Abstract
The medial prefrontal cortex (mPFC) has been implicated in the processing of emotionally significant stimuli, particularly the inhibition of inappropriate responses. We examined the role of the mPFC in regulation of fear responses using a differential fear conditioning procedure in which the excitatory conditioned stimulus (CS+) was paired with an aversive footshock and intermixed with the inhibitory conditioned stimulus (CS-). In the first experiment, using rats as subjects, muscimol, a gamma-amino-butyric acid type A (GABAA) receptor agonist, or artificial cerebrospinal fluid (aCSF) was infused intracranially into the mPFC across three conditioning sessions. Twenty-four hours after the last conditioning session, freezing response of the rats was tested in a drug-free state. Neither the muscimol nor the aCSF infusion had any effect on differential responding. In the second experiment, the same experimental procedure was used except that the infusion was made before the testing session rather than the conditioning sessions. The results showed that muscimol infusion impaired differential responding: the level of freezing to CS- was indiscriminable from that to CS+. Taken together, these results suggest that the mPFC is responsible for the regulation of fear response by inhibiting inappropriate fear expressions.
Keywords: medial prefrontal cortex, differential fear conditioning, extinction, inhibitory learning
INTRODUCTION
Since the historical case of Phineas Gage, a railroad worker whose brain damage drastically changed his personality, the medial prefrontal cortex (mPFC) has been implicated in the regulation of emotion [1-3]. For example, the mPFC is critical for the extinction of fear conditioned response (CR) where the associative link between the conditioned stimulus (CS) and the aversive unconditioned stimulus (US) is suppressed following repeated presentations of the CS without the US [4-6]. Converging evidence suggests that extinction involves post-learning modulation rather than erasure or forgetting of learned fear response [7]. Lesions in the mPFC caused retardation of extinction process, delayed recall of extinction, and deficits in the long-term retention of extinguished fear [6, 8, 9]. Moreover, results from human neuroimaging studies found correlation between mPFC activation and strength of extinction memory [10, 11].
The mPFC regulates fear response by modulating amygdala activity. The amygdala has been implicated in initial acquisition of CS-US association [12], expression of learned fear response [13], and extinction of fear response [14, 15]. Interactions between the mPFC and amygdala, therefore, are indispensible for fear expression and inhibition [16-18]. Recent studies focused more on local networks within the mPFC and their connections with the amygdala. For example, Vidal-Gonzalez et al. proposed that excitatory and inhibitory interactions within the mPFC regulates fear response through modulation of amygdala activity [19]. Specifically, activity in the prelimbic subregion (PL) of the mPFC is critical for the expression of learned fear, whereas the infralimbic subregion (IL) is involved in the acquisition of extinction memory but not the expression of fear CRs [20]. Changes in intrinsic excitability of the IL regulate fear expression even before extinction [21]. In addition, Laviollete et al. showed that canabinoid receptor blockade in the mPFC interferes with the acquisition and expression of olfactory fear conditioning [16].
In a typical laboratory investigation of fear extinction, sessions of fear conditioning (acquisition) and extinction are clearly separated and serially presented. Interestingly, the interval between acquisition and extinction seems to change the behavioral outcome quite dramatically. Extinction training which would normally produce significant reduction of fear response was not effective when the interval was shortened. This phenomenon, called immediate extinction deficit (IED) [22], has been proposed to result from lack of mPFC activation due to the short acquisition-to-extinction interval [23]. Taken together, when and how the mPFC modulates fear response through expression and inhibition of the fear circuit is still controversial.
A feasible but different approach to the problem of mPFC regulation of fear response is to present excitatory and inhibitory trials within the same session. Among variations of fear conditioning procedure, a differential conditioning paradigm involving the conditioned stimulus that signals an upcoming aversive or threatening US (CS+) and a stimulus that signals the absence of such stimulus (CS-) can be a useful model to investigate the role of excitatory and inhibitory processes in regulation of emotional responses [24]. Although some early studies using the differential procedure showed that mPFC lesion produced decreased discrimination between the CS+ and CS- which is primarily caused by increased responding to the CS- [25, 26], no prior studies have employed pharmacological inactivation of the mPFC to dissect its functional involvement across different stages. Therefore, we examined the role of the mPFC in differential fear conditioning using microinfusion of the GABAA receptor agonist muscimol. Due to high density of GABAA receptors [27], muscimol has been proved to be an efficient technique to transiently inactivate the mPFC [13].
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats (225~250 g) were used as subjects. All experiments were conducted in accordance with the National Institutes of Health guideline for animal care and welfare. The animals were housed individually on a 12-h light/dark cycle with lights on at 9:00 A.M. They were maintained at temperature of 21~24℃ and given ad libitum access to food and water.
Apparatus
Differential fear conditioning and retention testing were conducted in one of two different conditioning chambers contained within a sound-attenuating cubicle. Each conditioning chamber rested on a load-cell platform (DACELL Co., Ltd., Chung-buk, Korea) that recorded chamber displacement in response to each rat's motor activity. During retention test, these chambers were modified for context shift, which included lining the side wall with a white board and treating with a different odor (Canadian balsam or cinnamon; Sigma). In addition, the whole chamber and cubicle were also switched with each other. The US was delivered through a grid floor attached to a shock generator (E13-14, Coulbourn Instruments, Allentown, PA, USA). Delivery of the CS and the US was controlled by a custom-made software written in LabView (National Instruments, Austin, TX). Background noise (65dB) was provided by ventilation fans. A small red light (12V, 4W) was continuously on within the cubicle to provide dim illumination. The apparatus was cleaned with 70% ethanol after each use.
Surgery for cannula implant
The rats were anesthetized with pentobarbital sodium (50mg/kg, i.p.) with supplemental injections given as needed. Stainless steel 26-guage guide cannulae (Plastics One, Roanoke, VA, USA) were bilaterally implanted targeting the medial prefrontal cortex using coordinates from Paxinos and Watson [28]: 2.2 mm posterior to bregma, ±0.5 mm lateral to the midline and 3.5 mm ventral from dura for Experiment 1 using a dual guide cannula with 1-mm center-to-center distance. For Experiment 2, two single cannulae were implanted at an angle of 15° (2.9 mm posterior to bregma, ±1.8 mm lateral to the midline and 3 mm ventral from dura). Implanted cannulae were secured by dental acrylic cemented over the anchoring screws attached on the skull. To prevent clogging of the guide cannulae, 33-guage dummy wires were inserted, which was temporally removed and replaced by injection cannulae for drug infusion.
Drug and intracranial infusions
Muscimol (Sigma, St. Louise, MO, USA) was dissolved in artificial cerebrospinal fluid (aCSF, pH 7.0~7.5), to a concentration of 0.1 µg /0.3 µl. A total amount of 0.3 µl/side of muscimol or aCSF was infused into each mPFC with 33-gauge infusion cannulae at a rate of 0.1 µl/min controlled by a programmable syringe pump (model 101, KD Scientific, Hollistion, MA). Following the infusion, rats were returned to their home cage and waited for 20~30 min in vivarium before undergoing a behavioral procedure.
Behavioral procedure and drug infusion
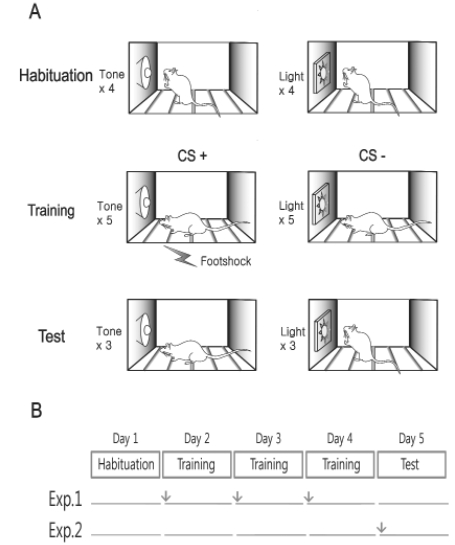
Fig. 1 shows schematized training and testing procedure. To prevent startle reactions to the CS, all rats received a habituation session composed of 8 alternating presentations of the CSs (4 for each CS). The CS was either a tone (CS1; 1 kHz, 70 dB, 30 s) or a light (CS2; white LED illumination, 3,200 mcd, 30 s). The inter-trial interval (ITI) was randomly varied between 250~350 s. Twenty-four hours after the habituation session, all rats received a drug infusion (muscimol or artificial cerebrospinal fluid: aCSF) followed by a conditioning session 30 min later (Exp. 1). The conditioning sessions included 10 randomly-mixed presentations of CS1 and CS2. Only one of the CSs was paired with the footshock US (0.5 mA, 1 s) that co-terminated with the CS (CS+). The other was presented alone (CS-). The assignment of the stimuli to CS+ or CS- was counterbalanced between subjects. The conditioning was repeated for three days. Twenty-four hrs after the third conditioning session, the retention test was performed in the switched chamber where the rats received three presentations of CS+ and CS- in an alternating sequence without the US (total of 6). To reduce the variability, the CS duration was extended to 180 s. For Exp. 2, the procedure was identical to Exp. 1 except that the drug infusion was performed 30 min before the retention test session. Conditioned fear response was measured by freezing which was defined as absence of all movements except for respiration. The cumulative time spent freezing was measured by two experienced experimenters who were blind to the group assignment. The discrimination ratio was calculated as the index of differential responding to the CSs using the following formula.
Fig. 1.
Schematic diagram of the experimental procedures. (A) Flow of experimental treatments. Rats were first given cannulae implantation targeted at the mPFC. After recovery, they were trained using differential fear conditioning procedure. The retention test was conducted 24 hrs after the last conditioning session. (B) Pre-conditioning vs. Pre-testing drug infusion. In Exp.1, muscimol or aCSF was infused before each conditioning session but not on the 24-hr retention test. In Exp. 2, rats were conditioned without drug infusion, and then muscimol or aCSF was infused only before the retention test. Arrows indicate drug infusion.
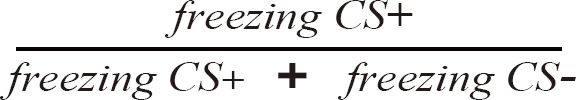
According to this formula, a discrimination ratio of 1 indicates complete discrimination between CS+ and CS-, whereas 0.5 indicates no discrimination.
Histology
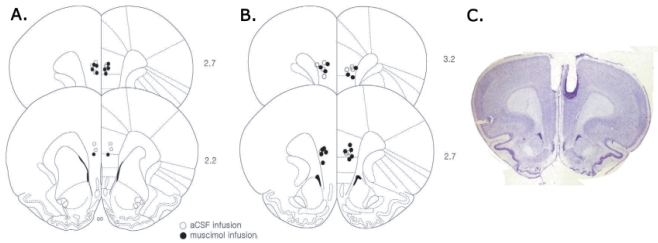
Following testing, rats were anesthetized with a lethal overdose of chloral hydrate (400 mg/kg) and perfused with saline followed by formalin. The brains were then removed, post-fixed in 10% formalin solution and transferred into 30% sucrose solution. When the brain sank to the bottom in the sucrose solution, coronal sections (50 µm thickness) were cut using a freezing microtome (Leica cryostat 1720, Germany). The sections then were mounted on gelatin-coated slides, stained with cresyl violet and coverslipped with Permount (Sigma). The slides were examined under a microscope for verification of the cannulae tip location. After histological reconstruction and close examination of brain sections, only the animals with injection sites within mPFC were included in the final analysis (Fig. 2).
Fig. 2.
Reconstruction of microinjection sites. Cannula placements for muscimol and aCSF groups in Exp. 1 (A) and Exp. 2 (B) are depicted on illustrated coronal sections of mPFC (modified from Paxinos and Watson, 1998). Numbers on the right represent anterior-posterior distance from bregma. (C) Photograph of a representative coronal section showing the location of guide cannulae in the mPFC.
Statistical analysis
All data were reported as means and standard errors of the mean (S.E.M) and analyzed with repeated measures analysis of variance for comparing day-to-day response and independent samples t-test for average response during retention test and paired samples t-test for differential freezing responses to the CSs within the subjects during retention test. An α level of 0.05 was used as a criterion of statistical significance.
RESULTS
Experiment 1. Pre-conditioning muscimol infusion into the mPFC
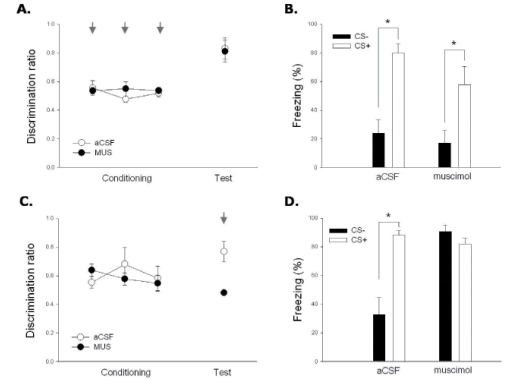
Experiment 1 was conducted to examine the role of the mPFC in the acquisition of differential fear response. Total of 15 rats (aCSF, n=8; muscimol, n=7) were microinfused with muscimol or aCSF into the mPFC just before each training session. There was no significant difference between groups on the discrimination ratio throughout the conditioning sessions (Fig. 3A: F(1,8)=0.346, not significant [n.s.]), repeated measures ANOVA). In the 24-hr retention test with no drug treatment, the aCSF group and the muscimol group showed differential responding between CS+ and CS- (Fig. 3B). Both groups froze significantly more to the CS+ than to the CS- (aCSF group, t(7)=-5.685, p<.05 ; muscimol group, t(6)=-2.835, p<.05, paired t-test). The level of differential responding was also similar, such that the discrimination ratio was not different between groups (Fig. 3A: t(8)=.191, n.s., independent t-test).
Fig. 3.
Effect of muscimol infusion on differential conditioning. (A, B). Pre-conditioning drug infusion (Exp. 1). (A) No significant effect of drug infusion on discrimination ratio was found (see Results for detail). (B) Freezing levels to the CS+ and CS- during the drug-free retention test showed differential responding regardless of drug condition. (C, D) Pre-testing drug infusion (Exp 2). (C) Muscimol infusion significantly reduced discrimination ratio on the retention test. (D) Freezing levels to the CS+ and CS- during the retention test showed that inhibitory responding to CS- was impaired only in the muscimol infusion group. Data are expressed as mean ± S.E.M. Arrows indicate drug infusion (muscimol or aCSF).
To determine whether muscimol infusion into the mPFC altered pain sensitivity, reactivity to footshock was measured during the presentations of the aversive US on the first conditioning trials in Exp. 1. Statistical analysis confirmed that there was no significant difference between the muscimol- and aCSF-infused groups (t(10)=-1.018, n.s., independent t-test).
Experiment 2. Pre-testing muscimol infusion into the mPFC
The purpose of Exp. 2 was to examine the role of the mPFC in the expression of differential fear response. In Exp. 2, rats underwent the same differential conditioning as Exp. 1 but without any drug infusion. Thirteen rats (aCSF, n=5; muscimol, n=8) were microinfused with muscimol or aCSF into the mPFC just before the retention test. Both groups showed no significant difference on any of the conditioning sessions (Fig. 3C: F(1,11)=0.052, n.s., repeated measures ANOVA). In the 24-hr retention test, however, only the aCSF group showed differential responding between CS+ and CS- (aCSF group, t(4)=-5.692, p < .05; muscimol group, t(7)=1.347, n.s., paired t-test). The aCSF group significantly froze more to CS+ than to CS-. In contrast, freezing to CS- in the muscimol group was greater than the aCSF group (Fig. 3D: t(4.599)=-4.467, p < .05, independent t-test). Also, the level of differential responding was different, such that the discrimination ratio was higher in the aCSF group than in the muscimol group (Fig. 3C: t(4.324)=3.987, p < .05, independent t-test).
DISCUSSION
The current study tested the role of the mPFC in the acquisition and expression of differential fear responding. In Exp.1, both the muscimol-infused and aCSF-infused rats showed differential responding to the CSs indicating that formation of excitatory associations between CS+ and the US and inhibitory association between CS- and the US were not dependent on the mPFC. On the other hand, in Exp. 2, when the same drug was applied before the test session, the differential responding was attenuated. That is, the rats with muscimol infusion showed as much freezing to the CS- as they did to the CS+, indicating that mPFC inactivation selectively impaired inhibitory responding to the CS- while sparing the excitatory responding. Taken together, the mPFC is particularly involved in regulation of fear expression based on associative memory of selective stimuli contingency [29, 30].
The current results are consistent with the studies of fear extinction in that extinction also involves inhibition of an existing response. In fact, Bouton claimed that extinction is a special case of conditioned inhibition [31]. Whereas conditioned fear response develops with increased cellular responding in the amygdala [32, 33], extinction involves cortical modulation of the excitatory circuit within the amygdala. For example, extinction modulates amygdala activity by activating inhibitory interneurons in the lateral nucleus (LA) [34] or intercalated cell mass in the central nucleus (CE) [17, 35]. Increased cellular activity within the mPFC was observed after extinction and stimulation of the mPFC enhanced extinction memory [19, 36]. Moreover, increased synaptic efficacy between the mPFC and the amygdala is highly predictive of the emergence and reduction of the conditioned fear response [37]. Remarkably, extinction was induced even in anesthetized rats as long as the activation of the mPFC accompanies repeated stimulation of the CS input pathway into the amygdala [38]. The current results also showed that inhibition of fear response was impaired by the inactivation of the mPFC (Exp. 2). The resurgence of fear response to CS- despite the preceding extinction training could have resulted from blockade of inhibitory projection onto the fear network within the amygdala. Excitatory responding to CS+ was intact which is believed to depend on the amygdala itself [39].
Considering the critical role of the mPFC in extinction learning and fear inhibition, questions remain regarding the intact acquisition despite the inactivation of the mPFC during the differential conditioning (Exp. 1). One interpretation of the result is that neural plasticity for response inhibition could be established in an alternative structure if the mPFC activation is blocked. To support, studies employing a differential fear conditioning procedure have reported that manipulation of the mPFC did not influence initial association of discrimination [40, 41] and neuronal activity in the amygdala increased to the CS+ relative to the CS- [42, 43]. In addition, mPFC lesion did not impair discriminatory conditioning with food reinforcement [44]. Taken together, neuronal plasticity responsible for inhibitory association could be produced outside the mPFC while the structure is unavailable.
Recent studies emphasized the interaction between the mPFC and the amygdala through functionally distinct regions such as the IL and PL for post-acquisition modulation of fear response [20]. Specifically, the IL is involved in retrieval of extinction memory whereas the PL is closely linked with expression of fear response [19, 20]. The histological reconstruction of our data shows that most of the cannula tips located in the middle area, suggesting that the drug must have spread over to the IL and PL (Fig. 2). If the mPFC is involved in both fear expression and inhibition as some previous models have suggested, then the differential responding to the CS+ and CS- should have been equally attenuated. However, the current result indicates that the mPFC is involved more in down-regulation rather than up-regulation or maintenance of fear response. One possibility is that the procedural difference between extinction and differential conditioning procedure might have resulted in different types of learning which require the IL and PL to a varying degree. An extinction session is always presented serially sometime after the acquisition. In contrast, inhibitory trials were intermixed with excitatory trials within the same session in our experiment. In sum, further study is needed to elucidate how the mPFC process and utilize inhibitory and excitatory stimuli and interact with parallel structures to generate an appropriate emotional response.
ACKNOWLEDGEMENTS
This research was supported by a grant(2011K000288) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, the Republic of Korea and by Korea University Grant.
References
- 1.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SW, Damasio H, Tranel D, Damasio AR. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. 2000;18:281–296. doi: 10.1207/S1532694202Anderson. [DOI] [PubMed] [Google Scholar]
- 3.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 4.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 5.Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 7.Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- 8.Lebrón K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 9.Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CH, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16:62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. J Neurosci. 2010;30:832–837. doi: 10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38:807–815. [PubMed] [Google Scholar]
- 25.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 26.Powell DA, Watson K, Maxwell B. Involvement of subdivisions of the medial prefrontal cortex in learned cardiac adjustments in rabbits. Behav Neurosci. 1994;108:294–307. doi: 10.1037//0735-7044.108.2.294. [DOI] [PubMed] [Google Scholar]
- 27.Dunn E, Fritschy JM, Carter DB, Merchant KM. Differential distribution of gamma-aminobutyric acidA receptor subunit (alpha 1, alpha 2, alpha 3, alpha 5 and beta 2 + 3) immunoreactivity in the medial prefrontal cortex of the rat. Neurosci Lett. 1996;210:213–217. doi: 10.1016/0304-3940(96)12678-1. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 29.Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- 30.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. J Exp Psychol Anim Behav Process. 1994;20:51–65. [PubMed] [Google Scholar]
- 32.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 33.Seo DO, Lee YK, Choi JS. Making of fear: a systematic dissection of Pavlovian fear conditioning. Korean J Exp Psychol. 2006;18:1–19. [Google Scholar]
- 34.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vouimba RM, Maroun M. Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–2285. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Choi JS. Long-term synaptic changes in two input pathways into the lateral nucleus of the amygdala underlie fear extinction. Learn Mem. 2010;17:23–34. doi: 10.1101/lm.1482910. [DOI] [PubMed] [Google Scholar]
- 39.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 40.Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett. 1998;257:151–154. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- 41.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Collins DR, Paré D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(-) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]