which is a plausible means of generating asymmetry intracellularly (Levin and Palmer, 2007). PCP originates intracellularly and PCP proteins are known to interact with – and cause biases in – the cytoskeleton (Aw and Levin, 2009; Djiane et al., 2005; Li et al., 2008; Mirkovic and Mlodzik, 2006). In fact, a recent study of Xenopus oocytes demonstrates that alterations in either ABP or PCP signaling disrupt the microtubule cytoskeleton; only when ABP and PCP proteins are normally expressed can the stable architecture of the oocyte be maintained (Cha et al., 2011). For this reason, we propose that the polarity machinery used in ABP and PCP could be a widely conserved intracellular mechanism used by vertebrates to orient the LR axis.
The cytoskeleton has been shown to be integral to the process of establishing the body axes as well as asymmetries. In C. elegans embryos, the sperm entry point provides polarity cues specifying the anterior-posterior axis, which is maintained by several Par molecules (Goldstein and Hird, 1996; Guo and Kemphues, 1996), and the microtubule-dependent localization of ion channels is crucial for generating consistent neuronal asymmetry (Chang et al., 2011). The sperm centrosome has therefore been proposed as a cytoskeletal mechanism to set up asymmetric cell divisions in the worm (Goldstein and Hird, 1996). In Xenopus, the sperm entry point marks the ventral side of the embryo (Scharf and Gerhart, 1980); after fertilization, cortical rotation localizes elements to the dorsal side of the embryo that are needed for dorsal structures to form (Chang et al., 1996; Nagano et al., 2000). Only when this process occurs is the dorsal-ventral axis established, and the LR axis is then oriented with respect to the other two.
Considering our data and the findings from studies of single cells (Xu et al., 2007), we propose that ABP proteins could be involved in the earliest steps to define the LR axis (Figure 7B). ABP and PCP proteins are therefore plausible candidates to act as the theoretical ‘F-molecule’ that has been proposed to tether the LR axis with the dorsal-ventral and anterior-posterior axes (Brown and Wolpert, 1990). Previous studies implicate the cytoskeleton as the origin of LR asymmetry (Aw et al., 2008; Danilchik et al., 2006; Qiu et al., 2005), and the localization of the Par6 complex during early development of Xenopus embryos suggests that ABP proteins play a role in generating asymmetry during the first cell divisions (Choi et al., 2000) via regulation of the cytoskeleton (Brazil and Hemmings, 2000). Loss-of-function experiments depleting ABP proteins in Xenopus oocytes support this conclusion (Cha et al., 2011). Additionally, CDC42, which is activated by the Par6/aPKC complex, is required for orientation of the microtubule organizing center (Tzima et al., 2003), providing an additional link between ABP proteins and the cytoskeleton.
Our previous results showed that a late-induced organizer can only orient its LR axis properly when an original organizer was present during the first cell cleavages, indicating that the early organizer transmits positional information to the secondary organizer (Vandenberg and Levin, 2010a). Here, our results implicate ABP and PCP signaling in the transfer of information from one twin to the other; expression of either DNPar6 or Vangl2MO disrupted direction of heart looping in twins resulting from late-induced organizers (Figure 6). Importantly, even disruption of PCP or ABP signaling in a small number of cells in the induced twin was sufficient to alter LR patterning.
Early Xenopus embryos have the benefit of large blastomeres that are all in contact, allowing for the direct flow of morphogens and proteins between cells, while many species appear to establish the LR axis when thousands of cells are present. In these animals, the architecture of the embryo precludes the ability to establish the LR axis by intracellular transport of determinants across the midline (Levin, 2004). The conjoined twin model (where a secondary organizer is induced de novo in a multicellular field) is a good analog of the environment for patterning of the LR axis in such species: with large numbers of cells present when the second organizer is induced, each cell does not intrinsically know whether it is on the left or right side of the embryo, but must orient itself within a field of cells. Our results position ABP and PCP signaling as integral in this process (Figure 7C); together with other studies showing a role for Vangl in LR patterning in many animals, our findings support a role for these proteins in a widely conserved intracellular mechanism used by vertebrates with different embryonic architectures to orient the LR axis.
Conclusions
We show a requirement for ABP and PCP signaling to properly orient the LR axis. Disruption of either pathway, via over- or mis-expression of a large panel of mRNAs or a Vangl2 morpholino, randomized organ placement in Xenopus tadpoles. Importantly, expression of several of these constructs outside of the GRP was sufficient to disrupt LR patterning, suggesting that the roles of PCP and ABP in LR patterning are cilia-independent. Altered ABP and PCP signaling also affected asymmetric expression of Xnr-1, altered tight junction integrity, and prevented the specific localization of 5HT in cleavage stage embryos. Finally, both ABP and PCP are required for the instructive ‘big brother effect’ observed when late-induced organizers are properly oriented within their own LR axis by an early organizer. Polarity proteins are therefore involved in several aspects of asymmetry generation, and may be useful in orienting the LR axis in a variety of species with different embryonic architectures at the time of axes specification.
Materials & Methods
Animal rearing
Xenopus laevis embryos were collected and fertilized in vitro according to standard protocols (Sive et al., 2000) in 0.1X Modified Marc’s Ringers (MMR) pH 7.8 + 0.1% Gentamycin. Xenopus embryos were housed at 14–18°C and staged according to (Nieuwkoop and Faber, 1967). All experiments were approved by the Animal Care and Use Committee at Tufts University and were conducted according to the Guidelines for the Care and Use for Laboratory Animals.
Microinjection of molecular constructs (mRNAs and morpholinos)
Constructs included: DNPar6, a human Par6 construct missing the N-terminus, i.e. the region that interacts with aPKC (Etienne-Manneville and Hall, 2001; Tzima et al., 2003); DNaPKC, a rat PKCζ construct lacking the kinase domain (Xu et al., 2007); RSG1-GFP, a human RSG1 construct fused to a GFP sequence (Gray et al., 2009); DNCDC42, a Xenopus dominant negative Cdc42 mutant T17N (Broman et al., 2006); constitutively active human CDC42 mutant Cdc42-L61 (Lamarche et al., 1996); Disheveled-GFP, a wildtype Xenopus Disheveled fused to GFP (Rothbacher et al., 2000; Wallingford et al., 2000); and wildtype mouse Diversin (Moeller et al., 2006). All constructs except RSG1-GFP were subcloned into pCS2 vectors; RSG1-GFP was maintained in CS107, a related vector. Linearized template was transcribed using a mMessage mMachine SP6 transcription kit (Ambion).
For all microinjections of mRNA constructs, capped synthetic mRNAs (Sive et al., 2000) were dissolved in water containing a lineage tracer and injected into embryos in 3% Ficoll using standard methods (50–100 msec pulses with borosilicate glass needles calibrated for a bubble pressure of 50–70 kPa in water) at the specified stage. For induction of conjoined twins, XSiamois was injected into a left ventral vegetal blastomere at the 16-cell stage (Nascone and Mercola, 1997). At stage 22, twin embryos were examined under fluorescence and sorted for left- and right-side induced twins; only left-sided twins were used for analysis (Vandenberg and Levin, 2010a). At stage 45, conjoined twin embryos were further sorted into “complete” twins, i.e. twins with two fully developed hearts and heads, and “incomplete” twins, i.e. twins with only one heart and an underdeveloped second head.
Vangl2 morpholinos (MO) were obtained from GeneTools using the sequences previously found to achieve effective knockdown (Antic et al., 2010; Mitchell et al., 2009). The morpholino was diluted in distilled water and approximately 40ng was injected. The Vangl2MO was constructed with a FITC tag, allowing lineage tracing after injection.
Laterality assay
At stage 45, Xenopus embryos were analyzed for position (situs) of the heart, stomach and gall bladder according to (Levin and Mercola, 1998b). Heterotaxia was defined as the reversal in position of one or more organs. Only embryos with a normal dorsoanterior index (DAI=5) were scored to prevent confounding of randomization caused by midline defects (Danos and Yost, 1995). Conjoined twins were analyzed at stage 45 for situs of the heart for both twins.
Percent heterotaxia was calculated as number of heterotaxic embryos divided by the total number of scorable embryos. A χ2 test with Pearson correction for increased stringency was used to compare absolute counts of heterotaxic embryos. Percent toxicity was calculated as the number of unscorable (dead or DAI ≠ 5) divided by the total number of treated embryos.
In situ hybridization
Whole mount in situ hybridization was performed using standard protocols (Harland, 1991). In situ hybridization probes against Xnr-1 (the Xenopus nodal) mRNAs (Sampath et al., 1997) were generated in vitro from linearized template using DIG labeling mix (Roche, Branford, CT).
Immunohistochemistry
4-cell embryos were fixed in MEMFA (Sive et al., 2000), embedded, and sectioned on a Leica M2255 microtome (paraffin) or Leica VT1000S vibratome (gelatin-albumin). For fluorescence microscopy, either cross-sections or sections along the plane of the animal-vegetal axis were collected to allow comparisons of protein expression between the four blastomeres as well as along the animal-vegetal axis. The samples were blocked, incubated with primary antibodies, washed, and incubated with secondary antibodies (see Table 1 for details). Controls included primary-only and secondary-only conditions. All sections were incubated for the same durations in all experiments.
Table 1.
Antibodies and concentrations used for immunohistochemistry
| Antibody | Primary Concentration | Secondary | Secondary Concentration | Detection | Type of section |
|---|---|---|---|---|---|
| Dsh2 (BioMol) | 1:50 in 5% goat serum + 1.5% milk | Goat anti-rabbit, conjugated to Alexa 647 (Jackson Immunoresearch) | 1:200 in PBS + 1.5% BSA | Direct via fluorescent secondary antibodies | Paraffin, 10μm |
| 5HT (Millipore) | 1:500 in 10% goat serum | Goat anti-rabbit, HRP conjugated (Jackson Immunoresearch) | 1:500 in 10% goat serum | HRP substrate (Moss Biologicals) | Gelatin-albumin, 100 μm |
| ZO-1 (Invitrogen) | 1:50 in 10% goat serum | Goat anti-rabbit, conjugated to Alexa 647(Jackson Immunoresearch) | 1:100 in 10% goat serum | Direct via fluorescent antibodies | Whole GRP from dorsal explants |
| Acetylated tubulin(Sigma) | 1:50 in 10% goat serum | Goat anti-mouse, conjugated to Alexa555(Jackson Immunoresearch) | 1:100 in 10% goat serum | Direct via fluorescent antibodies | Whole GRP from dorsal explants |
Cilia positioning assays were performed on st. 16–17 embryos according to previous methods (Antic et al., 2010). Briefly, immunohistochemistry was performed for cilia and the location of cilia in GRP cells was determined to be in the anterior, middle, or posterior third of the cell. 110–225 cells were examined in at least 4 embryos for each treatment.
Tight Junction Assay
An assay using cell membrane impermeable biotin (Aw et al., 2010) was used to determine whether tight junction integrity was maintained. Briefly, at st. 6, control and injected embryos were cooled to 10°C and incubated in a fresh solution of 1 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce) in 0.1X MMR with 10mM HEPES (pH 7.8) for 12 minutes. Embryos were then washed, fixed, embedded in paraffin, and sectioned at 5μm on a Leica M2255 microtome. Biotin was visualized using anti-strepavidin-Alexa 555 antibodies (Molecular Probes).
Cell lineage tracer
For some experiments, mRNA encoding β-galactosidase (β-gal) was co-injected with other mRNAs or injected alone into specific blastomeres at the stages indicated. At stage 45, tadpoles were scored for heterotaxia, overdosed with tricaine, fixed for 30 min in MEMFA, washed, and stained with X-gal (Roche Applied Sciences, Indianapolis, IN) at 37°C.
Microscopy and image collection
For immunohistochemistry and the visualization of FITC signals in Vangl2MO-injected twins, an Olympus BX-61 with a Hamamatsu ORCA AG CCD camera, controlled by MetaMorph software, was used for imaging. For all other experiments, images were collected with a Nikon SMZ1500 dissection microscope with a Retiga 2000R camera and ImageQ software. Photoshop™ was used to orient, scale, and improve clarity of images. Data were neither added nor subtracted; original images are available on request.
Supplementary Material
As expected from previous studies of DNaPKC (Chalmers et al., 2005), expression of DNPar6 decreased expression of the apical marker cytokeratin and increased expression of the basolateral marker β1-integrin. In all panels, arrows indicate normal and arrowheads abnormal expression.
A)Constructs were injected into one blastomere at the 4-cell stage. This localization was verified by expression of a lineage marker (β-gal) at the GRP, or absent from the GRP. Panels of st. 16–17 embryos show β-gal localization on the exterior neural folds (left panels) and specifically at the GRP on dissected dorsal explants (right panel). For all injections detailed in Table 2, β-gal localization was verified at st. 45 (examples shown). B) Injections into the left dorsal blastomere (red) target the GRP and contribute to LR-relevant flow, and injections into the right ventral blastomere (green) do not contribute to the GRP or LR-relevant flow. Blastomeres indicated by gray may make minor contributions to the GRP and/or LR-relevant flow. Regardless of which blastomere is targeted by these molecular constructs, significant levels of heterotaxia were observed. For all constructs, all blastomeres are significantly different from control embryos injected with β-gal alone (p<0.01). Significant differences were not observed when comparing the individual blastomeres pair-wise for each molecular treatment. Experiments for each blastomere and each construct include at least 50 embryos.
A) In the first set of twin experiments, molecular constructs were injected at the 1-cell stage, and a secondary organizer was induced via injection of XSiamois at the 16-cell stage. Here, localization of the FITC-tagged Vangl2MO demonstrates that this molecular reagent was expressed throughout the developing twin. B) In the second set of twin experiments, molecular constructs were co-injected with XSiamois at the 16-cell stage. Here, localization of the FITC-tagged Vangl2MO demonstrates that this reagent was limited to a small number of cells in the induced twin. In all figures, yellow arrows indicate the original twin and blue arrows indicate the induced twin. For clarity, we have outlined the FITC signal in each twin with a white line.
Acknowledgments
Funding: This work was supported by American Heart Association Established Investigator Grant 0740088N and NIH grant R01-GM077425 to ML, and NRSA grant 1F32GM087107 to LNV.
The authors gratefully acknowledge Joan Lemire for assistance with molecular constructs and for comments on the manuscript, Punita Koustubhan and Amber Currier for Xenopus husbandry and general laboratory assistance, Claire Stevenson for histology, and members of the Levin Lab for helpful discussions. For sharing their molecular constructs, we thank J. Xu for DNPar6 and DNaPKC, J. Wallingford for RSG1-GFP and disheveled-GFP, T. Sharma for DN-CDC42, N. Lamarche for constitutively-active CDC42, N. Nascone for XSiamois, and C. Wright for Xnr-1. This work was supported by American Heart Association Established Investigator Grant 0740088N and NIH grant R01-GM077425 to ML, and NRSA grant 1F32GM087107 to LNV. These funders had no role in the study design or the collection and analysis of the data.
Literature Cited
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armakolas A, Koutsilieris M, Klar AJ. Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development. Curr Opin Cell Biol. 2010;22:81–87. doi: 10.1016/j.ceb.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H, K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster JC, Pearson W, Nichols CG, Shi NQ, Carneiro K, Levin M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol. 2010;346:39–53. doi: 10.1016/j.ydbio.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. What’s left in asymmetry. Dev Dyn. 2008;237:3453–3463. doi: 10.1002/dvdy.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, McNeill H. Coupling planar cell polarity signaling to morphogenesis. The Scientific World Journal. 2002;2:434–454. doi: 10.1100/tsw.2002.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Cilia: multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn. 2009;238:1215–1225. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Cell polarity: scaffold proteins par excellence. Curr Biol. 2000;10:R592–594. doi: 10.1016/s0960-9822(00)00635-7. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Dev Biol. 2001;230:217–229. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Broman MT, Kouklis P, Gao X, Ramchardran R, Neamu RF, Minshall RD, Malik AB. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res. 2006;98:73–80. doi: 10.1161/01.RES.0000198387.44395.e9. [DOI] [PubMed] [Google Scholar]
- Brown NA, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Burgess DR. Cytokinesis and the establishment of early embryonic cell polarity. Biochem Soc Trans. 2008;36:384–386. doi: 10.1042/BST0360384. [DOI] [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. Two rights make a wrong: human left-right malformations. Human Molecular Genetics. 1998;7:1565–1571. doi: 10.1093/hmg/7.10.1565. [DOI] [PubMed] [Google Scholar]
- Casey B, Hackett BP. Left-right axis malformations in man and mouse. Current Opinion in Genetics & Development. 2000;10:257–261. doi: 10.1016/s0959-437x(00)00085-x. [DOI] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138:3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development. 2005;132:977–986. doi: 10.1242/dev.01645. [DOI] [PubMed] [Google Scholar]
- Chang C, Hsieh YW, Lesch BJ, Bargmann CI, Chuang CF. Microtubule-based localization of a synaptic calcium-signaling complex is required for left-right neuronal asymmetry in C. elegans. Development. 2011;138:3509–3518. doi: 10.1242/dev.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, LeGuellec K, Houliston E. Immunodetection of cytoskeletal structures and the Eg5 motor protein on deep-etch replicas of Xenopus egg cortices isolated during the cortical rotation. Biol Cell. 1996;88:89–98. [PubMed] [Google Scholar]
- Choi S-C, Kim J, Han J-K. Identification and developmental expression of par-6 gene in Xenopus laevis. Mech Dev. 2000;91:347–350. doi: 10.1016/s0925-4773(99)00281-6. [DOI] [PubMed] [Google Scholar]
- Chung S, Vining MS, Bradley PL, Chan CC, Wharton KJ, Andrew DJ. Serrano (sano) functions with the planar cell polarity genes to control tracheal tube length. PLoS Genetics. 2009;5:e10000746. doi: 10.1371/journal.pgen.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE, Riegert K. Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development. 2006;133:4517–4526. doi: 10.1242/dev.02642. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development. 1995;121:1467–1474. doi: 10.1242/dev.121.5.1467. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Esser AT, Smith KC, Weaver JC, Levin M. A Mathematical Model of Morphogen Electrophoresis through Gap Junctions. Developmental Dynamics. 2006;235:2144–2159. doi: 10.1002/dvdy.20870. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCz. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Biol. 2003;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Romio L, Castro S, Collins JE, Goulding DA, Stemple DL, Woolf AS, Wilson SW. Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum Molec Genet. 2009;18:289–303. doi: 10.1093/hmg/ddn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Hird SN. Specification of the anterior-posterior axis in C. elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- Gray R, Abitua P, Wlodarczyk B, Szabo-Rogers H, Blanchard O, Lee I, Weiss G, Liu K, Marcotte E, Wallingford J, Finnell R. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–944. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. Molecular genetics of asymmetric cleavage in the early Caenorhabditis elegans embryo. Curr Opin Genet Dev. 1996;6:408–415. doi: 10.1016/s0959-437x(96)80061-x. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical uses in Cell and Molecular Biology. San Diego: Academic Press; 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Kosaki K, Casey B. Genetics of human left-right axis malformations. Seminars in Cell & Developmental Biology. 1998;9:89–99. doi: 10.1006/scdb.1997.0187. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lee JD, Migeotte I, Anderson KV. Left-right patterning in the mouse requires Epb4.1I5-dependent morphogenesis of the node and midline. Dev Biol. 2010;346:237–246. doi: 10.1016/j.ydbio.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Battini L, Gusella GL. Cilium, centrosome and cell cycle regulation in polycystic kidney disease. Biochim Biophys Acta. 2011;1812:1263–1271. doi: 10.1016/j.bbadis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Motor protein control of ion flux is an early step in embryonic left-right asymmetry. Bioessays. 2003;25:1002–1010. doi: 10.1002/bies.10339. [DOI] [PubMed] [Google Scholar]
- Levin M. The embryonic origins of left-right asymmetry. Crit Rev Oral Biol Med. 2004;15:197–206. doi: 10.1177/154411130401500403. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Evolutionary conservation of mechanisms upstream of asymmetric Nodal expression: reconciling chick and Xenopus. Developmental Genetics. 1998a;23:185–193. doi: 10.1002/(SICI)1520-6408(1998)23:3<185::AID-DVG4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left right asymmetry. Dev Biol. 1998b;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang L, Hays TS, Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J Cell Biol. 2008;180:31–38. doi: 10.1083/jcb.200707007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve C, Guilleret I, Vick P, Weber T, Andre P, Beyer T, Blum M, Constam DB. Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development. 2009;136:3019–3030. doi: 10.1242/dev.038174. [DOI] [PubMed] [Google Scholar]
- Manner J. Does an equivalent of the “ventral node” exist in chick embryos? A scanning electron microscopic study. Anatomy & Embryology. 2001;203:481–490. doi: 10.1007/s004290100183. [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Kai M, Hernandez V, Osborn DP, Tada M, Beales PL. Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish. Dev Biol. 2010;345:215–225. doi: 10.1016/j.ydbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harb Perspect Biol. 2009;1:a001370. doi: 10.1101/cshperspect.a001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Mirkovic I, Mlodzik M. Cooperative activities of Drosophila DE-Cadherin and DN-Cadherin regulate the cell motility process of ommatidial rotation. Development. 2006;133:3283–3293. doi: 10.1242/dev.02468. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci U S A. 2006;103:15900–15905. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HA, Hausen P. Epithelial cell polarity in early Xenopus development. Dev Dyn. 1995;202:405–420. doi: 10.1002/aja.1002020410. [DOI] [PubMed] [Google Scholar]
- Nagano T, Ito Y, Tashiro K, Kobayakawa Y, Sakai M. Dorsal induction from dorsal vegetal cells in Xenopus occurs after mid-blastula transition. Mech Dev. 2000;93:3–14. doi: 10.1016/s0925-4773(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Nascone N, Mercola M. Organizer induction determines left-right asymmetry in Xenopus. Dev Biol. 1997;189:68–78. doi: 10.1006/dbio.1997.8635. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Ohkawara B, Niehrs C. An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev Dyn. 2011;240:188–194. doi: 10.1002/dvdy.22500. [DOI] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Oteiza P, Koppen M, Krieg M, Pulgar E, Farias C, Melo C, Preibisch S, Muller D, Tada M, Hartel S, Heisenberg C-P, Concha ML. Planar cell polarity signalling regulates cell adhesion properties in progenitors of the zebrafish laterality organ. Development. 2010;137:3459–3468. doi: 10.1242/dev.049981. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. Gap junctions provide new links in left-right patterning. Cell. 2007;129:645–647. doi: 10.1016/j.cell.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet. 2006;49:349–362. doi: 10.1016/j.ejmg.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–189. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Reynolds A, McDearmid JR, Lachance S, De Marco P, Merello E, Capra V, Gros P, Drapeau P, Kibar Z. VANGL1 rare variants associated with neural tube defects affect convergent extension in zebrafish. Mech Dev. 2010;127:385–392. doi: 10.1016/j.mod.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO Journal. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development. 1997;124:3293–3302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Santos N, Reiter JF. Tilting at nodal windmills: planar cell polarity positions cilia to tell left from right. Dev Cell. 2010;19:5–6. doi: 10.1016/j.devcel.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC. Determination of the dorsal-ventral axis in eggs of Xenopus laevis: complete rescue of UV-impaired eggs by oblique orientation before first cleavage. Dev Biol. 1980;79:181–198. doi: 10.1016/0012-1606(80)90082-2. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase lepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK. Alterations in heart looping induced by overexpression of the tight junction protein Claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev. 2006;123:210–227. doi: 10.1016/j.mod.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H, Grainger RM, Harland R. Early development of Xenopus laevis. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Petzoldt AG, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Current Opinion in Genetics & Development. 2007;17:351–358. doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Tabin C. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J Mol Histol. 2005;36:317–323. doi: 10.1007/s10735-005-9000-y. [DOI] [PubMed] [Google Scholar]
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized Cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. The Journal of Biological Chemistry. 2003;278:31020–31023. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Perspectives and open problems in the early phases of left-right patterning. Seminars in Cell & Developmental Biology. 2009;20:456–463. doi: 10.1016/j.semcdb.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development. 2010a;137:1095–1105. doi: 10.1242/dev.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev Dyn. 2010b;239:3131–3146. doi: 10.1002/dvdy.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoven MK, Bauer Huang SL, Albin SD, Bargmann CI. The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron. 2006;51:291–302. doi: 10.1016/j.neuron.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol. 2009;331:281–291. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Wallingford J, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wan LQ, Ronaldson K, Park M, Taylor G, Zhang Y, Gimble JM, Vunjak-Novakovic G. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc Natl Acad Sci U S A. 2011;108:12295–12300. doi: 10.1073/pnas.1103834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton KJ. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A. 2007;104:9296–9300. doi: 10.1073/pnas.0703153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ. Establishment of left-right asymmetry. International Review of Cytology. 2001;203:357–381. doi: 10.1016/s0074-7696(01)03011-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis. 2009;47:719–728. doi: 10.1002/dvg.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As expected from previous studies of DNaPKC (Chalmers et al., 2005), expression of DNPar6 decreased expression of the apical marker cytokeratin and increased expression of the basolateral marker β1-integrin. In all panels, arrows indicate normal and arrowheads abnormal expression.
A)Constructs were injected into one blastomere at the 4-cell stage. This localization was verified by expression of a lineage marker (β-gal) at the GRP, or absent from the GRP. Panels of st. 16–17 embryos show β-gal localization on the exterior neural folds (left panels) and specifically at the GRP on dissected dorsal explants (right panel). For all injections detailed in Table 2, β-gal localization was verified at st. 45 (examples shown). B) Injections into the left dorsal blastomere (red) target the GRP and contribute to LR-relevant flow, and injections into the right ventral blastomere (green) do not contribute to the GRP or LR-relevant flow. Blastomeres indicated by gray may make minor contributions to the GRP and/or LR-relevant flow. Regardless of which blastomere is targeted by these molecular constructs, significant levels of heterotaxia were observed. For all constructs, all blastomeres are significantly different from control embryos injected with β-gal alone (p<0.01). Significant differences were not observed when comparing the individual blastomeres pair-wise for each molecular treatment. Experiments for each blastomere and each construct include at least 50 embryos.
A) In the first set of twin experiments, molecular constructs were injected at the 1-cell stage, and a secondary organizer was induced via injection of XSiamois at the 16-cell stage. Here, localization of the FITC-tagged Vangl2MO demonstrates that this molecular reagent was expressed throughout the developing twin. B) In the second set of twin experiments, molecular constructs were co-injected with XSiamois at the 16-cell stage. Here, localization of the FITC-tagged Vangl2MO demonstrates that this reagent was limited to a small number of cells in the induced twin. In all figures, yellow arrows indicate the original twin and blue arrows indicate the induced twin. For clarity, we have outlined the FITC signal in each twin with a white line.