Abstract
Alzheimer’s disease (AD) has been well characterized by the presence of reactive microglia, often associated with Aβ-plaque deposition. The oligomeric form of Aβ peptide (Aβo) has neurotoxic effects in the presence of microglia and is suggested to potentiate proinflammatory changes in microglia in AD. Primary murine microglia cultures stimulated with Aβo displayed increased protein phospho-tyrosine and secreted TNF-α levels which were attenuated by the Src/Abl inhibitor, dasatinib. Intracerebroventricular infusions of Aβo into C57BL6/J mice stimulated increased microgliosis and protein phospho-tyrosine levels that were also attenuated by dasatinib administration. The rodent findings were validated in human AD brains versus age-matched controls demonstrating reactive microglial association with Aβo deposits and increased microglial protein phospho-tyrosine and phospho-Src levels. These data suggest a role for Aβo in microglial activation through a tyrosine kinase-dependant pathway both in rodent models and human disease. Use of a selective non-receptor tyrosine kinase inhibitor such as dasatinib to attenuate microglial-dependent proinflammatory changes may prove to be an important step towards developing anti-inflammatory treatments for AD.
Keywords: amyloid beta oligomer, tyrosine kinase, microglia activation
1. Introduction
The major histopathological markers of Alzheimer’s disease (AD) are neurofibrilary tangles and β-amyloid (Aβ) peptide containing plaques. Involvement of Aβ in the pathogenesis of AD is supported by the amyloid cascade hypothesis that has remained controversial over the years (Hardy and Higgins, 1992). It suggests that mutations in APP, PS1, and PS2 proteins and abnormalities in APP metabolism may lead to accumulation and deposition of Aβ plaques and Aβ dependent synaptic and neuritic injury, altered signaling events, and neuron dysfunction subsequently lead to tangles, cell death, and dementia (Hardy and Selkoe, 2002; Hardy, 2006). Because Aβ peptide forms a fibrillar core of the senile plaques in both sporadic and autosomal dominant disease it is hypothesized that fibrillar Aβ deposition is mechanistically critical for disease (Jarrett et al., 1993; Pike et al., 1993).
Besides the accumulation of plaques and tangles it is well described in human disease and its transgenic mouse models that reactive microglia numbers are increased compared to non-demented controls and wild type mice, respectively (McGeer et al., 1987; Akiyama and McGeer, 1990; Cras et al., 1990; Styren et al., 1990; Frautschy et al., 1998; Stalder et al., 1999; Wegiel et al., 2001; Sasaki et al., 2002; Wegiel et al., 2003). The activated microglia are morphologically distinct from resting microglia and are commonly associated with Aβ fibril containing plaques (Itagaki et al., 1989; Mattiace et al., 1990; Perlmutter et al., 1990; Mackenzie et al., 1995; Sasaki et al., 1997; Akiyama et al., 1999; Benzing et al., 1999; Morgan et al., 2005; Meyer-Luehmann et al., 2008). Numerous in vitro studies have demonstrated that Aβ fibrils can directly stimulate microglia to acquire a pro-inflammatory phenotype (Banati et al., 1993; Del Bo et al., 1995; Giulian et al., 1995; Klegeris et al., 1997; Combs et al., 2000; Combs et al., 2001a; Combs et al., 2001b).
However, recent data suggests that nonfibrillar, oligomeric Aβ conformations may be more reliable indices of disease progression. For example, levels of soluble forms of Aβ have been shown to directly correlate with cognitive impairment and synaptic loss (Lue et al., 1999; McLean et al., 1999). Much like their fibrillar derivatives, these soluble oligomers are neurotoxic, stimulate gliosis, produce cognitive dysfunction, and decrease long-term potentiation both in vitro and in vivo (Roher et al., 1996; Cleary et al., 2005; Klyubin et al., 2005; Lesne et al., 2006). Indeed, up to 70% of diffuse plaques in nondemented aged individuals contain microglia (Sasaki et al., 1997) suggesting that microglial interaction with nonfibrillar peptide is common. Work from primates even indicates that gliosis precedes fibrillar plaque deposition (Martin et al., 1994). Therefore, the fibrillar, insoluble form of the peptide may not be the only species mediating neuronal death/dysfunction. More importantly, the oligomeric peptide may represent a target for early disease therapy.
We previously demonstrated in vitro that Aβ oligomers stimulate a tyrosine kinase based signaling response in microglia leading to acquisition of a reactive, neurotoxic secretory phenotype (Sondag et al., 2009). In this work, we continue to define the ability of Aβ oligomers to activate microglia. Using primary murine microglia cultures we demonstrate that the Src/Abl inhibitor dasatinib is sufficient to attenuate the oligomer stimulated increased in protein phospho-tyrosine changes and increased cytokine secretion. More importantly, dasatinib was also able to attenuate the ability of oligomer to increase protein phospho-tyrosine changes and microgliosis in vivo during intracerebroventricular infusion of the oligomeric species. Our study indicates that use of the FDA approved cancer drug, dasatinib, or related non-receptor tyrosine kinase inhibitors, may be useful to attenuate Aβ oligomer-dependent proinflammatory changes in Alzheimer’s disease.
2. Methods
2.1. Materials
Anti-oligomer antibody (I11) and anti-fibril antibody (O.C.) were previously described (Kayed et al., 2007). The anti-oligomer antibody (A11) was purchased from Invitrogen (Camarillo, CA). Anti-Aβ, clones 6E10 and 4G8 were from Covance (Emeryville, CA). The Anti-Lyn antibody, anti-Src, anti-α-tubulin antibodies and horseradish peroxidase conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse TNF-α Elisa kit was obtained from R&D Systems (Minneapolis, MN). Anti-phospho-tyrosine (4G10) antibody was from Upstate (Temecula, CA), HLA-DR Ab-1 (LN3) antibody was from Neomarkers (Fremont, CA) and anti-pLyn (396) antibody was purchased from Abcam (Cambridge, MA). The Vector SG and Vector NovaRed kits were from Vector Laboratories Inc. (Burlingame, CA). Anti-CD68 was obtained from Serotec (Raleigh, NC) and anti-pSrc antibody was purchased from Cell Signaling Technology (Danvers, MA). The non-receptor tyrosine kinase inhibitor, dasatinib was obtained from LC Laboratories (Woburn, MA). The transgenic mouse line, strain 005864 B6.Cg-Tg(APPswe,PSEN1dE9)85Dbo/J was obtained from the Jackson Laboratory (Bar Habor, Maine).
2.2. Preparation of peptides
Aβ1-42 was purchased from Bachem (Torrance, CA) or American Peptide (Sunnyvale, CA) for fibril and oligomer preparations, respectively. Aβ1-42 peptide was dissolved in 1:1 acetonitrile-water, aliquoted and dried. The aliquoted peptides were stored at −20 degree C until use. For oligomer preparation, each tube was dissolved in 50 μL HFIP and then diluted with 175 μL of sterile water and stirred at room temperature for 48h. The peptide was then spun at 14,000 rpm, for 10 min and the supernatant quantified using the method of Bradford(Bradford, 1976). For Aβ fibril preparations, Aβ1-42 peptide was dissolved in deionized water and incubated for a week at 37 degrees C. Before use, the fibril was mixed well and quantified using the Bradford assay.
2.3. Structural peptide analyses
For Western blot analysis of SDS-stable multimeric forms of oligomers, the peptide was diluted to different concentrations, separated by 15% SDS-PAGE and analyzed by Western blot using 6E10 (anti-Aβ) as the primary antibody. For dot-blot analyses of oligomers and fibrils, 1μg each of Aβ oligomer or fibril were dot blotted onto polyvinylidene flouride (PVDF) membrane and incubated with A11 (anti-oligomer) and 6E10 (anti-Aβ) antibodies and analyzed via enhanced chemiluminescence (GE Healthcare, Piscataway, NJ).
2.4. Tissue culture
Microglia cultures were derived from the brains of postnatal day 1–3 C57BL/6J mice. Briefly, cortices were removed and trypsinized. The trypsin was inactivated in microglial growth media (DMEM/F-12 with L-glutamine (Invitrogen) containing 10% heat-inactivated FBS, 5% heat-inactivated horse serum, and antibiotics, penicillin, streptomycin and neomycin (Gibco, Invitrogen). The tissue was triturated and plated into tissue culture flasks. After 24 hours all media and cellular debris was replaced with fresh media. After 7 more days one half of the media was replaced and cells were maintained as a mixed glia culture until day 14. At 14 days in vitro, microglia were shaken from the mixed glial culture at 200 rpm for 45 min and collected for use.
2.5. Cell stimulation
Microglia were placed into serum-free DMEM/F12 media for stimulations with Aβ oligomers or fibrils. To inhibit tyrosine kinases, cells were pretreated with drug or vehicle (DMSO) for 30 min before adding the appropriate concentrations of peptides. For ELISA analysis, cells were stimulated in 96-well plates (20,000 cells/well, 75 μL serum-free DMEM/F12) for 24 hours with 8 replicates per condition repeated 4 independent times. Experiments for Western blot analyses were for 5 minutes. After stimulation, cells were lysed in RIPA buffer (20mM Tris, pH 7.4, 150mM NaCl, 1mM Na3VO4 10mM NaF, 1mM EDTA, 1mM EGTA, 0.2mM phenylmethylsulfonyl fluoride, 1% Triton X-100, 0.1% SDS, and 0.5% deoxycholate) and quantified from five independent experiments.
2.6. Enzyme linked-immuno-sorbent assay (ELISA)
Media was collected from microglia following 24 hour stimulation for ELISA analysis. Levels of mouse TNF-α in the media were determined using commercially available ELISA kits according to the manufacturer’s protocol (R&D Systems).
2.7. Cell viability assay
The MTT reduction assay was performed to assess changes in cell survival. In brief, the media was removed from cells stimulated with or without Aβ and dasatinib and replaced with media containing 3[4,5-dimethylthiazol-2-y1]-2,5-diphenyltetrazolium bromide (MTT, 100μg/mL) for 4 hours. The media was aspirated and the reduced formazan precipitate was dissolved in isopropanol and absorbances read at 560/650nm via plate reader.
2.7. Western blot analysis
Cell lysates from 5 minute stimulations were resolved by SDS-PAGE and transferred to PVDF membranes. Western blots were blocked and incubated in anti-phospho-tyrosine (4G10) antibody as primary antibody with α-tubulin as the loading control. Blots were washed followed by incubation with HRP-conjugated secondary antibodies and antibody binding was detected via enhanced chemiluminescence.
2.8. Intracerebroventricular infusion of Aβ 1-42 oligomers and dasatinib
Aβ oligomers with or without dasatinib were infused into the right ventricle of C57BL/6 female mouse brains at 12 months of age. Mice were anesthetized with sodium pentobarbital (Nembutal, 70mg/kg) and a scalp incision made for stereotaxic placement of the cannula. Blunt end dissection caudally from the base of the scalp incision were performed to generate a small subcutaneous pocket for placement of an Alzet (model 1004, 0.25μL/hour delivery rate, Cupertino, CA) osmotic pump in the sub-scapular region. A cannula (Brain infusion kit, Alzet) was stereotaxically placed into the right lateral cerebral ventricle at coordinates −1.0 mm mediolateral and −0.5 mm anterioposterior from Bregma; −1.5 mm dorsal-ventral from skull. The cannulas were connected to subscapularly placed miniosmotic pumps (Alzet, model 1004) delivering either Aβ oligomers (1.6μg/day) or vehicle (4mM Hepes, 250μg/mL human high density lipoprotein) with or without dasatinib (500ng/kg/day) for 14 days. At the end of the infusion period, mice were euthanized, brains perfused with PBS-CaCl2 and rapidly collected. The right hemispheres were collected for cryosectioning and the left hemispheres were flash frozen in liquid nitrogen and lysed in RIPA buffer for biochemical analysis.
2.9. Immunostaining mouse tissue
Right brain hemispheres were immersion fixed in 4% paraformaldehyde and all hemispheres per condition were embedded in a 15% gelatin matrix (in 0.1M phosphate buffer) and again fixed in paraformaldehyde followed by exchanges of 30% sucrose in PBS over a several day time period. The fixed gelatin blocks were then flash frozen using a combination of dry-ice and isomethylpentane, and 40μm sections were cut using a freezing microtome. Serial sections were collected and immunostained with the desired antibodies. Anti-phosphotyrosine (4G10) antibody was used at a dilution of 1:1000, CD68 at 1:500, anti-Aβ (4G8) at 1:500, A11 antibody at 1:250, O.C. antibody at 1:4000, and I11 antibody was used at 1:500 dilution. Primary antibody incubations were overnight at 4 degree C followed by 2 hour room temperature incubation with biotinylated secondary antibodies (Vector labs) at 1:2000 dilution. Immunoreactivity was visualized using visible light chromogens VIP, Vector Blue, and DAB (Vector Laboratories). For immunohistochemical quantification, 3 consecutive serial sections, (40μm apart) throughout the hippocampal region were chosen for densitometric analysis. Optical densities from the CA1 region were measured using Adobe Photoshop software (Adobe Systems, San Jose, CA). The optical density of the entire CA1 region from a representative section was selected via marquee. The same size marquee was applied to all sections across all conditions to allow comparison of optical densities independent of area changes. All sections were immunostained at the same time to minimize background variability and background values in an unstained area of tissue for each section was set to zero before measuring optical density values. The values for each section were averaged (3sections/brain, 5–6 brains per condition) and plotted.
2.10. Double Label Immunofluorescence
For double label immunofluorescence staining, tissue was incubated in the desired primary antibodies and then corresponding texas red and FITC-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Tissue was counterstained with DAPI and visualized using a Zeiss LSM-510 META Confocal Microscope (Thornwood, NY).
2.11. Thioflavin staining
Mouse brain sections from Aβ oligomer and vehicle infused animals were stained with thioflavin (0.1g/10mL) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen) to visualize the nucleus. To quench lipofuscin autofluorescence, the sections were then incubated in 0.1% Sudan Black for 30 min, rinsed with PBS and cover slipped using PBS-glycerol (1:1).
2.12 Human tissue double staining
Human tissue was obtained from University of Washington Alzheimer Disease Research Center (ADRC, NIH P50AG05136) and was cut via freezing microtome (40μm) for immunostaining using DAB or Vector Red (Vector Laboratories, Burlingame, CA, US) as the first chromagen. The sections were then stripped using 0.2N HCl, 5 min. and incubated with the second primary antibody for immunostaining using Vector Blue or Vector SG (Vector Laboratories) as the second chromagen.
2.13. Statistical analysis
Data are presented as mean +/− standard deviation. For in vitro and in vivo analyses, values statistically different from controls were determined using one-way ANOVA. The Turkey-Kramer multiple comparisons post test was used to determine p values. For statistical comparison of human AD tissue with age-matched controls, a paired t test was performed.
3. Results
3.1. Aβo oligomer stimulated increased total protein phospho-tyrosine levels in primary microglia cultures that were attenuated by a non-receptor tyrosine kinase inhibitor, dasatinib
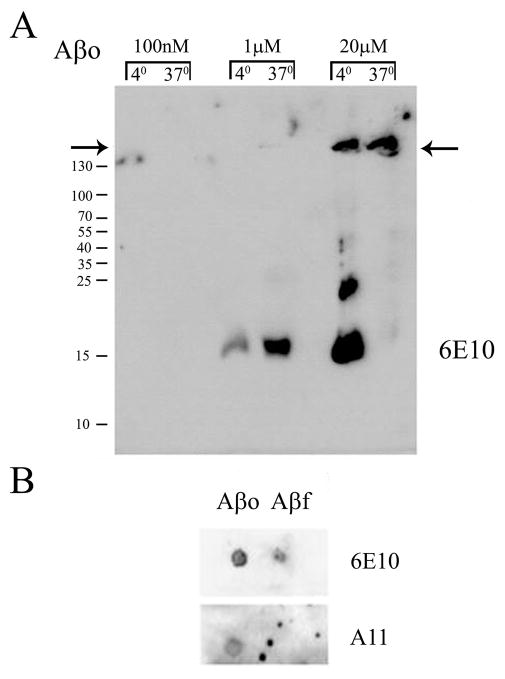
In order to characterize the form of Aβ oligomer used in our studies, we first performed a structural analyses of the prepared peptide. Preparations were separated via 15% SDS-PAGE to demonstrate that the major detergent resistant multimer of Aβ migrated with an apparent molecular weight loosely correlating with a trimeric/tetrameric form of the peptide that was stable even after incubation at 37 degree C (Fig. 1A). Larger amounts of loaded peptide demonstrated larger molecular weight multimers that were lesser species (Fig. 1A). This low molecular weight migration pattern correlates approximately with the multimeric confirmations of Aβ oligomers isolated from human cerebral cortex as well as cerebrospinal fluid in which dimer/trimer forms of Aβ have been the primary forms observed (Klyubin et al., 2008; Shankar et al., 2008; Villemagne et al., 2010; Shankar et al., 2011). This demonstrates that several detergent resistant species of oligomers are present in our preparation validating their use for subsequent cellular stimulations. In order to further confirm that the peptides were in a stable oligomeric conformation, dot blot analyses were performed after a period of 48h at 37 degree C. Oligomeric peptides were compared to fibrillar peptide. As expected, both fibrils and oligomers were immunodetected with an anti-Aβ antibody 6E10 (Fig. 1B). However, only the oligomeric preparation was detected by the anti-oligomer antibody A11 (Kayed et al., 2003) (Fig. 1B).
Figure 1. Structural characterization of Aβ1-42 oligomers (Aβo) and Aβ1-42 fibrils (Aβf).
The peptide (A) Aβo was prepared and stored overnight at 4 or 37 degree C to assess multimer stability. Increasing concentrations of peptide were separated by 15% SDS-PAGE and Western blotted with anti-Aβ antibody, 6E10. (B) Alternatively, prepared Aβo and Aβf were dot blotted (1μg each) onto PVDF and incubated with anti-Aβ antibody, 6E10, or anti-oligomer antibody, A11. Arrows indicate border between stacking and resolving gels for 6E10 Western blots.
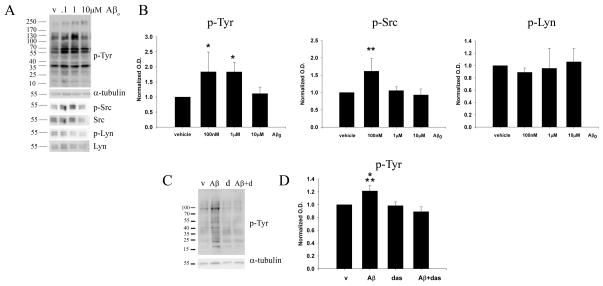
We next determined the appropriate concentration of Aβo for stimulating microglia. Our prior studies demonstrated that oligomeric peptide was sufficient to increase primary murine microglia total protein phospho-tyrosine levels although the concentrations employed were above the physiologic range and included a larger range of multimeric species in the preparation (Sondag et al., 2009). Primary murine microglia cultures were treated with varying concentrations of Aβo for 5 minutes in order to assess the signaling response stimulated by peptide interaction. Oligomeric peptide stimulated an increase in total protein phospho-tyrosine levels (Fig. 2A) that was significantly different from controls even as low as 100nM (Fig. 2B). Importantly this concentration is similar to that reported from human CSF and human cell culture conditioned medium (Seubert et al., 1992; Podlisny et al., 1995). Due to the fact that different proteins are recognized with anti-phospho-tyrosine antibodies, it is very common to see different overall Western blot banding patterns with different concentrations of ligands and different time courses of stimulation since protein phosphorylation/dephosphorylation is dynamically regulated during all of the signaling events initiated during any acute stimulation. Indeed, as can be seen from Figure 2, some phospho-tyrosine immunoreactive bands steadily increase with dosage while others demonstrate a biphasic response. To further validate this point, we examined the changes of two specific tyrosine kinases in these experiments. The non-receptor tyrosine kinase, Src, was elevated in its active, phosphorylated form at 100mM Aβo stimulation, while there was no change in the levels of active, Lyn kinase levels with either of the Aβo doses (Fig. 2B). Because of this complex, dynamic change we focused on the concentration that provides a maximal change for overall tyrosine phosphorylation rather than any particular molecular weight species.
Figure 2. Oligomeric Aβ1-42 stimulated increased microglial protein phospho-tyrosine levels that were attenuated by the tyrosine kinase inhibitor, dasatinib.
(A) Primary microglia were vehicle treated (v), or stimulated for 5 min with 0.1μM, 1μM and 5μM Aβo. Cells lysates were resolved by 10% SDS-PAGE and Western blotted using anti-phospho-Tyr (4G10), anti-pSrc, anti-pLyn antibodies or anti-α-tubulin, anti-Src and anti-Lyn (loading controls) antibodies. A Representative blot from 5 independent experiments is shown. (B) Densitometric analyses of the Western blots was performed normalizing protein phospho-tyrosine, pSrc and pLyn levels against their respective α-tubulin, Src and Lyn controls and averaging +/−SD. Percent fold changes in phospho-tyrosine levels were plotted (*p< 0.05 from vehicle). (C) Primary microglia were stimulated for 5 min with 1μM Aβo with or without 30 min pretreatment with 100pM dasatinib (d). A representative Western blot from 3 independent experiments is shown. (D) Densitometric analyses of Western blots were performed for protein phosphotyrosine levels normalized to their respective α-tubulin control (*p<0.05 from vehicle and dasatinib, **p<0.01 from Aβ+dasatinib).
In order to determine whether this Aβ stimulated increase in protein phospho-tyrosine levels could be inhibited pharmacologically, a clinically relevant non-receptor tyrosine kinase Src/Abl inhibitor, dasatinib, was used to assess its ability to attenuate the oligomer stimulated change in total protein phospho-tyrosine levels (Shah et al., 2004; Das et al., 2006) (Fig. 2C). As expected, dasatinib pretreatment significantly attenuated the Aβo stimulated increase in total protein phospho-tyrosine levels (Fig. 2D). This data indicates that Aβo is able to stimulate increased tyrosine kinase activity in primary murine microglia at physiologically relevant concentrations and this can be attenuated through the use of a non-receptor tyrosine kinase inhibitor, dasatinib.
3.2. Aβo oligomer stimulated increased TNF-α secretion from primary microglia cultures
To assess cell viability in presence of oligomeric Aβ, primary microglia cultures were treated with different concentrations of Aβo to validate that the 1μM concentration which demonstrated the most robust changes in protein phosphotyrosine levels was not toxic. The 10μM Aβo proved to be slightly toxic to the cells by 24 hr of stimulation (Fig. 3A) justifying the use of 1μM for subsequent experiments and perhaps partially explaining the decrease in levels of protein phosphotyrosine observed after 10μM Aβo stimulations (Fig 2A). In order to determine whether the increase in tyrosine kinase activity was responsible for a change in secretory phenotype, microglia were stimulated with Aβ oligomers and fibrils with or without increasing concentrations of dasatinib for 24 hours. A concentration of 1μM Aβo was used for the stimulations based upon the fact that a robust stimulatory change in protein phospho-tyrosine levels was observed with this concentration (Fig. 2A) as well as the toxicity data from MTT analyses of microglia (Fig. 3A). In addition, this represents a concentration closer to the levels found in brain (Seubert et al., 1992; Podlisny et al., 1995) offering a more physiologic impression of oligomer rather than fibril effects of Aβ during the progression of disease. Both fibrillar and oligomeric Aβ stimulated a significant increase in TNF-α secretion that was attenuated in a dose dependent fashion (Fig. 3B). Based upon the fact that dasatinib attenuated TNF-α secretion maximally at the lowest concentration employed, 100pM, suggests that the drug concentration curve employed was already above the IC50 (Johnson et al., 2005). This data demonstrated that oligomer Aβ stimulated a proinflammatory, cytokine secretory phenotype that was dependent upon propagation of the tyrosine-kinase based signaling response. The cell viability MTT reduction assay demonstrated that neither the peptide nor drug treatments were toxic to the microglia (Fig. 3C).
Figure 3. Oligomeric Aβ1-42 stimulated increased microglial secretion of the proinflammatory cytokine, TNF-α, that was attenuated by dasatinib.
(A) Primary microglia were untreated (control), vehicle treated, or stimulated with 1μM and 10μM Aβo for 24 hr to assess cell viability using the MTT assay. Absorbance values (560/650nm) were averaged+/−SD. (*p<0.05 from vehicle). (B) Primary microglia were unstimulated (control), DMSO vehicle treated (v), or stimulated for 24 hours with 1μM Aβo or 10μM Aβf in the presence/absence of 100pM, 1nM and 10nM dasatinib. Media was collected from treated cells and used to quantify changes in TNF-α secretion via ELISA. Secreted values were averaged +/−SD (*p< 0.05 from control and vehicle). (C) After media removal, the treated cells were used to assess cell viability via the MTT reduction assay. Absorbance values (560/650nm) were averaged+/−SD.
3.3. Aβo oligomers stimulated tyrosine kinase dependent microgliosis in vivo
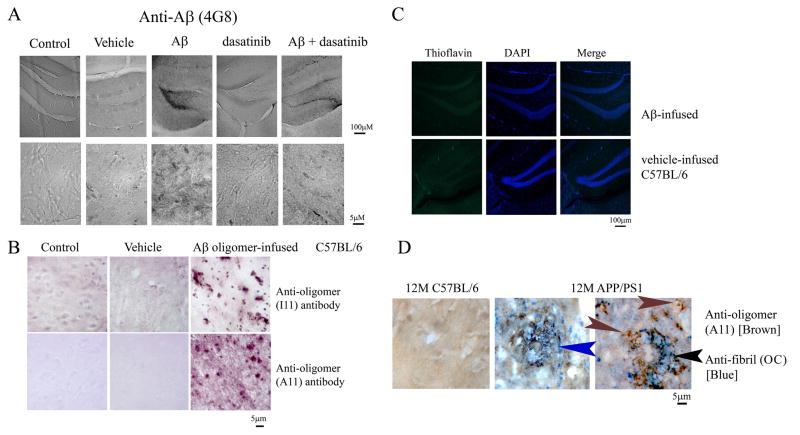
In order to validate the relevance of the in vitro findings, we next determined whether Aβo could stimulate a tyrosine kinase associated microgliosis in vivo. The Aβo were intracerebroventricularly infused into the right lateral ventricle of 12 month old C57BL/6 mice for 14 days to stimulate gliosis in the absence or presence of co-administered dasatinib. Aβo infused animals demonstrated a robust increase in microglial-like phospho-tyrosine immunoreactivity that spatially correlated with increased reactive microglial CD68 immunoreactivity (Fig. 4A). Densitometric analysis of the immunostaining demonstrated that dasatinib significantly attenuated the Aβo-dependent increase in both phospho-tyrosine (Fig. 4B) and CD68 (Fig. 4C) immunoreactivity. To validate the correlation between increased phosphotyrosine immunoreactivity and microgliosis, Aβo-infused tissue was double labeled using anti-phosphotyrosine (4G10) antibody and CD68 antibody, along with control brain sections. CD68-positive microglia co-localized with phosphotyrosine immunoreactivity in the Aβ-infused mouse brains (Fig. 4D). In order to compare this acute model of Aβ oligomer stimulated increase in protein phospho-tyrosine immunoreactivity to more chronic gliosis paradigms, microgliosis in infused brains was compared to that observed in a common transgenic mouse model of AD. This particular APP/PS1 model is a double transgenic line that expresses chimeric mouse/human amyloid precursor protein (Ms/Hu APPswe) and a mutant human presenilin 1 (PS1dE9), allowing the mice to secrete human Aβ peptide. This mouse model of AD shows a progressive increase in Aβ deposition with age (Garcia-Alloza et al., 2006). As expected, CD68 positive microglia in this model but not C57BL/6J controls correlated with increased phospho-tyrosine immunoreactivity (Fig. 4D). This demonstrated that elevated phosphotyrosine immunoreactivity was a common presentation of reactive microglia across disease model paradigms including both acute and transgenic models.
Figure 4. Intracerebroventricular infusion of oligomeric Aβ1-42 stimulated increased protein phospho-tyrosine and CD68 immunoreactivity that was attenuated by dasatinib.
Aβ oligomer (1.2mg/day) or vehicle control (human HDL in artificial CSF) in the presence or absence of drug (500ng/kg/day) were infused into the right lateral ventricle of 12 month old male C57BL6/J mice for 14 days (n=6). Brains were fixed, sectioned, and immunostained using anti-phospho-tyrosine (4G10) and CD68 antibodies. (A) Representative images from the dentate gyrus of the right hippocampus are shown. The arrow indicates the region imaged for the high magnification insets. Optical density (O.D.) of immunoreactivities for (B) phospho-tyrosine and (C) CD68 were quantified from serial sections of the CA1 region. (*p< 0.001 from c, v, dasatinib (das) and Aβ+dasatinib). (D) Aβ oligomer infused brains along with 12-month APP/PS1 and control mouse brains were double-immunostained using anti-phospho-tyrosine, 4G10 (red) and anti-CD68 (green) antibodies and counterstained with DAPI. Images shown are 40× magnification.
However, it was not clear whether the phospho-tyrosine immunoreactive, activated microglia in the Aβ oligomer infused brains correlated with oligomeric Aβ deposition. In order to determine whether microgliosis in the infusion model correlated with oligomeric Aβ deposits, immunohistochemical analyses of Aβ in infused brains was performed. Staining with the anti-Aβ antibody, 4G8, demonstrated immunoreactivity (Fig. 5A) broadly correlating with the same localization pattern of CD68 and phospho-tyrosine immunoreactivity observed in the peptide infused animals (Fig 4A). To determine whether the infused, immunodetected Aβo had remained oligomeric rather than converting to fibrils, sections of treated brains were stained using anti-oligomer antibodies, A11 and I11 to detect oligomeric species. Both A11 and I11 antibodies demonstrated positive immunoreactivity in the Aβo infused brains compared to vehicle-infused and control animals (Fig. 5B). This was supported by the fact that Aβo infused animals were negative for thioflavin staining and comparable to the vehicle treated group, suggesting that the immunodetected Aβ in infused brains had not fibrillized (Fig. 5C). To further validate the use of anti-oligomer antibodies at recognizing non-fibril confirmations in situ, again, the transgenic APP/PS1 model was used. Immunostaining of brains from 12 month old animals demonstrated robust immunoreactivity with anti-fibril antibody, OC, compared to control animals (Fig. 5D). However, abundant immunoreactivity of non-fibril, oligomeric Aβ was also observed using A11, anti-oligomer antibody in these brains. Indeed, although OC and A11 immunoreactivity co-localized to the same plaque structures, each antibody often displayed unique immunoreactivities within plaques (Fig. 5D). This not only indicates that plaques contain heterogeneous Aβ conformations but also suggests that anti-oligomer antibodies are useful for immunodetecting peptide conformations in situ, particularly in the infusion model employed.
Figure 5. Brains of Aβ oligomer-infused animals were thioflavin negative but displayed immunoreactivity with anti-Aβ, 4G8, and anti-oligomer, A11 and I11 antibodies.
(A) Brains from wild-type control, vehicle-infused, and Aβ oligomer-infused animals (+/−) dasatinib were immunostained with anti-Aβ (4G8) antibody. Representative images from the dentate gyrus of the right hippocampus are shown. (B) Brains were also immunostained with anti-oligomer antibodies, A11 and I11, to detect infused peptide. Representative images of control, vehicle-infused, and Aβ oligomer-infused animals are shown. (C) Brains from vehicle-infused and Aβ oligomer- infused C57BL/6J mice were stained with thioflavin to identify the presence of any fibrils and counterstained with DAPI as a nuclear stain and Sudan black to quench autofluorescence. Representative images from the dentate gyrus are shown from 6 animals per group. (D) Brains from 12 month old C57BL/6J control and APP/PS1 mice were immunostained with A11 and OC antibodies to visualize fibrillar and oligomeric Aβ peptide deposition. Representative double label with A11 (brown) and OC (blue) is shown. Arrows indicate only A11 immunoreactivity (brown), only OC immunoreacitvity (blue) and co-localization (black).
3.4. AD brains demonstrated increased protein phospho-tyrosine, Aβ, active Src kinase and active Lyn kinase levels compared to age matched controls
To further establish the correlation between Aβ oligomer levels and tyrosine kinase dependent microgliosis during disease, we compared human AD tissue with age matched non-demented controls. As seen in the in vitro and in vivo rodent data, examination of temporal cortex from human AD brains demonstrated a significant increase in overall protein phospho-tyrosine levels (Fig. 6A,B). This correlated with a significant increase is phosphorylated active forms of the tyrosine kinase, Src, as well as Lyn kinase, in diseased brains versus their corresponding age- matched controls suggesting that the protein phospho-tyrosine changes could be related to increased activity of the Src family of kinases (Fig. 6C,D). AD and control tissue lysates were also analyzed via Western Blot analyses to examine levels of multimeric forms of Aβ oligomers. Densitometric analysis of the multimeric SDS-stable species (Fig. 1A) demonstrated that AD brains had a significantly higher level than age-matched controls (Fig. 6A,B) correlating well with the changes in protein phospho-tyrosine, active Src and active Lyn levels.
Figure 6. AD brains had elevated protein phospho-tyrosine and active phospho-Src levels compared to age-matched controls.
AD and age-matched control (c) temporal lobe lysates (n=6) were resolved by 10% SDS-PAGE and Western blotted using (A) anti phospho-Tyr (4G10), anti-Aβ (6E10), anti-α-tubulin (loading control) antibodies, (C) anti-Lyn, anti-phospho-Lyn (Tyr396), anti-Src, and anti-phospho-Src antibodies. Optical density of (B) Total phospho-Tyr or Aβ multimers and (D) pLyn and pSrc from 6 AD and age-matched control brain blots were normalized to their respective α-tubulin blots or total protein levels (Lyn/Src) then averaged and graphed (+/−SD). (*p< 0.001)
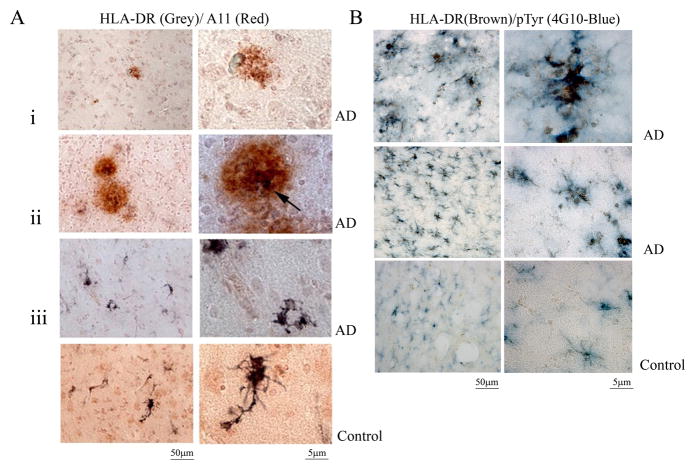
3.5. HLA-DR positive microglia from AD brains demonstrated increased phospho-tyrosine immunoreactivity and heterogeneously localized with oligomeric Aβ
In order to verify whether the changes in protein phospho-tyrosine and Aβ oligomers correlated with microglial activation in the AD brains, immunostaining of fixed AD tissue was compared to age-matched controls. As expected, AD brains demonstrated defined, plaque-like deposits that were immunoreactive with the anti-oligomer (A11) antibody (Fig. 7A). Although A11 positive deposits were not uniformly associated with activated microglia there were clear instances of HLA-DR (LN3) positive activated microglia directly associated with oligomeric deposits (Fig. 7A). This supported the in vitro (Fig. 2) and in vivo (Fig. 4) rodent observations of the ability of oligomeric Aβ to stimulate microglia and suggested that a similar activation occurs during disease. To further support the rodent observations, the AD and control brain sections were immunostained to determine whether HLA-DR positive, reactive microglia demonstrated increased protein phospho-tyrosine levels indicative of increased tyrosine kinase activity. AD brains demonstrated increased immunostaining for reactive microglia compared to age-matched controls (Fig. 7B). More importantly, reactive HLA-DR positive microglia co-localized with anti-phospho-tyrosine immunoreactivity in both diffuse and clustered cell patterns supporting the idea that tyrosine kinase-dependent changes were a component of the microglial activation process during disease (Fig. 7B).
Figure 7. A population of AD brain microglia was phospho-tyrosine immunoreactive and localized to Aβ oligomers.
AD and control (c) temporal lobe sections were immunostained using anti-HLA-DR antibody to identify microglia, anti-oligomer antibody, A11, to visualize prefibril peptide, and anti-phospho-Tyr (4G10) antibodies. (A) A11/HLA-DR double label is shown with (i) only A11 (red) immunoreactivity, (ii) double label (black arrow) and (iii) only HLA-DR (grey) immunoreactivity in the AD brain. (B) A double label for HLA-DR (brown)/phospho-Tyr (blue) is also shown. Images are representative of three cases each.
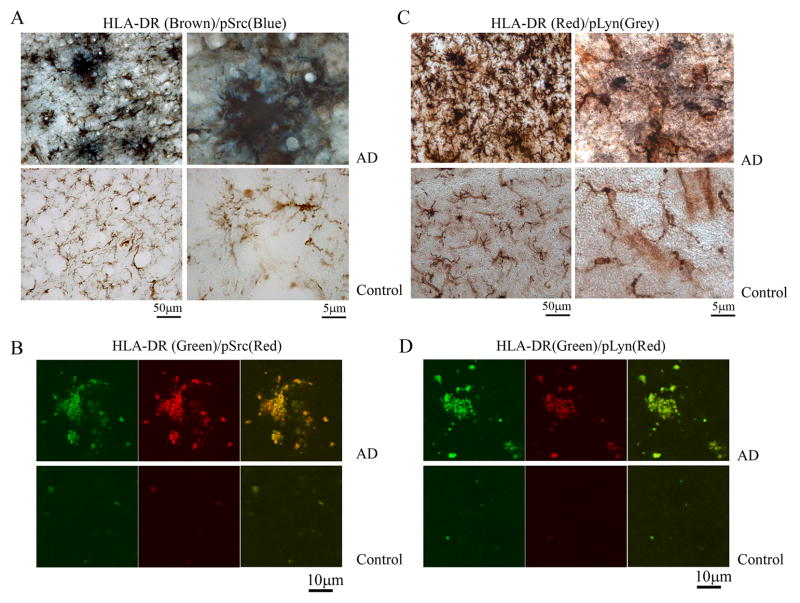
3.6. HLA-DR positive microglia from AD brains displayed increased immunoreactivity for active, phosphorylated Src and Lyn kinases
To begin identifying the specific enzymes responsible for the increased protein phospho-tyrosine levels, additional immunostaining of activated microglia in AD versus age-matched control brains was performed. Based upon the Western blot analysis of increased levels of active phosphorylated Src in AD brains (Fig. 6) immunostaining of active Src was again compared to the related family member, Lyn. Double label immunostaining using anti-HLA-DR to identify reactive microglia verified that a heterogeneous portion of the activated microglia also demonstrated increased phosphorylated, active Src immunoreactivity compared to age-matched control brains (Fig. 8A). A small amount of reactive microglia in AD versus control brains also demonstrated immunoreactivity for phosphorylated active, Lyn (Fig. 8C). The immunostaining correlated well with the Western blot analysis changes (Fig. 6) and supported the idea that tyrosine kinase-dependent activation of microglia occurred in AD brains involving at least the non-receptor kinase, Src. Dual label immunoflourescence and confocal imaging confirmed the co-localization of reactive microglia (using anti-HLA-DR antibody) and active Src immunoreactivity (Fig. 8B) as well as active Lyn immunoreactivity (Fig. 8D)
Figure 8. A population of AD brain microglia was immunoreactive for phospho-Lyn and phospho-Src.
AD and control temporal lobe sections were immunostained using anti-HLA-DR antibody to identify microglia, anti-phospho-Lyn-396 antibody to identify activated Lyn kinase and anti-phospho-Src antibody to identify activated Src kinase. (A) p-Src (blue)/HLA-DR (brown) double label and (C) p-Lyn (grey)/HLA-DR (red) double label images are shown. Images are representative of three cases each. AD and Control temporal lobe sections were also double-labeled using immunoflourescence using (B) anti-HLA-DR (green) and anti-pSrc (red) antibodies or (D) anti-HLA-DR (green) and anti-pLyn (red) antibodies.
4. Discussion
Our findings demonstrate that Aβ oligomers stimulate microglia activation in vitro via a tyrosine kinase associated pathway that results in increased secretion of the proinflammatory cytokine, TNF-α. It is possible to attenuate the oligomer-stimulated microglial phenotype change using a non-receptor tyrosine kinase inhibitor, dasatinib. The in vitro observations were validated in vivo demonstrating that intracerebroventricular infusion of oligomeric Aβ also stimulated increased microgliosis via a tyrosine kinase-dependent mechanism that was attenuated by co-delivery of dasatinib. Finally, the rodent findings correlated with reactive microglial increase in protein phospho-tyrosine, p-Lyn and p-Src levels in AD brains compared to controls. To the best of our knowledge microglial pSrc and pLyn immunoreactivity in AD brains has not been reported prior thus identifying possible kinase targets for anti-inflammatory strategies. Also, the ability of oligomeric Aβ to stimulate these microglial changes in an in vivo mouse model now offers a system for addressing drug efficacy. Finally, we provide the first evidence that dasatinib, selected to target a particular tyrosine kinase, can be used to attenuate oligomeric Aβ-dependent microgliosis. Collectively, these findings suggest that Aβo can serve as a microglial activating ligand that leads to a proinflammatory phenotype and use of non-receptor tyrosine kinase inhibitors may be an effective strategy to limit microglial-mediated changes during disease.
Although much of the data relies upon phospho-tyrosine immunoreactivity, we appreciate that this type of assessment represents changes in a large number of diverse proteins. Indeed, it is likely that phosphatase activities are activated in parallel with kinase activities resulting in the cumulative presentation of protein phosphotyrosine levels observed with the Western blots or the immunostains. With both histology and Western blot analysis of phospho-tyrosine immunoreactivity, it is difficult to predict what proteins are being assessed. In addition, the absolute profile of particular phospho-tyrosine immunoreactive banding patterns by Western blot is very difficult to exactly reproduce from experiment to experiment since it can literally differ by collection times that vary by a few seconds. Indeed, although we perform all experiments as precisely identical as possible, there are sometimes differences even in basal levels of protein phospho-tyrosine banding patterns. For this reason, we are careful not to place emphasis on any particular molecular weight species when performing phospho-tyrosine Western blots but rather on overall differences always compared to their appropriate vehicles/controls.
In addition, we appreciate that immunostaining quantitation employed in the current study could benefit from additional means of quantitation. We have relied on immunostaining to perform the quantitation in this study based upon our impression from other studies in the lab that the hemisphere that receives the infusion has the more robust response compared to the contralateral side. Therefore, quantitative analysis is usually performed on only the infused side which we collected by fixation for this study. Future work could include an additional means of quantitation of microgliosis associated changes including ELISA, kinase activity assays, and Western blotting.
It is important to point out that although the microgliosis observed from the in vivo component of the data resulted from infusion of oligomeric Aβ, we have not demonstrated that the microglial changes observed are due to a direct stimulation of microglia by Aβ. This is an intrinsic limitation of the in vivo approach and the staining is necessarily correlative with the in vitro data set. Nevertheless, we feel that the histologic observations of the infused brains do offer some insight into possible events that may occur during disease even though we can not definitively state that oligomers are directly stimulating a microglial increase in tyrosine kinase activity. Since there is, to the best of our knowledge, no accepted stimulus for the increase in phosphotyrosine immunoreactivity in the AD mouse models or the human disease we offer the suggestion that oligomeric Aβ may be one of the ligands involved. In addition, the oligomeric infusion paradigm at least allows us to state that the increase in protein phosphotyrosine immunoreactivity is associated with an oligomeric stimulation in vivo which is mechanistically a bit more rigorous that immunostaining transgenic or human diseased brains and co-localizing Aβ and phosphotyrosine immunoreactivity and hypothesizing that Aβ is the stimulus responsible for the immunoreactivity change.
We have found that an oligomeric preparation of Aβ corresponding roughly to the size of SDS-stable trimer/tetramers at initial stimulation can drive microgliosis via a tyrosine kinase-dependent mechanism that correlates with a similar change in tyrosine phosphorylation in human diseased brains. In spite of the fact that the oligomeric Aβ was delivered in a HDL solution to ensure its stability as an oligomeric preparation while in the pump, it is possible that the multimeric species changed after infusion into the brain over the time course of 14 day administration resulting in higher molecular weight species. However, immunohistochemical analyses using anti-oligomer antibodies to observe for oligomeric Aβ immunoreactivity strongly suggests by the thioflavin negative, A11/Ill positive nature of the deposited Aβ that the peptide was retained in an oligomeric and not fibrillar form. We cannot exclude the possibility that higher order multimers and possibly some undetected fibrils did accumulate in the tissue during the infusion period along with, perhaps, co-deposition of endogenous Aβ. With the understanding that there may be some multimeric species of Aβ involved, along with the SDS-stable trimeric/tetrameric infused Aβ, we suggest that the changes observed in microgliosis and the ability of dasatinib to attenuate those changes remain novel and relevant to Aβ-dependent microglial changes during disease. With regard to human disease, we certainly do not exclude the likelihood that fibrillar deposits of Aβ are also potent stimuli of microglia. Increased protein phopho-tyrosine staining from both AD brains and transgenic mouse models (Wood and Zinsmeister, 1991; Frautschy et al., 1998) have characterized microglial activation in association with Aβ plaques. Furthermore, prior work has demonstrated that fibrils also stimulate microglia in vitro via tyrosine kinase-mediated mechanisms (McDonald et al., 1997; Combs et al., 1999). However, our histologic analysis of human brains demonstrated that some reactive microglia were associated with oligomeric Aβ as well, a finding not widely reported. In addition, many phospho-tyrosine immunoreactive microglia in diseased brains were not associated with any observable Aβ deposits suggesting that non-deposited Aβ oligomers or other molecules could be activating these cells as well. Therefore, we propose that oligomers are one of the stimuli driving microgliosis during disease but recognize a likely role of fibrils as well as possible still unnamed ligands.
In spite of that fact that the specific stimuli driving microgliosis during disease may be heterogeneous, a common finding from our rodent in vitro and in vivo findings and AD brains is the increase in protein phospho-tyrosine levels. Our prior in vitro work has demonstrated that specific non-receptor tyrosine kinase activities including Lyn and Syk are involved in the oligomer-dependent activation of microglia (Sondag et al., 2009) which correlated well with the increased phospho-tyrosine immunoreactivity observed in the intracerebroventricular infused animals. We have not yet characterized the extent of specific kinase changes in the in vivo model but we are aware that numerous tyrosine kinases are expressed in microglia and it is likely that the temporal profile of kinase activities changes during chronic oligomer stimulation. During disease, fibril or other ligand stimulations may also initiate additional changes in specific tyrosine kinase activities that will be superimposed upon oligomer-mediated responses (Sondag et al., 2009). Therefore, we have not attached any crucial pro-inflammatory significance to a particular kinase activity at this point other than to validate that in human disease, active forms of non-receptor tyrosine kinases, Src and Lyn, are increased in reactive microglia consistent with the rodent data. Indeed, the microglial phospho-Src and phospho-Lyn immunoreactivity changes we observed in the AD brains is, to the best of our knowledge, a new contribution to the field by beginning to identify particular kinase activities that may be responsible for the increase in phosphotyrosine immunoreactivity. Future efforts to identify particular populations of microglia that are immunoreactive for activated tyrosine kinases in correlation with co-localization to oligomeric or fibrillar Aβ may offer insight into mechanistic reasons for microglial heterogeneity of activation during disease.
This common change of increased forms of active non-receptor tyrosine kinases in the rodent and human findings served as the rationale for selecting a somewhat broad specificity agent, dasatinib, for its ability to attenuate the function of several different kinases, in particular the Src family members (Lombardo et al., 2004; Das et al., 2006). The ability of dasatinib to limit protein phospho-tyrosine changes and cytokine secretion suggests that targeting kinase activity is a viable anti-inflammatory strategy for treating AD. It is interesting to note that limiting tyrosine kinase-dependent microgliosis is not the only attractive reason for targeting these enzymes. For instance tau phosphorylation is modulated via activity of the tyrosine kinase Abl modulating Cdk5 activation in a transgenic mouse model of AD (Alvarez et al., 2004; Cancino et al., 2009). Very recently, another tyrosine kinase inhibitor anti-cancer drug, imatinib, has been shown to target gamma-secretase activating protein as a therapeutic approach for Alzheimer’s disease in a rodent model (He et al., 2010). Not surprisingly, recruitment of Src and Abl tyrosine kinases to APP has been demonstrated (Trommsdorff et al., 1998). Our prior work has also verified an increased association of Src with APP in human diseased brains (Austin et al., 2009). Although we have focused on a specific oligomer-stimulated mechanism of proinflammatory microgliosis in AD that is non-receptor tyrosine kinase dependent, it appears that the heterogeneous mixture of changes during disease offers the possibility that inhibition of this class of kinases will be attractive from a number of different therapeutic perspectives.
Validation that an orally available, FDA approved drug, dasatinib, is efficacious in our in vivo study supports the idea of therapeutic tyrosine kinase inhibition for AD. Dasatinib (commercially, Sprycel) is used for treating chronic myeloid leukemia (Shah et al., 2004) and has the ability to cross the blood brain barrier (Porkka et al., 2008). It attenuates proinflammatory microgliosis and inhibits disease relevant non-receptor tyrosine kinases. Further studies involving transgenic mouse models of AD and use of dasatinib and/or other non-receptor tyrosine kinase inhibitors will shed light on the mechanism(s) of microglial activation during disease and confirm that tyrosine kinase activity is an important target for attenuating disease.
Acknowledgments
This publication was supported by NIH/NCRR 1P20 RR17699, NIH/NIA 1R01AG026330 UND Intercollegiate Academic Fund and Office of the Provost, and NSF grant # EPS-0814442. We thank Dr. Eric J. Murphy for help during the Aβ oligomer preparation and Drs. Linda Van Eldik, Jeff Craft, Thad Rosenberger, and Lalida Rojanathammanee and Ms. Keiko Murase for expert advice and assistance with animal surgeries. We are grateful to Dr. Rakez Kayed for generously supplying A11, I11, and OC antibodies and offering many helpful suggestions and comments for the immunostaining procedure.
Footnotes
Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gunjan Dhawan, Email: gdhawan@medicine.nodak.edu.
Angie M. Floden, Email: angela.floden@med.und.edu.
References
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL. Occurrence of the diffuse amyloid beta-protein (Abeta) deposits with numerous Abeta-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia. 1999;25:324–331. doi: 10.1002/(sici)1098-1136(19990215)25:4<324::aid-glia2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Alvarez AR, Sandoval PC, Leal NR, Castro PU, Kosik KS. Activation of the neuronal cAbl tyrosine kinase by amyloid-beta-peptide and reactive oxygen species. Neurobiol Dis. 2004;17:326–336. doi: 10.1016/j.nbd.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Austin SA, Sens MA, Combs CK. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci. 2009;29:14451–14462. doi: 10.1523/JNEUROSCI.3107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cancino GI, Perez de Arce K, Castro PU, Toledo EM, von Bernhardi R, Alvarez AR. cAbl tyrosine kinase modulates tau pathology and Cdk5 phosphorylation in AD transgenic mice. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Combs CK, Bates P, Karlo JC, Landreth GE. Regulation of beta-amyloid stimulated proinflammatory responses by peroxisome proliferator-activated receptor alpha. Neurochem Int. 2001a;39:449–457. doi: 10.1016/s0197-0186(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001b;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cras P, Kawai M, Siedlak S, Mulvihill P, Gambetti P, Lowery D, Gonzalez-DeWhitt P, Greenberg B, Perry G. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer’s disease. Am J Pathol. 1990;137:241–246. [PMC free article] [PubMed] [Google Scholar]
- Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49:6819–6832. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and beta-amyloid production in cultures. Neurosci Lett. 1995;188:70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Walker DG, McGeer PL. Interaction of Alzheimer beta-amyloid peptide with the human monocytic cell line THP-1 results in a protein kinase C-dependent secretion of tumor necrosis factor-alpha. Brain Res. 1997;747:114–121. doi: 10.1016/s0006-8993(96)01229-2. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Hao C, Munoz DG. Role of microglia in senile plaque formation. Neurobiol Aging. 1995;16:797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pardo CA, Cork LC, Price DL. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994;145:1358–1381. [PMC free article] [PubMed] [Google Scholar]
- Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R, Wild R, Luo R, Arnan M, Brethon B, Eccersley L, Hjorth-Hansen H, Hoglund M, Klamova H, Knutsen H, Parikh S, Raffoux E, Gruber F, Brito-Babapulle F, Dombret H, Duarte RF, Elonen E, Paquette R, Zwaan CM, Lee FY. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaguchi H, Ogawa A, Sugihara S, Nakazato Y. Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol. 1997;94:316–322. doi: 10.1007/s004010050713. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Shoji M, Harigaya Y, Kawarabayashi T, Ikeda M, Naito M, Matsubara E, Abe K, Nakazato Y. Amyloid cored plaques in Tg2576 transgenic mice are characterized by giant plaques, slightly activated microglia, and the lack of paired helical filament-typed, dystrophic neurites. Virchows Arch. 2002;441:358–367. doi: 10.1007/s00428-002-0643-8. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Welzel AT, McDonald JM, Selkoe DJ, Walsh DM. Isolation of low-n amyloid beta-protein oligomers from cultured cells, CSF, and brain. Methods Mol Biol. 2011;670:33–44. doi: 10.1007/978-1-60761-744-0_3. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondag CM, Dhawan G, Combs CK. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J Neuroinflammation. 2009;6:1. doi: 10.1186/1742-2094-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styren SD, Civin WH, Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer’s disease brain. Exp Neurol. 1990;110:93–104. doi: 10.1016/0014-4886(90)90054-v. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Perez KA, Pike KE, Kok WM, Rowe CC, White AR, Bourgeat P, Salvado O, Bedo J, Hutton CA, Faux NG, Masters CL, Barnham KJ. Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer’s disease. J Neurosci. 2010;30:6315–6322. doi: 10.1523/JNEUROSCI.5180-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Imaki H, Wang KC, Wronska A, Osuchowski M, Rubenstein R. Origin and turnover of microglial cells in fibrillar plaques of APPsw transgenic mice. Acta Neuropathol. 2003;105:393–402. doi: 10.1007/s00401-002-0660-3. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22:49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Wood JG, Zinsmeister P. Tyrosine phosphorylation systems in Alzheimer’s disease pathology. Neurosci Lett. 1991;121:12–16. doi: 10.1016/0304-3940(91)90637-9. [DOI] [PubMed] [Google Scholar]