Abstract
Prolonged subjection to unstable work or lighting schedules, particularly in rotating shift-workers, is associated with an increase risk of immune-related diseases, including several cancers. Consequences of chronic circadian disruption may also extend to the innate immune system to promote cancer growth, as natural-killer (NK) cell function is modulated by circadian mechanisms and plays a key role in lysis of tumor cells. In order to determine if NK cell function is disrupted by a model of human shift-work and jet-lag, Fischer (344) rats were exposed to either a standard 12:12 light-dark cycle, or a chronic shift-lag paradigm, consisting of ten repeated 6 hour photic advances occurring every 2 days, followed by 5–7 days of constant darkness. This model resulted in considerable circadian disruption as assessed by circadian running wheel activity. NK cells were enriched from control and shifted animals, and gene, protein, and cytolytic activity assays were performed. Chronic shift-lag altered the circadian expression of clock genes, Per2 and Bmal1, and cytolytic factors, perforin and granzyme B, as well as the cytokine, IFNγ. These alterations were correlated with suppressed circadian expression of NK cytolytic activity. Further, chronic shift-lag attenuated NK cell cytolytic activity under stimulated in-vivo conditions, and promoted lung tumor growth, following intravenous injection of MADB106 tumor cells. Together, these findings suggest chronic circadian disruption promotes tumor growth by altering the circadian rhythms of NK cell function.
Introduction
The multi-oscillator circadian system adapts to changing internal and external states in order to optimize the timing of physiological processes. Temporal coordination among multiple physiological systems is essential for homeostatic regulation, while circadian disruption may negatively impact health. Several large-scale epidemiological studies on rotating shift-workers have reported working during the night is a major risk factor for several types of cancer, including non-Hodgkin’s lymphoma (1), breast (2–4), endometrial (5), prostate (6, 7), and colon (8, 9) cancers. Further, cancer risk is positively related to the frequency of night shifts an individual had worked, highlighting the potential detrimental effects of prolonged unstable work and light-exposure schedules (4, 9). In addition, robustness of circadian rhythms in salivary cortisol and rest-activity cycles are significant predictors for survival in cancer patients (10, 11). Other diseases, such as obesity, diabetes, and cardiovascular problems, are highly prevalent among shift-workers as well (12–14). Despite growing evidence from human studies linking circadian disruption or desynchony, to disease, an understanding of the essential mechanisms involved in the transition or promotion of particular disease states, such as tumor development, are lacking.
Since the initial reports in humans associating shift-work and cancer, a majority of animal studies have primarily focused on the consequences of disrupted molecular clocks on cellular proliferation pathways to promote tumor growth (15–19). Although these pathways are important for cancer development and progression, the relationship between circadian disruption and cancer may extend beyond perturbations of cell cycle processes. For example, inflammatory response by macrophages is dysregulated by disruption to cellular clocks (20, 21). However, the underlying mechanism by which altered circadian immune function may promote the transition to particular disease states is unknown, especially in relation to cancer. There is little evidence implicating disruption to circadian mechanisms in impaired immune function, and tumor growth.
The role of natural killer (NK) cells as critical mediators of cancer immunosurveillance is well established (22, 23). In mice, depletion of NK cells is associated with increased tumor growth in spontaneous and induced tumor models (24, 25). Similarly low NK cell activity is associated with increased risk for cancer in human populations (26, 27). In order to kill tumor cells, NK cells recruit proinflammatory cytokines, release cytolytic granules, and activate receptors on target cells (23, 28, 29). Of particular importance are the cytolytic factors, granzyme B and perforin, and cytokines tumor necrosis factor (TNF) and interferon gamma (IFNγ), which are critical factors involved in regulating NK cell mediated killing of tumor cells (28–30). Decreases in any of these factors are associated with increased risk of developing infections and tumors (24, 25, 31–33).
The negative health consequences of circadian disruption are likely to involve alterations in NK cell function. Circadian mechanisms may optimize an immunological response by coordinating function between peripheral tissues and immunocompetent cells. Previously, we have reported that NK cell function, including cytokines, cytolytic factors, and cytolytic ability, is tightly controlled by the circadian system (34). Signals directly or indirectly derived from the circadian pacemaker, which is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, are transmitted to peripheral tissues by neural and endocrine pathways in order to synchronize and coordinate body physiology (34–36). Rhythmic sympathetic input to the spleen modulates NK cell function by regulating components of the molecular clock in these cells (37). Maximal cytotoxicity at specific circadian phases is postulated to be driven by molecular clock coordinated expression of IFNγ, TNFα, perforin and granzyme B (38, 39).
Nevertheless, there is little data indicating potential relationships between disruption of clocks in immune cells, and the promotion of tumor growth. Therefore, the main goal of this study was to determine whether NK cells were susceptible to circadian disruption by exposing animals to a chronic shift-lag paradigm in which the daily light-dark (LD) cycle was repeatedly phase-shifted in order to experimentally mimic the disruptive effects of human shift-work and jet-lag. Under the shift-lag schedule, circadian disruption was assessed by continuously monitoring of voluntary running-wheel activity. We then examined circadian rhythms in NK cell function at the gene, protein, and physiological levels under both basal and stimulated conditions. Finally, we sought to determine if alterations in NK cell function due to repeated shifting of the LD cycle were related to an increased risk of tumor development using an induction model of NK-sensitive adenocarcinoma cells (MADB106) (40, 41). MADB106 cancer cells typically metastasize in the lungs following intravenous injection and the method is well established for exploring the role of NK cell function in tumor growth (41–43). We hypothesized chronic shifting would suppress NK cell function in response to tumor cells by altering the coordinated circadian expression of key immune factors. We also postulated these changes in NK cell function would promote tumor growth.
Materials and Methods
Animals and apparatus
Male Fischer (F344) rats were obtained from Charles River at about 50 days of age. Upon arrival, animals were acclimated to the housing facility and maintained individually in running-wheel cages (wheel diameter: 34 cm; Mini Mitter Co., Bend, OR), placed within light- and sound-attenuating climate-controlled cubicles. Running-wheel activity was recorded by a computer and stored for later analysis of circadian parameters using the ClockLab software package (Actimetrics Co., Wilmette, IL). Animals were given free access to food and water for the duration of the experiment. All experimental procedures and animal treatment protocols were approved by Rutgers Animal Care and Facilities Committee and complied with National Institutes of Health policies.
Procedures
Animals were maintained initially under a standard 12:12 LD cycle with lights on at 6:00 h and lights off at 18:00 h for a 1–2 week acclimation period in order to stabilize running-wheel activity, and then assigned randomly to either remain under the standard LD cycle (controls) or to undergo the chronic shift-lag protocol (n = 90 per lighting regimen used for various assays, see below). The shift lag protocol consisted of 6-h LD phase advances repeated every 2 days for a total of 10 shifts (see Fig. 1A–Cfor a schematic representation of the control and shift-lag lighting regimens). For example, the first advance consisted of the lights coming on 6 h earlier than the previous day (lights on at 24:00 h), which resulted in a shortening of the dark period on the following day. Repeated phase advances were chosen due to extensive prior data indicating that advances evoke greater circadian disruption than do phase delays. In order to determine whether the chronic shift-lag paradigm resulted in alterations of circadian expression of cytolytic factors, cytokines, and cytolytic capabilities in NK cells, a set of animals (n = 36 per lighting regimen) were sacrificed by decapitation at six time points (n = 6 per time point) 5–7 days into DD, which corresponded to circadian times (CT) 3, 7, 11, 19, and 23. To avoid the potential masking effects of light on these circadian rhythms, animals were sacrificed 5–7 days into DD following the last advance of the LD cycle. Actograms were used to determine CTs by defining CT12 as the onset of running-wheel activity. Immediately upon sacrifice, tissues were collected and NK cells were separated from spleens for gene, protein, and cytotoxicity assays. In order to determine the effects of chronic shift-lag on NK cell cytotoxicity in response to tumor cells and the possible consequences on tumor growth, separate groups of animals (n = 30 per lighting regimen) were inoculated with MADB106 cells at CT19 in DD. Half of these animals (n = 10 per lighting regimen) were sacrificed 24 h after inoculation in order to assess immediate NK cell response to the presentation of tumor cells (40, 43). The remaining animals (n = 20 per lighting regimen) were placed for 6–8 weeks under the same standard LD cycle as during the acclimation period, and then sacrificed for lung extraction and inspection of tumor metasases.
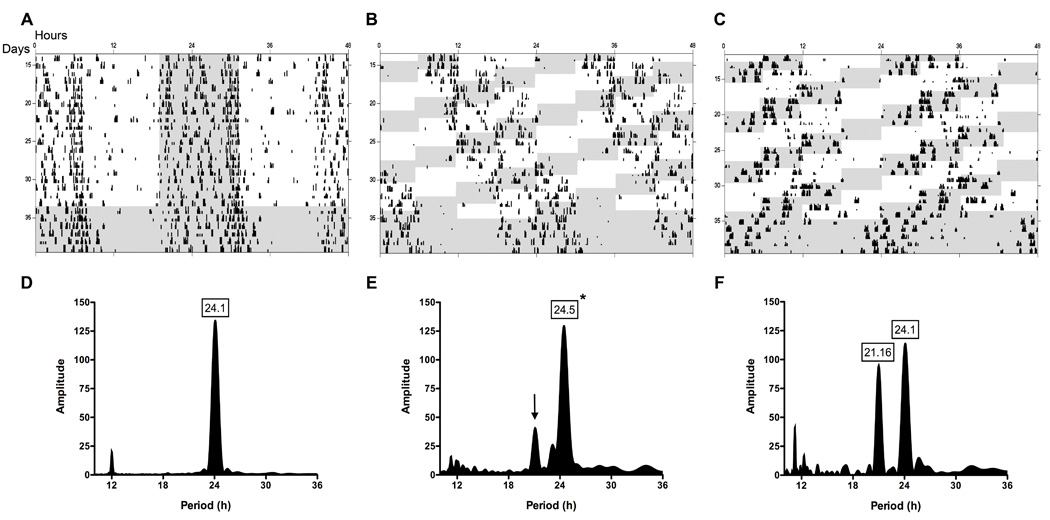
Figure 1. Effects of chronic shift-lag on circadian wheel-running activity rhythms.
Circadian wheel-running activity rhythms depicted as standard raster-style double-plotted actograms from several representative animals from control (A) and shifted (B,C) groups. Repeated shifts of the LD cycle resulted in different behavioral patterns of reentrainment among animals. Shift-lagged animals tended to essentially “ignore” (B), or reentrain (C) to changing environmental light cues. LS periodogram analysis for control (D) and shifted (E) animals of rhythm period and amplitude during standard or shifted LD cycles revealed shifted animals tend to remain rhythmic with a significantly longer period (*; p < 0.0001). Large secondary peaks are evident in the shifted group (arrow), which with further analysis revealed particular animals displaying shorter periods. Robust periods shorter in length were also common in the shifted group, which is represented by an actogram (C) and periodogram (F) of an individual animal during the shifting paradigm. No differences were found in rhythm amplitude during standard or shifting paradigms. Shaded regions represents dark phase of LD cycle.
Enrichment of NK cells from spleen
Individual spleen tissue was processed as described previously (44). RBCs and granulocytes were removed from splenocyte suspensions by density centrifugation using Histopaque 1083 (Sigma-Aldrich). Splenocytes (~10 × 107 cells per spleen), were extracted from the middle layer, washed with RPMI (Invitrogen), and resuspended in buffer (PBS, 0.5% BSA). Splenocytes were incubated with primary antibodies conjugated to FITC (BD Biosciences), anti-CD6 (OX52; T-cells), anti-CD45RA (OX-33; mature B-cells), and anti-RT1B (OX-6; dendritic cells and macrophages), followed by secondary incubation with anti-FITC microbeads per manufacturer’s instructions (Miltenyi Biotec). NK cells were then enriched by magnetic separation (negative selection) using an AutoMACS Magnetic Separator (Miltenyi Biotec). The enriched fraction consistently yielded ~5 × 106 cells per spleen with a purity of ~80–90%, which was assessed by flow-cytometry. CD-3+ and CD-8+ cells in the enriched fraction was consistently below 3%, and the remaining 10–20% of cells are believed to be a mixture of premature hematopoietic and endothelial cells. Following enrichment, NK cells were divided and lysed in appropriate buffers for RNA and protein analyses, or used for cytotoxicity assays.
RNA extraction, RT-PCR, and real-time RT-PCR
Total RNA was isolated from ~2 × 106 NK cells per spleen using the RNeasy Mini Kit (Qiagen). Using Superscript III First-Strand Synthesis SuperMix (Invitrogen) for RT-PCR, 100 ng of total RNA was reverse transcribed and relative quantification of mRNA levels were performed by real-time RT-PCR (SYBR Green and TaqMan gene expression assays, Applied Biosystems), using an ABI Prism 7700 Sequence Detector. The following primer sequences were used: Per2 F 5’-GCAGCCTTTCGATTATTCTTC-3’, R 5’-GCTCCACGGGTTGATGAAG-3’; Bmal1 F 5’-TCCGATGACGAACTGAAACAC-3’, R 5’-CTCGGTCACATCCTACGACAA-3’; perforin F 5’-GCATCGGTGCCCAAGCCAGTG-3’, R 5’-GCCAGCGAGCCCCTGCTCATCA-3’, and specifically designed probes were used for granzyme-B and IFN-γ (Applied Biosystems). Analyses were performed using the standard curve method with GADPH (Applied Biosystems) as the normalizing endogenous control. Relative mRNA expression levels were calculated as the percentage of the maximum value over the 24 h period (45). The sample with the maximum ratio was used to compute percentages for all other samples by dividing each ratio by the maximum and converting to a percentage. Values at each CT represent a mean and standard error of these values.
Western blot
For protein analyses, enriched NK cells (~2 × 106 cells per spleen) were lysed with a buffer containing protease and phosphatase inhibitors (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, and 5% glycerol with Pierce Halt protease inhibitor). Protein (50 µg) from total lysate was loaded and separated on a 4–20% SDS-PAGE and transferred overnight to polyvinylidene difluoride membranes. Membranes were incubated with primary antibody for 12 h at 4°C in blocking buffer 5% w/v nonfat dry milk in Tris-buffered saline and 0.1% Tween-20. Primary antibodies were used as follows: anti-BMAL1 rabbit polyclonal (1:500; Millipore), anti-PER2 mouse monoclonal (1:100; BD Biosciences), anti-perforin rabbit polyclonal (1:250), anti-granzyme B mouse monoclonal (1:200), and anti-IFN-γ mouse monoclonal (1:250) antibodies (Santa Cruz Biotechnology). Membranes were washed and incubated with peroxidase conjugated secondary antibodies (1:5000; Vector Laboratories) for 1 h at room temperature. Membranes were incubated with ECL Western blot chemiluminescence reagent (Pierce), exposed to x-ray film, developed, and densitometry was performed using Image J analysis software (NIH). Each protein was normalized to corresponding intensities for β-actin. Relative protein expression values were calculated as the percentage of the maximum value over the 24 h period (46). The sample with the maximum ratio was used to compute percentages for all other samples by dividing each ratio by the maximum and converting to a percentage. Values at each CT represent a mean and standard error of these values.
NK cell cytotoxicity
The cytotoxicity of enriched NK cells collected at each time point was determined by calcein-AM assays using YAC-1 murine lymphoma cells (ATCC) as target cells. YAC-1 cells were grown and maintained in RPMI-1640 without phenol red (Cellgro) containing 1% antibiotics (Sigma-Aldrich) and 10% fetal bovine serum (Sigma-Aldrich). YAC-1 cells were washed and incubated with 5 mM calcein-AM (Sigma-Aldrich) in serum-free RPMI for 10 min at 37°C. Labeled YAC-1 cells were washed and plated into U-bottom 96-well plates (Falcon) at a concentration of 5 × 104 cells per well. NK cells were added at various effector to target cell (E:T) ratios in triplicates. YAC-1 cells in RPMI alone were to determine spontaneous calcein-AM release, while maximal release was achieved by lysing target cells in buffer (0.1% Triton X-100). NK cells collected over the circadian timescale were pre-incubated for 12 h with IL-2 at 37°C (100 ng/ml; R&D Systems) prior to 4 h incubation with YAC-1 target cells. IL-2 was used to increase assay efficiency due to the comparatively selective nature of this cytokine to increase cytotoxicity in NK cells (47). NK cells collected from animals inoculated with MADB106 cells at CT19 were incubated at 37°C for 4 h with YAC-1 cells in the absence of IL-2. All assays were analyzed using a fluorescence plate reader (Tecan). The percent cytotoxicity for each sample was calculated as follows: % cytotoxicity = [(experimental well – spontaneous well) / (max lysed well – spontaneous well)] × 100. The percentages at each E:T ratio were converted to lytic units per 107 effector cells and based on 20% specific cell lysis (48).
Tumor cell inoculation and metastases
Following control and chronic shift-lag protocols, animals were placed under DD for 5–7 days and inoculated at CT19 with MADB106 tumor cells (1 × 105 cells/0.2 ml/rat) into the jugular vein under isoflurane anesthesia (Henry Schein). As noted above, CT19 was determined by wheel-running data for each animal. This time was chosen based on previous studies and our pilot data showing maximal response of NK cells during the dark phase of the LD cycle (45, 46). Isoflurane was used to minimize the amount of time the animal was anesthetized. Small incision sites (~1 cm) were closed with a surgical clip and rats were placed back into their homecage. Surgical and inoculation procedures took ~5–7 mins to complete per animal. Animals were sacrificed 6–8 weeks after tumor cell inoculation and each animal was inspected for the presence of visible tumors. As expected, tumors were located primarily in the lungs. Lungs were collected and placed for 24 h in Bouin’s solution (72% saturated picric acid solution, 23% formaldehyde 37% solution, and 5% glacial acetic acid). Lungs were washed with ethanol and two individuals separately counted surface tumor metastases.
Plasma analysis of corticosterone
Blood samples (~80–100 µL) were collected at CT7 and CT19 in 15 µL of EDTA and centrifuged for plasma. Plasma was analyzed for corticosterone levels by a competitive enzyme-linked immunosorbent assay (ELISA; IBL, USA) according to manufacturer’s recommendations. All samples from both groups were run on a single 96-well plate to limit variability. Standard curves were used to determine corticosterone levels.
Data analysis
Two-way ANOVA with Bonferroni post-hoc tests was used to determine differences between control and shift-lag groups at each circadian phase. The gene and protein expression data were tested for significant circadian rhythmicity using CircWave v1.4 software (21, 49) to determine the best-fitting linear harmonic regression with an assumed period of 24 h and with alpha set at 0.05. The center of gravity (COG) of each best-fitting wave-form, which is comprised of both sine and cosine components, in CircWave was used as the circadian acrophase and the associated estimation error was used as the standard deviation. In higher order wave-forms, the COG is a reliable estimator of phase of peak expression (50, 51). One-way ANOVA with Newman-Keuls multiple comparison tests were used to compare acrophases between groups for each gene or protein. Student t-tests were used to compare group means for circadian acrophases of NK cell activity circadian acrophases, tumor frequency data and circadian periods. Mann-Whitney U test was used to compare tumor frequencies between different defined chronotypes of the chronic shift-lag animals. Circadian period and robustness of running-wheel activity rhythms were derived using Lomb-Scargle periodograms during and following the control and shift-lag protocols (ClockLab).
Results
Circadian wheel-running activity rhythms
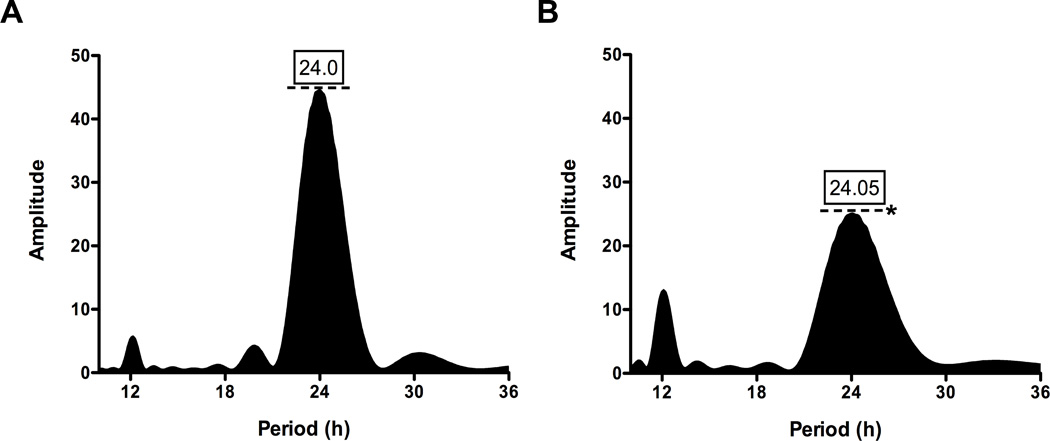
Control animals under the standard LD cycle displayed typical circadian entrainment patterns (Fig. 1A) with an approximate period of 24 h (Fig. 1D). Under the chronic shift lag protocol, rats exhibited different patterns of locomotor activity rhythms. For most shift-lag animals, periodogram analysis revealed two distinct peaks, one corresponding to the mean period of the shift-lag schedule (i.e., ~21 h) and one corresponding to the typical, longer-then-24 h free-running period normally shown by rats under free-running conditions (Fig. 1E,F). However, there was considerable individual variation among shift-lag animals in the relative prominence of these peaks, and a clear correspondence between the periodogram results and the actigraphic record within individuals (Fig. 1B,C). In DD following the shifting paradigm, rhythm amplitude was significantly reduced with no observable change in period (Fig. 2A,B; p < 0.0001). Thus, the shift-lag protocol induced considerable circadian disruption and also resulted in light exposure during the animals’ subjective night.
Figure 2. Circadian wheel-running activity rhythm amplitudes are decreased following chronic shift lag.
LS periodograms analysis restricted to the initial 5 days in DD for control (A) and shifted (B) groups. No differences for period were demonstrated between groups in DD. Rhythm amplitude levels (dotted lines) were significantly decreased in DD following the chronic shifting paradigm (*; p < 0.0001). Shifted animals remained comparatively rhythmic with a dampening of circadian rhythm.
Chronic shift lag alters the circadian expression of clock genes in enriched NK cells
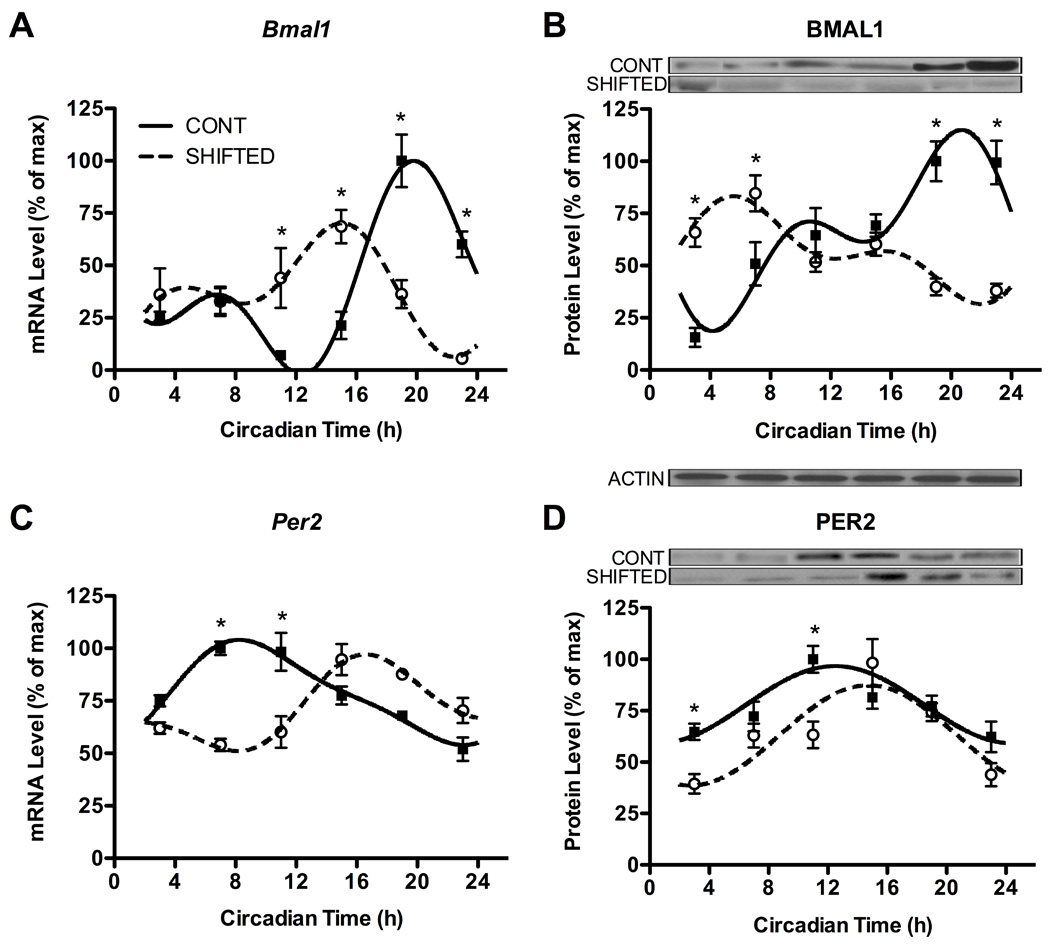
In order to determine whether chronic shifting affected the molecular clock in peripheral immune cells, we examined gene and protein expression of two canonical clock genes, Per2 and Bmal1 (52), from enriched NK cells collected during DD at 4-h intervals across the circadian cycle. In control animals, both Per2 and Bmal1 gene and protein expression displayed robust circadian rhythmicity (Fig. 3A–D; all p’s < 0.01). Acrophase estimates (estimate ± SD) derived from fitted curves indicated Per2 and Bmal1 expression peaks were out of phase with one another at both the gene (Fig. 3A,C) and protein (Fig. 3B,D) levels (Table 1), as expected from previous studies (45). In addition, as hypothesized, chronic shift lag altered the circadian expression of both clock genes and proteins; nevertheless, these displayed significant rhythmicity in shift-lag animals (Fig. 3A–D; all p’s < 0.01). Comparison of acrophases for Per2 and Bmal1 revealed expression rhythms were shifted in NK cell from shift-lagged animals compared to controls (Table 1; Fig. 3A,C; p’s < 0.05), while BMAL1, but not PER2, phase of peak expression was affected by shift-lag (Table 1; Fig. 3B,D). Moreover, two-way ANOVAs indicated significant group by time interactions (p’s < 0.05) for Per2 and Bmal1 gene and protein expression rhythms, which were further explored using Bonferroni post-hoc tests to indicate significant changes in levels of expression at each CT. Levels of expression were significantly altered at particular CTs for Per2 and Bmal1 (Fig. 3), further indicating chronic shift lag affected the rhythmic expression of core clock genes, with the most profound effects on BMAL1 expression.
Figure 3. Chronic shift lag alters the circadian expression of clock genes and proteins in enriched NK cells.
Expression levels of Bmal1 and Per2 genes (A,C) and associated proteins (B,D) in NK cells separated from animals at different time points in DD following either control or shifted paradigms. Representative immunoblots are shown above respective protein plots across all CTs. Densitometric quantification of protein levels was done by Image J software. Sine wave fits using linear harmonic regression with an assumed period of 24 h for control and shifted groups (solid and dotted lines, respectively) are superimposed with group means ± SEMs for each CT. All curve fits are significant (p < 0.01). *, p < 0.05, significant difference between groups at CT.
Table 1. Chronic shift-lag alters the acrophases of gene, protein, and cytolytic activity rhythms in NK cells.
Acrophases were determined from curves fitted to gene and protein expression and NK cell activity levels over the circadian cycle. Acrophases (± SD) represent estimations of the circadian phase corresponding to the peak of the rhythm. One-way ANOVA with Newman-Keuls multiple comparison tests were used to compare acrophases between control and shifted groups (n = 6 per lighting regimen) by gene and protein, or in the case of NK cell activity (n = 4 per lighting regimen) a Student’s t-test was used.
| Gene | Protein | |||
|---|---|---|---|---|
| Control | Shifted | Control | Shifted | |
| Per2 | 9.54 ± 3.26a | 17.71 ± 3.30* | 14.2 ± 3.49a | 15.28 ± 3.25 |
| Bmal1 | 20.88 ± 2.21 | 13.03 ± 2.77* | 19.05 ± 2.54 | 9.38 ± 3.32* |
| Perf | 22.24 ± 3.23 | - | 18.85 ± 3.18 | - |
| Gran B | 20.66 ± 1.76 | 13.66 ± 3.46* | 18.90 ± 3.10 | 19.32 ± 2.96 |
| IFNγ | 18.97 ± 2.46 | 13.95 ± 3.00* | 20.90 ± 3.32 | 20.93 ± 3.12 |
| Cytolytic Activity | ||||
| Control | Shifted | |||
| NK Cells | 17.35 ± 3.04 | 3.78 ± 3.25* | ||
p < 0.05, significant differences between groups;
p < 0.05, significant differences between Per2 and Bmal1.
Chronic shift lag alters the circadian expression of the cytokine IFNγ and cytolytic factors perforin and granzyme B in enriched NK cells
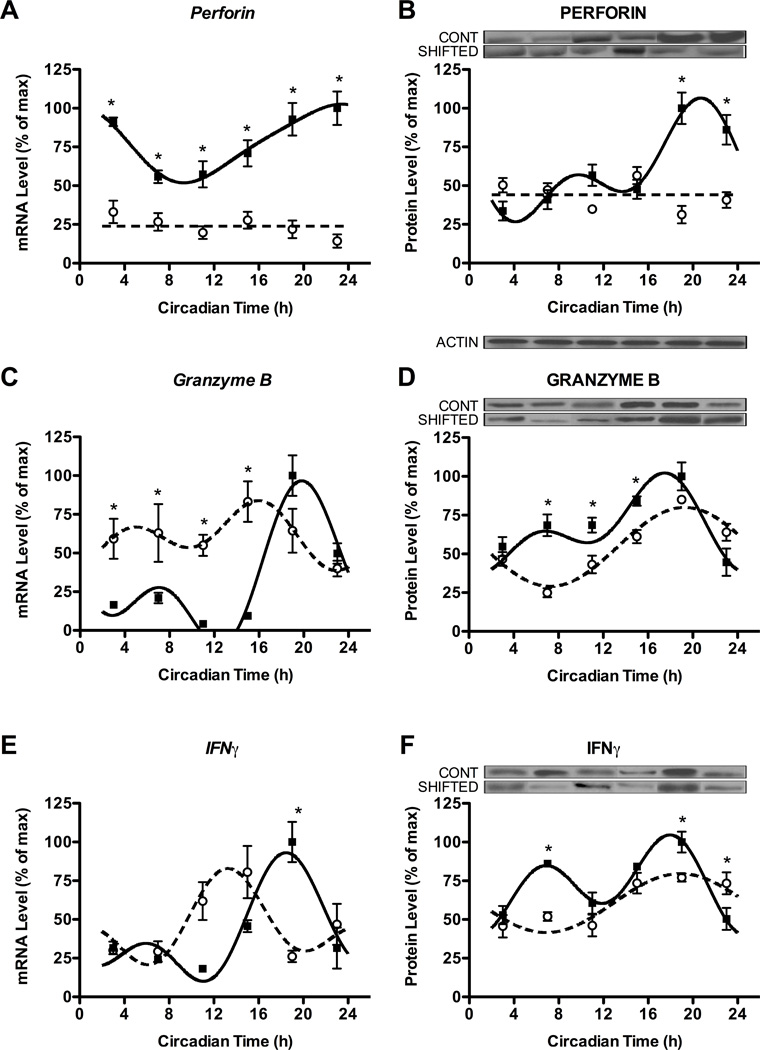
Alterations in circadian expression of core components of the molecular clock were paralleled by changes in expression rhythms of cytokines and cytolytic factors in NK cells. Consistent with previous results (37, 38), NK cells from control animals displayed robust circadian rhythms of gene and protein expression of perforin, granzyme B and IFNγ (Fig. 4A–F; assumed period of 24 h, all p’s < 0.01), with expression peaks during the active phase (Table 1). In line with our hypothesis, chronic shift-lag altered the rhythmic gene and protein expression of perforin, granzyme B, and IFNγ (Fig. 4A–F). Chronic shift-lag completely abolished the gene and protein rhythms of perforin in NK cells, as indicated by a lack of significant curve fitting, and decreased gene expression across the entire circadian cycle (Fig. 4A,B; compared to controls, all CTs, p < 0.05). Although gene expression rhythms remained intact for granzyme B and IFNγ in animals undergoing shift-lag (p’s < 0.01), these gene rhythms were shifted earlier in the circadian cycle as indicated by changes in acrophase (Table 1), and changes in expression levels at particular phases (Fig. 4C,E). The protein expression of granzyme B and IFNγ did not show a similar shift in peak expression (Table 1; Fig. 4D,F). In general, alterations in rhythms of these factors due to shift-lag resulted in reductions of gene and protein levels near or at phases of peak expression in control animals (Fig. 4; p’s < 0.05). Therefore, chronic shift-lag appeared to differentially alter gene and protein expression of cytokines and cytolytic factors by either shifting the circadian rhythm to earlier in the cycle, or attenuating levels at phases of peak expression.
Figure 4. Chronic shift lag alters the circadian expression of cytokines and cytolytic factors in enriched NK cells.
Expression levels of genes (left) and proteins (right) for cytolytic factors perforin (A,B) and granzyme B (C,D), and cytokine IFNγ (E,F) in NK cells separated from animals at different time points in DD following either control or shifted paradigms. Representative immunoblots are shown above respective protein plots across all CTs. Densitometric quantification of protein levels was done by Image J software. Sine wave fits using linear harmonic regression with an assumed period of 24 h for control and shifted groups (solid and dotted lines, respectively) are superimposed with group means ± SEMs for each CT. Straight line (i.e., perforin) denotes no significant curve fit, and thus a complete lack of rhythm. All curve fits are significant (p < 0.01). *, p < 0.05, significant difference between groups at CT.
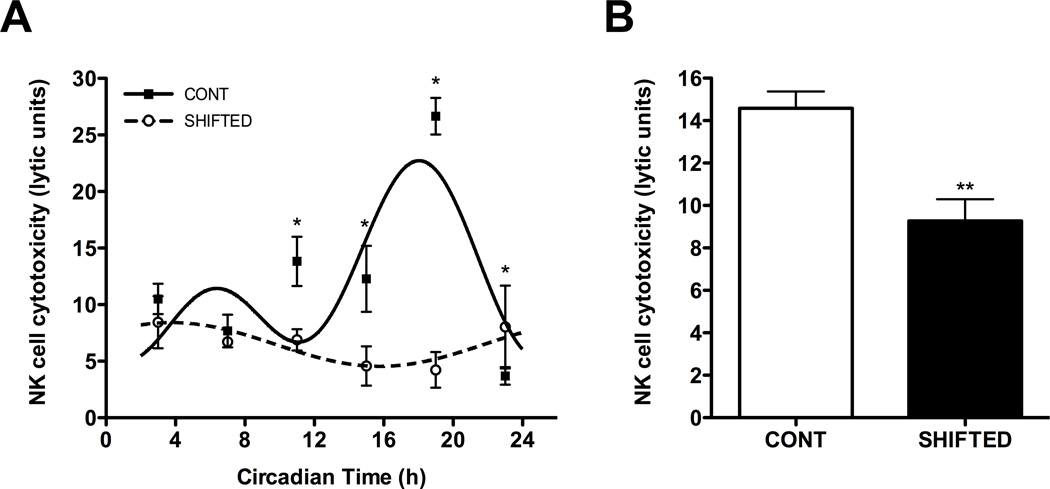
Chronic shift lag alters the circadian rhythmicity of NK cell cytolytic function
In an effort to investigate whether changes in circadian rhythms of cytokines and cytolytic factors due to chronic shift-lag had consequences on NK cell function, we completed a series of NK cell cytotoxicity assays across the circadian cycle. The cytotoxic function of NK cells displays a robust circadian rhythm with a peak during the subjective night (Table 1; Fig. 5A; p < 0.001), which is in line with our previous results (46). In shifted animals, NK cell cytotoxicity remained rhythmic with a shift in the rhythm by ~16 h (Table 1; Fig. 5A; p < 0.05). Importantly, it is evident that peaks of NK cell cytotoxicity observed in controls animals were dramatically reduced in the shifted group (Fig. 5A; p < 0.05). From this data, it is suggested coordinated circadian peaks of expression of perforin, granzyme B, and IFNγ are important for NK cell mediated killing.
Figure 5. Cytotoxicity of NK cells is suppressed in animals undergoing chronic shift lag.
NK cell cytotoxicity expresses a robust circadian rhythm, which is significantly suppressed during peak times by chronic shift lag (A). Under immune stimulated conditions, NK cell cytotoxicity is decreased in animals undergoing chronic shift-lag (B). Sine wave fits using linear harmonic regression with an assumed period of 24 h for control and shifted groups (solid and dotted lines, respectively) are superimposed with group means ± SEMs for each CT. All curve fits are significant (p < 0.01). *, p < 0.05, significant difference between groups at CT; **, p < 0.01, significant difference between groups.
To determine whether chronic shift-lag altered NK cell function in-vivo, enriched NK cells were collected from animals injected with MADB106 tumor cells. Under the premise that circadian activity of NK cell function is critical for immune response to cancer, tumor cells were injected at CT19—the phase of maximal cytolytic activity (Table 1; Fig. 5A). As expected, NK cells from shifted animals collected 24 h following the intravenous introduction of tumor cells displayed significantly reduced cytolytic activity (Fig. 5B; p < 0.01). We interpret that shift-lag significantly altered the circadian rhythm of NK cell cytolytic function and the NK cells’ response to tumor challenge.
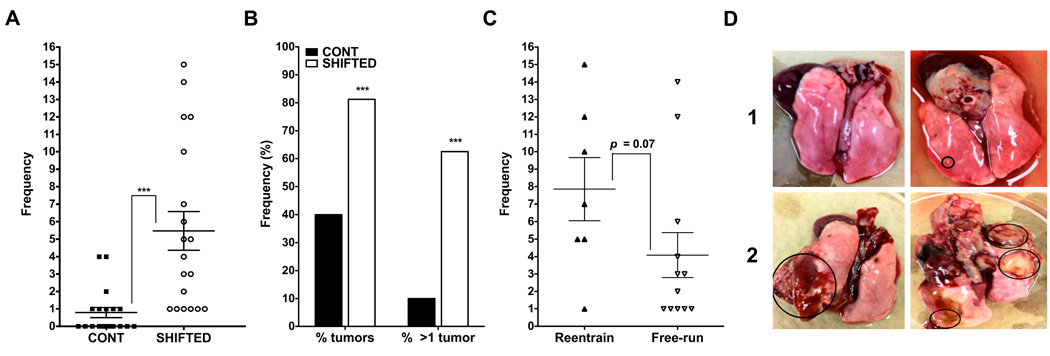
Decreased innate immune function in chronically shifted animals is associated with increases in lung tumor frequency and prevalence
Control and shift-lag animals injected at CT19 with MADB106 tumor cells were left for 6–8 weeks to determine tumor frequency or prevalence. In line with our hypothesis, chronic shift-lag promoted tumor growth indicated by increased frequency and prevalence of tumors in the lungs (Fig. 6A,B; p < 0.001). In addition, tumor load was compared between animals displaying two different chronotypes in response to the shifting protocol—animals that exhibited either free-running activity rhythms, or reentrainment (Fig. 1B,C, respectively). The shifted group that displayed repeated reentrainment tended to have more tumors relative to the group that did not successfully synchronize to the shift-lag schedule, but this difference did not reach significance (Fig. 6C; p < 0.07). Representative lungs from control and shifted (Fig. 6D, rows 1 and 2, respectively) animals are pictured. Two animals from the chronic shift-lag group were removed from the analysis because tumor growth was so invasive to the lungs and surrounding organs each required sacrificing 3 weeks following the injection (not included in the analysis). No control animals required sacrificing prior to the planned 6–8 weeks.
Figure 6. Chronic shift lag increases tumor frequency and incidence in the lungs.
Lung tumor frequency (A) and percentage of animals with tumors (B) is increased in shifted animals. Animals displaying reentrainment during the repeated shifting schedule tended to have an increased tumor load compared to shifted animals displaying free-running activity rhythms (C; p = 0.07). Pictures of lungs with multiple tumor loci (circled) from representative animals in control (D, row 1) and shifted (C, row 2) groups. ***, p < 0.0001, significant difference between groups.
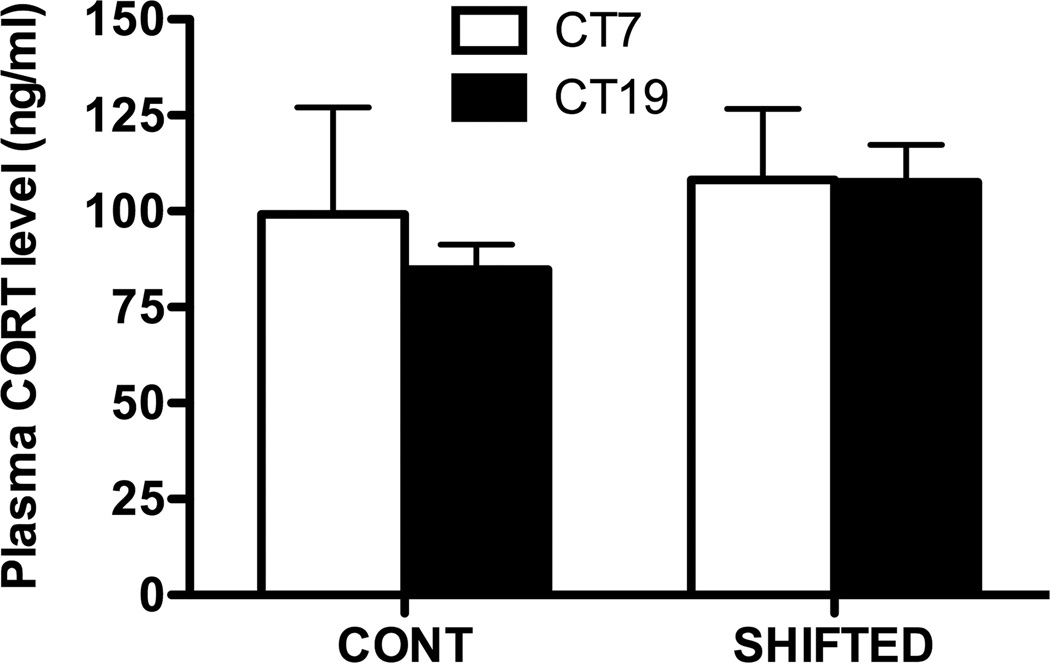
Suppressed NK cell function in animals undergoing chronic shift lag may not be due to increases in plasma corticosterone
Other studies have shown repeated shifting of the LD cycle either shifts the rhythm or transiently increases concentration of plasma corticosterone (15, 53). In order to determine if shifting increased plasma corticosterone, levels were measured at two time points CT7 and CT19 at the time of sacrifice (5–7 days into DD; n = 4 per time point). Corticosterone levels did not differ between groups at either time point (Fig. 7). At CT19, time of maximal NK cytolytic activity in controls, corticosterone levels were not increased in shifted animals suggesting attenuation of NK function may not be due to activation of the stress axis.
Figure 7. Plasma corticosterone levels in control and shifted animals.
Corticosterone was measured at two circadian phases, CT7 and CT19, at the time of sacrifice 5–7 days into DD. No differences were observed between groups at either time point. Suppressed NK cell function may not be due to increases in corticosterone levels.
Discussion
The circadian system regulates numerous cellular and physiological processes, including immune function (34). At the top of circadian hierarchy lies the SCN, which coordinates timing of peripheral clocks by hormonal and autonomic nervous system signals (54). For example, immune tissues receive rhythmic sympathetic input that modulates clock gene expression and other circadian functions within immunomodulatory cells, including splenic NK cells (37, 55, 56). NK cell function seems to be pervasively regulated by clock mechanisms, as clock genes, cytokines, and cytolytic factors all oscillate in a circadian fashion (38, 45). The present study demonstrates that circadian coordination of the immune response may influence the degree to which the innate immune system initially reacts to circulating tumor cells. During a state of chronic circadian desynchrony, the cytolytic activity of NK cells is almost completely suppressed, quite possibly by disrupting of the coordinated expression of multiple cytolytic factors. We postulate that this mechanism is responsible for the increased incidence of lung tumors in animals undergoing chronic shift-lag.
A number of epidemiological studies have linked disruptions in circadian rhythms to cancer susceptibility and clinical course, although the genetic and physiological mechanisms underlying these findings are poorly understood. Much attention has been placed on the role of the molecular clock in cell proliferation, DNA damage and repair, and apoptotic pathways within the developing tumor cells (16, 57–59). In contrast, the present study shows that the tumorigenic effects of circadian disruption depend on tumor surveillance mechanisms of NK cells. Specifically, chronic shift-lag altered the expression of clock genes and the immunomodulators, IFNγ, perforin, and granzyme B in enriched NK cells at both the gene and protein levels. Thus, the functional activity of NK cells was significant decreased at normal phases of peak expression compared to control animals. Further, gene expression rhythms of granzyme B and IFNγ displayed a shift by ~4 h to earlier in the circadian cycle, but similar shifts were not observed at the protein level. Perforin expression appeared the most affected by chronic shift-lag, as gene and protein rhythms were completely abolished in NK cells. It is plausible that these reductions of perforin hamper NK cell mediated apoptosis and lysis of tumor cells in shifted animals (60–62), especially since granzyme B and IFNγ were also reduced, which has been associated with increased tumor susceptibility (31).
Circadian coordination of NK cell function appears to be important for generating an efficient immune response. Consistent with our previous findings, circadian rhythms of perforin, granzyme B and IFNγ expression peak at similar phases during the subjective night correspond to the circadian peak of NK cell cytotoxicity (45, 46). In an effort to determine whether circadian coordination of NK cell function may have functional significance, we injected control and shifted animals with tumor cells at CT19 under the hypothesis that chronic shift lag would cause impairment of NK cell function, ultimately producing a shift in the rhythms of cytotoxicity. Somewhat surprisingly, however, chronic shift lag dramatically attenuated NK cell function across the entire circadian cycle, suggesting that similar tumor frequencies might have been observed in shifted animals regardless of phase of tumor cell injection. The apparent discoordination of cytokines, cytolytic factors, and cytotoxicity, along with the attenuation of cytotoxicity following an in-vivo tumor challenge, suggests circadian disruption compromises the ability of NK cells to kill tumor cells. In our model, this could lead to the promotion of tumor growth by increasing the probability of tumor cell retention following inoculation.
In order to assess circadian disruption at the whole-animal level, we monitored circadian activity rhythms throughout the experimental protocols. Because rhythmic locomotor activity is a reliable indicator of circadian pacemaker functioning, changes in amplitude and/or period due to chronic shift-lag can be inferred to reflect SCN activity. Inspection of actograms from our shifted animals yielded two different chronotypes. While some shifted animals exhibited an apparent free-running period, essentially ignoring the changing light onsets, others displayed, short circadian periods as a consequence of partial synchronization to the advancing shift-lag schedule. Using a similar shifting protocol with hamsters, the former has been observed (53). Nevertheless, despite shifting induced alterations in rhythm amplitude and period, our animals remained completely rhythmic during the experiment, and thus, suggest the SCN maintains coherent rhythmicity even during rapidly changing environmental cues (53). Studies examining the effects of photic phase shifts on SCN clock gene expression have also demonstrated the SCN maintains rhythmicity with differing periods of reentrainment depending on frequency and direction of the shifts (63).
Furthermore, examination of circadian activity rhythms during the exposure to the shift-lag schedule revealed that repeated reentrainment may have led to increased tumorigenesis relative to animals expression stable free-running rhythms under the same conditions. Since reentrainment is associated with internal circadian desynchrony at both the molecular and physiological levels (64, 65), these preliminary observations support the hypothesis that dissociation among multiple circadian clocks may indeed underlie the pathophysiological effects of shift-lag exposure.
Nevertheless, while rhythmicity is maintained in the SCN, major SCN outputs that relay temporal information to other brain areas and peripheral tissues are compromised under shift-lag conditions (63, 64). Clocks in peripheral tissues reentrain at a slower rate compared to the SCN following a single advance or delay, including those in the spleen (63, 65). Sympathetic input to the spleen may be a major output pathway by which the SCN coordinates NK cell function (37). It is interesting to speculate that chronic shift-lag impedes temporal coordination of the immune system by attenuating SCN-derived output signals to “pre-autonomic” neurons (66). Indeed, homeostasis of the sympathetic nervous system is disrupted in chronically shifted animals (17).
The molecular clock in NK cells was altered by chronic shift-lag, which was evident by advances in the rhythms and reductions of Bmal1 gene and protein expression, and a delay in the expression rhythms of Per2. Bmal1 rhythms were slightly attenuated, but remained rhythmic. The delay of the Per2 gene was much more dramatic (~6 h) than the pattern observed in the protein rhythm (~1 h). Although shifting-induced reductions in NK cell function were correlated with changes in clock gene expression, these data can only suggest the involvement of molecular clocks in NK cells to regulate cytolytic abilities. Similarly, in macrophages, disruptions to clock genes due to repeated shifting has been associated with dysregulation of inflammatory responses (20). In order to determine the extent to which molecular clocks within NK cells regulate cell specific functions, further studies are necessary to tease apart the interactions between clock and immune-related pathways. At the molecular level, a potential intersection could be clock modulation of NF-κB, a key transcription factor involved in cellular immune response (67). When activated, NF-κB can promote gene transcription of IFNγ (68), perforin (69), and granzyme B (70) by direct binding to enhancer elements.
Other factors could be involved in mediating the effects of chronic shift-lag on NK cell function beyond involvement of circadian mechanisms. Circulating glucocorticoids and melatonin oscillate in anti-phase, peaking during the early and late circadian cycle, respectively, and are known modulators of NK cell function (71, 72). However, several points of evidence suggest glucocorticoids and melatonin may not necessarily be critical for maintaining immune rhythms. In macrophages and NK cells, functional rhythms persist despite the absence of glucocorticoid rhythms (73, 74), or lack of melatonin production (73, 75). Furthermore, consistent with a previous result (20), our study suggests dysregulation of NK cell function induced by chronic shift-lag is not due to increased circulating corticosterone, especially during times of peak functioning. Corticosterone increases induced by chronic shifting paradigms may be transient and play a role in resynchronization of the circadian system (49, 53). Nevertheless, at the time points of NK cell collection, corticosterone was not increased in our shifted animals.
In conclusion, we have demonstrated that chronic states of circadian desynchrony severely attenuated NK cell function possibly by altering the circadian rhythms of cytokines, cytolytic factors, and cytotoxicity. In addition, suppressed NK cell function was observed during an in-vivo immune stimulated condition in shifted animals, suggesting circadian disruption has substantial consequences on the tumor response of NK cells. Importantly, loss of rhythms in NK cell cytolytic activity was associated with an increased frequency of tumors. More detailed studies are required to further understand the integration of physiological and molecular changes in immune function caused by disruption to the circadian system. For example, whether the chronic shift-lag model used here alters noradrenergic signaling of the sympathetic nervous system to impact NK cell function has yet to be determined (17). Molecular studies, such as large-scale gene expression arrays in primary and secondary immune tissues, of wild-type and clock mutant mice may also provide a wealth of information regarding the molecular links between circadian and immune systems. Understanding these intersections could be invaluable to developing novel therapeutics to restore circadian functioning in immune cells, which could aid in preventing disease progression (59, 76, 77).
Footnotes
This work is supported by NIH R01 HL088041 and R37 award AA08757.
References
- 1.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int. J. Cancer. 2008;123:2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 3.Hansen J. Light at night, shiftwork, and breast cancer risk. J. Natl. Cancer Inst. 2001;93:1513–1515. doi: 10.1093/jnci/93.20.1513. [DOI] [PubMed] [Google Scholar]
- 4.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J. Natl. Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan AN, Schernhammer ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281:1–7. doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 7.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am. J. Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 8.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol. Int. 2009;26:108–125. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- 9.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J. Natl. Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 10.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Lellouch J, Misset JL, Touitou Y, Levi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 11.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 12.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the 'Quebec en Forme' Project. Int. J. Obes. (Lond) 2006;30:1080–1085. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 13.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand. J. Work Environ. Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- 14.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup. Environ. Med. 2006;63:451–455. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 16.Filipski E, Li XM, Levi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control. 2006;17:509–514. doi: 10.1007/s10552-005-9007-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pendergast JS, Yeom M, Reyes BA, Ohmiya Y, Yamazaki S. Disconnected circadian and cell cycles in a tumor-driven cell line. Commun. Integr. Biol. 2010;3:536–539. doi: 10.4161/cib.3.6.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, Cocco L, Vitale M. NK cells and cancer. J. Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 23.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 27.Konjevic G, Radenkovic S, Srdic T, Jurisic V, Stamatovic L, Milovic M. Association of decreased NK cell activity and IFNgamma expression with pSTAT dysregulation in breast cancer patients. J. Buon. 2011;16:219–226. [PubMed] [Google Scholar]
- 28.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 29.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 30.Cifone MG, D'Alo S, Parroni R, Millimaggi D, Biordi L, Martinotti S, Santoni A. Interleukin-2-activated rat natural killer cells express inducible nitric oxide synthase that contributes to cytotoxic function and interferon-gamma production. Blood. 1999;93:3876–3884. [PubMed] [Google Scholar]
- 31.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 33.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 34.Logan RW, Sarkar DK. Circadian nature of immune function. Mol. Cell. Endocrinol. 2011 doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Albrecht U, Oster H. The circadian clock and behavior. Behav. Brain. Res. 2001;125:89–91. doi: 10.1016/s0166-4328(01)00288-1. [DOI] [PubMed] [Google Scholar]
- 36.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 37.Logan RW, Arjona A, Sarkar DK. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav. Immun. 2011;25:101–109. doi: 10.1016/j.bbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav. Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Arjona A, Sarkar DK. Are circadian rhythms the code of hypothalamicimmune communication? Insights from natural killer cells. Neurochem. Res. 2008;33:708–718. doi: 10.1007/s11064-007-9501-z. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Eliyahu S, Page GG, Yirmiya R, Taylor AN. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat. Med. 1996;2:457–460. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- 41.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth. Analg. 2003;97:1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 42.Page GG, Ben-Eliyahu S. A role for NK cells in greater susceptibility of young rats to metastatic formation. Dev. Comp. Immunol. 1999;23:87–96. doi: 10.1016/s0145-305x(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar DK, Zhang C, Murugan S, Dokur M, Boyadjieva NI, Ortiguela M, Reuhl KR, Mojtehedzadeh S. Transplantation of {beta}-endorphin neurons into the hypothalamus promotes immune function and restricts the growth and metastasis of mammary carcinoma. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyadjieva N, Dokur M, Advis JP, Meadows GG, Sarkar DK. Chronic ethanol inhibits NK cell cytolytic activity: role of opioid peptide beta-endorphin. J. Immunol. 2001;167:5645–5652. doi: 10.4049/jimmunol.167.10.5645. [DOI] [PubMed] [Google Scholar]
- 45.Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 46.Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J. Immunol. 2004;172:2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- 47.Wang KS, Ritz J, Frank DA. IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J. Immunol. 1999;162:299–304. [PubMed] [Google Scholar]
- 48.Bryant J, Day R, Whiteside TL, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J. Immunol. Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-u. [DOI] [PubMed] [Google Scholar]
- 49.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J. Clin. Invest. 2010;120:2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hut RA, Scheper A, Daan S. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J. Comp. Physiol. A. 2000;186:707–715. doi: 10.1007/s003590000124. [DOI] [PubMed] [Google Scholar]
- 51.GJ K. Center-of-gravity of circadian activity and its relation to free-running period in two rodent species. J. Interdiscip. Cycle Res. 1980;11:1–8. [Google Scholar]
- 52.Reppert SM. Cellular and molecular basis of circadian timing in mammals. Semin. Perinatol. 2000;24:243–246. doi: 10.1053/sper.2000.9122. [DOI] [PubMed] [Google Scholar]
- 53.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalsbeek A, Yi CX, Cailotto C, la Fleur SE, Fliers E, Buijs RM. Mammalian clock output mechanisms. Essays Biochem. 2011;49:137–151. doi: 10.1042/bse0490137. [DOI] [PubMed] [Google Scholar]
- 55.Boyadjieva NI, Ortiguela M, Arjona A, Cheng X, Sarkar DK. Beta-endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol Clin. Exp. Res. 2009;33:931–937. doi: 10.1111/j.1530-0277.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dokur M, Boyadjieva N, Sarkar DK. Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J. Neuroimmunol. 2004;151:148–157. doi: 10.1016/j.jneuroim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat. Rev. Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 58.Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15:351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 59.Ramsey MR, Ellisen LW. Circadian function in cancer: regulating the DNA damage response. Proc. Natl. Acad. Sci. USA. 2011;108:10379–10380. doi: 10.1073/pnas.1107319108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trapani, A. J, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 61.van den Broek MF, Kagi D, Zinkernagel RM, Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur. J. Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- 62.Wallin RP, Screpanti V, Michaelsson J, Grandien A, Ljunggren HG. Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur. J. Immunol. 2003;33:2727–2735. doi: 10.1002/eji.200324070. [DOI] [PubMed] [Google Scholar]
- 63.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur. J. Neurosci. 2009;29:171–180. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- 64.Yan L. Structural and functional changes in the suprachiasmatic nucleus following chronic circadian rhythm perturbation. Neuroscience. 2011;183:99–107. doi: 10.1016/j.neuroscience.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 66.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 68.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J. Biol. Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 69.Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang C, Bi E, Hu Y, Deng W, Tian Z, Dong C, Sun B. A novel NF-kappaB binding site controls human granzyme B gene transcription. J. Immunol. 2006;176:4173–4181. doi: 10.4049/jimmunol.176.7.4173. [DOI] [PubMed] [Google Scholar]
- 71.Krukowski K, Eddy J, Kosik KL, Konley T, Janusek LW, Mathews HL. Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav. Immun. 2011;25:239–249. doi: 10.1016/j.bbi.2010.07.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC. Melatonin, immune function and aging. Immun. Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J. Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 74.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 76.Filipski E, Levi F. Circadian disruption in experimental cancer processes. Integr. Cancer Ther. 2009;8:298–302. doi: 10.1177/1534735409352085. [DOI] [PubMed] [Google Scholar]
- 77.Innominato PF, Levi FA, Bjarnason GA. Chronotherapy and the molecular clock: Clinical implications in oncology. Adv. Drug Deliv. Rev. 2010;62:979–1001. doi: 10.1016/j.addr.2010.06.002. [DOI] [PubMed] [Google Scholar]