Abstract
Malaria control in the impoverished, highly endemic settings of sub-Saharan Africa remains a major public health challenge. Successes have been achieved only where sustained, concerted, multi-pronged interventions have been instituted. As one of the world’s poorest countries, Malawi experiences malaria incidence rates that have remained high despite a decade of gradually expanding and more intensive prevention efforts. The Malawi International Center for Excellence in Malaria Research (ICEMR) is beginning work to augment the knowledge base for reducing Plasmodium transmission and malaria morbidity and mortality. Among ICEMR goals, we intend to better assess patterns of infection and disease, and analyze transmission by Anopheles vector species in both urban and rural ecological settings. We will evaluate parasite population genetics and dynamics, transmission intensities and vector ecologies, social and environmental determinants of disease patterns and risk, and human-vector-parasite dynamics. Such context-specific information will help to focus appropriate prevention and treatment activities on efforts to control malaria in Malawi. In zones of intense and stable transmission, like Malawi,, elimination poses particularly thorny challenges - - and these challengers are different from those of traditional control and prevention activities. Working toward elimination will require knowledge of how various interventions impact on transmission as it approaches very low levels. At present, Malawi is faced with immediate, context-specific problems of scaling-up prevention and control activities simply to begin reducing infection and disease to tolerable levels. The research required to support these objectives is critically evaluated here.
Keywords: Malaria eradication, Plasmodium transmission, Anopheles ecology, Africa infectious disease, vector control, public health policy
1. Introduction
Malaria eradication is again under serious debate, reignited in part due to activities of the Gates Foundation and the World Health Organization (WHO) (Roberts and Enserink, 2007). While most consider global eradication unrealistic within the next few decades, if ever, local elimination is now being seriously proposed and pursued in some settings (Greenwood, 2009). Regional or national elimination has become a goal in specific contexts such as island countries of the Pacific and some nations of southern Africa. WHO has produced a field manual for malaria elimination in low and moderate endemic countries (World Health Organization, 2007). However, the knowledge base and logistical capacity needed for elimination efforts vary with different geographies, economies, cultures and infrastructures. The issues include technical, operational and financial aspects (Moonen et al., 2010b; World Health Organization, 2008), and vary with contextual and epidemiological conditions (Tatem et al., 2010; World Health Organization, 2008).
Here, we present features of the epidemiology of malaria in Malawi to help judge whether and how elimination might be considered and pursued. To that end, we discuss knowledge gaps that the Malawi ICEMR might help fill, including research designed to focus on individuals at risk and to determine which interventions seem most likely to reduce infection and disease. Using the framework of the series of reports by the malERA groups (Alonso et al., 2011), we explore some of the questions concerning exposure, surveillance and vector biology, as well as health systems and infrastructure that will help guide interventions in Malawi.
Tatem et al.’s (2010) evaluation of Malawi’s relatively strong technical but weak operational capacities, in the face of intense malaria transmission, suggest that elimination is not a realistic possibility, at least in the short terms. By virtually every economic metric, the people of Malawi are among the world’s most destitute, facing enormous disease burdens from various infectious agents, widespread under-nutrition, and limited natural resources. Malaria, almost entirely due to Plasmodium falciparum, but with P. malariae and P. ovale present, is endemic throughout all of this land-locked, sub-Saharan country. The Malawi Ministry of Health (MOH) estimates that about six million malaria episodes occur each year (National Malaria Control Program, 2010) among a 2010 population of 15 million people (United Nations Development Program, 2010). Malaria accounts for nearly half of all outpatient clinic visits, and is the number one cause of hospital admissions among children under five years of age. Despite considerable progress over the past few decades in understanding the patterns, underlying risk factors, appropriate prevention and effective treatment of Plasmodium infection and malaria disease, malaria remains one of the major impediments to improved health and economic development in Malawi.
The Malawi International Center for Excellence in Malaria Research (ICEMR) was developed with the goal of enhancing knowledge of Plasmodium transmission and malaria disease patterns and prevention in order to reduce morbidity and mortality. Research is being undertaken with the goal of generating data that will be useful to NMCP, including enhanced surveillance that will be integrated into routine activities. We are specifically trying to avoid project-specific data collection that will cease when funding ends.
Details of past research and interventions aimed at reducing Malawi’s malaria burden have been presented by Mathanga et al.(2011), and are briefly summarized below. The rationale for expanded research under the Malawi ICEMR program, and the implications for malaria control and possible elimination are presented subsequently.
1.1. Environmental, demographic and economic setting
Malawi, a small, ecologically diverse country in south-eastern Africa bordered by Mozambique, Tanzania and Zambia, is one of the most densely populated nations in Africa. Possessing few natural resources, Malawi ranked 153 of 169 countries in the Human Development Index, with the 2008 GDP per capita at US $902 PPP, making it among the poorest countries in the world (United Nations Development Program, 2010). Income inequalities are extreme. Poverty reduction remedies involve various initiatives including political, social, health, security and education activities, but progress has been limited. Malawi has experienced an unchanged Human Development Index rank since 1995, even though many health infrastructure measures (e.g. infant mortality rate, potable water access, literacy) have improved (United Nations Development Program, 2010).
Malawi’s child mortality rate is among the highest in the world. The 2010 nationwide infant mortality rate was 73 per 1,000 live births, with an under-five mortality rate of 127 per 1,000 (National Statistical Office, 2010) HIV/AIDS, low educational attainment and deforestation are among many serious challenges.
Administratively, the country is divided into 28 districts, most of which are considered predominantly rural. Malawi’s estimated annual national population growth rate (2005–2010) was 2.5%, but is more than double that (5.2%) in areas classified as urban (United Nations Human Settlement Programme, 2008). Two-thirds of all urban dwellers in Malawi live in slum conditions. “Urbanisation in Malawi has become synonymous with poverty and slum growth” (United Nations Human Settlement Programme, 2008). Indeed, at 6.3% per year, Malawi currently has the highest urbanization rate of any African country. Although the situation of Malawians living in urban settings is considered somewhat better than that of rural residents if judged by standard measures of economic development (Bowie, 2006), urban life is still extremely unhealthy. Among Malawi’s urban dwellers, less than one-third have electricity, one in seven have piped water arriving at the house, and more than three-quarters only have access to pit-toilets. This urban context is one where malaria transmission is reported (Mathanga et al., 2006), but poorly understood.
Health services are provided by the Government of Malawi through a system of hospitals, health centers and health posts at no cost to patients. There is a parallel system of private practice, and many itinerant shops and pharmacies sell medications over-the-counter. Numerous NGOs also function in various health and disease-prevention contexts throughout the country. Thus, despite extreme poverty, there is a public health infrastructure that has been supporting malaria control and prevention activities.
1.2. Malaria burden and eco-epidemiological patterns
Malaria is found throughout Malawi, with generally greater transmission in the south than the north, near the shores of Lake Malawi, and in rural than in urban settings (Mathanga et al., 2011). However, despite efforts to improve diagnosis and treatment, and expand use of insecticide-treated nets (ITNs), indoor residual spraying (IRS), and other interventions, there was no decrease in disease incidence during the decade beginning in 2000 (World Malaria Report 2010). Malawi’s routine passive surveillance of malaria at health facilities, in which a febrile illness is classified as “malaria”, even in the absence of parasitological diagnosis, indicated that cases increased from ~3.7 million in 2005 to ~6.8 million in 2009 (Malawi Ministry of Health, 2005a, 2010b). These data were gathered during a period of scaled-up anti-malaria interventions, mostly directed at children less than five years of age, who experience an estimated average annual incidence of ~one episode per child per year (Malawi Ministry of Health, 2010b). Transmission occurs year-round, with more cases appearing during January–April, during/after the warmer, rainy season (Figure 2). While there may have been changes in diagnostic and reporting accuracy during this period, there appears to have been little national improvement in malaria control, and among the findings of an evaluation of Malawi MOH health sector programs during 2004–2010 (Malawi Ministry of Health, 2010a) was the concern that “(p)rogress in the malaria programme… is disappointing.”
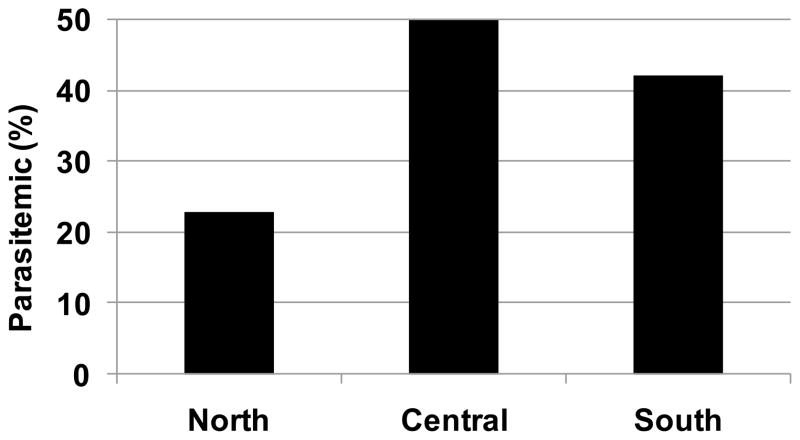
Figure 2.
Prevalence of Plasmodium parasitemia among Malawians residing in three regions of the country during 2010. Data are from systematic, nationwide surveillance by the Malaria Indicator Survey (National Malaria Control Program, 2010)
Distribution of conventional ITNs was provided under the auspices of the National Malaria Control Program, with funding and technical assistance from the President’s Malaria Initiative (PMI) and the Global Fund, beginning in 2006 (National Malaria Control Program, 2010). Results from a 2010 nationwide Malaria Indicator Survey (MIS) (Malawi Ministry of Health, 2010c) showed that 60% of households owned one net, and 54% of children under five years of age had slept, during the night preceding the survey, under a long-lasting insecticide treated net (LLIN). In addition, 49% of pregnant women had slept under an ITN during the preceding night. An increase in the number of reported cases coincident with a scale-up of control activities seems paradoxical. A potential contributing factor may be that, over the same period, ACTs were introduced as first-line treatment, and drugs were made available, free of charge, but only at government-sponsored health facilities. This policy may have increased use of these facilities.
This same MIS showed a Plasmodium infection prevalence in April 2010 of 43% among under-five children (National Malaria Control Program, 2010). Furthermore, roughly one-quarter of children hospitalized in Blantyre’s Queen Elizabeth Central Hospital (QECH) during 2001 through 2009 were diagnosed with malaria (Kwizombe et al. 2011), a prevalence that, along with the number of outpatient malaria cases, has not changed during the decade. Similarly, community surveys between 2005 and 2009 showed little decline in either parasitemia or anemia among children under 30 months of age (Mathanga et al., 2010). By contrast, in nearby Zambia where transmission intensity, population density, and socio-economic conditions are somewhat different, implementation of malaria control across a range of fronts dropped malaria prevalence to very low levels compared to pre-intervention levels (Chizema-Kawesha et al., 2010; Steketee and Campbell, 2010). Whether such efforts can produce the same impact in Malawi cannot be properly evaluated until all planned anti-malaria efforts, including IRS, are fully implemented.
2. Current Malaria Control and Prevention Activities
Efforts to reduce malaria morbidity and mortality in Malawi are currently designed to treat cases, lessen incidence of severe disease in mothers and children, and reduce Plasmodium transmission through modern prevention methods (Mathanga et al., 2011). The Malawi government pursued a strategic plan for 2005–2010 (Malawi Ministry of Health, 2005b) that involved prompt access to artemisinin-based combination therapy (ACT) for all, widespread use of intermittent preventive treatment in pregnancy (IPTp), distribution of ITNs and LLINs, and localized use of indoor residual spraying (IRS) in two districts (Nkhotakota and Salima). Through various donors, including PMI/USAID, the UK Department for International Development (DFID), and the Global Fund, funding for malaria treatment, prevention and control has increased considerably, particularly during the past half-decade.
Prevention of infection currently relies heavily on free distribution of LLINs to pregnant women and mothers of newborns. Despite efforts to expand the number of women and children who sleep under nets, only about half reported doing so in the most recent survey (National Malaria Control Program, 2010). Although use of IPTp began in 1993, efforts in the past few years at expanding preventive treatment through antenatal clinics have had only limited success (Mathanga et al., 2011). Other prevention activities that could be directed at reducing vector abundance (IRS, environmental management) have not been widely attempted, let alone aggressively pursued. Lack of IRS use may reflect inadequate resources, insufficient political will, and an under-appreciation of its effectiveness, as is likely also the case with environmental control efforts. In general, Malawi suffers from a paucity of knowledge concerning the local ecological and entomological drivers of malaria risk, such as the agricultural context. This is aggravated by the serious shortage of well trained vector biologists in the country.
Treatment that is intended to be prompt and effective, particularly focused on under-five year olds, has been a part of Malawi’s new anti-malaria strategy, including introduction of ACT in 2007. Despite efforts to expand treatment, the proportion of under-five year old children who were promptly treated remained unchanged at about one-quarter of that population during 2004 through 2010 (Mathanga et al., 2011).
3. Current research efforts in Malawi aimed at improving malaria control
This backdrop of extreme poverty, limited resources, widespread, year-round, and intense transmission, and continued high rates of disease in Malawi, raises challenging issues about the knowledge needs for improving malaria control, let alone pursuing regional elimination. Context-specific information and knowledge are critical to help focus appropriate prevention and treatment activities on people at highest risk and areas that are most important in transmission. Limited research has been carried out to date to provide such information.
3.1. Assessing patterns of infection and disease
Efforts to diminish malaria incidence benefit from enhanced active surveillance, since many cases are missed if only passive, clinic-based records are used (Moonen et al., 2010a). Passive reporting of malaria cases (febrile illness with or without parasitologic confirmation) occurs in MOH facilities and is summarized in annual reports by the Health Management Information System (HMIS) (Malawi Ministry of Health, 2010b). Although not research per se, these data represent potentially useful information, even if most Malawians do not seek care for fever (Mathanga et al., 2011). However, most cases are diagnosed clinically at government health centers, as few facilities have a laboratory capacity. Furthermore, only those people who are treated at such clinics or health posts are recorded, thus excluding infections that are self-diagnosed/treated or asymptomatic. For these reasons, such health facility-based data need to be considered incomplete (Rowe et al., 2009). An incentive for seeking care at government facilities is that lumefantrine-artemether (LA), the first line ACT, is available for free within government health facilities, but must be purchased in the private sector.
Active surveillance, which addresses different questions and provides different data, has been generated by specific research projects, most of which are of limited duration. We are unaware of any systematic, population-based, active case detection studies designed to estimate Malawi’s disease burden. The Malawi MIS (Malawi Ministry of Health, 2010c), conducted in March–April 2010 by the National Malaria Control Program (NMCP) and various partner agencies, provides the most thorough, nation-wide summary of infection, self-reported disease and prevention efforts to date. National information from all 28 districts and diverse ecological and social settings represents a cross-sectional benchmark for evaluating future change, although to date, only limited details, presented in summary format, have been made available.
3.2 Treatment seeking and diagnosis
Reducing the burden of malaria and shortening the infectious period can only occur if infections and disease are promptly diagnosed and properly treated. Clinicians staffing the free MOH clinics, recognize and treat most uncomplicated febrile illnesses as malaria, Prompt and appropriate health-seeking behavior, however, is affected by cultural and socio-economic barriers as has been shown in one study in Malawi (Chibwana et al., 2009), suggesting the importance of behavioral change messages to address local beliefs about causes of fever and appropriate health care.
Effective field performance of malaria rapid diagnostic tests (RDTs) and use of these results in treatment was recently evaluated to suggest how this tool might best be used in national scale-up efforts (Chinkhumba et al., 2010). The 7% false negative rate and $0.80 – $1.00 cost per test (Chinkhumba pers. comm.) were both high, and represent impediments to rapid implementation of this approach, especially given the limited resources of the National Malaria Control Programme. One of our ICEMR activities will be to assess the implementation of RDTs in the context of the surveillance activities at our study sites.
3.3. Disease treatment and prevention
Cerebral malaria (CM) has recently been studied in Malawi, producing suggestions for improvements in diagnosis using retinopathy (Lewallen et al., 2008), as well as reducing the developmental impacts of CM (Birbeck et al., 2010). Other studies have characterized the timing and frequency of P .falciparum infection on pregnancy outcomes including low birth weight and maternal anemia (Kalilani et al., 2010). Risk of preterm delivery and low birth weight was shown to be reduced by treating pregnant women monthly with sulfadoxine-pyrimethamine (SP) and two azithromycin doses (Luntamo et al., 2010).
Use of ITNs depends on availability and acceptability. Mathanga et al. (2009) have demonstrated that distribution of ITNs as part of routine immunization services in Malawi was effective in improving coverage among women attending these clinics. Similarly, IPTi was shown to be more acceptable in Malawi and other sites in Africa when presented in the context of EPI vaccination of newborns (Gysels et al., 2009). Another study in southern Malawi suggested that community-based distribution of SP for IPTp improved coverage during pregnancy, but reduced attendance at antenatal clinics (Msyamboza et al., 2009). These recent reports demonstrate considerable capacity in Malawi for such research.
Prevention of transmission aimed at Anopheles vectors depends on an understanding of species-specific ecologies, abundances, behaviors and susceptibilities to insecticides, all of which are evolving differently in different parts of sub-Saharan Africa (SSA). Unfortunately, in Malawi there has been very little published research on components of malaria risk related to mosquito vectors (Mathanga et al. 2011). As anti-vector interventions intensify, studies on their effectiveness, insecticide resistance, and vector behavior will become increasingly important.
4. Planned ICEMR-based research goals and potential impacts
Malaria control effectiveness in Malawi will depend on an understanding of the specific ecological, cultural, behavioral and infrastructural contexts of transmission in different settings of the country. The research challenges that Malawi ICEMR investigators have identified, when considered in the context of malaria control activities in Malawi and elsewhere in SSA, reflect an appreciation of the enormous variety of factors that complicate malaria control efforts in this highly endemic setting. New research into the parasite, vector, and human interactions are intended to inform malaria treatment and control efforts, and to complement and expand upon recent studies described above. These efforts and their potential contributions to control efforts are briefly outlined below.
4.1. Parasite population dynamics, evolution and treatment
The genetic diversity of P. falciparum strains in Malawi is an important characteristic of malaria risk that may influence the acquisition of immunity, immune responses and treatment effectiveness (Escalante et al., 1998; Takala and Plowe, 2009). As control efforts are expanded and scaled-up, P. falciparum genetic complexity may decline, creating smaller subpopulations of the parasite (Anthony et al., 2005). Decreased effective population size is likely to alter parasite population parameters (e.g. population differentiation, linkage disequilibrium), but this may occur differently in different regions of Malawi. Molecular genetic studies of Plasmodium strains from individuals with malaria illnesses and from those with asymptomatic parasitemia will help to address this possibility, and also inform how the geographic distribution of the parasite strains change over time
The prevalence of SP-resistant strains of P. falciparum may decline now that SP has been replaced by LA as first-line treatment for malaria illnesses. The initial report of this phenomenon in one area of Malawi (Laufer et al., 2010) will be expanded to other sites in an attempt to determine how widespread and important this might be. The prevalence of molecular markers of SP-resistance in specimens gathered from cohort and cross-sectional human studies (described below) will help evaluate the extent and pace of the return of SP-susceptible parasites, and may have an impact policies relating to IPTp, in particular.
4.2. Transmission intensity and comparative vector ecology
Understanding the ecological determinants of Plasmodium transmission patterns is an important component of malaria prevention efforts, and is even more important if elimination is contemplated via vector control-mediated reduction in transmission (Ferguson et al., 2010). Existing methods of vector control, including IRS and ITN use, have never completely prevented all vector-human contact, and various lines of evidence suggest that changes in vector behavior and insecticide sensitivity evolve with continued use of such interventions (Raghavendra et al., 2011; Rieckmann, 2006). The eco-epidemiology of when and where vectors feed, as well as their use of non-human hosts, is likely to change as new selective pressures are presented. Impacts on transmission, and effectiveness of vector-focused interventions, could be seriously compromised.
Another component of vector ecology involves the diversity of competent species in different habitats. Interventions that focus primarily on behaviors or habitats characteristic of one species may permit increased abundance or range expansion of other competent Anopheles. Ecological competition, but also density-dependence and predation particularly among larvae, represent important opportunities for manipulating vector abundance and human-vector contacts. Any credible effort to reduce malaria risk through mosquito vectors must address these fundamental ecological characteristics. They are all targets of potential interventions and critical drivers in the complex dynamics that will affect sustainability of other kinds of prevention and control activities.
Anopheles species are likely to exhibit different densities and behaviors across the range of ecological conditions in Malawi (Mzilahowa et al., 2008). Hence, fundamental observations are needed to understand these patterns in the context of changing conditions. Long-term observations, at multiple spatial scales (household to eco-regional), are essential to analyze vector abundance and behavior in response to shifting anti-vector interventions and human activities. Analyses of such space-time patterns can enhance understanding of interactions operating at different levels of effects (individual prevention, social movements, regional development), and can improve the evaluation of particular interventions. Such observations and experiments are planned to better comprehend vector community structure, malaria transmission intensity, and landscape influences relative to vector distribution in different eco-geographic zones. In settings of differing malaria transmission intensity, the contribution of species-specific vectorial capacity parameters should better define risk and focus control. By quantifying the spatial heterogeneity of larval and adult vector distributions, control measures can be more directed.
The effect of intense interventions on Malawi malaria vector populations may affect the evolution of specific behaviors (host preference, breeding site selection) and susceptibility to repellants or insecticides. By quantifying changes in population structure of malaria vectors in response to control activities, insights into the relevant evolutionary processes should facilitate decisions regarding the deployment of various interventions. Of particular concern are evolutionary changes in population structures of A. gambiae s.s. and A. arabiensis under conditions of intense control. Current entomological monitoring activities across the country have shown that A. funestus is a predominant malaria vector in a number of districts and environmental settings (Mzilahowa, unpublished) (Hunt et al., 2010). This species also exhibits widespread resistance to most pyrethroids and bendiocarb (Mzilahowa, unpublished) thereby posing a challenge to malaria control efforts. It may be that the widespread distribution and vectorial capacity of this species is partly responsible for the lack of anti-malaria successes (Coetzee and Fontenille, 2004). Even in regions of low to moderate transmission, simulation models have not always been successful in characterizing attempts to interrupt transmission (Gatton and Cheng, 2010). For example, stochastic events (e.g. weather, war, economic crisis) are likely to impact simulation success, age-specific responses will be different and vary over time, and changes in morbidity do not necessarily correspond to decreases in transmission. Quantifying these interactions should enhance the utility of simulations to model the impact of various interventions.
4.3. Human disease patterns and risk factors
Despite difficulties in establishing baseline measures needed to define malaria endemicity (Hay et al., 2008), Malawi MOH data show generally intense, year-round transmission with somewhat elevated incidence corresponding to the rainy season (December to March). Human disease surveillance, both passive and active, has begun to document the magnitude and space-time patterns of malaria in Malawi, but has not been accompanied by complementary observations of vector ecology. Active surveillance of human behavior patterns in relation to environmental, disease, and vector ecologies are needed to determine the relative contributions of known risk factors and to elucidate unrecognized risk factors for transmission.
Analyses are planned in the Malawi ICEMR that compare existing nation-wide, passive malaria surveillance data from Malawi’s 568 MOH health clinics (where most people are treated initially) with patterns of various environmental measures (land use, population density, elevation, precipitation, etc.). General patterns of environmental risk serve to identify the regions where interventions are most needed, and the environmental drivers that may explain risk patterns. Differences and similarities in urban, semi-urban, peri-urban and rural locations should be particularly instructive in setting the stage for detailed active surveillance of risk.
Active, systematic surveillance at selected hospitals and health centers for asymptomatic, uncomplicated, severe, and pregnancy-associated malaria are aimed to enhance understanding of spatial and environmental patterns of risk which are otherwise often based on questionnaire-based studies such as those from the Demographic and Health Surveys (DHS). One outcome of such active investigation should also be improved identification of demographic, geographic and temporal patterns of asymptomatic infection and under-recognized transmission. Plans for twice-yearly, cross-sectional surveys in each of the study districts will provide a better picture of the habitat heterogeneity (urban, highland and lowland) and its contribution to transmission – data that are critical to more focused control efforts.
Prospective, community-based, case-control household studies of socioeconomic and environmental factors that are hypothesized to affect risk of P. falciparum infection will take place at six urban and peri-urban government health facilities. Questionnaire-based data on SES, malaria KAP, travel history, and ITN use, as well as geo-location and construction of each house, will be explored using multilevel and spatial statistical approaches. Urban-rural differences and proximity to breeding sites will be investigated. Inferences regarding the location of transmission based on patterns of social variables, behaviors (travel and prevention) and exposure to vectors are intended to help focus control efforts. The rationale for comparisons across the urban-rural continuum of habitats (Robert et al., 2003) is based on 1) a lack of knowledge regarding where urban residents are becoming infected, 2) the recognition of different socio-ecological dynamics in urban vs. rural contexts, 3) our ignorance about the impact of travel between these settings on transmission. Regional elimination is cannot be achieved unless interventions are focused on all of the habitats in which transmission is occurring.
4.4. Human-vector-parasite interactions
Ultimately, effective malaria control must consider the dynamics of interactions among all three components of transmission. Determinants of human exposure to Anopheles vectors that transmit various Plasmodium genotypes in different environments, and under different prevention scenarios, set the stage for complex space-time analyses that will be helpful in illuminating both the risks of infection and of developing malaria disease.
Household variation in malaria cases does not necessarily reflect local transmission. Travel that results in P. falciparum infection at other locations will not be associated with local environmental factors, hence complicating exposure analyses. Thus, systematic analyses of adult Anopheles spp. abundance in and around houses of infected and non-infected people are planned, as are studies of adjacent larval breeding site ecologies. In addition, molecular analyses of P. falciparum isolates from people and from Anopheles mosquitoes may provide insight into population-level genotypic heterogeneity and locations of infection acquisition. This is particularly important to understanding the growing concern over “urban” malaria. If transmission is not occurring locally, as is often presumed, this would have important implications for control practices and policy.
The rapidly changing context of urban environments (biophysical, human density, number of non-immunes, climate variability) creates instability in malaria risk. In addition, interventions are now being scaled-up. Prospective studies of malaria risk that capture and characterize change over the course of many years will help to understand the effects of control. Time-series analyses, and more complex simulations of space-time patterns under changing control regimens, are needed if malaria control efforts are to be expanded in the longer-term direction of elimination.
4.5. Integration with ongoing malaria control
The Malawi ICEMR team is involved at all levels of Malawi’s malaria control activities. Every effort is being been made to embed ICEMR data collection activities into the day-to-day activities in the sentinel health centres and hospitals of our studies. If these pilot activities are deemed feasible and if the data generated are considered useful, the ICEMR model will be replicated elsewhere in Malawi.
The advisory group for the Malawi ICEMR includes “end users” such as the District Health Officers in the sentinel ICEMR sites, as well as representatives from the Malawi’s National Malaria Control Programme (NMCP) and MOH. This advisory group plans to meet at least two times per year. In addition, members of the ICEMR team attend the quarterly meetings of the NMCP, and thus are “in the loop” regarding ongoing and planned activities.
5. Implications for local elimination of malaria in Malawi
Malaria elimination, and even eradication, have again been proposed as feasible goals, stimulating new political commitments and resources to expand and coordinate interventions heretofore aimed at malaria prevention and control (Hommel, 2008; Tanner and de Savigny, 2008). As outlined in the Roll Back Malaria (RBM) Global Malaria Action Plan (Roll Back Malaria Partnership, 2008), scaling-up and maintaining intensive anti-malaria operations, with particular focus on the margins of endemic areas, will require research into new control tools and implementation. Scaling- up of IRS and LLINs is important, as is coordinated monitoring and surveillance of key activities. The World Health Organization (WHO) has produced a “field manual” for malaria elimination in countries with low to moderate transmission (World Health Organization, 2007), but, not surprisingly, no such document exists for the countries with high transmission, which encompass the vast majority of cases and deaths (World Health Organization, 2010).
Most of Malawi experiences high intensity, stable transmission. For such areas, the WHO considers that “(t)here is no evidence to-date to indicate that malaria transmission can be interrupted, nor that malaria elimination can be sustained in such areas with existing tools (Mendis et al., 2009).” Regardless of whether elimination is defined as the complete absence of local transmission, of non-introduced transmission, or a small number of epidemiologically-linked cases (Cohen et al., 2010), the pathway toward elimination in Malawi must be carefully evaluated given the context of very high transmission, moderate anti-malaria activities, and an underfunded health infrastructure.
5.1. Intervention needs in Malawi
For malaria elimination in Malawi to be seriously considered, a major expansion in current intervention activities is necessary. Evidence-based decisions regarding logistics, cost-effectiveness, feasibility and likelihood of success of various options will require more research. A recent analysis designated most countries in SSA, including Malawi, as having relatively weak operational and technical feasibility for malaria elimination (Tatem et al., 2010). Although it has been argued that elimination is achievable simply through more intensive and thorough use of the tools and methods currently being employed in control, this logic has been questioned (Cohen et al., 2010; Greenwood, 2008).
Elimination poses challenges that are different than control, an insight that was first articulated more than 50 years ago (World Health Organization, 1956). Intervention must occur in all transmission foci (partial coverage is unacceptable); transmission must be completely interrupted (temporary or fragmented is inadequate); active case detection is required (because of the need to identify untreated or self-treated infections); imported case recognition is critical (because transmission must cease entirely); integration with other health programs is desirable (yet elimination is specific and time-limited); and efficient/responsive program administration is essential (control programs can tolerate lapses, but elimination programs cannot) (World Health Organization, 2007).
Current plans to intensify the fight against malaria, whether aimed at better control or moving toward local elimination, advocate “scaling-up” of various anti-malaria activities. Obstacles to scale-up of interventions involve practices, programs and policies at various levels of organization, including individuals and families, communities, health care infrastructure, regional and national governments, and biophysical environmental contexts (Mills et al., 2008). In most of Malawi, accessibility of or engagement with malaria control are not important impediments. Health services (e.g. local clinics) are generally nearby (<20 km), even though preliminary analysis have suggested that ITN use, for example, declines with distance from health centers (Larson et al. unpublished). Furthermore, diagnoses and treatment are increasingly available and are usually free of charge. Thus, Malawi’s national malaria control program already appears to be surprisingly robust for a country that ranks near the bottom of most national economic or social development indicators (World Bank 2007). Centralized health policies combined with basic technical capacity and widespread cell phone communication, as well as political stability and security, all favor expanded scale-up of anti-malaria efforts. Nevertheless, the bio-physical environment and climate predispose Malawians to extremely high P. falciparum transmission.
5.2. Evaluating elimination feasibility in Malawi
A recent study characterized conditions for elimination, considering the interactions of technical, operational and financial feasibilities (Moonen et al., 2010b). Technical feasibility depends on measuring the basic biological and epidemiological vavariables of transmission (e.g. infection prevalence, EIR, clinical incidence, human movements) that determine key parameters (e.g. basic reproduction number (Ro) or force of transmission and importation rate). These, in turn, allow estimates of the anti-malarial coverage required. In Malawi, such basic technical information is largely lacking, except for disease incidence surveys and health-care facility reporting. Our research agenda for the next few years will address these data gaps by providing fundamental entomological and parasitological data, as well as information on human prevention behaviors and movement in three ecologically distinct areas (highland urban, lowland rural, highland rural). The ICEMR activities will generate the information required to establish control targets and appraise feasibility of regional malaria elimination within the study areas. Evaluation of operational and financial feasibility, while not directly included in our research agenda, nevertheless partly depends on results from that research. The magnitude and intensity of interventions needed to consider eventual elimination will require technical information to determine the extent of operations, inform barriers to scaling-up, evaluate costs and compare scenarios. It seems certain that “… we lack sufficient knowledge, systems and tools to eradicate malaria today” (Tanner and de Savigny, 2008), and it remains to be seen whether “… we do have a window of political will and financial resources to refocus on the goal of effective control through universal coverage of appropriate interventions” (Tanner and de Savigny, 2008).
Not only must costs be considered, but the cost-benefit of continued control versus real elimination needs evaluation. A recent study (Sabot et al., 2010) demonstrated that, while empirical economic data are scarce, and there is wide variability among countries, investment in elimination is generally more costly than long-term control. This is likely to be especially true in highly endemic settings such as Malawi where so little of the country’s own resources can be allocated to combating malaria. Even with increased donor investment, benefits have been difficult to appreciate. As Sabot et al. (2010) point out, however, elimination is unlikely to be sought solely on the basis of marginal cost or financial returns. Nevertheless, the costs of such effort need to be evaluated realistically, and the case studies in their report represent the first examination of cost-savings that might come from proposed elimination. Any attempt at designing an elimination program in Malawi, or elsewhere, should include rigorous economic analyses of that local context.
As Feachem et al. (2010) recently noted, a comprehensive feasibility assessment of malaria elimination is needed before embarking on a national policy. Such a policy for Malawi would have to consider long-term financing in the context of other health and development needs. Sustained anti-malaria interventions aimed at elimination cannot realistically be funded in this extremely poor country, without continued support from external donors far into the future. If this is not realistic, then elimination plans may need to be reconsidered.
5.3. Research needs for control and elimination in Malawi
Extensive analyses undertaken by nine expert panels, recently published as series of reports in PLoS Medicine (January 2011), reviewed the research agendas needed to advance malaria eradication. The lead article in this series (Alonso et al., 2011) identified a large body of critical research needs. They include laboratory studies (parasites, treatment drugs, prevention vaccines), field studies (e.g. vector control, community-level transmission assessment), and theoretical research (mathematical models of transmission dynamics, evaluation of intervention strategies). All of these research endeavors must be integrated, they declare, through better tools and strategies for monitoring and evaluation in the context of a nation’s larger health and social systems. This is a very tall order for a country such as Malawi where most people live on less than US $1 per day, and the total annual (2008) per capita health care expenditure (PPP international US$) was $49,of which $30 came from the government (World Health Organization, 2011). Data originating in each specific, underdeveloped, malarious setting are key , highlighting the need for capacity development in these endemic areas.
Among other suggestions, Alonso et al. (2011) propose that the research paradigm shift toward one that incorporates interactions among the parasite, mosquito, and human host, much as our ICEMR efforts have been designed. They argue that this integration requires “… in-depth population-based field studies…” that can bridge “…the artificial gap between bench-based research, preclinical research, clinical research, and population-based science…,” again consistent with our proposed studies. To suggest, however, that this is a logical direction for research strategies is not the same as arguing that this can lead toward local elimination, let alone eradication. Again, much of this research effort must be in collaboration with the scientists, policy makers, economists, and health workers of the affected countries.
5.3.1
Improved diagnoses and diagnostics were highlighted as important by another of these reports (The malERA Consultative Group on Diagnoses and Diagnostics, 2011), and the Malawi ICEMR also will be engaged in this area. Many activities involving screening efforts, detecting symptomatic infection, assessing immunity, and developing tools for underserved rural areas are critical for a high transmission country like Malawi.
5.3.2
Drug therapy and prevention (The malERA Consultative Group on Drugs, 2011) and vaccine development (The malERA Consultative Group on Vaccines, 2011) were analyzed for their importance in elimination, and both considered the role of the research communities in developing new compounds or approaches. The need to test these interventions in field settings is clear, but how they will interact with, complement, or possibly contradict other processes in transmission dynamics remains a complicated issue. While some of this complexity can be considered using simulation modeling, many of the pathological and epidemiological feedbacks or administration issues can only be evaluated in practice through empirical investigations. This is best illustrated by an emphasis on vaccines to reduce parasite transmission rather than focusing primarily on individual-level morbidity and mortality.
5.3.3
Coordinated and functional health systems that are backed by research and evaluation are essential to effective control, let alone elimination (The malERA Consultative Group on Health Systems and Operational Research, 2011). Our ICEMR activity plans to develop research ties with economists and policy analysts in Malawi to better understand the constraints that inhibit highly efficacious interventions from become maximally effective. Again, the focus will be to enhance control operations, whether or not they are thought of as leading to elimination. Past experience (Moonen et al., 2010a) suggests that many of the logistical issues important to control will change if ever an elimination goal seemed within reach. As the authors of this review panel point out, “…research questions need to be defined locally but are of relevance to all programmes engaged in control or elimination.”
5.3.4
Mathematical modeling can help guide malaria control activities by “…synthesizing information, quantifying uncertainty, and extrapolating current knowledge” (The malERA Consultative Group on Modeling, 2011). In addition to emphasizing the theme that modelers must work with end users and other stakeholders, no single, comprehensive model or analytical approach will yield solutions in all settings. Most models that address intense transmission in settings such as Malawi illustrate the challenge of control aimed at simply reducing transmission, let alone completely halting it. The value of these analyses to policymakers and health workers in this SSA region is particularly in helping to assess the technical, operational, and financial feasibility of interventions.
5.3.5
Although not especially glamorous, monitoring, evaluation, and surveillance may be among the most important components of malaria control aimed at eventual elimination (The malERA Consultative Group on Monitoring, 2011). Currently, Malawi needs enhanced measurements of the most basic metrics (morbidity and mortality) before it can consider shifting toward widespread detection of infection and transmission. In addition to assisting with this, our ICEMR program plans to help develop and use improved malaria distribution maps to guide control efforts. Detection and measurement of asymptomatic infections in adults (Harris et al., 2010; Ouedraogo et al., 2009; The malERA Consultative Group on Diagnoses and Diagnostics, 2011) could become an important activity if transmission were brought to very low levels in Malawi. At present, however, even standard surveillance of malaria disease is inadequate.
5.3.6
Vector control could be effectively aimed at elimination if our understanding of how vector populations contribute to the complex dynamics of parasite transmission is enhanced (The malERA Consultative Group on Vector Control, 2011). Because the details of vector species, geography, habitats, human contact patterns and control strategies vary so much, local knowledge is required to effect changes that reduce transmission and disease. Fundamentals of Anopheles species-specific biology, behavior, insecticide resistance patterns, and many other contributors to transmission are needed in Malawi and other countries in the region. Not only is a deeper understanding of these vector-related drivers critical to avoiding some of the shortcomings of previous global eradication efforts, but this understanding will increase appreciation of the magnitude of the problem, resulting in more realistic expectations of the timeframe and costs required for elimination efforts. Any lasting outcomes rest on the recognition that endpoints will be dynamic, reflecting the moving target that includes evolving ecologies of vectors along with the parasites and humans.
Malawi’s engagement with its similarly impoverished neighbors (Mozambique, Zambia and Tanzania) will be essential in long-range planning and collaborative activities. Effective national malaria reduction in settings where regional transmission is intense cannot realistically aim at elimination unless all countries in the region achieve such success. Except in the unusual island-nation contexts, cross-border cooperation is essential as part of any elimination plan.
The challenges of malaria control in Malawi are formidable, and will certainly be more effectively addressed as increased resources are directed at that problem. Progress will be slow, and will depend on using the available resources efficiently and effectively. Much of our research is directed toward this end. The goal of local elimination of malaria in Malawi seems far-fetched, but if Malawi is able to reduce transmission, the focus could expand and intensify to include local elimination. Necessary improvements in understanding and changes in operational endeavors will have to be addressed if that potential is ever to be realized.
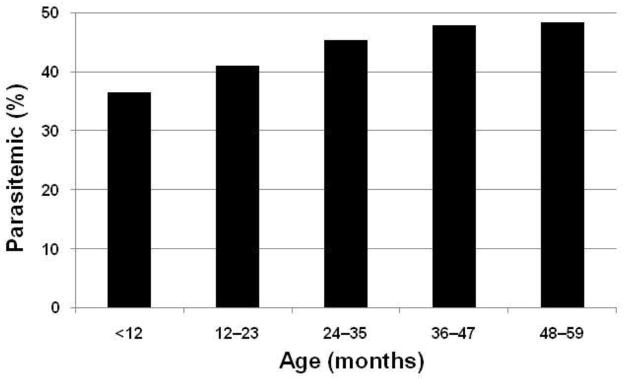
Figure 1.
Prevalence of Plasmodium parasitemia in Malawian children under five years old during 2010. Data are from systematic, nationwide surveillance by the Malaria Indicator Survey (National Malaria Control Program, 2010)
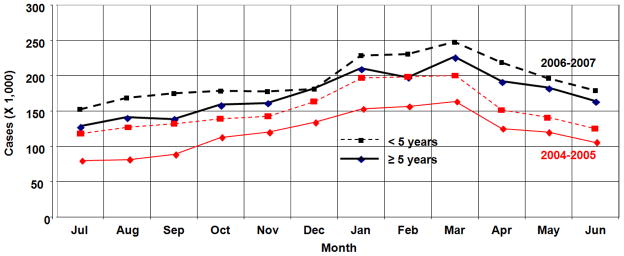
Figure 3.
Number of presumptive cases of malaria diagnosed throughout Malawi during July 2004–June, 2005 (red), and July 2006–June 2007 (black) grouped by ages less than 5 years old (dashed) and 5 or more years old (solid). Data are from Malaria Indicator Surveys (Malawi Ministry of Health, 2005a, 2010b)
Acknowledgments
Supported in part by NIAID grant 1U19AI089683-01 for the International Centers of Excellence for Malaria Research (ICEMR), and the African Studies Center at The University of Michigan (MLW). We thank Miriam K. Laufer, University of Maryland School of Medicine, and Peter S. Larson, University of Michigan, for helpful discussion of these ideas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, Conway DJ, Cox-Singh J, Matusop A, Ratnam S, Shamsul S, Singh B. Fragmented population structure of plasmodium falciparum in a region of declining endemicity. The Journal of infectious diseases. 2005;191:1558–1564. doi: 10.1086/429338. [DOI] [PubMed] [Google Scholar]
- Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C. Poverty and Health in Malawi. Malawi Medical Journal. 2006;18:5–12. [Google Scholar]
- Chibwana AI, Mathanga DP, Chinkhumba J, Campbell CH., Jr Socio-cultural predictors of health-seeking behaviour for febrile under-five children in Mwanza-Neno district, Malawi. Malar J. 2009;8:219. doi: 10.1186/1475-2875-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkhumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, San Joaquin M, Sande J, Ali D, Mathanga D. Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malaria J. 2010;9 doi: 10.1186/1475-2875-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizema-Kawesha E, Miller JM, Steketee RW, Mukonka VM, Mukuka C, Mohamed AD, Miti SK, Campbell CC. Scaling up malaria control in Zambia: progress and impact 2005–2008. The American journal of tropical medicine and hygiene. 2010;83:480–488. doi: 10.4269/ajtmh.2010.10-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee M, Fontenille D. Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochem Mol Biol. 2004;34:599–605. doi: 10.1016/j.ibmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Moonen B, Snow RW, Smith DL. How absolute is zero? An evaluation of historical and current definitions of malaria elimination. Malar J. 2010;9:213. doi: 10.1186/1475-2875-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante AA, Lal AA, Ayala FJ. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 1998;149:189–202. doi: 10.1093/genetics/149.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem RG, Phillips AA, Targett GA, Snow RW. Call to action: priorities for malaria elimination. Lancet. 2010;376:1517–1521. doi: 10.1016/S0140-6736(10)61500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton ML, Cheng Q. Interrupting malaria transmission: quantifying the impact of interventions in regions of low to moderate transmission. PLoS One. 2010;5:e15149. doi: 10.1371/journal.pone.0015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. Can malaria be eliminated? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(Suppl 1):S2–5. doi: 10.1016/j.trstmh.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Greenwood BM. Control to elimination: implications for malaria research. Trends Parasitol. 2008;24:449–454. doi: 10.1016/j.pt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gysels M, Pell C, Mathanga DP, Adongo P, Odhiambo F, Gosling R, Akweongo P, Mwangi R, Okello G, Mangesho P, Slutsker L, Kremsner PG, Grobusch MP, Hamel MJ, Newman RD, Pool R. Community response to intermittent preventive treatment of malaria in infants (IPTi) delivered through the expanded programme of immunization in five African settings. Malar J. 2009;8:191. doi: 10.1186/1475-2875-8-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson ML, Gatton ML, Shanks GD, Cheng Q. A large proportion of asymptomatic Plasmodium infections with low and submicroscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malaria J. 2010;9:254. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel M. Towards a research agenda for global malaria elimination. Malar J. 2008;7(Suppl 1):S1. doi: 10.1186/1475-2875-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R, Edwardes M, Coetzee M. Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasit Vectors. 2010;3:122. doi: 10.1186/1756-3305-3-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg. 2010;104:416–422. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–808. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:1089–1094. doi: 10.1016/j.trstmh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am J Trop Med Hyg. 2010;83:1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawi Ministry of Health. Health Management Information Bulletin, Annual Report (2004–2005) 2005a. pp. 1–36. [Google Scholar]
- Malawi Ministry of Health. Malaria Strategic Plan 2005–2010: Scalig up Malaria Control Interventions. Lilongwe, Malawi: 2005b. [Google Scholar]
- Malawi Ministry of Health. Final Evaluation of the Health Sector Programme of Work (2004–2010) Technical Report. 2010a;2:1–23. http://xa.yimg.com/kq/groups/16987450/284173746/name/Malawi+POW+achievements+TR+draft.pdf.
- Malawi Ministry of Health. Health Management Information Bulletin, Annual Report (2009–2010) 2010b. pp. 1–35. [Google Scholar]
- Malawi Ministry of Health. Malawi National Malaria Indicator Survey 2010. 2010c. pp. 1–87. [Google Scholar]
- Mathanga DP, Campbell CH, Jr, Vanden Eng J, Wolkon A, Bronzan RN, Malenga GJ, Ali D, Desai M. Comparison of anaemia and parasitaemia as indicators of malaria control in household and EPI-health facility surveys in Malawi. Malar J. 2010;9:107. doi: 10.1186/1475-2875-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathanga DP, Campbell CH, Taylor TE, Barlow R, Wilson ML. Socially marketed insecticide-treated nets effectively reduce Plasmodium infection and anaemia among children in urban Malawi. Trop Med Int Health. 2006;11:1367–1374. doi: 10.1111/j.1365-3156.2006.01684.x. [DOI] [PubMed] [Google Scholar]
- Mathanga DP, Luman ET, Campbell CH, Silwimba C, Malenga G. Integration of insecticide-treated net distribution into routine immunization services in Malawi: a pilot study. Trop Med Int Health. 2009;14:792–801. doi: 10.1111/j.1365-3156.2009.02295.x. [DOI] [PubMed] [Google Scholar]
- Mathanga DP, Walker ED, Wilson ML, Ali D, Taylor TE, Laufer MK. Malaria control in Malawi: current status and directions for the future. Acta Tropica. 2011 doi: 10.1016/j.actatropica.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH. From malaria control to eradication: The WHO perspective. Trop Med Int Health. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J. 2008;7(Suppl 1):S11. doi: 10.1186/1475-2875-7-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010a;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen B, Cohen JM, Tatem AJ, Cohen J, Hay SI, Sabot O, Smith DL. A framework for assessing the feasibility of malaria elimination. Malar J. 2010b;9:322. doi: 10.1186/1475-2875-9-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msyamboza KP, Savage EJ, Kazembe PN, Gies S, Kalanda G, D’Alessandro U, Brabin BJ. Community-based distribution of sulfadoxine-pyrimethamine for intermittent preventive treatment of malaria during pregnancy improved coverage but reduced antenatal attendance in southern Malawi. Trop Med Int Health. 2009;14:183–189. doi: 10.1111/j.1365-3156.2008.02197.x. [DOI] [PubMed] [Google Scholar]
- Mzilahowa T, Ball AJ, Bass C, Morgan JC, Nyoni B, Steen K, Donnelly MJ, Wilding CS. Reduced susceptibility to DDT in field populations of Anopheles quadriannulatus and Anopheles arabiensis in Malawi: evidence for larval selection. Med Vet Entomol. 2008;22:258–263. doi: 10.1111/j.1365-2915.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- National Malaria Control Program. Malawi National Malaria Indicator Survey. Ministry of Health; Lilongwe Malawi: 2010. [Google Scholar]
- National Statistical Office. Malawi Demographic and Health Survey 2010. 2010. [Google Scholar]
- Ouedraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nebie I, Roeffen W, Verhave JP, Luty AJ, Sauerwein R. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One. 2009;4:e8410. doi: 10.1371/journal.pone.0008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra K, Barik TK, Reddy BP, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108:757–779. doi: 10.1007/s00436-010-2232-0. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH. The chequered history of malaria control: are new and better tools the ultimate answer? Ann Trop Med Parasitol. 2006;100:647–662. doi: 10.1179/136485906X112185. [DOI] [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- Roberts L, Enserink M. Malaria. Did they really say ... eradication? Science. 2007;318:1544–1545. doi: 10.1126/science.318.5856.1544. [DOI] [PubMed] [Google Scholar]
- Roll Back Malaria Partnership. Global Malaria Action Plan. 2008. p. 271. [Google Scholar]
- Rowe AK, Kachur SP, Yoon SS, Lynch M, Slutsker L, Steketee RW. Caution is required when using health facility-based data to evaluate the health impact of malaria control efforts in Africa. Malaria J. 2009;8:209. doi: 10.1186/1475-2875-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabot O, Cohen JM, Hsiang MS, Kahn JG, Basu S, Tang L, Zheng B, Gao Q, Zou L, Tatarsky A, Aboobakar S, Usas J, Barrett S, Cohen JL, Jamison DT, Feachem RG. Costs and financial feasibility of malaria elimination. Lancet. 2010;376:1604–1615. doi: 10.1016/S0140-6736(10)61355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee RW, Campbell CC. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malaria J. 2010;9:299. doi: 10.1186/1475-2875-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31:560–573. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M, de Savigny D. Malaria eradication back on the table. Bull World Health Organ. 2008;86:82. doi: 10.2471/BLT.07.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010;376:1579–1591. doi: 10.1016/S0140-6736(10)61301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Diagnoses Diagnostics. A Research Agenda for Malaria Eradication: Diagnoses and Diagnostics. PLoS Med. 2011;8:e1000396. doi: 10.1371/journal.pmed.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Drugs. A Research Agenda for Malaria Eradication: Drugs. PLoS Med. 2011;8:e1000402. doi: 10.1371/journal.pmed.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Health Systems Operational Research. A Research Agenda for Malaria Eradication: Health Systems and Operational Research. PLoS Med. 2011;8:e1000397. doi: 10.1371/journal.pmed.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Modeling. A Research Agenda for Malaria Eradication: Modeling. PLoS Med. 2011;8:e1000403. doi: 10.1371/journal.pmed.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Monitoring E Surveillance. A Research Agenda for Malaria Eradication: Monitoring, Evaluation, and Surveillance. PLoS Med. 2011;8:e1000400. doi: 10.1371/journal.pmed.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Vaccines. A Research Agenda for Malaria Eradication: Vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Vector Control. A Research Agenda for Malaria Eradication: Vector Control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Program. Human Development Report 2010: The Real Wealth of Nations: Pathways to Human Development. 2010. [Google Scholar]
- United Nations Human Settlement Programme. Country Programme Document 2008 – 2009 - Malawi. 2008. pp. 1–22. [Google Scholar]
- World Health Organization. WHO Expert Committee on Malaria. Sixth report. 1956:10–11. [PubMed] [Google Scholar]
- World Health Organization. Malaria elimination: A field manual for low and moderate endemic countries. 2007. World Health Organization; Geneva, Switzerland: 2007. pp. 1–85. [Google Scholar]
- World Health Organization. Global malaria control and elimination: Report of a technical review. 2008. 47 pages. [Google Scholar]
- World Health Organization. World malaria report. World Health Organization; Geneva, Switzerland: 2010. p. v. [Google Scholar]
- World Health Organization. World Health Statistics 2011. 2011. p. 170. [Google Scholar]