Abstract
The study sites for the West African ICEMR are in three countries (The Gambia, Senegal, Mali) and are located within 750 km of each other. In addition, the National Malaria Control Programmes of these countries have virtually identical policies: 1] Artemisinin Combination Therapies (ACTs) for the treatment of symptomatic Plasmodium falciparum infection, 2] Long-Lasting Insecticide-treated bed Nets (LLINs) to reduce the Entomololgic Inoculation Rate (EIR) and 3] Sulfadoxine-Pyrimethamine for the Intermittent Preventive Treatment of malaria during pregnancy (IPTp). However, the prevalence of P. falciparum malaria and the status of malaria control vary markedly across the four sites with differences in the duration of the transmission season (from 4–5 to 10–11 months), the intensity of transmission (with EIRs from unmeasurably low to 4–5 per person per month), multiplicity of infection (from a mean of 1.0 to means of 2–5) and the status of malaria control (from areas which have virtually no control to areas that are at the threshold of malaria elimination). The most important priority is the need to obtain comparable data on the population-based prevalence, incidence and transmission of malaria before new candidate interventions or combinations of interventions are introduced for malaria control.
Keywords: Artemisinin combination therapies, Long-lasting insecticide treated bed nets: Entomologic inoculation rate, Intermittent preventive treatment of malaria during pregnancy, transmission intensity, multiplicity of infection
1. Introduction
1.1 Overview of the History of Malaria and Malaria Control in sub-Saharan Africa
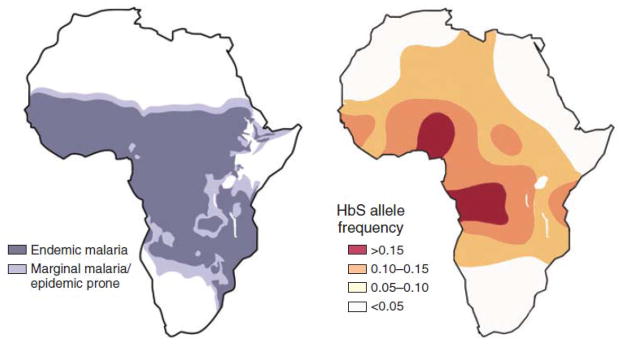
As evidenced by the overlapping distributions of Plasmodium falciparum malaria and the sickle cell gene in sub-Saharan Africa (Figure 1; Wellems & Fairhurst, 2005), P. falciparum has had a profound effect on the survival of Africans for millennia (Allison, 1954; Beet, 1946). In addition, beginning in the 19th century, malaria in Africa has exerted similar effects on Europeans and other visitors (Shah, 2010). More recently (in the 1950s and 1960s), a “global” malaria eradication campaign supported by the World Health Organization, the World Bank and others actually ignored malaria in sub-Saharan Africa (Cohen, et al., 2010; Pampana, 1969). As a result, the focus of malaria control in sub-Saharan Africa since that time has been to minimize morbidity (e.g., cerebral malaria, severe malarial anemia) and mortality, but not to interrupt transmission (WHO, 2000). However, current thinking about malaria control has recently been transformed by a substantial increase in support and by data suggesting that it may possible to control malaria (i.e., to reduce its prevalence by ≥ 50% and interrupt transmission) using interventions available today (Ceesay, et al., 2008). Thus, if effective malaria control can be achieved using technology that is available now, the benefits of control and the opportunity for malaria elimination may not need to be postponed 10–15 years or more for the development of a safe and effective vaccine (Jongwutiwes, et al., 2010) or post-artemisinin antimalarials.
Figure 1.
Distributions of Plasmodium falciparum and the HbS Allele in Sub-Saharan Africa
Reproduced with permission (TE Wellems & RM Fairhurst, 2005; Nat Genet).
1.2 The Recent Global Commitment to Malaria Control and Elimination
Today, more resources than ever before are available for the control and potential elimination of malaria (Roberts and Enserink, 2007) based on support from private foundations (Gates Foundation, Malaria Foundation, Medicines for Malaria Venture, Wellcome Trust, Burroughs-Wellcome Fund) (McCoy, et al., 2009), international programs and agencies (Roll-Back Malaria, The Global Fund, President’s Malaria Initiative, World Health Organization Special Programme for Research and Training in Tropical Diseases [TDR] and the World Bank) and national programs: National Institutes of Health (including the ICEMR Program), the Centers for Disease Control and Prevention (CDC) and the President’s Malaria Initiative (PMI) in the U.S. and the Medical Research Council (MRC) in the U.K.
1.3 The Conceptual Transition: moving from the Reduction of Morbidity and Mortality to the Interruption of Transmission and Potential Elimination
Previous thinking about malaria control in Africa has focused on the prevention of life-threatening illness and death – on reducing morbidity and mortality (Chuma, et al., 2009; WHO, 2000). This is because reductions in morbidity and mortality can be achieved using health center-based strategies to identify persons with symptomatic infections (who are at the greatest risk of morbidity and mortality; especially children ≤ 5 years of age and pregnant women). In contrast, in order to interrupt transmission, the individuals of greatest interest may be those with asymptomatic infections who are living in the community and infecting the anopheline mosquito pool while they remain parasitemic and asymptomatic (which may be 6–8 months or longer; especially for children ≥ 10 years of age and adults). Although it will always be important to prevent morbidity and mortality by treating individuals with symptomatic infections, when the programmatic objective changes from reducing morbidity and mortality to the interruption of transmission and the potential elimination of malaria (Feachem, et al., 2010 and 2008; Moonen, et al., 2010; Sabot, et al., 2010; Tatem, et al., 2010a & 2010b; Greenwood, 2009; Hay, et al., 2008; Mendis, et al., 2009; Águas, et al, 2008; Mills, et al., 2008), the strategic priorities inevitably shift from symptomatic to asymptomatic infection and from the clinic to the community.
1.4 The Potential for Malaria Control and Elimination
The previous malaria eradication campaign failed because resistance developed to the strategies on which it was based: 1] treatment of bloodstream infection with chloroquine to reduce the reservoir of human infection and 2] spraying residual insecticides such as DDT under the eaves of houses to interruption transmission by killing the anopheline vector as it rested after taking a blood meal (Cohen, et al., 2010; Pampana, 1969). Because resistance to antimalarial drugs and insecticides remains a serious problem (Wongsrichanalai, et al., 2002; Cortes, et al., 2003; Brown, 1958), there has been concern about whether it is feasible or wise to propose a second campaign for global malaria control and elimination (Roberts and Enserink, 2007). Although justifiable skepticism remains, the most important new information in the past 5–10 years is a series of reports describing improvements in malaria control using interventions that are available today such as insecticide (permethrin)-impregnated bed nets and artemisinin-combination therapies (ACTs) (Feachem, et al., 2010; O’Meara, et al., 2010; Greenwood, 2009; Mendis, et al., 2009; Águas, et al., 2008; Ceesay, et al., 2008; Nahlen & Low-Beer, 2007).
1.5 Policy Consensus on the Approach to Malaria Control
Based on these reports (Feachem, et al., 2010 and 2008; Moonen, et al., 2010; Sabot, et al., 2010; Tatem, et al., 2010a & 2010b; Greenwood, 2009; Hay, et al., 2008; Mendis, et al., 2009; Águas, et al, 2008; Ceesay, et al., 2008; Mills, et al., 2008)), which often describe ≥ 50% decreases in morbidity and mortality, most international and national malaria control programmes now endorse the same three malaria control strategies (Table 1): 1] treatment of symptomatic infection with ACTs, 2] use of long-lasting insecticide (permethrin)-treated bed nets (LLINs) and 3] intermittent preventive treatment during pregnancy (IPTp) with sulfadoxine + pyrimethamine. However, because malaria control policies are virtually identical across the countries of West Africa, an obvious question is why the results (the levels of malaria control) are so different in countries that appear similar, are geographically close and have essentially identical malaria control policies?
Table 1.
Current Consensus Malaria Control Policies in West Africa
| 1] | Artemsinin-combination treatments (ACTs) for the treatment of persons with symptomatic uncomplicated Plasmodium falciparum infection using either Coartem (artemether + lumefantrine; The Gambia, Senegal, Mali), Falcimon or Arsucam (artesunate + amodiaquine; The Gambia, Senegal, Mali), or Duocotexin (dihydroartemisinin + piperaquine; Senegal), |
| 2] | Long-Lasting Insecticide-treated bed Nets (LLINs) to reduce the frequency of malaria transmission from the anopheline vector to humans for pregnant women and their infants (Mali, Senegal) or the entire population (The Gambia), |
| 3] | Intermittent Preventive Treatment with pyrimethamine + sulfadoxine (Fansidar) during Pregnancy (IPTp) to reduce the frequency of placental malaria and its consequences for the newborn by using therapeutic doses (75 mg pyrimethamine, 1500 mg sulfadoxine) of Fansidar during both the second and third trimesters. |
1.6 Rationale for Creation of the ICEMR Network
The National Institute of Allergy and Infectious Diseases (NIH) has supported basic research on malaria for decades with less emphasis on field studies which have typically involved CDC, USAID, NGOs and other international agencies (NIAID Malaria Research Program, 2011). In contrast, the rationale for the ICEMR Network was to link basic and applied studies of malaria by obtaining data on the epidemiology and transmission of malaria at multiple field sites and using those data to identify: 1] the current obstacles to malaria control and 2] the basic science questions that will need to be resolved in order to eliminate the current obstacles to malaria control and its potential elimination.
As described in the original RFA (RFA-09-017), the roles of the individual ICEMRs are to obtain and analyze those data, but not to perform prospective, controlled studies of individual interventions or combinations of interventions. For that reason, these studies will require baseline data from each of the participating field sites before new interventions are implemented in order to assess the effects of those interventions in subsequent years. Likewise, the inclusion of sites with different intensities of transmission within each ICEMR (as required by the RFA) will also pose major challenges in terms of interpreting the results obtained.
In addition, in this case (the West African ICEMR), the essentially identical malaria control policies in The Gambia, Senegal and Mali (Table 1) potentially provide an opportunity to evaluate the impacts of differences in malaria endemicity (intensity of transmission) and implementation on the outcomes of those policies.
2. Baseline Data on the Epidemiology and Transmission of Malaria in West Africa (The Gambia, Senegal and Mali)
2.1 Geographic and Climatic Similarities and Differences
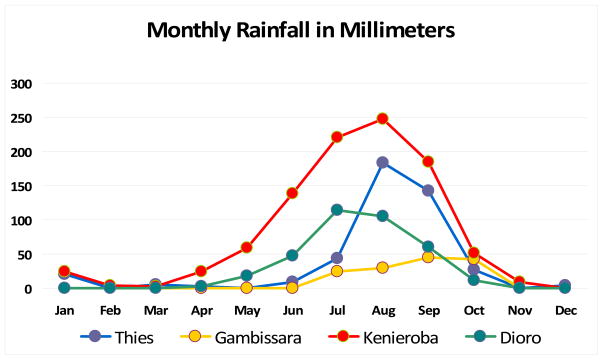
From the perspectives of geography and climate, the countries of greatest interest because of their involvement in the West African ICEMR (The Gambia, Senegal, Mali) are contiguous (Figure 2), have similar geography (lush regions in areas with high rainfall, savana regions and arid sahelian regions with little or no rainfall), similar seasons (rainy season from July or August to October or November) (Figure 3) and similar African populations (Bambara, Fulani, Jola, Mandinka, Songhai, Wolof). As a result, the transmission, incidence and prevalence of P. falciparum malaria vary seasonally in parallel across the four study sites (October–November peaks in the rainy season; May–June low points at the end of the dry season).
Figure 2.
Map of West Africa with ICEMR-Participating Countries
Reproduced with permission (Amy K. Bei)
Figure 3.
2.2 Epidemiologic and Transmission-based Differences among Study Sites
The prevalence of malaria and the intensity of transmission vary strikingly across these sites despite similar seasonality (Table 2) and relatively short distances between them (≤ 750 km). Two of the three rural sites have a low prevalence of infection associated with the microhabitat-based transmission observed commonly with Anopheles gambiae (Sogoba, et al., 2007; Okech, et al., 2004). In contrast, the third rural site (Dioro in Mali, Figure 4) has virtually year-round transmission (June–July to May) from a combination of seasonal rainfall (June–July to October–November) and irrigation from the Niger River to grow rice (flooding of an initial 8,000 hectares from August to December and a second 8,000 hectares from January to May). Because these large expanses of stagnant water provide abundant breeding sites, the human biting rates in such irrigated regions are high (Sogoba, et al., 2007). As a result, the intensity of transmission (as estimated from the Entomologic Inoculation Rate=EIR and the multiplicity of infection=MOI) in areas such as Dioro are higher than in areas with microhabitat-based transmission. The fourth site (an urban site at a clinic in the city of Thies in Senegal) illustrates three of the unresolved issues related to urban malaria at the beginning of a decade when urbanization is increasing, and at the end of which ≥ 50% of the African population is expected to be urban rather than rural: 1] large numbers of persons seeking care for symptomatic P. falciparum infection, 2] limited data on the prevalence of asymptomatic infection in the community, and 3] limited information on breeding sites and the areas where transmission is occurring. In addition, these complexities are exacerbated by urban referral patterns, which may inadvertently lead to higher frequencies of drug-resistant parasites at urban referral centers than rural field sites.
Table 2.
Prevalence of Malaria and the Intensity of Transmission across the Four Field Sites
| Individual Study Sites: | Dioro | Kenieroba | Thies | Gambissara |
|---|---|---|---|---|
| Country: | MALI | MALI | SENEGAL | GAMBIA |
| Location (GIS coordinates and description) | 13.45 N, −6.27 W 385 km from Bamako |
12.11 N, −8.33 W 73 km from Bamako |
14.81 N, −16.92 W 72 km from Dakar |
13.32 N, − 14.22 W 500 km from Banjul |
| Population (size of the relevant community) | 13,605 in 8 study villages | 6,000 total | 400,000 catchment population | 182,586 catchment population |
| Ecological environment | Sahel (rural) Niger River flood plain, inland delta |
South-Savanna (rural) Niger River, microhabitats, scattered breeding sites |
Urban Coastal rainfall scattered breeding sites |
Savanna (rural) Gambia River many breeding sites |
| Transmission (endemicity, seasonal vs. year-round) | Virtually year-round Hyperendemic June–July to May | Seasonal Hyperendemic July–November | Seasonal Hypoendemic Sept–December | Seasonal, Hypoendemic August to November |
| Peak Rainy season EIR (/person/mo) | 4.8 per month | 1.74 [0.93–2.79] | 1.0 per month | 0.93 per month |
| Dry season EIRs (/person/mo) | Not available | 0.01 [0.00–0.08] | Not available | Not available |
| Peak Biting rates (mean/person/night) | 200–300 per night | 58.7 [54.5–63.2] | < 3.0 per night | 0.5 per night |
| Relevant anopheline vector(s), non-gambiae vectors, … | An. gambiae min An. funestus | An. gambiae min An. funestus | An. gambiae EIR < 5 per year | An. gambiae An. arabiensis |
| Rainy Season Prevalence | 84% for 2–9 year olds | 41%–62% all ages | 10.6% all ages | 2.7% all ages |
| Dry Season Prevalence ethnicity of the population) | Not available Bambara |
17%–22% all ages Malinke |
Not available Woloff, Sereres mostly, also Toucouleur |
Not available Saharahule, Fula, Mandinka |
| Current intervention strategies: (bed net usage) | ACTs for treatment, SP IPT in pregnancy, Bed nets (ITNs, high) | ACTs for treatment, SP IPT in pregnancy, Bed nets (ITNs, moderate) | ACTs for treatment, SP IPT in pregnancy, Bed nets (ITNs) | ACTs for treatment, SP IPT pregnancy, Bed nets (ITN, high) |
| Economic activity and occupational status, | Irrigation for growing rice, | Subsistence agriculture: non-irrigated land, farming, fishing | Urban occupations | Subsistence farming: rice, other crops |
| Ages most affected by malaria | Children ≤ 5 years Morbidity 74% in ≤ 5 years 54% in general population |
Children ≤ 5 years | Ages 4–20 years | Historically ≤ 5 years, now 11–15 years |
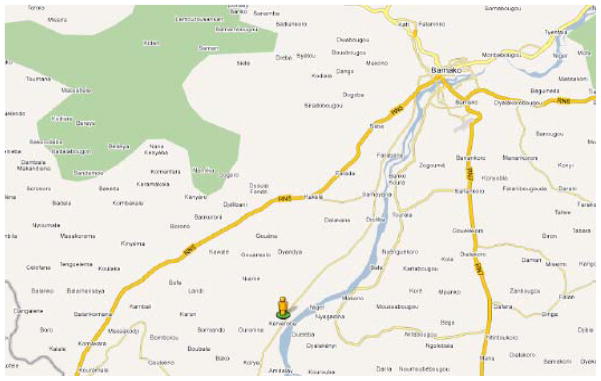
Figure 4.
Location of the Dioro (Segou) Irrigation Scheme in Mali
Reproduced with permission (Google.com).
2.3 Policies for Malaria Control and their Implementation
The increased support for malaria control now being provided by PMI, the U.S. Agency for International Development, the Global Fund, the World Bank, Roll Back Malaria, World Health Organization, Gates Foundation, Wellcome Trust and other agencies offers an unprecedented opportunity to develop and implement rational approaches to malaria control. As a result, malaria control policies are now remarkably uniform across West Africa. However, there may be less uniformity in the implementation of those policies. In addition, with the resources now available, there is increasing interest in strategies with the potential to improve malaria control and simultaneously address unresolved scientific questions (especially questions related to the elimination of malaria).
2.4 Study Design and Data Analysis Challenges
Based on the need for effective antimalarial drugs the greatest concerns in West Africa today are about the effectiveness of antimalarial treatment (ACTs) and bed nets (LLINs). To address those questions, it will be necessary to perform comparative studies using one or more malaria control regimens known to be effective and one or more alternative regimens. In contrast to studies of investigational drugs, studies of public health interventions typically involve regimens that have been FDA-, EMEA- or host country-approved and are therefore not investigational. In terms of study design, non-inferiority study designs are often the most appropriate.
In terms of study design, there is also a need for randomization to control for as yet unknown variables with the potential to cause confounding. Although the randomization of symptomatic subjects is relatively straightforward when they present individually (e.g., in comparative studies of antimalarial treatment regimens), it may be considerably more difficult (and can be close to impossible) in community-based studies of strategies such as ITNs, LLINs or IRS. In those situations, it may be realistic (even necessary) to allocate subjects within the same households or communities to the same preventive strategies.
3. Sahel: Effects of Combinations of Malaria Control Interventions
3.1 Community-based Study with pre- and post-Intervention Evaluations
Because large areas of land (8,000 hectares at a time) are flooded each year to grow rice in the commune of Dioro near Segou in Mali (Figure 4), malaria transmission begins with the annual rains in mid-June or early July (Figure 3) and continues for 10–11 months through May of the following year. In order to examine the effects of three interventions on malaria, a census was obtained and baseline malaria studies were performed in October 2006 in collaboration with the Millennium Village Project. These studies were followed one month later by the introduction of three interventions: 1] ACTs (artemether + lumefantrine=Coartem) for the treatment of symptomatic P. falciparum infection, 2] universal coverage with LLINs based on one net for every 1.8 individuals, and 3] IPTp with sulfadoxine + pyrimethamine (Fansidar) during the second and third trimesters of pregnancy. One year later (December 2007), the baseline studies of malaria infection, disease and transmission performed in October 2006 were repeated to test for changes from baseline which had persisted for ≥ 12 months.
The results of this study demonstrated that interventions now available can reduce the frequency of infection and disease in humans, and can simultaneously reduce transmission from mosquitoes to humans (reduce the EIR). In comparison to other reports, the strength of this study is that the same evaluations were performed one month before and one year after the introduction of the control interventions – thus permitting one to infer a cause-and-effect relationship between the introduction of these three interventions and subsequent reductions in the prevalence, incidence and transmission of malaria.
3.2 Intensity of Human Exposure to Mosquitoes with Year-round Irrigation and the Effects of Intervention on the Entomologic Inoculation Rate
One of the inadvertent results of irrigation to grow rice on flooded land is the creation of breeding sites for the mosquito vector, Anopheles gambiae (Figure 5). As a result, human biting rates in regions with irrigation may reach 200–300 per person per night (Sogoba, et al., 2007). In light of these biting rates, the results of the three control interventions in 2006–2007 (virtual elimination of mosquitoes inside houses, > 40% reduction in the EIR) are highly encouraging.
Figure 5.

Creation of Breeding Sites with Irrigation to Grow Rice
4. Savana: Microhabitat-based Rainy Season Transmission
4.1 Overview of Transmission and Morbidity of Malaria in Kenieroba, Mali
Kenieroba (70 km south of Bamako and 4–5 km from the Niger River in Mali) (Figure 6) provides an example of microhabitat-based rainy season transmission by Anopheles gambiae. Recent data from Diakité, Fairhurst and their colleagues in 2008 suggest that annual attack rates for uncomplicated P. falciparum malaria are ~60% among children less than 18 years of age in this community (773 slide-confirmed cases of uncomplicated P. falciparum malaria among 1300 children within the 5 month transmission season from July to November).
Figure 6.
Village of Kenieroba on the Niger River 60 km south of Bamako in Mali
Reproduced with permission (Google.com).
4.2 Natural Selection for Red Blood Cell Polymorphisms
Data obtained from Kenieroba during the past several years illustrate the continuing selective pressure(s) exerted by P. falciparum malaria in West Africa. For example, 60% of children ≤ 10 years of age in the village of Kenieroba have one or more red blood cell polymorphisms, that protect against malaria by inhibiting the invasion of the red blood cell by the parasite or its replication and thus reduce the frequency of symptomatic uncomplicated P. falciparum malaria in the population (Personal communication: RM Fairhurst, CA Long).
4.3 Persistent Dry Season Transmission
In addition, recent studies by Sogoba, Doumbia and their colleagues have examined the issue of persistent dry season transmission. At times when pyrethrum spray catches (PSCs) and human landing rates have found no evidence of adult mosquitoes in Kenieroba, their studies suggest there are previously undetected breeding sites along the banks of the Niger River. If those breeding sites persist during the dry season, they may account for previously unexplained dry season transmission (new human infections) in villages such as Kenieroba at times when adult anopheline mosquitoes are rare or undetectable.
5. Riverine: Decreasing P. falciparum Infection in The Gambia
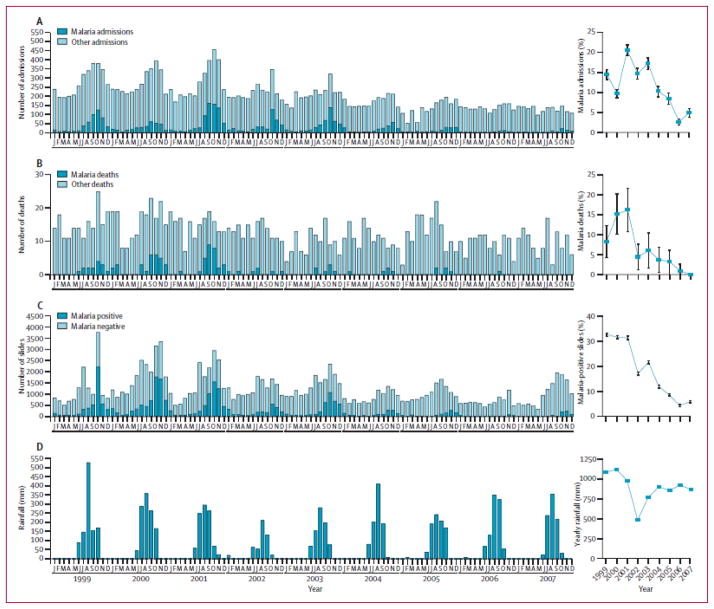
5.1 Decreases in Malaria Admissions, Deaths and Positive Thick Smears
From 1999 to 2007, there were significant decreases in the numbers of in-patient admissions, deaths and positive thick smears reported from health centers and hospitals in The Gambia (Ceesay et al., 2008; Figure 7). These data are striking; they suggest that malaria control is feasible today in The Gambia and raise the possibility that malaria elimination may be feasible in sub-Saharan Africa. However, multiple interventions and changes have been introduced by the Gambian National Malaria Control Programme during the past decade. As a result, it is difficult to attribute these decreases in human infection and disease to any one intervention or combination of interventions.
Figure 7.
Decreased Malaria in The Gambia, from 1999 to 2007
Reproduced with permission (Ceesay et al., 2008; The Lancet).
5.2 Recent Changes in Malaria Endemicity during the 2010 Transmission Season
With increased rains and flooding during the 2010 transmission season, there has been a resurgence of malaria in The Gambia during the past year (Gambian National Malaria Control Programme), which has likewise been observed – although not yet reported – in the neighboring countries of Senegal and Mali.
5.3 The Need for Population-based Data
The decreases in malaria cases, deaths and positive smears in data from health centers and hospitals in The Gambia (Figure 8) from 1999 to 2007 (Ceesay, et al., 2008) and recent evidence for year-to-year variability highlight the need for population-based data on the prevalence, incidence and transmission of malaria in order to monitor and evaluate the effects of individual interventions and combinations of interventions over time. For this reason, baseline studies of the prevalence, incidence and transmission of malaria are a priority in order to obtain population-based data that can serve as a comparator for the evaluation of candidate interventions in subsequent years.
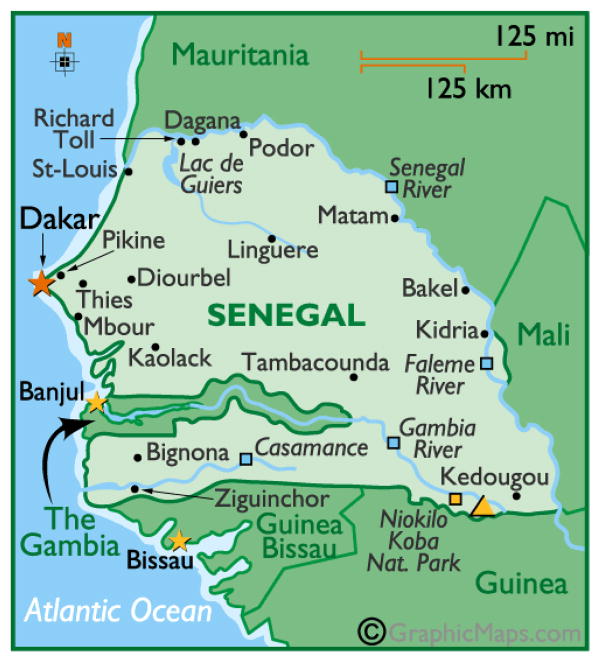
Figure 8.
Location of the City of Thies in Senegal
Reproduced with permission (GraphicMaps.com).
6. Urban Malaria: the City of Thies in Senegal
6.1 The Increasing Importance of Urban Malaria in Sub-Saharan Africa
The consensus of demographers is that the urbanization already in progress across sub-Saharan Africa will continue during the coming decade, such that ≥ 50% of the population will be urban by the end of 2020 (Keiser, et al., 2005). For this reason, we have included an urban site in the city of Thies (which has ~400,000 inhabitants and is 70 km east of Dakar, Figure 8) as a comparator for the rural site at Gambissara in The Gambia and the two rural sites at Dioro and Kenieroba in Mali.
In contrast, the advantages of urban sites are that they provide access to larger populations than can be followed prospectively in rural areas, and permit one to sample segments of the population with greater access to proprietary antimalarial drugs and private medical care. Conversely, the major disadvantage of urban sites is that they rely almost exclusively on passive case detection (persons seeking help because they believe they may have malaria) because of the larger populations, which make community-wide surveys for population-based data more expensive.
6.2 Results of Preliminary Studies performed at the Thies Urban Site
During each of the past four years, an average of 2,-3,000 persons have been tested for P. falciparum infection at the Thies urban site under the auspices of the University Cheikh Anta Diop and the Ministry of Health. On average, 10–25% of the persons tested have been positive by thick smear and have therefore been treated free of charge with an ACT (Coartem [artemether + lumefantrine], Falcimon or Arsucam [artesunate + amodiaquine] or Duocotexin [dihydroartemisinin + piperaquine]) based on the policies of the MOH, PMI and the National Malaria Control Programme of Senegal.
6.3 Ex Vivo Testing of P. falciparum Isolates from Infected Subjects
In addition, during the past several years, Ndiaye and his colleagues have examined parasite isolates from infected symptomatic subjects in Thies for their ability to replicate in vitro using an assay based on a fluorescent dye [4-6-diamidino-2-phenylindole=DAPI] rather than radioisotopes to measure nucleic acid synthesis (Ndiaye, et al., 2010). Those studies (Figure 9) indicate that isolates with high IC50s for chloroquine and artemisinin are present among infected subjects in this region of Senegal.
Figure 9.
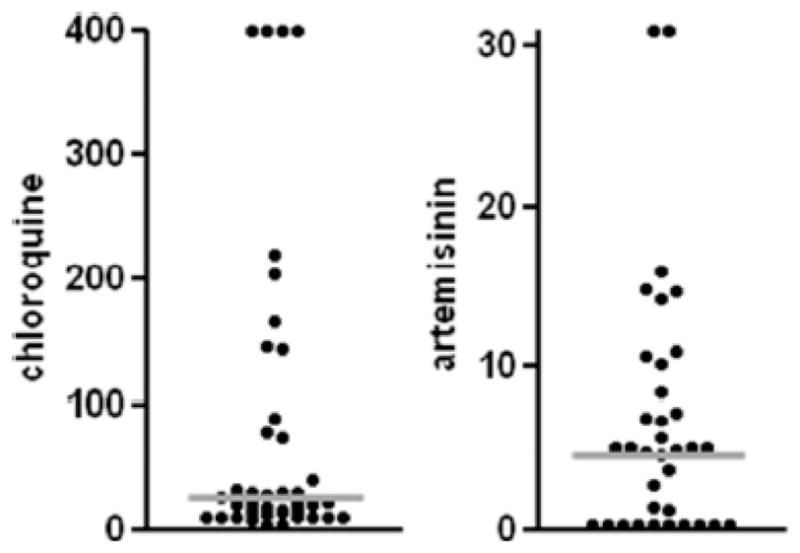
Plots of IC50 Values for Chloroquine and Artemisinin
Reproduced with permission (Ndiaye, et al., 2010; Am J Trop Med Hyg).
7. Discussion and Conclusions
7.1 Ranges of Study Sites and Intervention Strategies
The three rural sites and one urban site we have identified represent a broad range of environments (sahel, savanna, riverine and urban), human impact (from infection in a majority of the population in Dioro to infection as an uncommon event in The Gambia), transmission seasons (microhabitat-based transmission for 4–5 months per year to 10–11 months of transmission per year with irrigation) and malaria control (from limited control to the threshold of elimination) (Table 2). In contrast, the malaria control policies that have been established in The Gambia, Senegal and Mali by PMI and the three respective National Malaria Control Programmes are virtually identical (ACTs, LLINs, IPTp) (Table 1).
7.2 The Relationship between Malaria Control Interventions and Outcomes
As noted above, the relationship between combinations of malaria control interventions and outcomes in The Gambia, Senegal and Mali is unclear because of potentially confounding differences across the four study sites and the region, and because policies that are identical on paper may be substantially different in practice.
7.3 Potential Monitoring and Evaluation Strategies and Challenges
The strategies available for monitoring and evaluation are unlikely to include the randomization of communities to different combinations of control interventions, and each of the study sites will also change over time. In fact, even the timing of the malaria season varies across the study sites because June and July are the start of the transmission season in Dioro and Kenieroba, but not in Gambissara or Thies where transmission begins in August and September.
Therefore, the only way to evaluate and interpret the effects of control interventions at the different study sites is to obtain baseline data for use as comparators after the introduction of new interventions in subsequent years. For this reason, the major initial goal is to obtain baseline data on the epidemiology, human impact and transmission of malaria at the four study sites during the first 1–2 years of support.
Acknowledgments
The studies described here have been supported by a cooperative agreement from the National Institutes of Allergy and Infectious Diseases (NIAID U19 AI 089696) for an international center of excellence in malaria research (ICEMR) award to a consortium involving the University of Bamako in Mali, the University Cheikh Anta Diop in Senegal, the Medical Research Council (MRC) Laboratories in The Gambia, Harvard School of Public Health, Boston College, London School of Hygiene and Tropical Medicine and the Tulane School of Public Health and Tropical Medicine. We thank our many colleagues for administrative and financial support (Abdoulie Barry, Ron Cail, Salif Camara, Rosie Chavez, Abou Alassane Diallo, Khady Touré Diop, Ramona Gonski, Shannon Joyce, Dembo Kanteh, Glen McGugan, Carmen Mejia, Papa Alioune Ndao, Seybatou Magatte Ndaw, Peter Noble, Malla Rao, Brenda Rodriguez, Mamkumbah Sanneh, Moussa Dien Sarr, IP Singh, Paula Strickland, Don Van Noy, Joan Vivestomas, Tonu Wali, James Clint Welty, Kijuana Yarls), advice and mentoring (Tumani Corrah, Abarahmane Dia, Amadou Diallo, Souleymanne Mboup, Saliou Ndiaye, Omar Ndir), information technology and computing support (Christopher Whalen, Brian Moyer), public health and malaria control partnerships (Moussa Thior, Mrs. Adam Jagne Sonko, Claude-Emile Rwagacondo), and for generous access to their personal expertise (Jennifer Anderson, Thomas P. Eisele, Joseph Keating, Ivo Foppa, Nicholas Manoukis, Meredith McMorrow, David Parker, Robert Perry, Margaret Pinder, Jon Eric Tongren, Sixte Zigirumugabe).
Abbreviations
- ACT
Artemisinin-Combination Treatment
- CDC
Centers for Disease Control (and Prevention)
- DDT
DichloroDiphenylTrichloroethane
- EIR
Entomologic Inoculation Rate
- EMEA
European Medicines Agency
- FDA
Food and Drug Administration
- ICEMR
International Center of Excellence for Malaria Research
- IPTp
Intermittent Preventive Treatment of malaria during Pregnancy
- ITN
Insecticide Treated bed Net
- IRS
Indoor Residual Spraying
- LLIN
Long-Lasting Insecticide-Treated bed Nets
- MOI
Multiplicity Of Infection
- MRC
Medical Research Council
- NGO
Non-Governmental Organization
- NIAID
National Institutes of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- PMI
President’s Malaria Initiative
- PSC
Pyrethrum Spray Catch
- TDR
World Health Organization Special Programme for Research and Training in Tropical Diseases
- USAID
United States Agency for International Development
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Águas R, White LJ, Snow RW, Gomes MGM. Prospects for malaria eradication in sub-Saharan Africa. PLoS One. 2008;3(3):e1767. doi: 10.1371/journal.pone.0001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg. 1954;48:312–318. doi: 10.1016/0035-9203(54)90101-7. [DOI] [PubMed] [Google Scholar]
- Beet EA. Sickle cell disease in the Balovale District of Northern Rhodesia. E Afr Med J. 1946;23:75–86. [PubMed] [Google Scholar]
- Brown AWA. The insecticide-resistance problem: a review of developments in 1956 and 1958. Bull WHO. 1958;18(3):309–321. [PMC free article] [PubMed] [Google Scholar]
- Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJC, Sesay SSS, Abubakar I, Dunyo S, Sey O, Palmer A, Fofana M, Corrah T, Bojang KA, Whittle HC, Greenwood BM, Conway DJ. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372(9649):1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma J, Abuya T, Memusi D, Juma E, Abhwale W, Ntwiga J, Nyandigisi A, Tetteh G, Shretta R, Amin A. Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J. 2009;8(10):243. doi: 10.1186/1475-2875-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JM, Moonen B, Snow RW, Smith DL. How absolute is zero: an evaluation of historical and current definitions of malaria elimination. Malar J. 2010;9:213. doi: 10.1186/1475-2875-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes A, Felger I, Beck HP. Molecular parasitology of malaria in Papua New Guinea. Trends Parasitol. 2003;19(6):246–249. doi: 10.1016/s1471-4922(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Malaria Elimination 1: Shrinking the malaria map: progress and prospects. Lancet. 2010;376(9752):1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem RGA, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371(9624):1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- Greenwood BM. Can malaria be eliminated? Trans R Soc Trop Med Hyg. 2009;103 (Suppl 1):S2–5. doi: 10.1016/j.trstmh.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8(6):369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S, Putaporntip C, Hughes AL. Bottleneck effects on vaccine-candidate antigen diversity of malaria parasites in Thailand. Vaccine. 2010;28(18):3112–3117. doi: 10.1016/j.vaccine.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Utzinger J, Caldas de Castro M, Smith TA, Tanner M, Singer BH. Urbanization in sub-Saharan Africa and implications for malaria control. Am J Trop Med Hyg. 2005;71(2 Suppl):118–127. [PubMed] [Google Scholar]
- McCoy D, Kembhavi G, Patel J, Luintel A. The Bill & Melinda Gates Foundation’s grant-making programme for global health. Lancet. 2009;373(9675):1645–1653. doi: 10.1016/S0140-6736(09)60571-7. [DOI] [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood BM, Wernsdorfer WH. From malaria control to eradication: the WHO perspective. Trop Med Int Health. 2009;14(7):802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J. 2008;7 (Suppl 1):S11. doi: 10.1186/1475-2875-7-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Malaria Elimination 3: Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376(9752):1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlen BL, Low-Beer D. Building to collective impact: the Global Fund support for measuring reduction in the burden of malaria. Am J Trop Med Hyg. 2007;77(Suppl 6):321–327. [PubMed] [Google Scholar]
- Ndiaye D, Patel V, Demas A, LeRoux M, Ndir O, Mboup S, Lakshmanan V, Daily JP, Wirth DF. A non-radioactive DAPI-based high-throughput in vitro assay to assess Plasmodium falciparum responsiveness to antimalarials – increased sensitivity of P. falciparum to chloroquine in Senegal. Am J Trop Med Hyg. 2010;82(2):228–230. doi: 10.4269/ajtmh.2010.09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAID Malaria Research Program. 2011 October 14th;2011 www.niaid.nih.gov/topics/malaria/Pages/default.aspx.
- Okech BA, Gouagna LC, Walczak E, Kabiru EW, Beier JC, Yan G, Githure JI. The development of Plasmodium falciparum in experimentally infected Anopheles gambiae (Diptera: Culicidae) under ambient microhabitat temperature in western Kenya. Acta Tropica. 2004;92(2):99–108. doi: 10.1016/j.actatropica.2004.06.003. [DOI] [PubMed] [Google Scholar]
- O’Meara WP, Mangeni JN, Steketee RW, Greenwood BM. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10(8):545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- Pampana EJ. A Textbook of Malaria Eradication. 2. Oxford; London: 1969. pp. 1–510. [Google Scholar]
- Roberts L, Enserink M. Did they really say … eradication? Science. 2007;318(5856):1544–1545. doi: 10.1126/science.318.5856.1544. [DOI] [PubMed] [Google Scholar]
- Sabot O, Cohen JM, Hsiang MS, Kahn JG, Basu S, Tang L, Zheng B, Gao Q, Zou L, Tatarsky A, Aboobakar S, Usas J, Barrett S, Cohen JL, Jamison DT, Feacham RGA. Malaria Elimination 4: Costs and financial feasibility of malaria elimination. Lancet. 2010;376(9752):1604–1615. doi: 10.1016/S0140-6736(10)61355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. The Fever: How Malaria has ruled Humankind for 500,000 Years. Sarah Crichton; New York: 2010. pp. 1–241. [Google Scholar]
- Sogoba N, Doumbia SO, Vounatsou P, Bagayoko MM, Dolo G, Traoré SF, Maïga HM, Touré YT, Smith T. Malaria transmission dynamics in Niono, Mali: the effect of the irrigation systems. Acta Tropica. 2007;101(3):232–240. doi: 10.1016/j.actatropica.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proc Natl Acad Sci USA. 2010a;107(27):1222–12227. doi: 10.1073/pnas.1002971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Malaria Elimination 2: Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010b;376(9752):1579–1591. doi: 10.1016/S0140-6736(10)61301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Fairhurst RM. Malaria-protective traits at odds in Africa? Nat Genet. 2005;37(11):1160–1162. doi: 10.1038/ng1105-1160. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) The Abuja declaration on Roll Back Malaria in Africa by the African Heads of States and Governments; 25th April; Abuja, Nigeria. Geneva: WHO; 2000. [Google Scholar]
- Wongsrichanali C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2(4):209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]