Abstract
Research has shown that sensory feedback modulates locomotor behavior in intact as well as spinal adult animals. Here we examined if locomotor activity (“stepping”) in newborn rats is influenced by cutaneous and proprioceptive feedback. One-day-old rats were treated with the serotonergic receptor agonist quipazine (3.0 mg/kg) to induce air-stepping behavior or with saline (vehicle control). During stepping, a substrate/floor (elastic, stiff, or none) was placed beneath their limbs so that the feet could make plantar surface contact with a substrate. Pups treated with quipazine showed significantly more alternated fore- and hindlimb steps and plantar paw contact with the substrate, compared to pups treated with saline. Pups also made proportionately less contact with the stiff substrate versus the elastic substrate during stepping. Different types of movements made on the substrate (paw pushes, taps, swipes, and stances) were also characterized. These results indicate that sensory feedback modulates locomotor mechanisms and behavior in perinatal rats.
Keywords: development, locomotion, motor coordination, perinatal, serotonin
1. Introduction
Both behavioral [4,9] and neurophysiological studies [25,29,36,40] have provided evidence that the neural mechanisms for locomotion begin developing before birth in the rat. However, weak and unstable postural control limits the expression of locomotor behavior during the early postnatal period [57], before the adult pattern of postural control is obtained by postnatal day 21 (P21) [22]. Early forms of spontaneous locomotion by the rat include pivoting and crawling behavior, which appear late during the first postnatal week and early during the second postnatal week, respectively [1]. During the first three to four postnatal weeks, locomotor behavior gradually improves until the adult form of locomotion is obtained [1,15].
Because rats do not typically exhibit episodes of spontaneous walking until at least two weeks after birth, locomotor behavior is often assessed in these young subjects in vivo by experimentally evoking locomotor-like patterns of limb coordination called “stepping” behavior. An alternated stepping pattern can be reliably evoked in newborn rats using pharmacological agents (i.e, serotonergic or catecholaminergic receptor agonists) [32,10] or strong sensory stimulation [1,19,26,38]. In vitro experiments on the isolated spinal cord of the perinatal rat have suggested that the mechanisms for generating the rhythmic alternating locomotor rhythm in the hindlimbs are located in the lower thoracic (T12/13) and upper lumbar (L1/L2) spinal cord [12,13,16,28] whereas the mechanism for coordinating interlimb patterned activity may be in the lower segments of the lumbar cord (L3–L5) [5]. Presumably, pharmacological stimulation of neurotransmitter systems and forms of sensory-induced stepping in the immature rat either directly or indirectly activate spinal locomotor mechanisms. In the case of stepping behavior induced by 5-HT agonists, such as quipazine, it appears that 5-HT receptors may directly engage spinal locomotor networks: a midthoracic spinal cord transection, which effectively eliminates communication between the brain and caudal levels of the spinal cord, does not eliminate quipazine-induced hindlimb stepping in the gestational day 20 rat fetus [9] or P5 rat pup [32]. Overall, findings from quipazine and L-DOPA induced stepping in the perinatal rat provide evidence that interlimb coordination during locomotor-like behavior is relatively immature and unstable early on, but does exhibit developmental improvement [10]. Studies conducted with precocial newborn chicks within the first few days after hatching suggest that developmental improvements in locomotor coordination are dependent upon experience and biomechanical constraints [33,34,46].
Afferent modulation of locomotion is well documented in adult animals [e.g., 7,11,39,42,44]. However, few studies have examined the extent to which sensory feedback modulates the expression of locomotor behavior during early development in young mammals. Experiments with human infants suggest that infants respond to sensory feedback during stepping on a treadmill [30,35,41,52]. And a study with spinalized rabbits showed that immature rabbits during the second and third postnatal week developed gait patterns that were consistent with the form of daily hindlimb motor training (alternated or synchronized) they received [56]. But in general, the issue of if and how sensory feedback affects locomotor coordination during the formative perinatal period has received little attention. This is an important issue to consider, for example, in rehabilitation efforts to improve motor function in infants with congenital motor and neurological disorders (e.g., Spina Bifida or Down Syndrome) [53,58]. Research in newly hatched chickens suggests a role for sensory experience in the development of locomotion [e.g., 33,34,46], but research with mammals on this topic is scant and remains unclear.
In the present study, we investigated the role of sensory feedback in the regulation of locomotor behavior during early motor development in a newborn altricial mammal. One-day old rat pups were suspended in the air by a sling and treated with the 5-HT agonist quipazine to induce air-stepping behavior [10,32]. A substrate then was placed beneath their limbs so that the feet could make plantar surface contact with a substrate, providing both cutaneous and altered proprioceptive feedback to the limbs. This kind of sensory stimulation is typical during normal bouts of weight bearing walking in older animals, but not during experimentally-induced air-stepping behavior in immature subjects [31,55]. Thus, rats were held in the sling in order to examine sensory effects on stepping under non-weight bearing conditions. Pups were tested on an elastic or stiff substrate (or no substrate for comparison), to examine which substrate type was most effective in influencing their stepping behavior and if pups responded to characteristics of the substrate.
2. Materials and Methods
2.1 Subjects
Subjects were the offspring of adult Sprague-Dawley rats bred in the Animal Care Facility at Idaho State University. They were allowed to stay in their home cage with the dam until the time of testing, which occurred approximately 24 hours after birth (postnatal day 1; P1). All subjects were healthy and had fed recently, as evidenced by a milk band across the abdomen. Care and use of animal subjects were in accordance with guidelines established by the Institutes of Laboratory Animal Resources and the National Institutes of Health [37], and the Idaho State University Animal Welfare Committee.
A total of 48 P1 rat pups served as subjects in this study. Half of the subjects were male. No more than one subject per litter was assigned to any particular treatment group. A sample of 8 subjects was assigned to each drug and substrate condition (2 drugs × 3 substrates × 8 subjects = 48 subjects total).
2.2 Behavioral observation
On P1, subjects were tested individually inside an infant incubator that controls humidity and temperature (35° C). Thirty minutes before the start of the experiment, the subject was placed in a plastic dish with 3 siblings inside the incubator to permit acclimation to incubator conditions. After the 30 min acclimation period, the subject was secured in a prone posture on a soft, vinyl-coated horizontal bar. Subjects were gently secured to the bar with two thin straps, thus creating a sling: one strap extended across the abdomen and the second strap extended across the neck. The straps did not interfere with limb movements made by the pup, but did prevent the body of the pup from moving side to side and out of camera view. A micro-camera was located inside the incubator, positioned laterally to the subject (with all 4 limbs in view), and was connected to an outside DVD recording unit. The entire 50-min observation session was recorded onto DVD.
2.3 Experimental design
For all subjects, the observation session began with a 5 min Baseline period. Immediately following this, a drug injection was administered and a substrate (or not, for the control condition) was placed below the subject at a distance of 80% of limb length. This started the 45-min Test portion of the experimental session. Time-code was impressed on the video recordings throughout the 50 min experimental session.
2.4 Pharmacology
Subjects received one 50 μl intraperitoneal injection with a 30 gauge needle: 3.0 mg/kg quipazine maleate (2-(1-piperazinyl)quinoline maleate salt (a serotonergic agonist obtained from Sigma-Aldrich (St. Louis, MO)) or 0.9% (wt/vol) saline (vehicle control). The dose of quipazine used in this study previously has been shown to effectively induce stepping behavior in the perinatal rat [9,10].
2.5 Substrate apparatus
Subjects were tested in one of three different substrate conditions: stiff, elastic, or none. Subjects in the stiff substrate condition had a hard Plexiglas plate placed below their limbs. Subjects in the elastic substrate condition had a rubber dental dam that was stretched over an embroidery hoop (thus a “trampoline”) placed below their limbs. The dental dam was stretched over the hoop in a way that maintained the elasticity of the substrate (which is similar to a small water balloon): it was sufficiently taut to avoid sagging but also allowed the pups to push down and deform the substrate. We determined that a force of 4.76 grams was necessary to deform/stretch the elastic substrate a distance of 5 mm (measurements were averaged over 10 trials), using a 5 mm surface area of depression. Pups were observed to deform the substrate, as illustrated in Figure 1. The distance of the substrate (Plexiglas plate or dental dam) from the ventrum of the pup’s body was approximately 80% of the length of the limbs at full extension. Thus, if the limbs were extended in a ventral position from the body, the paws would very likely touch the substrate. However, at the distance the substrate was placed, the paws did not necessarily touch the substrate (i.e., the limbs could remain more flexed and close to the body, or the limbs could extend more rostrally, caudally, or laterally, rather than ventrally). Subjects in the none/no substrate condition had nothing placed below their limbs. During limb movement, subjects in this condition did not come into contact with any substrate.
Figure 1.
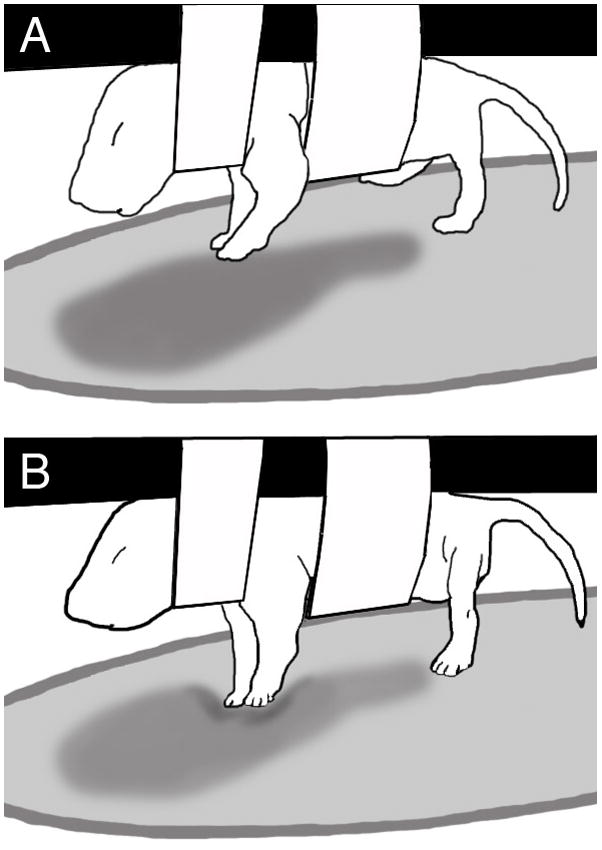
Illustration of a P1 rat pup interacting with the elastic substrate. (Top panel) The pup is suspended in the air by a sling, with the elastic substrate placed beneath the limbs. Notice that the forelimbs are slightly flexed and not touching the substrate. (Bottom) In the next video frame, the forelimbs are extending and pressing down on the elastic substrate using the plantar side of the foot, thus exhibiting what was called a forepaw push.
2.6 Behavioral scoring
Behavioral scoring was done during normal or reduced speed video playback of the Baseline and Test periods. Both limb activity and types of paw-substrate interactions were scored (see below). Four separate viewing passes of the video records were made: one for scoring forelimb activity and one for scoring hindlimb activity, and one for scoring paw-substrate interactions in the hindpaws, as well as the forepaws. Events were entered into an event recorder program (JWatcher™) that records the category of behavior and time of entry (±0.01 s). During behavioral scoring, an effort was made to keep the experimenter blind to the subject’s group association. This was possible for the type of drug treatment (quipazine or saline), but not substrate condition. Inter- and intrarater reliability for scoring was > 90%.
2.6.1 Stepping behavior
All occurrences of stepping behavior, as well independent limb movements, were scored in separate viewing passes for the forelimbs and the hindlimbs. In this study, alternated stepping is considered to be a bilateral pattern, involving alternated step cycles of both right and left homologous limb pairs. Thus alternated stepping was scored when two consecutive and alternating extensions and flexions of homologous limb pairs were exhibited by the subject [9]. Synchronous stepping was scored when consecutive and synchronous flexion and extension of homologous limb pairs was exhibited by the subject. All other limb movements that were not engaged in bilateral stepping behavior were scored as independent limb movements (i.e., spontaneous limb twitches, single limb steps, etc.). Additionally, whether or not the subject made contact with the substrate (touch vs. no touch) also was scored for each movement category.
2.6.2 Paw-substrate interactions
To characterize the quality of movement when touching the substrate, paw-substrate interactions were scored. Paw-substrate interactions were scored for all movements made in contact with the substrate, and for both the forelimbs and hindlimbs. Four categories of interaction were scored: push—a dorsal-ventral movement of the paw on the substrate, in which the limb was extended as far as possible; tap—a dorsal-ventral movement of the paw on the substrate, in which the limb was not completely extended and the paw made only brief contact; swipe—a rostral-caudal movement of the paw sliding across the substrate; and stance—a paw held in contact with the substrate with no movement. Additionally, two types of paw positions were assessed in the push, tap and stance categories. This included a dorsal paw touch and a plantar (ventral) paw touch, where the dorsal surface or the pad of the foot was in contact with the substrate, respectively.
2.7 Statistical approach
Stepping behavior and paw-substrate interactions were compared among groups using analysis of variance (ANOVA) tests, with post hoc comparisons of means following main or interaction effects using Fisher’s Protected Least Significant Difference. Analyses were conducted separately for the forelimbs and hindlimbs, in the case that the limbs adapted differently to the presence of a substrate. Instances of stepping were first summarized in 5 min bins across the 50 min observation session. Frequency counts and percentages of stepping behavior were analyzed by repeated measures ANOVA, with time as the repeated measure. Independent variables were drug and substrate condition. Types of paw-substrate interactions were summarized across the entire period in which the subject was exposed to a substrate (the 45 min Test period), and comparisons of group means were examined with a two-way ANOVA. Statistical tests were performed using StatPlus® (AnalystSoft), and a 5% significance level was adopted for all tests.
3. Results
3.1 Effects on forelimb stepping
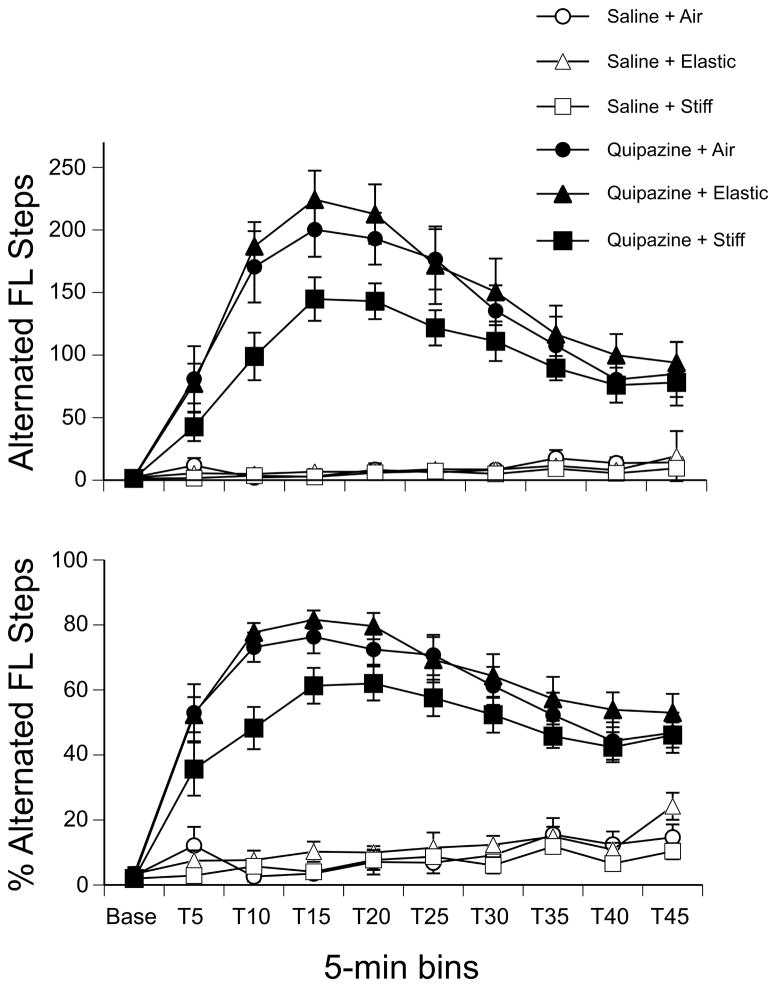
A three-way repeated measures ANOVA (2 drug × 3 substrate × 10 time bins) revealed a significant effect of drug (F(1,42) = 178.22, p < 0.001), a significant effect of time (F(9,378) = 50.27, p < 0.001), and a significant interaction of these two factors (F(9,378) = 50.58, p < 0.001) on the number of alternated forelimb steps. As shown in Figure 2, pups treated with quipazine showed more alternated forelimb steps compared to those treated with saline at all time points following drug injection (all ps < 0.001, except at baseline). Pups treated with saline, as well as all pups during baseline, generally showed more spontaneous limb twitches (data not shown), which have been shown to be indicative of active sleep in newborn rats [6,27].
Figure 2.
Alternated forelimb stepping by drug treatment and substrate condition. (Top panel) The mean number and (Bottom) the mean percentage of alternated forelimb steps during the 5-min Baseline and 45-min Test period. Immediately after Baseline, subjects were treated with quipazine or saline, and an elastic, stiff, or no substrate was placed below their feet for the remainder of the Test session. Points show means; vertical lines depict SEM.
The analysis also showed that there was not a main effect of substrate on alternated forelimb steps (p = 0.06). However, because we were specifically interested in the effects of substrate on quipazine-induced stepping, we examined the effect of substrate in quipazine-treated pups during the peak of stepping behavior. The peak of stepping was identified as the two continuous 5-min bins with the highest frequency of steps for both the forelimbs and hindlimbs: T15 and T20 (stepping peak = data collapsed across these 2 time bins). A one-way ANOVA revealed a significant main effect of substrate during this 10-min period (F(2,21) = 3.90, p = 0.04). Post hoc analysis of group means indicated that quipazine-treated pups stepped with their forelimbs significantly more when they were on the elastic substrate as compared to the stiff substrate.
Because individual pups may show different levels of overall activity, we examined the percentage of alternated steps as a function of total movements. Total movements include all steps (alternated or synchronous, multiplied by 2 since both limbs move during these patterns) and independent limb movements. Thus, this analysis normalizes the amount of activity across subjects. A comparison of the percentage of alternated forelimb steps in a three-way repeated measures ANOVA (2 drug × 3 substrate × 10 time bins) revealed significant effects of drug (F(1,42) = 411.08, p < 0.001), substrate (F(2,42) = 6.15, p < 0.01), time (F(9,378) = 52.75, p < 0.001), and an interaction of drug by time (F(9,378) = 44.96, p < 0.001). The pattern of effects is very similar to that of absolute number of steps. In addition, follow-up tests indicated that the percentage of forelimb steps was significantly lower for pups tested on the stiff substrate (Fig. 2).
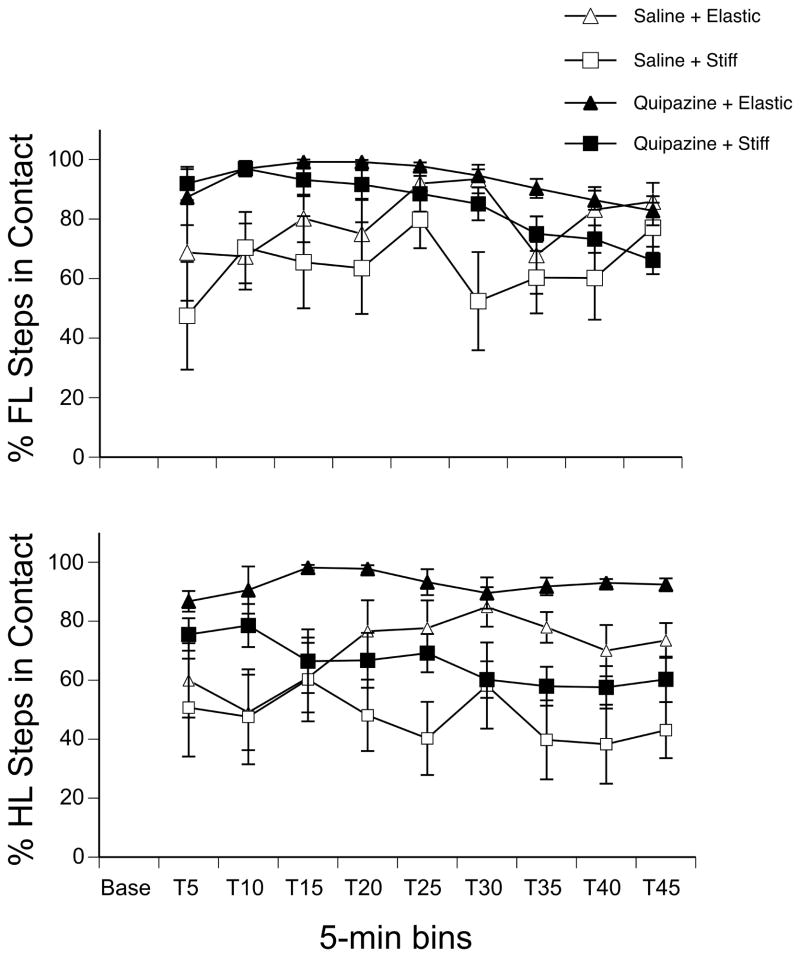
Since the substrate was positioned so that pups were not forced to make contact with it, we examined the percentage of alternated forelimb steps that did make or did not make contact with the substrate. Because pups tested in the air condition had no opportunity to make contact with a substrate, this analysis was limited to subjects in the elastic and stiff substrate conditions. A three-way repeated measures ANOVA indicated a main of drug (F(1,28) = 17.08, p < 0.001), a main effect of substrate (F(1,28) = 8.05, p < 0.01), and an interaction between drug and time (F(8,224) = 2.03, p = 0.04). As shown in Figure 3, pups treated with quipazine stepped in contact with the substrate more often than pups in the saline condition in the beginning of the test session (follow-up tests were significant at p ≤ 0.01 for all time points before T25), and pups tested on the stiff substrate made contact less consistently with the substrate than pups tested on the elastic substrate. We observed that pups in the stiff substrate condition tended to show greater limb flexion during the swing phase of the locomotor cycle and greater limb extension in the caudal direction during the stance phase, thus appearing to avoid touching the substrate.
Figure 3.
Effect of drug treatment and substrate on the percentage of alternated steps made in contact with the elastic or stiff substrate. (Top panel) The mean percentage of forelimb and (Bottom) hindlimb steps made in contact with the substrate during the 5-min Baseline and 45-min Test period. Points show means; vertical lines depict SEM.
Comparison of synchronous forelimb steps in a three-way repeated measures ANOVA revealed significant effects effect of drug (F(1,42) = 73.39, p < 0.001), time (F(9,378) = 7.85, p < 0.001), and an interaction of these two factors (F(9,378) = 7.40, p < 0.001). Pups treated with quipazine showed significantly more synchronous steps than pups treated with saline following drug injection. However, the average number of synchronous forelimb steps remained relatively low for each group: saline-treated subjects showed an average of 1.10 ± 0.27 synchronous forelimb steps per 5 min time bin during the test session, whereas quipazine-treated subjects showed an average of 8.65 ± 1.00 (values indicate Mean ± SEM). This is consistent with previous data [9,32], suggesting that quipazine mainly induces alternated, and not synchronous, stepping activity.
3.2 Effects on hindlimb stepping
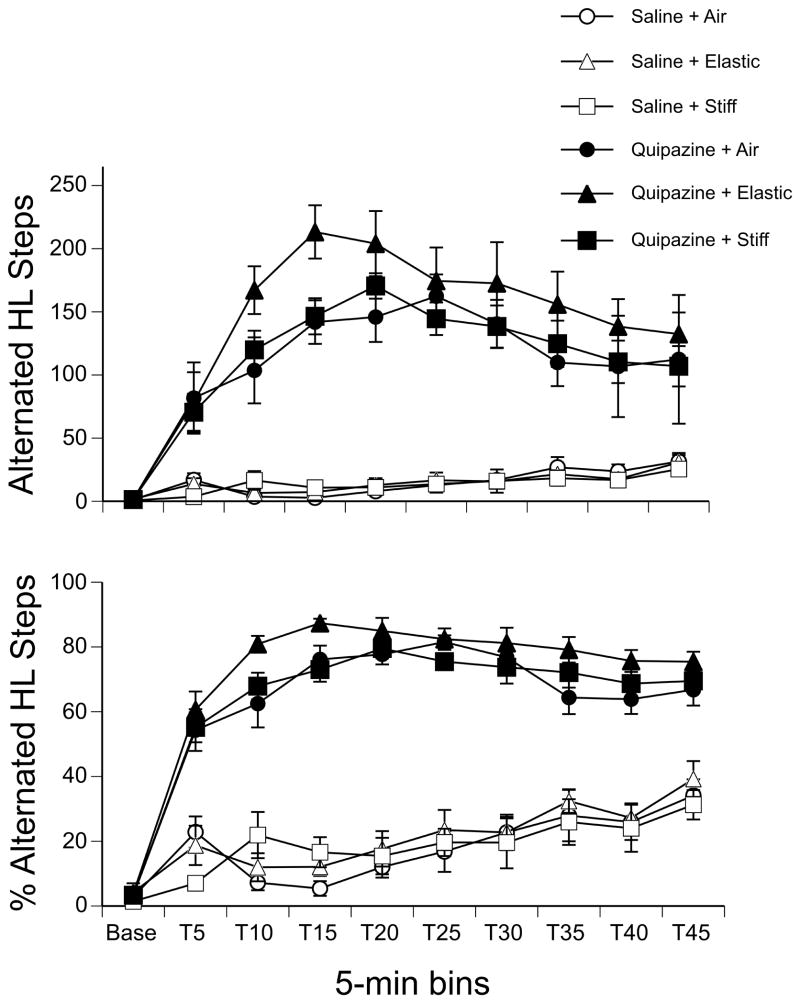
Analysis of alternated hindlimb steps in a three-way repeated measures ANOVA (2 drug × 3 substrate × 10 time bins) revealed significant effects of drug (F(1,42) = 149.11, p < 0.001), time (F(9,378) = 52.20, p < 0.001), and an interaction of these two factors (F(9,378) = 43.03, p < 0.001). As shown in Figure 4, pups treated with quipazine showed more alternated hindlimb steps compared to those treated with saline at all time points following drug injection (all ps < 0.001, except at baseline). Although there was no effect of substrate in the overall analysis, a one-way ANOVA examining the effect of substrate in quipazine-treated subjects at the peak of stepping (T15 and T20) revealed a significant main effect of substrate during this 10-min period (F(2,21) = 3.84, p = 0.04). Post hoc analysis of group means indicated that quipazine-treated pups stepped with their hindlimbs significantly more when they were on the elastic substrate as compared to the stiff substrate or when tested in the air with no substrate, during the peak of stepping.
Figure 4.
Alternated hindlimb stepping by drug treatment and substrate condition. (Top panel) The mean number and (Bottom) the mean percentage of alternated hindlimb steps during the 5-min Baseline and 45-min Test period. Points show means; vertical lines depict SEM.
Mirroring the pattern of effects for the frequency of alternated hindlimb steps, a three-way repeated measures ANOVA found significant effects of drug (F(1,42) = 399.90, p < 0.001), time (F(9,378) = 88.89, p < 0.001), and an interaction of these two factors (F(9,378) = 39.95, p < 0.001) on the percentage of alternated hindlimb steps (Fig. 4).
For the percentage of alternated steps that did or did not touch the substrate for pups in the elastic and stiff substrate conditions, we found an effect of drug (F(1,28) = 10.90, p < 0.01), an effect of substrate (F(1,28) = 14.93, p < 0.001), and an interaction of substrate by time (F(8,224)=2.31, p = 0.02). Post hoc tests showed that pups treated with quipazine made significantly more contact with the substrate than pups treated with saline, and that pups in the elastic condition made contact significantly more than pups in the stiff condition. To explore the substrate by time interaction, we examined the simple main effect of substrate at each time bin during the test session. As shown in Figure 3, a clear difference between the substrates emerged at T20 and remained during the rest of the test session (all ps < 0.001): pups tested on the elastic substrate touched the substrate significantly more during alternated hindlimb stepping than pups tested on the stiff substrate, for subjects in either drug group. As with the forelimbs, we observed that the hindlimbs also showed greater flexion during swing and greater limb extension in the caudal direction for pups tested on the stiff substrate.
Comparison of synchronous hindlimb steps in a three-way repeated measures ANOVA revealed a significant effect of time (F(9,378) = 4.65, p < 0.001) and an interaction of drug and time (F(9,378) = 7.37, p < 0.001). As with the forelimbs, the average number of synchronous hindlimb steps remained relatively low for each group: saline-treated subjects showed an average of 2.33 ± 0.42 synchronous hindlimb steps per 5 min time bin during the test session, whereas quipazine-treated subjects showed an average of 2.31 ± 0.51 (values indicate Mean ± SEM). Only at T5, 5 minutes after the drug injection, was there a simple main effect of drug: quipazine-treated pups showed more synchronous hindlimb steps (7.08 ± 1.26) than saline-treated pups (1.79 ± 0.34).
3.3 Forepaw-substrate interactions
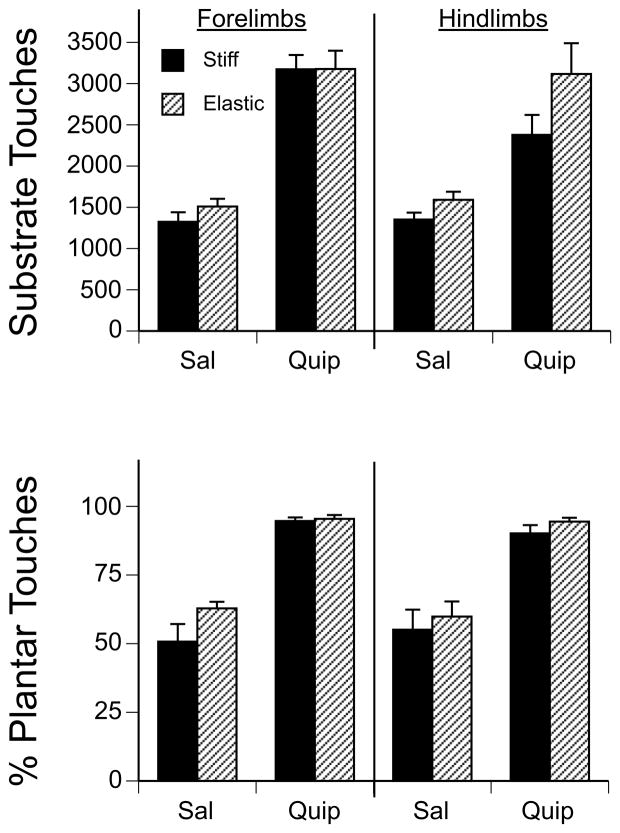
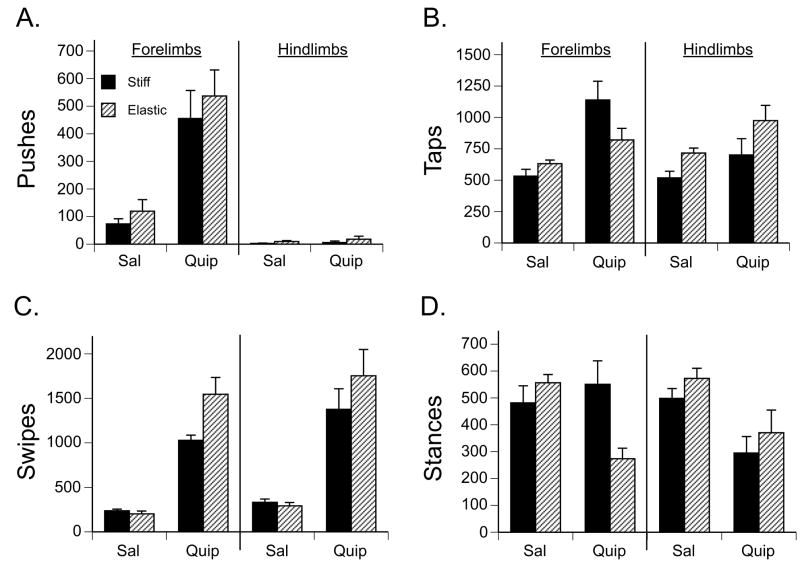
In terms of the overall number of forepaw touches made on the substrates, there was a significant main effect of drug (F(1,28) = 140.47, p < 0.001), but no effect of substrate or interaction between the two factors. Pups treated with quipazine showed more than twice as many forepaw touches on the substrate compared to saline-treated pups (Fig. 5).
Figure 5.
Paw-substrate interactions by drug treatment and substrate condition. The mean number of (A) paw pushes, (B) taps, (C) swipes, and (D) stances in the forelimbs and hindlimbs during the 45-min Test period for subjects in the elastic or stiff substrate conditions. Bars show means; vertical lines depict SEM.
We noticed that during contact with the substrate, pups sometimes were using the dorsal side of the paws during pushes, taps and stances, but at other times were using the plantar side of the paws. Therefore, we also examined the frequency of dorsal and plantar paw touches during the different types of movements. (We did not examine this for swipes, as swipes by definition involved a rostral-caudal movement of the paw across the substrate. The paws tended to make contact with the substrate on the plantar and lateral sides during a swipe.) To examine the relative frequency of plantar and dorsal forepaw touches on the substrate, we calculated the percentage of plantar forepaw touches as a function of total plantar and dorsal paw touches. A two-way ANOVA showed a significant main effect of drug on the percentage of plantar paw touches (F(1,28) = 82.94, p < 0.001), but no significant main effect of substrate or an interaction between the two factors. Subjects treated with quipazine exhibited a significantly higher percentage of plantar paw touches than did saline-treated subjects. As shown in Figure 5, nearly all of the paw-substrate interactions were made with the plantar surface of the paw in subjects treated with quipazine, whereas only about half of the paw-substrate interactions were made with the plantar side of the paw in subjects treated with saline.
Because subjects spent a considerable amount of time during the test session touching the substrate, we wanted to know how the subjects were using the substrate, or the types of movements they were making on the substrate. Therefore, we examined the quality of paw-substrate interactions for both the forepaws and hindpaws in both the stiff and elastic substrate conditions.
A two-way ANOVA (2 drug × 2 substrate) showed that there was a significant main effect of drug on the number of forepaw pushes (F(1,28) = 33.64, p < 0.001), but no significant main effect of substrate or an interaction between the two factors. As shown in Figure 6A, pups treated with quipazine exhibited significantly more forepaw pushes (nearly 5 times more) than did saline-treated pups. When pups showed paw pushes on the elastic substrate, they typically deformed the substrate, resulting in greater limb extension than pups in the stiff substrate condition.
Figure 6.
Paw touches by drug treatment and substrate condition. (Top panel) The mean number of substrate touches and (Bottom) the percentage of plantar paw touches in the forelimbs and hindlimbs during 45-min Test period for subjects in the elastic or stiff substrate conditions. Bars show means; vertical lines depict SEM.
Figure 6B depicts the number of forepaw taps. A two-way ANOVA showed that there was a significant main effect of drug (F(1,28) = 16.37, p < 0.001) and a significant interaction of drug and substrate (F(1,28) = 4.51, p < 0.05), on the number of forepaw taps. A post hoc test showed that quipazine-treated subjects expressed significantly more forepaw taps compared to saline-treated subjects. Although the tests for simple main effects were not significant (ps = 0.10), the data showed that the effect of substrate was not the same in the different drug conditions: quipazine-treated pups showed more taps on the stiff substrate, whereas saline-treated pups tended to show more taps on the elastic substrate.
For forepaw swipes, a two-way ANOVA showed that there were significant main effects of drug (F(1,28) = 108.80, p < 0.001) and substrate (F(1,28) = 5.56, p < 0.05), and a significant interaction between the two factors (F(1,28) = 7.27, p < 0.05; Fig. 6C). In general, quipazine-treated pups showed significantly more forepaw swipes than saline-treated pups, and subjects swiped with their forepaws significantly more frequently on the elastic versus the stiff substrate. To follow up the interaction, the effects of substrate in the different drug conditions were examined. There was a simple main effect of substrate in the quipazine condition (p < 0.05): significantly more forepaw swipes happened on the stiff substrate compared to the elastic substrate.
A two-way ANOVA showed that there was a significant interaction between drug and substrate on the number of forepaw stances (F(1,28) = 9.00, p < 0.01). As can be seen in Figure 6D, subjects showed significantly more forepaw stances when tested on the elastic versus the stiff substrate, following treatment with quipazine (p = 0.01).
3.4 Hindpaw-substrate interactions
As with the forepaws, we found that quipazine-treated subjects showed significantly more hindpaw touches and plantar paw touches on the substrate than saline-treated subjects (both ps < 0.001). As shown in Figure 5, quipazine-treated subjects showed nearly double the amount of each compared to saline-treated subjects.
We also analyzed the effects of drug and substrate on types of hindpaw-substrate interactions. A two-way ANOVA (2 drug × 2 substrate) showed no main or interaction effects on the number of hindpaw pushes. As can be seen in Figure 6A, subjects in each group made extremely few hindpaw pushes.
A two-way ANOVA showed that there was a significant main effect of drug (F(1,28) = 6.23, p < 0.05) and a significant main effect of substrate (F(1,28) = 7.14, p < 0.05) on the number of hindpaw taps. Post hoc tests showed that quipazine-treated pups expressed significantly more hindpaw taps compared to saline-treated pups, and that pups made significantly more hindpaw taps on the stiff versus the elastic substrate (Fig. 6B).
For hindpaw swipes, a two-way ANOVA showed that there was a significant main effect of drug (F(1,28) = 53.87, p < 0.001). Figure 6C shows that pups treated with quipazine expressed significantly more hindpaw swipes than did saline-treated pups. Pups showed approximately 5 times more hindpaw swipes following treatment with quipazine compared to saline.
Likewise, there was a significant main effect of drug on hindpaw stances (F(1,28) = 15.67, p < 0.01), on the percentage of plantar paw touches (F(1,28) = 65.94, p < 0.001), and on the number of hindpaw touches made on the substrate (F(1,28) = 36.99, p < 0.001). As shown in Fig. 6D, subjects expressed significantly more hindpaw stances following treatment with saline.
4. Discussion
Results of the present study show that locomotor activity in one-day old rats is modulated by the presence and type of substrate pups step on during quipazine-induced stepping. Stepping behavior closely resembles locomotor behavior expressed by older animals, and therefore experimental induction of this behavior in the rat provides a window into the developing locomotor system in an in vivo mammalian model [14]. By examining the alternated stepping behavior of newborn rats in response to a substrate in a non-weight bearing (air-stepping) condition, we were able to characterize how cutaneous and altered proprioceptive stimulation influences the early expression of locomotor mechanisms without the confounds of poor postural control and weak muscles at this age. To our knowledge, this is the first study to examine the effects of sensory feedback on stepping behavior in the in vivo perinatal rat.
Our findings show that stepping was influenced by a substrate in different ways and at different times during the test session. During the peak of stepping, which occurred during the first half of the test session, quipazine-treated pups responded to the stiff substrate with a decrease in the number of forelimb steps, but responded to the elastic substrate with an increase in the number of hindlimb steps. Although there were no differences in the amount of forelimb or hindlimb steps among the groups near the end of the 45-min test session, by the middle to the end of the test session pups were in fact stepping differently on the two substrates: pups made proportionately fewer alternated forelimb and hindlimb steps in contact with the stiff substrate compared to the elastic substrate. This suggests that although pups in the stiff substrate condition were stepping the same amount as pups in the air and elastic substrate conditions, they were somehow avoiding the stiff substrate. Observations suggest that pups avoided the stiff substrate by showing greater limb flexion during the swing phase of the locomotor cycle, and greater limb extension in the caudal direction during the stance phase.
The reason why pups appeared to avoid the stiff substrate at times, but stepped more on the elastic substrate, remains to be determined. Yet it is interesting to speculate that perhaps their previous experience may have influenced their performance in the present testing situation. For instance, fetal fats move in a uterine environment, in which the myometrial tissue has been shown to have elastic properties [10] and thus can provide contingent movement-related feedback. Additionally, P1 rats have been observed to show stepping movements along the dam’s ventrum as they crawl to gain access to a nipple [18]. Presumably the skin on the dam’s ventrum also is somewhat elastic and not a rigid, hard substance. Thus in both the fetal and newborn environments, rat pups may experience overcoming resistance provided by moving in environments with elastic kinds of characteristics. In contrast, when pups try to move against a hard floor (i.e., the floor of the cage), movement may be inhibited as their weak muscles cannot support their bodies very well. The finding that pups responded differentially to the two substrates suggests that they are sensitive to differences in the quality of the substrates and sensory feedback. In general, these results corroborate reports of locomotor adaptations to biomechanical, proprioceptive, and cutaneous sensory stimulation in human infants [e.g., 17,59] and rabbits [56] and newly hatched chicks [33,34]. Together, they suggest an important role for sensory feedback in the early development of the locomotor system.
As suggested above, pups interacted with the substrates in different ways. For example, pups showed more paw pushes with the forelimbs compared to the hindlimbs, and more swipes on the elastic versus the stiff substrate. Additionally, after treatment with quipazine pups showed a much higher (nearly double) proportion of plantar paw touches on both the elastic and stiff substrates. This finding is very interesting as it suggests that quipazine facilitates plantar contact with a substrate, which is typical of normal walking locomotion. Quipazine also has been shown to enhance robotic training and facilitate weight bearing hindlimb steps in complete spinal transected (at T7–T9) adult mice [21], and promote plantar paw placement during stepping on a treadmill in adult spinal rats given concurrent epidural stimulation at the lumbosacral level [23,24]. Thus in the present study the increase in plantar paw contact, as well as other types of movement patterns (i.e., paw pushes, taps, etc.), may have been produced by quipazine alone or by a synergistic effect of quipazine and sensory feedback from stepping on the substrate. As the experiments with adult rodents suggest, these effects are likely mediated at a spinal level.
In fact, quipazine was chosen to induce stepping behavior in the present study precisely because of its role in activating spinal locomotor networks [9,21,32,54]. Quipazine-induced stepping in low thoracic transected mice can be blocked by pretreatment with a 5-HT2A antagonist [54], suggesting that the effects of quipazine in intact animals may at least be partly mediated by 5-HT2A receptors in the spinal cord as well. Quipazine is often used to increase locomotor performance in spinally injured cats [3] and rodents [e.g., 2,20,21]. In general, studies that have coupled quipazine treatment with some kind of physical therapy (e.g., treadmill training, robotic training, etc.) in spinal animals suggest that both pharmacological and sensory stimulation help facilitate locomotor and reflex function. Recently it was reported [24] that spinal rats treated with quipazine showed lower epidural stimulation thresholds to elicit muscle twitch, indicating that quipazine increased the sensitivity of the spinal cord and perhaps increased motoneuron excitability. Whether or not afferent input to the spinal cord was enhanced or motoneuron properties were modulated by quipazine in the current study is not clear. However, Pflieger et al. [43] showed that P3–P5 rat pups that were depleted of 5-HT since birth showed decreased motoneuron excitability, suggesting a role for 5-HT in the early development of motoneuron properties. Taken together, it is plausible that many of the effects reported in the current study are spinally mediated. Experiments are underway to determine if developing rats with complete low thoracic spinal cord transections respond to quipazine-induced stepping on a substrate in a similar fashion.
Given that studies conducted with newborn human infants have found a relationship between amount of stepping and arousal level, it is interesting to consider the role of arousal in the current study. When infants are held up-right with their feet placed on a surface, the number of steps expressed is directly related to level of behavioral arousal; thus, when infants show higher levels of behavioral arousal they generally show more steps [50,51]. However, the number of steps infants show on a treadmill has been shown to be independent of arousal [52]. Thelen and colleagues [51] have suggested that, at least in the up-right testing condition, behavioral arousal may increase motoneuron firing rates and/or recruitment, thus helping the infants to lift and move their legs. In the current study, it is likely that quipazine is having an effect on behavioral activation. For instance, we found that for quipazine-treated pups the vast majority of movements expressed were alternated steps, which is indicative of an awake state in newborn rats [6]. Likewise, previous research with immature rats [9,48] has demonstrated dramatic increases in behavioral activation, prominently including locomotor-type behavior (i.e., stepping, forelimb paddling, hindlimb treadling), following treatment with quipazine. However, hypersensitivity to sensory stimulation (usually taken as an indicator of increased arousal) following quipazine administration has not yet been demonstrated in neonatal rats, although treatment with quipazine has been shown to induce tail flick analgesia [47]. Because we found that quipazine induced such high levels of alternated stepping but only slightly increased synchronous stepping, our findings suggest that quipazine specifically promotes alternated limb coordination against a backdrop of behavioral activation, but not arousal per se.
In conclusion, the findings reported here show that stepping behavior in one-day old rats is modulated by sensory feedback. They also show that the air-stepping paradigm can be used to experimentally investigate the role of sensory input on the development and expression of locomotor mechanisms in a developing mammal. Further research needs to be done to determine the role of sensory feedback on the development of the locomotor system. Such research may provide important implications for activity-based rehabilitation treatments [53] for infants that show developmental delays in locomotion.
Highlights.
We examined how different substrates affect stepping in newborn rats.
Stepping in one-day-old rat pups was induced with the 5-HT agonist quipazine.
Quipazine induced plantar paw contact with a substrate.
Pups made more contact with elastic than stiff substrate during stepping.
Sensory feedback modulates locomotor mechanisms during early development.
Acknowledgments
This study was supported by NIH grant #1R15HD062980-01. Partial support was provided by NIH Grant #P20 RR0116454 from the INBRE Program of the National Center for Research Resources, grant #1010 to MRB from the Faculty Research Committee and grant #S09-13 to MMS from the Undergraduate Research Committee, Idaho State University, Pocatello, Idaho. We thank Sierra Harris, Hillary Swann, and Dr. Scott R. Robinson for providing helpful comments on earlier drafts of this manuscript, and Dr. Scott R. Robinson for making the illustration of the pup. Preliminary data have been presented in abstract form [8,45,49].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 2.Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–7. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 3.Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- 4.Bekoff A, Lau B. Interlimb coordination in 20-day-old rat fetuses. J Exp Zool. 1980;214:173–5. doi: 10.1002/jez.1402140207. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand S, Cazalets JR. The respective contribution of lumbar segments to the generation of locomotion in the isolated spinal cord of newborn rat. Eur J Neurosci. 2002;16:1741–50. doi: 10.1046/j.1460-9568.2002.02233.x. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Dev Psychobiol. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol. 2003;90:3625–39. doi: 10.1152/jn.00496.2003. [DOI] [PubMed] [Google Scholar]
- 8.Brumley MR, Roberto ME, Strain MM. Program No. 564.5. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Sensory feedback modulates quipazine-induced stepping in the neonatal rat. Online. [Google Scholar]
- 9.Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A, and α-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behav Neurosci. 2005;119:821–33. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- 10.Brumley MR, Robinson SR. Experience in the perinatal development of action systems. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 181–209. [Google Scholar]
- 11.Büschges A, Manira AE. Sensory pathways and their modulation in the control of locomotion. Curr Opin Neurobiol. 1998;8:733–9. doi: 10.1016/s0959-4388(98)80115-3. [DOI] [PubMed] [Google Scholar]
- 12.Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–51. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazalets JR, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci. 1996;16:298–306. doi: 10.1523/JNEUROSCI.16-01-00298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. [DOI] [PubMed] [Google Scholar]
- 15.Clarke KA, Williams E. Development of locomotion in the rat—spatiotemporal footfall patterns. Physiol Behav. 1994;55:151–5. doi: 10.1016/0031-9384(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 16.Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–59. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- 17.Davis DW, Thelen E, Keck J. Treadmill stepping in infants born prematurely. Early Hum Dev. 1994;39:211–23. doi: 10.1016/0378-3782(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 18.Eilam D, Smotherman WP. How the neonatal rat gets to the nipple: common motor modules and their involvement in the expression of early motor behavior. Dev Psychobiol. 1998;32:57–66. doi: 10.1002/(sici)1098-2302(199801)32:1<57::aid-dev7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Fady JC, Jamon M, Clarac F. Early olfactory-induced rhythmic limb activity in the newborn rat. Brain Res Dev Brain Res. 1998;108:111–23. doi: 10.1016/s0165-3806(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 20.Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res. 1999;55:87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–47. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisler HC, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiol Exp (Wars) 1993;53:517–23. [PubMed] [Google Scholar]
- 23.Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–36. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- 24.Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci Lett. 2008;438:281–5. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iizuka M, Nishimaru H, Kudo N. Development of the spatial pattern of 5-HT-induced locomotor rhythm in the lumbar spinal cord of rat fetuses in vitro. Neurosci Res. 1998;31:107–11. doi: 10.1016/s0168-0102(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 26.Jamon M, Clarac F. Early walking in the neonatal rat: a kinematic study. Behav Neurosci. 1998;112:1218–28. doi: 10.1037//0735-7044.112.5.1218. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson K, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–17. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- 28.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–94. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo N, Nishimaru H, Nakayama K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Prog Brain Res. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- 30.Lam T, Wolstenholme C, Yang JF. How do infants adapt to loading the limb during the swing phase of stepping? J Neurophysiol. 2003;89:1920–8. doi: 10.1152/jn.01030.2002. [DOI] [PubMed] [Google Scholar]
- 31.McCrea AE, Stehouwer DJ, Van Hartesveldt C. L-dopa-induced air-stepping in preweanling rats. I. Effects of dose and age. Brain Res Dev Brain Res. 1994;82:136–42. doi: 10.1016/0165-3806(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 32.McEwen ML, Van Hartesveldt C, Stehouwer DJ. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci. 1997;111:825–33. doi: 10.1037//0735-7044.111.4.825. [DOI] [PubMed] [Google Scholar]
- 33.Muir GD, Chu TK. Posthatching locomotor experience alters locomotor development in chicks. J Neurophysiol. 2002;88:117–23. doi: 10.1152/jn.2002.88.1.117. [DOI] [PubMed] [Google Scholar]
- 34.Muir GD, Gowri KS. Role of motor and visual experience during development of bipedal locomotion in chicks. J Neurophysiol. 2005;94:3691–7. doi: 10.1152/jn.01121.2004. [DOI] [PubMed] [Google Scholar]
- 35.Musselman KE, Patrick SK, Vasudevan EV, Bastian AJ, Yang JF. Unique characteristics of motor adaptation during walking in young children. J Neurophysiol. 2011;105:2195–203. doi: 10.1152/jn.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama K, Nishimaru H, Kudo N. Developmental changes in 5-hydroxytryptamine-induced rhythmic activity in the spinal cord of the rat fetus in vitro. Neurosci Lett. 2001;307:1–4. doi: 10.1016/s0304-3940(01)01913-9. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86-23) Washington, DC: U.S. Government Printing Office; 1996. [Google Scholar]
- 38.Norreel JC, Pflieger JF, Pearlstein E, Simeoni-Alias J, Clarac F, Vinay L. Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat. J Neurosci. 2003;23:1924–32. doi: 10.1523/JNEUROSCI.23-05-01924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norton JA, Mushahwar VK. Afferent inputs to mid- and lower-lumbar spinal segments are necessary for stepping in spinal cats. Ann N Y Acad Sci. 2010;1198:10–20. doi: 10.1111/j.1749-6632.2010.05540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaki S, Yamada T, Iizuka M, Nishimaru H, Kudo N. Development of locomotor activity induced by NMDA receptor activation in the lumbar spinal cord of the rat fetus studied in vitro. Brain Res Dev Brain Res. 1996;97:118–25. doi: 10.1016/s0165-3806(96)00139-3. [DOI] [PubMed] [Google Scholar]
- 41.Pang MY, Lam T, Yang JF. Infants adapt their stepping to repeated trip-inducing stimuli. J Neurophysiol. 2003;90:2731–40. doi: 10.1152/jn.00407.2003. [DOI] [PubMed] [Google Scholar]
- 42.Pearson KG. Plasticity of neuronal networks in the spinal cord: modifications in response to altered sensory input. Prog Brain Res. 2000;128:61–70. doi: 10.1016/S0079-6123(00)28007-2. [DOI] [PubMed] [Google Scholar]
- 43.Pflieger JF, Clarac F, Vinay L. Postural modifications and neuronal excitability changes induced by a short-term serotonin depletion during neonatal development in the rat. J Neurosci. 2002;22:5108–17. doi: 10.1523/JNEUROSCI.22-12-05108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossignol S, Bouyer L, Langlet C, Barthélemy D, Chau C, Giroux N, Brustein E, Marcoux J, Leblond H, Reader TA. Determinants of locomotor recovery after spinal nerve injury in the cat. Prog Brain Res. 2004;143:163–72. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- 45.Roberto ME, Strain MM, Brumley MR. Does tactile feedback influence stepping behavior in the postnatal day one rat? Dev Psychobiol. 2009;51:591. [Google Scholar]
- 46.Sindhurakar A, Bradley NS. Kinematic analysis of overground locomotion in chicks incubated under different light conditions. Dev Psychobiol. 2010;52:802–12. doi: 10.1002/dev.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spear LP, Enters EK, Aswad MA, Louzan M. Drug and environmentally induced manipulations of the opiate and serotonergic systems alter nociception in neonatal rat pups. Behav Neural Biol. 1985;44:1–22. doi: 10.1016/s0163-1047(85)91121-5. [DOI] [PubMed] [Google Scholar]
- 48.Spear LP, Ristine LA. Quipazine-induced behavior in neonatal rat pups. Pharmacol Biochem Behav. 1981;14:831–4. doi: 10.1016/0091-3057(81)90369-5. [DOI] [PubMed] [Google Scholar]
- 49.Strain MM, Roberto ME, Brumley MR. A descriptive analysis of how newborn rats interact with different substrates during locomotor behavior. Dev Psychobiol. 2009;51:600. [Google Scholar]
- 50.Thelen E, Fisher DM, Ridley-Johnson R. The relationship between physical growth and a newborn reflex. Infant Behav Dev. 1984;7:479–93. [Google Scholar]
- 51.Thelen E, Fisher DM, Ridley-Johnson R, Griffin NJ. Effects of body build and arousal on newborn infant stepping. Dev Psychobiol. 1982;15:447–53. doi: 10.1002/dev.420150506. [DOI] [PubMed] [Google Scholar]
- 52.Thelen E, Ulrich BD. Hidden skills: a dynamic systems analysis of treadmill stepping during the first year. Monogr Soc Res Child Dev. 1991;56:1–98. [PubMed] [Google Scholar]
- 53.Ulrich BD. Opportunities for early intervention based on theory, basic neuroscience, and clinical science. Phys Ther. 2010;90:1868–80. doi: 10.2522/ptj.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ung RV, Landry ES, Rouleau P, Lapointe NP, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci. 2008;28:2231–42. doi: 10.1111/j.1460-9568.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- 55.Van Hartesveldt C, Sickles AE, Porter JD, Stehouwer DJ. L-dopa-induced air-stepping in developing rats. Brain Res Dev Brain Res. 1991;58:251–5. doi: 10.1016/0165-3806(91)90012-8. [DOI] [PubMed] [Google Scholar]
- 56.Viala D, Viala G, Fayein N. Plasticity of locomotor organization in infant rabbits spinalized shortly after birth. In: Goldberger ME, Gorio A, Murray M, editors. Development and plasticity of the mammalian spinal cord. Padova: Liviana Press; 1986. pp. 301–10. [Google Scholar]
- 57.Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Brain Res Rev. 2002;40:118–29. doi: 10.1016/s0165-0173(02)00195-9. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo-Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with Down syndrome after different treadmill interventions. Exp Brain Res. 2008;186:261–72. doi: 10.1007/s00221-007-1230-7. [DOI] [PubMed] [Google Scholar]
- 59.Yang JF, Lam T, Pang MY, Lamont E, Musselman K, Seinen E. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol. 2004;82:662–74. doi: 10.1139/y04-070. [DOI] [PubMed] [Google Scholar]