Abstract
Since ion channels move electrical charge during their activity, they have traditionally been studied using electrophysiological approaches. This was sometimes combined with mathematical models, for example with the description of the ionic mechanisms underlying the initiation and propagation of action potentials in the squid giant axon by Hodgkin and Huxley. The methods for studying ion channels also have strong roots in protein chemistry (limited proteolysis, the use of antibodies, etc). The advent of the molecular cloning and the identification of genes coding for specific ion channel subunits in the late 1980’s introduced a multitude of new techniques with which to study ion channels and the field has been rapidly expanding ever since (e.g. antibody development against specific peptide sequences, mutagenesis, the use of gene targeting in animal models, determination of their protein structures) and new methods are still in development. This review focuses on techniques commonly employed to examine ion channel function in a electrophysiological laboratory. The focus is on the KATP channel, but many of the techniques described are also used to study other ion channels.
Introduction
ATP-sensitive (KATP) channels are expressed in diverse cell types [1]. Their activity is characteristically and principally regulated by the concentrations of intracellular nucleotides. A decrease in cytosolic ATP:ADP ratio activates the channel. Molecularly, KATP channels are composed of pore-forming subunits belonging to the inward rectifying K+ (Kir) channel family, as well as regulatory sulfonylurea receptors (SUR), belonging to the ABC transporting family of membrane proteins [2]. The KATP channel is thought to be a hetero-octameric complex of four Kir6 subunits (Kir6.1 or Kir6.2, encoded by the KCNJ8 and KCNJ11 genes), and four SUR subunits: SUR1 (encoded by ABCC8 gene) or SUR2A/SUR2B (encoded by the ABCC9 gene) [3]. Although the cardiac ventricular KATP channel is considered to be composed of Kir6.2/SUR2A subunits (Table 1), an increased importance to other subunits (e.g. Kir6.1 and SUR1) has recently emerged [4–5]. The purpose of this treatise is not to review the physiology, molecular or disease aspects of KATP channels and the reader is referred to recent reviews on these topics [3, 6]. Instead, this review will focus on the methodology commonly used to measure KATP channel function and composition.
Table 1.
Subunit composition of KATP channel subunits in various cell types of the cardiovascular system.
Electrophysiological approaches
Patch clamp recordings are most frequently used to investigate KATP channel properties and function. Microscopic recordings of unitary channel events provide much information regarding the channel, including its unitary conductance, dwell time kinetics and bursting behavior. Macroscopic KATP channel activity can also be recorded in the whole-cell recordings patch clamp configuration. Each of these recording techniques has their own advantages and disadvantages. Isolated patch experiments provide the most detailed information of the channel properties, but should be weighed against the shortcoming that data are obtained in a cell-free environment and in the absence of physiological modifiers of channel behavior. The opposite argument is true for cell-attached or whole-cell recordings. These techniques are best combined to approximate the physiological function of the channel.
Biophysical properties
The biophysical characteristics of the KATP channel are best obtained at the unitary channel level (excised or cell-attached patches). The analysis of unitary currents is described in some detail in a chapter [7] of a book that is a mandatory addition to the bookshelf of any cellular electrophysiologist, and full details will not be repeated here.
Measurement of the unitary conductance
The unitary current amplitude, defined as the current flowing when a single KATP channel opens, is most often measured with patch-clamp recordings made in the inside-out or cell-attached configurations. By plotting the unitary current as a function of the pipette potential (which can be changed by step increments or by using a slow voltage ramp protocol), one can estimate the unitary conductance. Since the KATP channel has weak inward rectifying properties, the unitary conductance is often determined as the slope conductance in a negative (and linear) range of membrane potentials. This method is advisable and is preferred over the practice of estimating the unitary conductance as the chord conductance (i.e. calculating the conductance from channel openings at a single, fixed voltage), which may not accurately account for rectifying properties of the channel (see also [8]). At any given voltage, the unitary current is most easily obtained by constructing an all-points histogram. This function is standard in most commercial patch clamp analysis software. This method is rather crude but is much more accurate than estimating the unitary current using cursors on a computer screen, which is subject to considerable variation due to the presence of noise in the recordings. The data trace (usually 10–20s of recording) should be baseline subtracted to remove baseline drift before analysis proceeds. The all-points histogram is subjected to curve fitting to a multiple Gaussian function and the unitary current is estimated as the differences between peak values. The unitary conductance of the ventricular KATP channel has been estimated to be ~78pS with high (140 mM) symmetrical K+ concentrations [9]. The dependence of the unitary conductance (γ) on the “extracellular” K+ concentration ([K+]o) can be approximated as:
| (1) |
The unitary current can also be estimated from macroscopic currents obtained in the whole-cell or excised patch configurations using a technique called “variance analysis”, but this is a poor substitute for the methods described above. Fully described elsewhere [10], the basis of this method is to record both the average (I) and variance (σ2) of the macroscopic current under conditions that vary the number of open channels. The unitary conductance is determined as the slope of a plot of the variance as a function of the mean current.
The open probability
The open probability (Po) is estimated as the fraction of time that a channel spends in the open state, divided by the total duration of the recording. The intra-burst open probability of the ventricular KATP channel is high (>0.9) directly after patch excision and declines progressively as the channel runs down, presumably due to membrane PIP2 depletion. The most accurate assessment of open probability (Po) is made after event detection [7]. This is only possible if (at most) only a few channels are active in a patch. The time (ti) spent at each current level corresponding to i = 0, 1, 2,… N open channels is used to calculate Po as follows (where N is the number of channels active in the patch and T is the duration of recording):
| (2) |
The open probability can also be estimated from all-points histograms constructed from segments of raw data recordings where individual channel openings (usually fewer than 5–6) can be discerned. It is imperative for baseline and drift correction to be performed before constructing the histogram and fitting to a sum of multiple Gaussian distributions. From the area (Ai) of each current level of channel opening (i from 0 …. N; with N the number of apparent open channels), the Po is calculated as:
| (3) |
It should be noted that open probability is often expressed as N.Po due to the uncertainty with the number of channels present in the patch that are available for opening. KATP channels are expressed at high density in cardiac myocytes (10–12 channels/patch [11] and in our experience many more, depending on the patch electrode resistance) and individual channel openings can therefore often not be discerned. Nevertheless, it is still possible to estimate N.Po in these patches by dividing the mean patch current by the unitary conductance (measured in a separate experiment or estimated from equation [1]).
Dwell time kinetics and busting behavior
Dwell time analysis should ideally be performed using recordings with only one active channel per patch. This is difficult to achieve with ventricular KATP channels because of their high expression density (3–5 channels/µm2). The use of high resistance patch electrodes (8–12 MΩ) may help to minimize the average number of channels per patch. Data should be filtered (8-pole, low-pass, Bessel response; e.g. at 2 kHz) before being acquired to disk. The sample rate should be 5–10 times higher than the filter frequency (e.g. 20 kHz) to avoid aliasing and to satisfy the Nyquist criterion. Channel event detection is performed using a 50% threshold method [7], making sure that the detection threshold is at least three times the standard deviation of the noise (not usually a problem for a large conductance channel such as the KATP channel). Due to the limited bandwidth (time resolution) of the recording system, short openings and closings cannot be reliably detected. If left uncorrected then the brief closings within a burst are missed, resulting in an overestimate of the mean open time. A correction for these missed events must therefore performed [12–14]. The “dead time” of the recording system can be directly measured using a waveform generator [15] or estimated as 0.253/f, where f is the filter cut-off frequency [16] (e.g. ~150 µs when filtering at 2 kHz).
Event detection produces a dataset consisting of a list of channel dwell (open and closed) times. A histogram is produced for each of the closed or open states, where the abscissa depicts event duration (binned) and the ordinate the number of events within that range. Note that the first bin should start at the time equal to the “dead time” (e.g. 150 µs; see above), since there are no observations present below this value. Channel dwell time is exponential in behavior and the resulting histogram can be subjected to curve fitting to a sum of multiple exponential functions. The time constants of the exponential distributions are the mean open/closed times. The mean open time of the cardiac KATP channel is estimated to be around 2.5 ms, whereas the closed state can best be described by a sum of multiple exponential functions, with time constants ranging from around 0.5 to 3500 ms [16–19].
Bursting behavior is a typical feature of KATP channel activity. Bursts are defined as groups of rapid opening and closings, separated by longer closing events. A critical time (tc), which separates bursts from each other, is specified [20–21], which is usually around 2.5–3.0 ms for KATP channels [17, 22–23]. The intraburst channel behavior (number of events per burst, mean burst duration) is calculated by omitting closed periods exceeding tc, and the interburst closed times are estimated by ignoring closing events shorter than the critical time.
Channel rundown
In excised patches, the activity of KATP channels diminishes with time after patches excision; a phenomenon named “rundown” [24]. The rate of run-down is variable and is partially prevented by MgATP [24–25]. The KATP channel rundown is by prolonged exposure to high MgATP, which is thought to act via membrane PIP2 alterations [3, 26]. In practice, run-down cannot be avoided, but can be minimized by using Mg2+-containing bath solutions or intermittently exposure to high MgATP concentrations after patch excision. We typically discard data from patches exhibiting >20% rundown. In other patches, the current reduction due to rundown should be corrected during analysis (e.g. by normalizing to the current recorded in zero ATP at various times during the recording [27]).
Regulation of channel activity
Regulation by nucleotides
A hallmark feature of KATP channels is their sensitivities to cytosolic nucleotides. Their activity is blocked by free ATP, but increased by MgATP and MgADP. ATP sensitivity is measured with excised patches in the inside-out configuration. Typically, different (6–8) ATP concentrations are applied with a device that allows rapid exchanges of solutions at the cytoplasmic side of membrane. Care should be taken to avoid channel rundown (by keeping recordings as short as possible) and/or correcting for any rundown that might have occurred. Current (I) is measured and expressed as a function of the maximal current recorded in the absence of ATP (Io), plotted as a function of the ATP concentration and data are subjected to curve fitting to a modified Hill equation:
| (4) |
Values for the ATP concentration that cause half-maximal inhibition (IC50) and the slope of the ATP response (n) are in the range of 15–100 µM and ~1.5–2.0 for ventricular myocytes [11, 28–29]. ADP also blocks the KATP channel, but concentrations needed (IC50 in the mM range; [29]) exceeds cytosolic levels and this mode of channel regulation is not likely to be pathophysiologically relevant. The KATP channel activity is powerfully stimulated by MgADP. Using methods similar to that describe above, the degree of ADP stimulation is typically measured in the presence of ATP (at a concentration that produces submaximal block) [30–31]. The net effect of MgADP therefore appears as a change of the ATP-sensitivity (rightward shift of the IC50).
Counting channels
Sometimes it is necessary to estimate the number of functional (open) KATP channels. A variety of techniques can be used, each with its own limitations.
Whole-cell recordings
The number of active KATP channels can be estimated from whole-cell recordings (or cell-attached patches) by measuring the KATP channel current density (current corrected for the electrically active cell surface; pA/pF). The idea is to open all available KATP channels at maximal open probability. However, this goal is not easily attained. Interventions commonly used include metabolic inhibitors (such as 2-deoxyglucose plus NaCN or dinitrophenol) [32–33], but is unclear whether these interventions activate all available KATP channels. A significant problem with this approach is that the KATP channel is very large and series resistance errors may prevent adequate patch clamp voltage control [34]. The KATP channel current may therefore be underestimated. Low resistance electrodes should be used, the access resistance should be low and every attempt should be made to electronically compensate the series resistance.
Excised patches
The KATP channel density can also be estimated from recordings made with excised membrane patches (inside-out configuration). In this case, the number of channels per patch can be estimated by Gaussian fitting of all-points amplitude histograms. In our experience, however, a typical membrane patch contains too many channels (upwards of 40) to make this method practical. By dividing the mean patch current by the unitary current, the value N.Po can be obtained, which reflects the number of channels (N) per patch (assuming a high, but constant open probability; Po). Given that the free patch area of a 2–3 MΩ pipette is estimated to be about 10µm2 [35], (or 0.01pF), the channel density can easily be calculated. The disadvantage of this approach is that Po is never unity and that it decreases over time as the channel runs down following patch excision. The channel density may therefore be underestimated.
Noise analysis
“Variance analysis” (see earlier) can also be employed as a technique to estimate the number of channels and/or the unitary conductance [10, 36]. Under conditions when channel opening is varied, the current variance (σ2) is plotted against the mean current (I), which results in a bell-shaped curve. The current (I) at the maximal variance is given by i.N/2, with i the unitary current and N the number of channels. The N can also be calculated when the variance becomes zero as N=I/i. Examples where non-stationary noise analysis has been used to estimate channel conductance and channel number exist for the smooth muscle KATP channel [37–39] as well as heterologously expressed KATP channels [40–41]. A direct comparison of this technique with unitary current analysis indicated that the unitary conductance (and therefore channel number) may be seriously underestimated using this technique [42].
Expression and biochemical modulation of KATP channel subunits
The electrophysiological properties, regulation and function of KATP channels are determined largely by their molecular composition. It is therefore often necessary to identify the tissue and cellular distribution and expression levels of the individual KATP channel subunits. Moreover, channel function is strongly modulated by post-translational modifications, which makes this an important topic of investigation.
Determining mRNA distribution and expression levels
Northern blotting
KATP channel subunit mRNA expression has been quantified by Northern blot analysis, in which mRNA is extracted from the tissue/cells of interest, fractionated by size with denaturing agarose gel electrophoresis and transferred to a membrane. The membrane is then hybridized with labeled DNA probes. Northern assays have the advantage to provide information regarding the transcript size, which is particularly relevant when examining splice variants. Additionally, it provides a direct comparison of mRNA abundance in different tissues or experimental conditions. A caveat is that the RNA should be of high quality. Northern assay has been successfully used for the detection of Kir6.1, Kir6.2, SUR1 and SUR2 subunits with probes consisting of either full-length cDNA [43] or fragments of DNAs [44–46].
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR is a sensitive method of detecting transcript expression. The mRNA is reverse transcribed and subunit and species-specific primers are used to amplify a region of the cDNA. The degree of amplification is proportional to the amount of starting material. A disadvantage of the method lies in the non-linear nature of the PCR reaction, which provides challenges when attempting to estimate comparative expression levels. This potential problem can be mitigated by keeping the number of PCR cycles low (to work within the exponential phase of amplification) or by using competitive RT-PCR for quantification purposes, for example as used to detect Kir6.2 and SUR mRNA expression levels in rat pancreatic islets [47]. An advantage includes the fact that the method allows for the detection of different size amplicons, which allows for duplex RT-PCR. It is also possible to use RT-PCR to distinguish between different splice variants, for example to distinguish between SUR2A and SUR2B subunits, which originate from a single gene (ABCC9) and differ from each other only in the C-terminal. With appropriate primer design, the presence of the alternatively spliced exon can readily be detected (for examples see Table 2). Consideration should be given on the variations of the SUR2 splicing in different species (see Figure 1). Semi-quantitative real-time RT-PCR is a more sensitive and reliable assay for detecting mRNA abundance than conventional RT-PCR [48]. Due to the fact that PCR efficiency is not perfect, primer-dependent and changing during the course of a PCR assay, care should be taken with both methods when comparing mRNA expression of different transcripts, unless a standard curve is generated for quantification purposes.
Table 2.
Primer sequences which have been used to discriminate between SUR2A and SUR2B transcripts by conventional RT-PCR
| Forward Primer | Reverse Primer | Product size (base pairs) |
Ref. |
|---|---|---|---|
| 5’-CCAGCTGAAGAATATGGTCAAA-3’ mouse exon 37 |
5’-CAGGTCTGCAGTCAGAATGG-3’ mouse exon 40 |
447 for SUR2A 271 for SUR2B |
[27] |
| 5’-ATCATGGATGAAGCCACTGC-3’ mouse exon 38 |
5’-AACGAGGCAAACACTCCATC-3’ mouse exon 40 |
357 for SUR2A 181 for SUR2B |
[58] |
| 5’-ATATGGTCAAATCTCTACCTGGAGG-3’ human exon 35 |
5’-GTTGGTCATCACCAAAGTGGAAAAG-3’ human exon 38 |
394 for SUR2A | [133] |
| 5’-ATATGGTCAAATCTCTACCTGGAGG-3’ human exon 35 |
5’-CATGTCTGCGCGAACAAAAGAAGC-3’ human exon 38 |
397 for SUR2B | [133] |
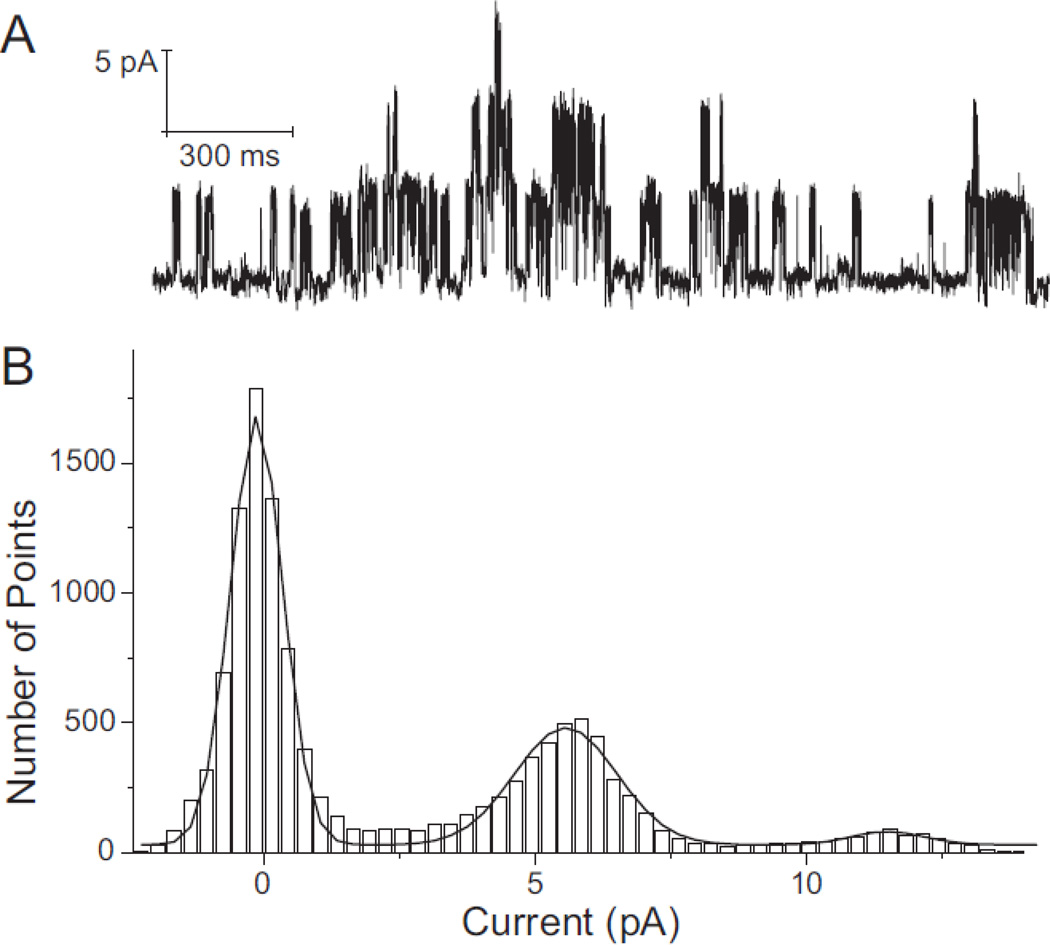
Figure 1.
Typical all-points amplitude histogram of the KATP channel. A: Recording of KATP channel activity in an inside-out membrane patch from a rat ventricular myocyte using symmetrically high (140mM) K+ concentrations in the pipette and bath solutions. The recording, which contains three active KATP channels, was made at room temperature at a membrane potential of −80 mV. Inward currents are shown as upward deflections. B: An all-points histogram of the current segment shown in Panel A, with bin width set at 0.3 pA. The solid line is a sum of three Gaussian functions fitted to the experimental data. The distance between peaks is ~5.8 pA, which represents the unitary amplitude of the KATP channel.
Microarray approaches
DNA microarray analysis utilizes thousands of DNA probes that are immobilized on a microchip. The mRNA of the tissue of interest is reverse transcribed to cDNA, labeled and hybridized with the probes. The probe-target hybridization is detected (usually with fluorescence) and quantified to provide information about the abundance of each target cDNA. Although this method does not specifically target KATP channel subunits, it can be used as an initial estimate of expression levels [49–50], which should then be followed by more robust methods. A major disadvantage is that microarrays selects against transcripts with low expression levels and ion channel subunits are often undetected due to the low signal-to-noise ratio inherent with this method.
Protein expression
Western blotting
Standard Western blotting techniques are commonly used to detect KATP channel subunit protein expression. Protein extracts are size fractionated using SDS PAGE, transferred to a membrane and immunoblotted with subunit-specific antibodies. KATP abundance in most tissues is relatively low and methods should be employed that allow for enrichment. Preparations commonly used are crude membranes [51–52], plasma membranes [33, 53] or organelles [54]. Our experience is that the common practice of boiling the sample before electrophoresis should be avoided (see also [55]), most likely because of precipitation or aggregation due to the hydrophobic nature of these proteins. In our experience, the use of wet transfer with a Tris/glycine buffer supplemented with 0.01% SDS gives the best transferring results for the SUR subunits. For increasing the sensitivity of the detection, transfer of proteins on PVDF membranes is usually preferred over nitrocellulose membranes. Both semi dry and wet transfer methods can be used, although wet transfer usually facilitates the transfer of proteins larger than ~100kD, such as the SUR subunits of the KATP channel.
Success of Western blotting is highly dependent on the specificity and sensitivity of the antibodies used to detect the KATP channel subunits. Development of highly specific KATP antibodies has been a great challenge for many years and a subject of debate among investigators of the field. Up to now, many antibodies are commercially available, although many laboratories prefer antibodies that they have produced and tested. We and others found the commercial G16 and M19 antibodies (Santa Cruz) respectively to detect the Kir6.2 and SUR2A subunits [56–62]. We have also had success using anti-Kir6.2 antibodies developed by us (e.g. W62 and C62 [43, 90]), an anti-Kir6.2 antibody developed by Dr. Hon-Chi Lee (Mayo Clinic, Rochester, MN) [27, 63–64] and the BNJ2 anti-SUR2A antibody developed by Dr Jon Makielski (University of Wisconsin, Madison, WI) [65]. Testing antibodies against mouse knockout tissues has revealed interesting observations. For example, we found that native Kir6.2 protein does not only migrate at the expected size of ~37kDa, but that several other bands at higher molecular weights are observed. These bands are specific since they are absent in cardiac membrane fractions prepared from a Kir6.2 KO mouse (Figure 3). The identities of those bands remain uncharacterized, but it is possible that they represent complexes of Kir6.2 with other proteins, that are resistant to the reducing and denaturating conditions used in our laboratory. Neuromab1 (a monoclonal antibody-generating resource funded by NIH) is now developing monoclonal KATP subunits antibodies, which should assist in a better characterization of KATP channel proteins.
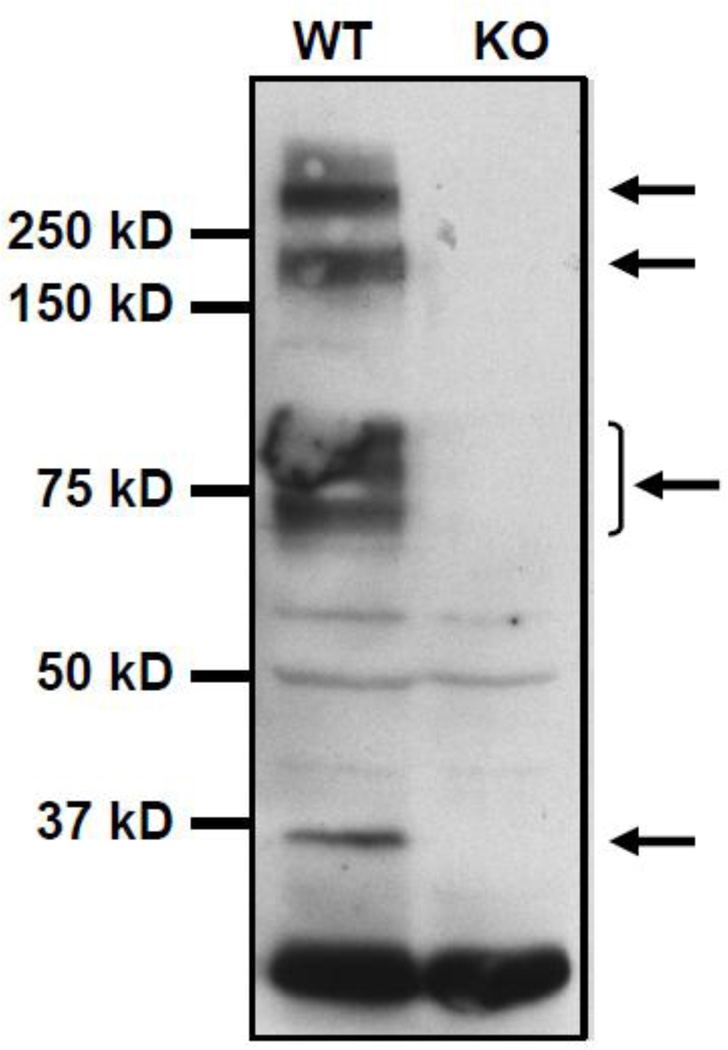
Figure 3.
Characterization of mouse cardiac Kir6.2 subunits by Western blotting. Heart membrane extracts from wild type (WT) and knock out (KO) mice were prepared and the expression of Kir6.2 was examined with SDS-PAGE using the C62 antibody developed by us against the peptide Ac-CVAKAKPKFSISPDSLS-OH (corresponding to the C-terminal 15 amino acids of human, mouse, rat, dog, bovine and horse Kir6.2. Note that samples were not heated before loading (see text). A specific band (i.e. present in the sample from the WT but not the KO mouse) was detected approximately at the level of 37 kD marker (Precision Plus Protein Kaleidoscope Standards, #161-0375, BioRad). This is a band commonly used for the study of this protein. However it is notable that higher order bands of high intensity were also present (arrows). These bands are specific since they were absent in cardiac membranes prepared from Kir6.2 knockout mice.
Binding to sulphonylureas
The levels of SUR subunits have also been estimated using sulphonylureas that are fluorescently labeled. Epifluorescence imaging of cells incubated with these substances followed by signal quantification analysis may provide information of SUR protein abundance in the cells [66] or subcellular localization of KATP channels [67]. The major advantage of this method versus Western blotting is that it is not dependent on antibody specificity. Disadvantages include the low quantification ability, the possible non KATP-specific binding of the labeled sulphonylureas and that it cannot discriminate among the different SUR subunits. Non-specific staining [68] and accumulation inside intracellular organelles of these dyes is problematic (e.g. BODIPY® FL glibenclamide is sold as a marker of the endoplasmic reticulum).
Post-translational modifications
The SUR1 subunit of the KATP channel has been shown to be subjected to N-linked glycosylation (N10 and N1050) in the Golgi, a modification that regulates its trafficking to the membrane [69–71]. There are at least three more types of post-translational modifications of the KATP channel reported. These include Serine /Theonine phosphorylation of Kir6.1, Kir6.2, SUR1 and SUR2B [72], S-nitrosylation of SUR1 (in heterologous expression systems [73] and S-glutathionylation of Kir6.1 subunit (in heterologous expression system and vascular smooth muscle cells [74]). Standard biochemical methods have been used for the detection of those modifications since KATP channel-specific tools are not yet available (such as phospho-specific antibodies).
Tissue distribution and subcellular localization
mRNA tissue distribution
mRNAs encoding Kir6.x and SURx isoforms have been detected in the heart using in situ hybridization techniques, including autoradiography on whole fixed and paraffin embedded mouse embryos [75] and riboprobe methodology on frozen sections of fixed tissues [76]. The latter method, however, may not be as sensitive, as it failed to show expression of SUR2B in myocardium, in contrast to the autoradiography study, Northern blot analyses, and immunolocalization studies.
Subcellular localization of proteins
Immunofluorescence and light microscopy
Native KATP channel subunits have been localized in dissociated cardiomyocytes or cultured neonatal myocytes by fluorescence and by confocal microscopy [53, 62, 77–81]. Prior to immunostaining using standard techniques, cells were fixed in paraformaldehyde and permeabilized with methanol or Triton X-100, depending on the properties of the antibodies used. Alternatively, cells were fixed/permeabilized in 1:1 methanol/acetone [77]. The major consideration in these and all other localization studies is the specificity and quality of the antibodies. KATP channels have also been localized in sections of heart tissue. Cryosections prepared from paraformaldehyde-fixed cardiac tissue have been stained for confocal microscopy using antibodies to Kir6.1 and Kir6.2 [61, 80]. Conventional horseradish peroxidase (HRP) staining methods have been applied to cryosections (anti-SUR antibodies) and paraffin sections (anti-Kir6.x antibodies) [78, 82]. One caveat is the poor resolution of HRP staining at the light microscopic level.
Immunoelectron microscopy
Cryosections stained with HRP-coupled secondary antibodies for light microscopy have also been post-fixed with osmium, embedded in resin, and sectioned for visualization at the EM level [61, 82]. In these studies, Kir6.x and SUR2x were found in the ER, mitochondria, and sarcolemma of cardiomyocytes. The major problem with this method is that HRP is not particularly quantitative at the EM level and can diffuse. A more quantitative method using ultrathin frozen sections of paraformaldehyde-fixed cardiac tissue has also been employed to localize KATP channels. The methods for preparing ultrathin frozen sections and immunostaining using colloidal gold coupled secondary antibodies are essentially the same as those used for other tissues [83]. The results of channel localization have been controversial as the sarcolemmal Kir6.1 and Kir6.2 subunits were reported also to be present in mitochondria in one study [61], while another study concluded that the localization of Kir6.1 to mitochondria was due to antibody cross-reactivity with mitochondrial proteins [80].
Modulation of KATP channel expression by siRNA
In many studies it is important to modulate the expression levels of the KATP channel subunits. The method of siRNA has been successfully employed to eliminate the expression of the KATP proteins in heart cells. This includes the use of small RNA molecules (siRNA oligonucleotides) that are designed to specifically bind the mRNA of the subunit of interest, resulting in the reduction of the translation of this transcript. The advantage of the direct down-regulation of the protein is that it may provide evidence for the functional roles of the protein under different experimental conditions. For example, siRNA oligonucleotides targeting Kir6.2 and Kir6.1 have been used to demonstrate a role for the KATP channel in hypoxic preconditioning in H9c2 cells [54] and silencing of SUR2 in rat neonatal cardiomyocytes has been used to demonstrate the role of KATP channel in the expression of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α [84]. Disadvantages include the possible non specific action of the siRNA oligonucleotides utilized and that the method can be applied only in in vitro models (cells in culture).
Trafficking of KATP channel subunits
Cell Fractionation
Cell Fractionation has been used to study the subcellular localization and trafficking of KATP channels both on ventricular tissue [33, 85] and on isolated cardiac myocytes [86]. Differential centrifugation or density gradient methods were used to separate plasma membranes from endosomes and mitochondria. The differential centrifugation method is based on the fact that mitochondria sediment at relatively low (4–10,000g) rate centrifugal force (RCF) while plasma membranes are recovered at intermediate (20–30,000g) and endosomes and microsomes at higher RCF [78, 85–87]. Preincubation of cardiac tissue in high salt buffers, which dissociate actin filaments and remove some peripheral membrane proteins, prior to homogenization improves separation [87]. Bao et al [33] combined high salt preincubation with an Optiprep step gradient protocol to separate sarcolemma and endosomal fractions [88]. Sucrose step gradients have also been used to prepare a sarcolemma membrane fraction containing KATP channels [62]. The purity of cellular organelles prepared from cardiac tissue in which actin/myosin filaments predominate has been a major challenge and the source of controversy in the field, especially with regard to whether sarcolemmal KATP channel subunits are present in mitochondria [see [89]].
Caveolae are cholesterol and sphingolipid-enriched invaginations of the plasma membrane involved in endocytic and signal transduction events. Because of their light density, they can be separated from most other cellular membranes on sucrose step gradients after removal of peripheral membrane proteins with sodium carbonate [53]. KATP channel subunits were shown to fractionate with caveolar membranes obtained from cardiomyocytes and to interact with caveolar component proteins.
Cell Surface Labeling Techniques
Because antibodies against extracellular epitopes of KATP channel subunits have not been available, detection of native channels at the cell surface using immunological techniques has not been feasible. However, it has been possible to label KATP channels and follow their internalization. Epitope tags or bungarotoxin binding sequences have been introduced into the extracellular loop of Kir6 or at the amino terminus or an internal extracellular sequence of SUR using molecular biological techniques that are beyond the scope of this review. The modified constructs are incorporated into expression vectors or recombinant adenovirus and the proteins expressed exogenously in cardiac myocytes and other cell types [90–92]. Quantitation can then be performed using ELISA assay [90] or the antibodies visualized by confocal microscopy [33, 90, 93–94]. Internalization and recycling of channels can also be observed by similar techniques [92, 95]. Biotinylation is another technique that has been used to quantify cell surface KATP channels, albeit in cells expressing high levels of the channels after transfection [95].
Pulse-chase labeling
Pulse-chase radiolabeling can be used to monitor the rate of transport of SUR subunits out of the endoplasmic reticulum (ER). Briefly, cells are pre-incubated in methionine- and cysteine-free medium (30 min – 2 h) and labeled in the same medium containing [35]S-met and cys (e.g., Translabel from ICN) for 30 min - 1 h. After washing, the cells are incubated in a medium supplemented with unlabeled met and cys (5 mM) for various periods of time, after which cells are solubilized in Tris EDTA buffer with detergents (e.g., 1% Triton X-100, see co-IP section). SUR subunits are visualized on a phosphorimager after immunoprecipitation and SDS-PAGE (8% polyacrylamide or a gradient gel). Because they are N-glycosylated, SUR1 subunits migrate as 2 bands on the gels – an initial band appearing after the pulse and representing the core glycosylated ER form as well as a second, slightly larger band appearing later and corresponding to the terminally glycosylated form that has traversed the Golgi apparatus. Pulse-chase labeling has been informative in characterizing the roles of hsp90 and syntaxin-1A in SUR1 trafficking out of the ER [96–97]. A similar protocol has been used to monitor rates of turnover of KATP channel subunits (e.g. [98]).
KATP channel complexes
It is getting increasingly acknowledged that native KATP channels consist, apart from the channel’s subunits Kir6.x and SURx, of a macromolecular protein complex that regulates their function. KATP interacting proteins can be identified using large scale proteomic assays (such as bacteria/yeast two hybrid systems or mass spectrometry) as well as smaller scale assays (such as co-immunoprecipitation).
Co-immunoprecipitation
This method includes immunoprecipitation (IP) of the subunit of interest under conditions which retain the interaction of the proteins, followed by detection of putative interacting proteins in the immunoprecipitate. The detection method often includes Western blotting, if the possibly interacting protein is known and antibodies against it are available [51], as well as mass spectrometry methods if the interacting protein is not known [99–100]. It is important that appropriate solubilization of the proteins is performed. The method used (detergents, sonication, etc.) should be carefully selected in order to effectively solubilize the proteins of interest without disturbing the possible interactions. For the cardiac KATP channel, solubilization in 1% Triton X100 has been widely used (for example [51, 53, 100]. For the same reason, incubations in high ionic strength buffers should not be included during the sample preparation and IP (a broadly used salt concentration for such experiments is approximately 150 mM, [51, 53, 100]. The success of this method is largely dependent on antibody quality (see also Western blot section). For the verification of the interaction, the use of multiple IP antibodies is suggested as well as use of the reciprocal IP.
Proteomics
Yeast/bacterial two hybrid systems
Two hybrid systems are based on transcription factors that consist of an activating and a DNA binding domain that can function only when they are in close proximity. Plasmids are produced that contain either the protein of interest (bait) fused with one part of the transcription factor or a group of target proteins (pray, often a large cDNA library) fused with the second part of the transcription factor. The bait and pray plasmids are simultaneously introduced into the yeast/bacteria and an interaction between proteins of the bait and pray will result in activation of the transcription factor and subsequent transcription of reporter genes. A bacteria hybrid system has been used to identify interacting proteins of the pore forming subunit of the cardiac KATP channel, Kir6.2 using a truncated version of the protein (residues 169–354) as a bait [51]. Moreover, a yeast two-hybrid system optimized for membrane protein interactions (MbYTH) has been used to verify the interaction of Kir6.2 with GAPDH [101]. Partial rat SUR1 (residues 598–1003) has been also used in screening a pancreatic cell cDNA library [102]. One of the advantages of these methods is that they are not antibody-dependent. However, controlling for false positive and negative reactions might be a point of criticism and the results are usually confirmed by other methods (such as co-IPs).
Mass spectrometry
Mass spectrometry methods are increasingly used in identification of protein interactions because of their sensitivity and the ability to provide quantitative results. When they are used in combination with co-IP assays they may lead to identification of unknown interacting partners of a protein. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analysis has been used to identify creatine kinase and the muscle form of LDH as interacting proteins cardiac KATP channel immunoprecipitates [99–100]. Recently, co-IP followed by tandem mass spectrometry was used to identify putative interacting proteins of the KATP channel in the rat ventricle [64]. Moreover, linear ion trap quadrupole-Orbitrap mass spectrometry was used in combination with two dimensional gel electrophoresis in identifying differentially expressed proteins in KATP channel deficient hearts under control or disease conditions [103–105].
Genetic approaches
Targeting KATP channels at the genetic level, either for overexpression or ablation, has been a powerful approach in studying the roles of diverse KATP channels in a variety of tissues. A full review of data obtained from these genetic approaches is beyond the scope of this paper and the reader is referred to other reviews for this information [106–107]. Two main approaches have been used to date: the first relies on the transgenic overexpression wild-type or mutant KATP channel subunits, and the second relies on gene targeting.
Transgenic mice
A transgenic animal is one in which a foreign gene (often a cDNA under the control of a tissue-specific promoter) has been deliberately inserted into its genome. The genomic insertion of the transgene is a random process. For practical reasons (well-defined genetics, small size, short breeding cycle and low housing costs in comparison to larger vertebrates) mice have become the principal species used for transgensis in biomedical research. Other advantages of transgensis are the lower cost and shorter time to produce offspring compared to other genetic approaches. Disadvantages include the fact that transgene insertion into the genome is random and control over expression is not possible. High expression levels of the transgene and the randomness of insertion may also disrupt the expression and/or function of other genes. Despite these limitations, transgensis has been successfully used to further our understanding of KATP channels in the intact animal (Table 3). For example, pancreatic expression of a pore-mutant, dominant negative or wild-type Kir6.2 subunits [108–109] was shown to affect pancreatic β-cell function. Cardiac-specific overexpression of dominant negative Kir6.x subunits was shown to reduce the tolerance of mice to exercise-induced stress [59]. Cardiac overexpression of ATP-insensitive or wild-type KATP channel subunits lead to diverse and complex phenotypical alterations in KATP channel activity and cardiovascular function [110–111]. We developed transgenic mice that allow tissue-specific expression of a pore-mutant Kir6 subunit (using the Cre-lox technology). These mice have been used to demonstrate that specific endothelial expression results in hypertension and abnormalities in endothelin-1 release [112] and that skeletal muscle-specific expression alters energetics [113].
Table 3.
Genetically modified mice in the study of the KATP channel.
| Transgenesis | ||||
|
Subunit targeted |
Type of modification |
Cell type targeted |
Functions modified/studied | References |
| Kir6.2 | Pore mutant | Pancreatic | Pancreatic β-cell function | [108–109] |
| Kir6.1, Kir6.2 | Pore mutant | Cardiac | Exercise stress, KATP activity | [59] |
| Kir6.2 | ATP insensitive | Cardiac | Cardiovascular function | [110] |
| SUR1, SUR2A | Overexpression | Cardiac | Cardiovascular function | [111] |
| Kir6 | Pore mutant | Endothelial | Hypertension, endothelial endothelin-1 release | [112] |
| Kir6 | Pore mutant | Smooth muscle | Energetics | [113] |
| Gene targeting | ||||
|
Subunit targeted |
Total number of exons |
Exon(s) targeted |
Functions modified/studied | References |
| Kir6.1 | 3 | 3rd | Vascular | [114] |
| Kir6.2 | 1 | 1st | Stress, ischemic preconditioning, insulin release | [115–117] |
| SUR1 | 39 | 1st | Insulin release | [118] |
| SUR1 | 39 | 2nd | Insulin release, cardiac ischemia, atrial KATP | [52, 119–120] |
| SUR2 | 41 | 12th to 16th | Vascular, cardiac ischemia | [65, 121–123] |
Exons are numbered according to the cited references.
Gene targeting
Gene targeting is an approach in which a specific gene is being altered by the process of homologous recombination. Gene targeting is almost exclusively performed in mice. The targeting can take the form of changing the entire gene or just a few key exons. These regions can be deleted (knockout) or replaced with another genetic element (knock-in). Examples of knock-ins include the replacement of a gene with a marker protein (useful for examining the tissue expression of a protein of interest) or with a mutant form of the same protein (useful for determining in-vivo effects of specific human mutations). A major advantage of gene targeting is that a specific gene is deleted, which should have less impact to dysregulate gene expression. For knock-ins, another advantage is that expression is under the control of endogenous promoters/enhancers, which allows for natural control of gene expression. A disadvantage is the high cost associated with gene targeting and the time it takes to generate these mouse models. For KATP channel subunits, the only published examples of gene targeting to date include the generation of knockout mice. Knockout mouse models have been developed in which expression of each of these KATP channel subunits has been eliminated. The availability of these animal models has done much to advance our knowledge of the functions of KATP channels in the in-vivo setting (Table 3). The KCNJ8 gene (Kir6.1) can be found on mouse chromosome 6 at location 142,522,146–142,528,581 and contains 3 exons. The coding region of Kir6.1 is contained in exons 2 and 3. In the Kir6.1 null mouse, the strategy was to delete coding region of exon 3 [114]. Phenotypically, these mice develop vascular problems (they lack vascular KATP channels and develop coronary artery spasms that resemble Prinzmetal (or variant) angina in humans. The KCNJ11 gene (Kir6.2) is found on mouse chromosome 25 at location 19,105,218–19,106,363. It has a single exon containing the entire coding region, which has been targeted for deletion [115]. These knockout mice largely lack the expected hyperglycemic phenotype, but have been used to demonstrate that Kir6.2 is a key regulator of glucose- and sulfonylurea-induced insulin secretion. These mice have also been used (amongst others) to demonstrate a role for sarcolemmal KATP channels in ischemic preconditioning [116] and that KATP channels protect against deleterious effects of stress [117]. The SUR1 subunit contains 17 transmembrane segments. The ABCC8 gene, which codes for SUR1, is found on mouse chromosome 25 at location 19,072,876–19,091,318. It is comprised of multiple exons and several SUR1 splice variants have been described. Two groups have produced SUR1-null mice by deleting exon 1, which contains promoter regions and the starting methionine [118], or exon 2 [119]. Although neither mouse model displayed the expected severe dysregulation of insulin release, they have been useful in helping us to understand the fine tuning of insulin release and other aspects of SUR1 function elsewhere in the body. For example, the SUR1-null mice unexpectedly are protected from cardiac ischemia [120] and they have been helpful in the identification of a novel subtype of KATP channels in mouse atria [52]. The SUR2 subunit is coded by the ABCC9 gene, which is found on mouse chromosome 6 at location 142,546,356–142,659,537. The mouse ABCC9 gene has 40 exons (who of which are untranslated). The two major splice variants (SUR2A and SUR2B) differ from each other in alternative use of exons 39 and 40. In the SUR2 null mice, two exons were targeted that code for regions in the first of the two intracellular nucleotide binding folds [121], which are involved in ATP/ADP binding. Similar to the Kir6.1-nulls, these mice are hypertensive and display coronary vasospasms [122]. These mice are paradoxically protected against ischemia [123], but the data interpretation is complicated by the presence of SUR2 isoforms that are still expressed in these mice [124].
Future directions
Although powerful, existing genetic mouse models have not been able to fully address questions related to the function of KATP channels. If anything, emerging data using these models have served to highlight that we do not fully understand the complexity of KATP channel function, and in particular, the important interactions that may occur between channel subtypes in different tissues. Several groups are currently working on strategies to conditionally knock out KATP channel subunits in a tissue-specific manner. Temporal control of gene deletion can also be useful in some experimental designs, but may be less of an issue since lethality has not been reported to be an issue with the existing knockout mice. Finally, it may be of interest to produce disease-causing knock-in mouse models (e.g. the E23K Kir6.2 variant) to examine the role of polymorphisms and mutations in disease [6].
Computer modeling
A full understanding of ion channel function is often reflected in the maturity of mathematical models describing their function and in the accuracy by which the computational model can predict known and unknown (yet to be tested) behaviors. Some channels are well characterized and excellent models exist; for example, modeling the L-type Ca2+ channel and its regulation by signalling cascades or Ca2+ movements within restricted spaces within a cardiac myocyte. The opening behavior of KATP channels has also been subject to numerical modeling. At the single channel level, KATP channels open in bursts. The closing time between bursts can be described by the summation of several closed states. This complicated state behavior of KATP channel channels is best simulated using Markovian models. The reader is referred to an excellent recent review, which provides a comparative analysis, strengths and weaknesses of several state-dependent numerical models of the KATP channel to describe its ATP-dependent and ATP-independent opening kinetics [125]. Existing models are classified into “independent” models, where at rest each of the four KATP channel pore-forming subunits independently alternate between the active/inactive states and the channel is closed when one or more of the channel subunits enter the inactive state [126–127]. The independent gating of subunits has been questioned [128] and the possibility exists that pore-forming subunits may interact with each other during the interburst open/closed transitions. This possibility is incorporated in the “concerted” models, which assume that all four Kir6.x subunits undergo a simultaneous state transition. These models provide a better description of some KATP channel characterizes, including inhibition of the channel by ATP [125]. Other aspects of KATP channel gating regulation are less well described numerically. For example, the biphasic effects of MgADP, which promotes KATP channel opening at low concentrations but inhibits the channel at higher concentrations, has been modeled for the cardiac KATP channel [129], but has not been widely adopted. Other aspects of KATP channel function, including changes in permeation (e.g. channel rectification induced by polyamines and monovalent cations), regulation by pH and signalling cascades) have largely not been implemented and await future efforts. Numerical models that do not attempt to simulate the KATP channel state dependence also exist [130]. The model of Ferrero et al [130] accounts for the regulation of the KATP channel by several intracellular factors ATP/ADP ratio, pH, Mg2+, and Na+) and is therefore often used in computational simulations of the metabolically impaired cardiac myocyte.
Highlights.
We review methods for measuring KATP channel in cardiac muscle
Methods covered include electrophysiological approaches and mRNA and protein expression
Other methods discussed include trafficking, complex formation and genetic approaches
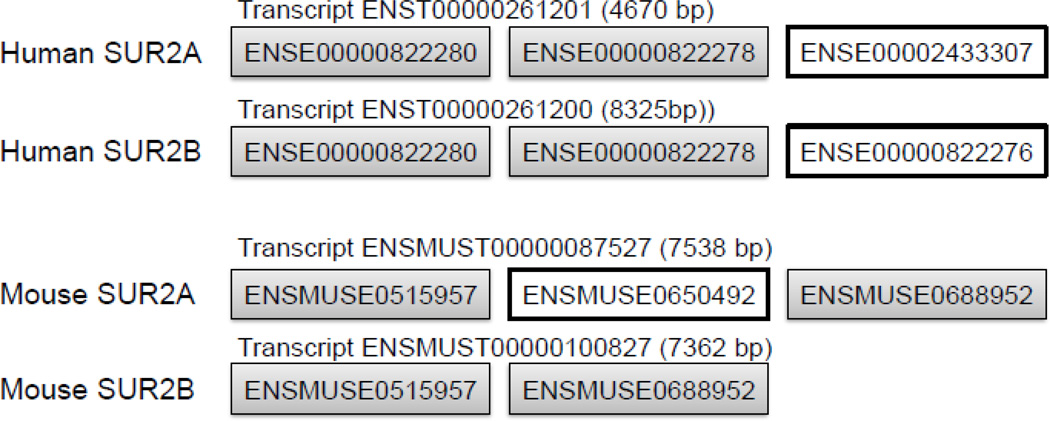
Figure 2.
Species specific considerations in separating the SUR2A and SUR2B transcripts by RT-PCR. The C-termini of the mature mRNA of SUR2A and SUR2B of human and mouse origin are shown (ENSEMBL exon numbering). In order to separate between the two transcripts, different sets of primers need to be used in the case of mRNA of human origin, with the reverse primers designed in the last exon. For the mouse, the same set of primers can discriminate between the two transcripts if the two primers are designed across the alternatively spliced exon. The rat Abcc9 gene has a similar exonic organization as the mouse (not shown).
Acknowledgments
This work was supported by National Institutes of Health grants HL105046 to LB and HL085820 & HL093563 to WAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Ashcroft FM. Adenosine 5'-triphosphate-sensitive potassium channels. Annual Review of Neuroscience. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 2.Coetzee WA, Amarillo Y, Chiu J, Chow A, McCormack T, Moreno H, et al. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440(7083):470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 4.Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea Receptor 1 Subunits of ATP-Sensitive Potassium Channels and Myocardial Ischemia/Reperfusion Injury. Trends Cardiovasc Med. 2009;19(2):61–67. doi: 10.1016/j.tcm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010 Oct;7(10):1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson TM, Terzic A. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010 Jul;460(2):295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigworth FJ, Colquhoun D. Fitting and Statistical Analysis of Single Channel Records. In: Sakmann B, Neher E, editors. Single Channel Recording. 2 ed. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- 8.Thompson SM. Relations between chord and slope conductances and equivalent electromotive forces. Am J Physiol. 1986 Feb;250(2 Pt 1):C333–C339. doi: 10.1152/ajpcell.1986.250.2.C333. [DOI] [PubMed] [Google Scholar]
- 9.Kakei M, Noma A, Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. Journal of Physiology (London) 1985;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinemann SH, Conti F. Nonstationary noise analysis and application to patch clamp recordings. Methods Enzymol. 1992;207:131–148. doi: 10.1016/0076-6879(92)07009-d. [DOI] [PubMed] [Google Scholar]
- 11.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K + channel activity in intact and permeabilized rat ventricular myocytes. Journal of Physiology (London) 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blatz AL, Magleby KL. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion-channel mechanisms. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1995. [Google Scholar]
- 14.Colquhoun D. Practical analysis of single channel records. In: Ogden DC, editor. Microelectrode techniques, The Plymouth workshop handbook. 2nd ed. Cambridge, UK: Company of Biologists; 1994. pp. 101–139. [Google Scholar]
- 15.Li L, Geng X, Drain P. Open state destabilization by ATP occupancy is mechanism speeding burst exit underlying KATP channel inhibition by ATP. J Gen Physiol. 2002 Jan;119(1):105–116. doi: 10.1085/jgp.119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu T, Hoshi T, Weintraub NL, Spector AA, Lee HC. Activation of ATP-sensitive K(+) channels by epoxyeicosatrienoic acids in rat cardiac ventricular myocytes. J Physiol. 2001 Dec 15;537(Pt 3):811–827. doi: 10.1111/j.1469-7793.2001.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alekseev AE, Kennedy ME, Navarro B, Terzic A. Burst kinetics of co-expressed Kir6.2/SUR1 clones: Comparison of recombinant with native ATP-sensitive K+ channel behavior. Journal of Membrane Biology. 1997;159:161–168. doi: 10.1007/s002329900279. [DOI] [PubMed] [Google Scholar]
- 18.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circulation Research. 1998;83(11):1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 19.Fan Z, Gao L, Wang W. Phosphatidic acid stimulates cardiac KATP channels like phosphatidylinositols, but with novel gating kinetics. Am J Physiol Cell Physiol. 2003 Jan;284(1):C94–C102. doi: 10.1152/ajpcell.00255.2002. [DOI] [PubMed] [Google Scholar]
- 20.Magleby KL, Pallotta BS. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clapham DE, Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillis KD, Gee WM, Hammoud A, McDaniel ML, Falke LC, Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1119–C1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- 23.Davies NW, Standen NB, Stanfield PR. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. Journal of Physiology (London) 1992;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findlay I, Dunne MJ. ATP maintains ATP-inhibited K+ channels in an operational state. Pflugers Archives-European Journal of Physiology. 1986;407:238–240. doi: 10.1007/BF00580683. [DOI] [PubMed] [Google Scholar]
- 25.Kozlowski RZ, Hales CN, Ashford MLJ. Dual effects of diazoxide on ATP-K + currents recorded from an insulin-cecreting cell line. British Journal of Pharmacology. 1989;97:1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie LH, Takano M, Kakei K, Noma A. Wortmannin, an inhibitor of phosphatidylinositol kinases, blocks the MgATP-dependent recovery of Kir6.2/SUR2A channels. Journal of Physiology (London) 1999;514(3):655–665. doi: 10.1111/j.1469-7793.1999.655ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao L, Kefalogianni E, Lader J, Hong M, Morley GE, Fishman GI, et al. Unique Properties of the ATP-Sensitive K+ Channel in the Mouse Ventricular Cardiac Conduction System. Circ Arrhythm Electrophysiol. 2011 Oct 9; doi: 10.1161/CIRCEP.111.964643. 2011; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Findlay I, Faivre JF. ATP-sensitive K + channels in heart muscle. FEBS letters. 1991;279:95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- 29.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 30.Findlay I. Effects of ADP on upon the ATP-sensitive K+ channel in rat ventricular myocytes. Journal of Membrane Biology. 1988;101:83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- 31.Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. Journal of Physiology (London) 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circulation Research. 1991;68:280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 33.Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H262–H270. doi: 10.1152/ajpheart.00857.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. In: Rudy B, Iverson LE, editors. Methods in Enzymology. New York: Academic Press; 1992. pp. 101–122. [DOI] [PubMed] [Google Scholar]
- 35.Sakmann B, Neher E. Geometric parameters of pipettes and membrane patches. In: Sakmann B, Neher E, editors. Single-channel recording. New York: Plenum Press; 1995. pp. 637–650. [Google Scholar]
- 36.Alvarez O, Gonzalez C, Latorre R. Counting channels: a tutorial guide on ion channel fluctuation analysis. Adv Physiol Educ. 2002 Dec;26(1–4):327–341. doi: 10.1152/advan.00006.2002. [DOI] [PubMed] [Google Scholar]
- 37.Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol. 1993 May;264(5 Pt 1):C1190–C1200. doi: 10.1152/ajpcell.1993.264.5.C1190. [DOI] [PubMed] [Google Scholar]
- 38.Russ U, Metzger F, Kickenweiz E, Hambrock A, Krippeit-Drews P, Quast U. Binding and effects of KATP channel openers in the vascular smooth muscle cell line, A10. Br J Pharmacol. 1997 Nov;122(6):1119–1126. doi: 10.1038/sj.bjp.0701514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bychkov R, Gollasch M, Ried C, Luft FC, Haller H. Effects of pinacidil on K+ channels in human coronary artery vascular smooth muscle cells. Am J Physiol. 1997 Jul;273(1 Pt 1):C161–C171. doi: 10.1152/ajpcell.1997.273.1.C161. [DOI] [PubMed] [Google Scholar]
- 40.Tammaro P, Ashcroft FM. A mutation in the ATP-binding site of the Kir6.2 subunit of the KATP channel alters coupling with the SUR2A subunit. J Physiol. 2007 Nov 1;584(Pt 3):743–753. doi: 10.1113/jphysiol.2007.143149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005 Sep;54(9):2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- 42.Teramoto N, Brading AF, Ito Y. Possible underestimation of the channel conductance underlying pinacidil-induced K+ currents using noise analysis in pig urethral myocytes. J Pharm Pharmacol. 2000 Nov;52(11):1395–1403. doi: 10.1211/0022357001777397. [DOI] [PubMed] [Google Scholar]
- 43.Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS letters. 1995 Dec 27;377(3):338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 44.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, III, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 45.Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, et al. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270(11):5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 46.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 47.Tokuyama Y, Fan Z, Furuta H, Makielski JC, Polonsky KS, Bell GI, et al. Rat inwardly rectifying potassium channel Kir6.2: cloning electrophysiological characterization, and decreased expression in pancreatic islets of male Zucker diabetic fatty rats. Biochem Biophys Res Commun. 1996 Mar 27;220(3):532–538. doi: 10.1006/bbrc.1996.0439. [DOI] [PubMed] [Google Scholar]
- 48.Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics. 2007;28(3):273–283. doi: 10.1152/physiolgenomics.00163.2006. [DOI] [PubMed] [Google Scholar]
- 49.Tivesten A, Barlind A, Caidahl K, Klintland N, Cittadini A, Ohlsson C, et al. Growth hormone-induced blood pressure decrease is associated with increased mRNA levels of the vascular smooth muscle KATP channel. J Endocrinol. 2004;183(1):195–202. doi: 10.1677/joe.1.05726. [DOI] [PubMed] [Google Scholar]
- 50.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, et al. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J. 2006 Aug;20(10):1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 51.Dhar Chowdhury P, Harrell MD, Han S, Jankowska D, Parachuru L, Morrissey A, et al. The glycolytic enzymes, glyceraldehyde, 3-phosphate dehydrogenase, triose phosphate isomerase and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. Journal of Biological Chemistry. 2005;280(46):38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, et al. Differential Structure of Atrial and Ventricular KATP: Atrial KATP Channels Require SUR1. Circulation Research. 2008;103(12):1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garg V, Jiao J, Hu K. Regulation of ATP-sensitive K+ channels by caveolin-enriched microdomains in cardiac myocytes. Cardiovasc Res. 2009 Apr 1;82(1):51–58. doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- 54.Jiao JD, Garg V, Yang B, Hu K. Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc Res. 2008 Jan;77(1):126–133. doi: 10.1093/cvr/cvm028. [DOI] [PubMed] [Google Scholar]
- 55.Crane A, Aguilar-Bryan L. Assembly, maturation, and turnover of K(ATP) channel subunits. J Biol Chem. 2004;279(10):9080–9090. doi: 10.1074/jbc.M311079200. [DOI] [PubMed] [Google Scholar]
- 56.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, et al. Is the molecular composition of K(ATP) channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the K ATP channel subunits Kir6.1 and Kir6.2. Journal of Molecular and Cellular Cardiology. 2001;33(8):1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida H, Feig J, Ghiu IA, Artman M, Coetzee WA. K(ATP) channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. Journal of Molecular and Cellular Cardiology. 2004;37(4):857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 58.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, et al. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatric Research. 2005;58(2):185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 59.Tong X, Porter LM, Liu G, Dhar Chowdhury P, Srivastava S, Pountney D, et al. Consequences of Cardiac Myocyte-Specific Ablation of KATP channels in Transgenic Mice expressing Dominant Negative Kir6 Subunits. Am J Physiol Heart Circ Physiol. 2006;291(2):H543–H551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tai KK, McCrossan ZA, Abbott GW. Activation of mitochondrial ATP-sensitive potassium channels increases cell viability against rotenone-induced cell death. J Neurochem. 2003 Mar;84(5):1193–1200. doi: 10.1046/j.1471-4159.2003.01625.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhou M, Tanaka O, Sekiguchi M, He HJ, Yasuoka Y, Itoh H, et al. ATP-sensitive K+-channel subunits on the mitochondria and endoplasmic reticulum of rat cardiomyocytes. J Histochem Cytochem. 2005 Dec;53(12):1491–1500. doi: 10.1369/jhc.5A6736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive K(ATP) activity. J Mol Cell Cardiol. 2008;44(1):188–200. doi: 10.1016/j.yjmcc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, et al. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006 Sep 1;575(Pt 2):627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong M, Kefaloyianni E, Bao L, Malester B, Delaroche D, Neubert TA, et al. Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex. FASEB J. 2011;25(7):2456–2467. doi: 10.1096/fj.10-176669. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, et al. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circulation Research. 2006;98(5):682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee S, Al Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285(4):C959–C967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- 67.Geng X, Li L, Watkins S, Robbins PD, Drain P. The insulin secretory granule is the major site of K(ATP) channels of the endocrine pancreas. Diabetes. 2003;52(3):767–776. doi: 10.2337/diabetes.52.3.767. [DOI] [PubMed] [Google Scholar]
- 68.Zunkler BJ, Wos-Maganga M, Panten U. Fluorescence microscopy studies with a fluorescent glibenclamide derivative, a high-affinity blocker of pancreatic beta-cell ATP-sensitive K+ currents. Biochem Pharmacol. 2004 Apr 15;67(8):1437–1444. doi: 10.1016/j.bcp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Raab-Graham KF, Cirilo LJ, Boettcher AA, Radeke CM, Vandenberg CA. Membrane topology of the amino-terminal region of the sulfonylurea receptor. J Biol Chem. 1999 Oct 8;274(41):29122–29129. doi: 10.1074/jbc.274.41.29122. [DOI] [PubMed] [Google Scholar]
- 70.Conti LR, Radeke CM, Vandenberg CA. Membrane targeting of ATP-sensitive potassium channel. Effects of glycosylation on surface expression. J Biol Chem. 2002 Jul 12;277(28):25416–25422. doi: 10.1074/jbc.M203109200. [DOI] [PubMed] [Google Scholar]
- 71.Masia R, Caputa G, Nichols CG. Regulation of KATP channel expression and activity by the SUR1 nucleotide binding fold 1. Channels (Austin) 2007 Jul–Aug;1(4):315–323. doi: 10.4161/chan.5083. [DOI] [PubMed] [Google Scholar]
- 72.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. Embo J. 1999 Sep 1;18(17):4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawano T, Zoga V, Kimura M, Liang MY, Wu HE, Gemes G, et al. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol Pain. 2009;5(12):12. doi: 10.1186/1744-8069-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem. 2010 Dec 3;285(49):38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular K ATP channels. Diabetes. 1996 Oct;45(10):1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 76.Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. Journal of Membrane Biology. 2003;196(1):61–69. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- 77.Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, et al. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J Mol Cell Cardiol. 2000;32(11):1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 78.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35(5):445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 79.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, et al. Immunolocalization of K(ATP) channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiology. 2005;5(1):1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foster DB, Rucker JJ, Marban E. Is Kir6.1 a subunit of mitoK(ATP)? Biochem Biophys Res Commun. 2008 Feb 15;366(3):649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kline CF, Hund TJ, Mohler PJ. Ankyrin regulates KATP channel membrane trafficking and gating in excitable cells. Channels (Austin) 2010 Jan;4(1):55–57. doi: 10.4161/chan.4.1.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou M, He HJ, Suzuki R, Liu KX, Tanaka O, Sekiguchi M, et al. Localization of sulfonylurea receptor subunits, SUR2A and SUR2B, in rat heart. J Histochem Cytochem. 2007 Aug;55(8):795–804. doi: 10.1369/jhc.6A7104.2007. [DOI] [PubMed] [Google Scholar]
- 83.Geuze HJ, Slot JW, Vanderley PA, Scheffer RCT. Use of Colloidal Gold Particles in Double-Labeling Immunoelectron Microscopy of Ultrathin Frozen Tissue-Sections. Journal of Cell Biology. 1981;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, et al. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008 Oct 24;103(9):1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards AG, Rees ML, Gioscia RA, Zachman DK, Lynch JM, Browder JC, et al. PKC-permitted elevation of sarcolemmal KATP concentration may explain female-specific resistance to myocardial infarction. J Physiol. 2009 Dec 1;587(Pt 23):5723–5737. doi: 10.1113/jphysiol.2009.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Budas GR, Jovanovic S, Crawford RM, Jovanovic A. Hypoxia-induced preconditioning in adult stimulated cardiomyocytes is mediated by the opening and trafficking of sarcolemmal KATP channels. FASEB J. 2004 Jun;18(9):1046–1048. doi: 10.1096/fj.04-1602fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuller W, Eaton P, Medina RA, Bell J, Shattock MJ. Differential centrifugation separates cardiac sarcolemmal and endosomal membranes from Langendorff-perfused rat hearts. Analytical Biochemistry. 2001;293(2):216–223. doi: 10.1006/abio.2001.5127. [DOI] [PubMed] [Google Scholar]
- 88.Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004 Apr;36(4):567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Zoratti M, De Marchi U, Gulbins E, Szabo I. Novel channels of the inner mitochondrial membrane. Biochim Biophys Acta. 2009 May;1787(5):351–363. doi: 10.1016/j.bbabio.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 90.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003 May 8;38(3):417–432. doi: 10.1016/s0896-6273(03)00256-3. 2003; 38: 417–32. [DOI] [PubMed] [Google Scholar]
- 91.Konstas AA, Dabrowski M, Korbmacher C, Tucker SJ. Intrinsic sensitivity of Kir1.1 (ROMK) to glibenclamide in the absence of SUR2B. Implications for the identity of the renal ATP-regulated secretory K+ channel. J Biol Chem. 2002 Jun 14;277(24):21346–21351. doi: 10.1074/jbc.M202005200. [DOI] [PubMed] [Google Scholar]
- 92.Bruederle CE, Gay J, Shyng SL. A Role of the Sulfonylurea Receptor 1 in Endocytic Trafficking of ATP-Sensitive Potassium Channels. Traffic. 2011 Jun 7; doi: 10.1111/j.1600-0854.2011.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem. 2010 Feb 19;285(8):5963–5973. doi: 10.1074/jbc.M109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith AJ, Sivaprasadarao A. Investigation of K(ATP) channel endocytosis by immunofluorescence. Methods Mol Biol. 2008;491:69–77. doi: 10.1007/978-1-59745-526-8_5. [DOI] [PubMed] [Google Scholar]
- 95.Smith AJ, Sivaprasadarao A. Investigation of K(ATP) channel endocytosis and cell surface density by Biotinylation and Western blotting. Methods Mol Biol. 2008;491:79–89. doi: 10.1007/978-1-59745-526-8_6. [DOI] [PubMed] [Google Scholar]
- 96.Yan FF, Pratt EB, Chen PC, Wang F, Skach WR, David LL, et al. Role of Hsp90 in biogenesis of the beta-cell ATP-sensitive potassium channel complex. Mol Biol Cell. 2010 Jun 15;21(12):1945–1954. doi: 10.1091/mbc.E10-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen PC, Bruederle CE, Gaisano HY, Shyng SL. Syntaxin 1A regulates surface expression of beta-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol. 2011 Mar;300(3):C506–C516. doi: 10.1152/ajpcell.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan F, Lin CW, Weisiger E, Cartier EA, Taschenberger G, Shyng SL. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem. 2004;279(12):11096–11105. doi: 10.1074/jbc.M312810200. %19. [DOI] [PubMed] [Google Scholar]
- 99.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB Journal. 2002;16(1):102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, et al. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO Journal. 2002;21(15):3936–3948. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 2005;6(9):848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2(11):805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 103.Zlatkovic J, Arrell DK, Kane GC, Miki T, Seino S, Terzic A. Proteomic profiling of KATP channel-deficient hypertensive heart maps risk for maladaptive cardiomyopathic outcome. Proteomics. 2009;9(5):1314–1325. doi: 10.1002/pmic.200800718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zlatkovic-Lindor J, Arrell DK, Yamada S, Nelson TJ, Terzic A. ATP-sensitive K(+) channel-deficient dilated cardiomyopathy proteome remodeled by embryonic stem cell therapy. Stem Cells. 2010 Aug;28(8):1355–1367. doi: 10.1002/stem.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arrell DK, Zlatkovic J, Kane GC, Yamada S, Terzic A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of heart disease susceptibility. J Proteome Res. 2009 Oct;8(10):4823–4834. doi: 10.1021/pr900561g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000 Mar;49(3):311–318. doi: 10.2337/diabetes.49.3.311. [DOI] [PubMed] [Google Scholar]
- 107.Minami K, Miki T, Kadowaki T, Seino S. Roles of ATP-sensitive K+ channels as metabolic sensors: studies of Kir6.x null mice. Diabetes. 2004;53 Suppl 3:S176–S180. doi: 10.2337/diabetes.53.suppl_3.s176. S176–S80. [DOI] [PubMed] [Google Scholar]
- 108.Miki T, Tashiro F, Iwanaga T, Nagashima K, Yoshitomi H, Aihara H, et al. Abnormalities of pancreatic islets by targeted expression of a dominant-negative KATP channel. Proceedings of the National Academy of Sciences of the United States of America. 1997 Oct 28;94(22):11969–11973. doi: 10.1073/pnas.94.22.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000 Mar 17;100(6):645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 110.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, et al. Tolerance for ATP-insensitive K(ATP) channels in transgenic mice. Circulation Research. 2001;89(11):1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 111.Flagg TP, Remedi MS, Masia R, Gomes J, McLerie M, Lopatin AN, et al. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal K(ATP) J Mol Cell Cardiol. 2005 doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Malester B, Tong X, Ghiu IA, Kontogeorgis A, Gutstein DE, Xu J, et al. Transgenic Expression of A Dominant Negative KATP Channel Subunit in the Mouse Endothelium: Effects on Coronary Flow and Endothelin-1 Secretion. FASEB Journal. 2007;21(9):2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- 113.Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, et al. Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell Metab. 2010 Jan;11(1):58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8(5):466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 115.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109(4):509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99(20):13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002 Oct 4;277(40):37176–37183. doi: 10.1074/jbc.M206757200. Epub2002Jul30. 2002; 277: 37176-83. [DOI] [PubMed] [Google Scholar]
- 119.Seghers V, Nakazaki M, DeMayo F, guilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275(13):9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 120.Elrod JW, Harrell MD, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, et al. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117(11):1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 121.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, et al. Disruption of Sur2-containing K(ATP) channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA. 2001;98(20):11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest. 2002;110(2):203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, et al. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J Mol Cell Cardiol. 2007 doi: 10.1016/j.yjmcc.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39(1):51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 125.Proks P, Ashcroft FM. Modeling K(ATP) channel gating and its regulation. Prog Biophys Mol Biol. 2009 Jan;99(1):7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 126.Enkvetchakul D, Loussouarn G, Makhina E, Shyng SL, Nichols CG. The kinetic and physical basis of K(ATP) channel gating: toward a unified molecular understanding. Biophys J. 2000 May;78(5):2334–2348. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005 May;6(5):470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang K, Csanady L, Chan KW. The N-terminal transmembrane domain (TMD0) and a cytosolic linker (L0) of sulphonylurea receptor define the unique intrinsic gating of KATP channels. J Physiol. 2006;576(Pt 2):379–389. doi: 10.1113/jphysiol.2006.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, et al. Coupling of cell energetics with membrane metabolic sensing Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002 Jul 5;277(27):24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 130.Ferrero JM, Jr, Siz J, Ferrero JM, Thakor NV. Simulation of action potentials from metabolically impaired cardiac myocytes - Role of ATP-sensitive K + current. Circulation Research. 1996;79:208–221. doi: 10.1161/01.res.79.2.208. [DOI] [PubMed] [Google Scholar]
- 131.Baron A, van Bever L, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circulation Research. 1999;85(8):707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- 132.Schnitzler MM, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol (Lond) 2000;525(Pt 2):307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26(1):135–143. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]