Abstract
Tethering genetically encoded peptide toxins or ligands close to their point of activity at the cell plasma membrane provides a new approach to the study of cell networks and neuronal circuits, as it allows selective targeting of specific cell populations, enhances the working concentration of the ligand or blocker peptide, and permits the engineering of a large variety of t-peptides (e.g., including use of fluorescent markers, viral vectors and point mutation variants). This review describes the development of tethered toxins and peptides derived from the identification of the cell surface nAChR modulator lynx1, the existence of related endogenous cell surface modulators of nAChR and AMPA receptors, and the application of the t-toxin and t-neuropeptide technology to the dissection of neuronal circuits in metazoans.
Introduction
During the last decade of research on neurotoxins and neuropeptides, an important number of specific inhibitors and modulators of ion channels and receptors have been identified. Thus, unique peptide venom toxins with characteristic cysteine backbones and selective affinities for voltage-gated sodium (Nav), calcium (Cav), and potassium (Kv) ion channels, and ligand-gated receptors, including nicotinic acetylcholine receptors (nAChRs) N-methyl-D-aspartate (NMDA) and G-protein coupled receptors (GPCRs) have been isolated and characterized. Likewise, endogenous neuropeptides released by distinct neuronal cell populations have been found to bind specific GPCRs, acting as specific signals between one population of neurons and another. In both cases, the high specificity of venom toxins and neuropeptides makes them ideal tools for deciphering the contributions of specific ionic and receptor-mediated signals in neuronal networks. However, given that these molecules are soluble, their activity cannot be restricted to a single cell population in a living organism, and their application requires constant administration to compensate for degradation and diffusion effects. To bypass these limitations, we developed genetically encoded tethered toxins (t-toxins) and tethered ligand peptides (t-peptides) that are bound to the cell surface by membrane-embedded tethers and act only on ion channels and receptors in the cell-population that expresses the t-toxin or t-peptide, and not on identical receptors present in neighboring cells that do not express the tethered modulator. In this review, we discuss the development of modular t-toxins and t-peptides with preserved pharmacological activity and specificity and their application to the genetic dissection of specific ionic and receptor-mediated signals that control the development and function of the CNS in metazoans.
Naturally occurring toxin-like tethered modulators
The tethered toxin strategy was developed by analogy to the features, structure, and mode of action of the cell-surface lynx1 prototoxin. lynx1 is an endogenous modulator of nicotinic acetylcholine receptors (nAChR) and is evolutionary related to snake venom α-neurotoxins [1,2]. lynx1 is tethered to the cell surface by a glycosylphosphatidylinositol (GPI) anchor and, like α-neurotoxins, contains a cysteine-rich region of 10 conserved cysteine residues with a characteristic spacing pattern that determines their three finger fold [1]. Other members of the ly6/α-neurotoxin family with nAChR modulatory activity include lynx2, SLURPs, PCSA and Pr-lynxes (Table and references therein). Related motifs to those present in the snake toxin α-bungarotoxin and in lynx1 have also been found in Sleepless, a Ly-6 protein that modifies Shaker-type K+ channels in Drosophila melanogaster [3] and in CKAMP44 a mammalian modulator of AMPA receptors. CKAMP44 contains a transmembrane segment that anchors a cysteine-rich region at the extracellular domain [4]. In this case the cysteine motif does not fold into a three finger structure but is similar to the cystine-knot motifs found in cone snail toxins that affect a number of voltage-gated channels [5] and in a conotoxin that modifies AMPA receptor function [6]. Altogether the existence of these toxin-like cell-surface modulators of ion channels is intriguing and raises the possibility that venom toxins have evolved from endogenous modulators.
Table 1.
Identified Ly6/α neurotoxins with cholinergic activity
| Ly6/neuro-toxin | Species | GPI- or secreted | length (aa) | cys (n) | Tested activity | References |
|---|---|---|---|---|---|---|
| α-Bgtx | Bungarus multicinctus | secreted | 74 | 8 | α7, α1β1δγ/ε | [21,22] |
| κ-Bgtx | Bungarus multicinctus | secreted | 87 | 8 | α3β2 | [23] |
| Lynx1 | Human, mouse , | GPI tethered | 116 | 10 | α4β2, α7, α3β2 | [1,2] |
| Lynx2=lypd1 | rat Human, mouse , rat | GPI tethered | 141 | 10 | α4β2/β4, α7 α1β1δγ/ε |
[24,25] |
| SLURP-1 | Human, mouse , rat | secreted | 103 | 10 | α7 | [26] |
| SLURP-2 | Human, mouse , rat | secreted | 97 | 10 | α3(β2/β4)+/−α5 | [27] |
| Pr-lynx1 | Pyrocoelia rufa (firefly) | GPI tethered | 120 | 10 | α3β4 | [28] |
| NI-lynx1 | Drosophila melanogaster | GPI tethered | 151 | hybrid Nlα1/β2 Nlα1/Nla2/β2 |
[29,30] | |
| NI-lynx2 | Drosophila melanogaster | GPI tethered | 148 | hybrid Nlα1/β2 Nlα1/Nlα2/β2 Nlα3/Nlα8/β2 |
[29,30] | |
| PCSA | Chicken | GPI tethered | 123 | α7 | [31] |
Design principles for engineering tethered ligand peptides
The first recombinant membrane-bound toxins were designed using the scaffold of the lynx1-like gene family, i.e., secretory signal and consensus sequences for GPI processing and recognition [7]. This design directs any bioactive peptide to the secretory pathway, where the signal sequence is cleaved and the GPI targeting sequence is substituted by a covalent bond to GPI, anchoring the peptide to the extracellular side of the plasma membrane of the cell in which it is expressed (Figure 1). Additional modules have been integrated into t-toxins and t-peptides to increased their activity and the ability to monitor their expression. These modifications have included the use of longer linkers [8], other membrane tethers, e.g. the transmembrane domains of the PDGF receptor [9] or herpes simplex virus glycoprotein C [10], as well as fluorescent markers (EGFP, mCherry and EBFP2) [9] and immunotags (e.g., Flag-tag and myc-tag) [7,11–13] (Figure 1).
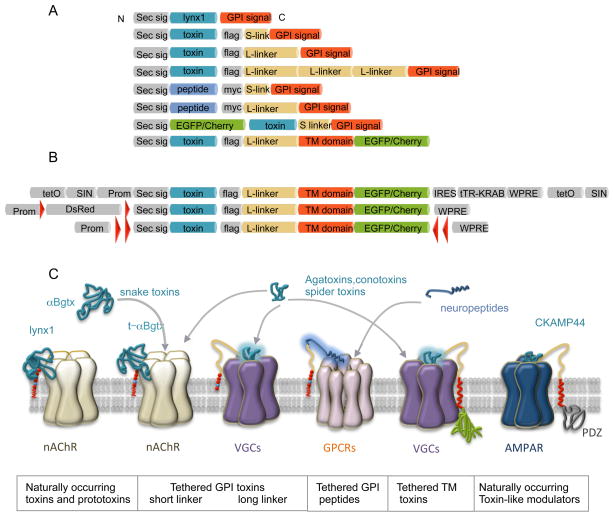
Figure 1. Scheme of the architecture and mode of binding of lynx1, derived engineered tethered toxins and neuropeptides and other naturally occurring toxin-like modulators.
(A) The tethered-peptide strategy uses the biological scaffold of lynx1 (cyan blue) to incorporate a secretory pathway signal sequence secretory signal (Sec sig: grey) and a GPI signal (red) to generate recombinant membrane-bound toxins and peptide ligands. Additional tethered-toxin and t-peptide variants consist of the secretory signal sequence (grey), toxin (cyan blue) or peptide (dark blue) ligand cassettes, fluorescence markers (EGFP or mCherry; in green), epitopes for immunostaining (flag-tag, myc-tag; in grey), flexible linker regions (beige), and distinct functional modules for membrane attachment (GPI signal or transmembranedomain TM; in red).
(B) Schematic of inducible and Cre-dependent viral vectors encoding t-toxins used for stereotactic injections in mice. Doxycycline inducible lentiviral vector containing tetO and tTR-KRAB cassettes [20]. Cre-dependent t-toxin viral vectors containing either loxP sites (red triangles) flanking DsRed [20], or a flip-excision (FLEX) switch with double loxP sites flanking the t-toxin. Prom: Promoter, WPRE: woodchuck post-transcriptional regulatory element.
(C) Illustration of lynx1 and GPI-tethered t-αBgtx binding to the nicotinic receptor (nAChR). Other peptide venom toxins, including agatoxins, conotoxins and spider toxins, and neuropeptides can be tethered to the membrane via a GPI anchor to cell specifically inactivate or modulate nAChRs, voltage gated channels (VGC) or G-protein couple receptors (GPCRs) depending on the selectivity of the t-toxin or t-peptide. T-toxins are also functional when tethered via a transmembrane (TM) domain. Curiously, CKAMP44, a naturally occurring toxin-like modulator of AMPAR [12] resembles TM t-toxins.
So far, approximately 40 different chimeric t-toxins derived from the venom of several predatory animals have been cloned and their activity has been characterized on voltage and ligand-gated ion channels in vertebrates and invertebrates [7–9,11–14]. Furthermore the t-peptide strategy has also been successfully extended to other bioactive peptides, including native peptide ligands of GPCRs [10,15], illustrating the general applicability of this approach for cell-surface modulation of receptors.
Expression and functional assays of these recombinant tethered effectors have revealed that several elements are critical to achieve robust expression on the cell-surface and steric availability for functional binding of the t-toxin/peptide to the receptor or channel of interest. The affinity of the bioactive peptide for its cognate ion channel or receptor has to be taken into account. Toxins with a strong affinity are potentially more effective. It is also important to consider the composition and length of the peptides to be tethered, i.e., charges and hydrophobicity of the amino acid residues, number of cysteine bonds in the case of toxins, and existence of non-canonical residues, or terminal amidations. Another relevant feature when designing t-peptide constructs is the linker sequence bridging the toxin peptide to the GPI anchor or TM domain (Figure 1). The distance of the t-toxin or t-peptide from the cell-surface has to be tailor-made for individual receptors and channels, and can be used for mapping active binding sites. Tethered constructs have been cloned using linkers consisting of glycine–asparagine repeats with lengths varying from 6 amino acids (aa) (short) to 20 aa (long), 40aa (2× long) or 60 aa (34 long) [7,8] (Figure 1). The longer flexible linker provides rotational freedom for the t-toxin to bind within the vestibule of voltage-gated channels or for ligand peptides to reach their binding site, such as onto class B1 GPCRs [10]. Experiments varying the length of the linker region of t-GID conotoxin indicate that a linker is necessary for inactivation of α7 nAChR currents. However, when the linker exceeds a certain length the inactivation is incomplete [8]. Similarly, the tethered form of the neuropeptide pigment dispersing factor (t-PDF) requires a shorter linker for greatest activity at its receptor [15].
The choice of membrane tether depends on the characteristics of the peptide as well as on the epitope-tags and markers to be used in combination (Figure 1). GPI anchors, which are less bulky than TM domains, may facilitate the mobility of the t-peptide in close proximity to its receptor within the plasma membrane. If the toxin or peptide does not require a free N-terminus for interacting with its cognate receptor, GPI versions containing EGFP followed by the t-toxin may be used [9]. However, GPI anchors are susceptible to cleavage by endogenous phospholipases, such as PI-PLC and phospholipase D. To avoid this potential problem, the GPI anchor can be replaced with a TM domain in t-toxins or t-peptides [9,10] (Figure 1). TM domains can be used to retain tethered modulators at the cell-surface and link fluorescent markers to the cytoplasmatic side of the plasma membrane avoiding hindrances between them [9].
General applicability of membrane t-toxins to analysis of animal physiology
Tethered toxins and peptides can be used for very diverse applications pertaining to experimental animal physiology. Several studies have shown that recombinant toxins as well as peptide ligands are not dispersed in solution and retain their high specificity for their cognate receptors, indicating that this approach can be used to restrict the site of neurotoxin or peptide ligand action to genetically targeted cells. For example, in vivo transgenic delivery of t-αBgtx in zebrafish using a muscle cell-specific promoter resulted in blockade of nAChR currents in muscle cells that expressed t-αBgtx but not in adjacent muscle fibers or in cells that expressed t-κBgtx, which has no activity on muscle-nAChRs [7]. Similarly, experiments in chicken employing a viral system to transduce ciliary neurons revealed that expression of t-αBgtx blocks calcium currents via nAChRs and prevents programmed cell-death of these neurons during early development [11]. Furthermore, given the tremendous power of Drosophila melanogaster fruit flies as a genetic system for cell-specific targeting of transgene expression in the nervous system, it was immediately apparent that the t-toxin system would likely work well for cell-specific modulation of particular ion channel subtypes in neuronal circuits of intact behaving flies. Accordingly, a library of transgenic fly strains was generated for GPI-tethered expression of any one of approximately two dozen previously characterized naturally occurring ion channel toxins from a variety of venomous predators, including cone snails, scorpions, bees, and spiders [13]. As a first test for bioactivity of these t-toxins in transgenic Drosophila, they were expressed pan-neuronally. Interestingly, only four of the toxins from this library exhibited detectable gross behavioral impairment, and all four of them were from the venoms of spiders [13]. This is perhaps not so surprising, as spiders are avid predators of flies. These four active t-toxins included inhibitors of N, P, Q-type and L-type Ca2+ channels, K+ channels, and Na+ channel inactivation [13].
In order to assess whether spider t-toxins could be expressed in cell-specific patterns within the nervous system as a tool for elucidating ion channel function in intact neural circuits, each of these four bioactive spider t-toxins was expressed solely in a group of approximately twenty circadian pacemaker neurons in the fly brain that secrete the neuropeptide PDF, known to be a key regulator of circadian rhythms of rest and activity [13,16]. Of these four spider t-toxins, three of them strongly disrupted circadian rhythms of locomotor activity, with only the inhibitor of N, P, Q-type Ca2+ channels having no effect. Using whole-cell patch-clamp electrophysiology, it was determined that inhibition of Na+ channel inactivation by t-toxin expression in these pacemaker neurons transformed their spontaneous firing pattern by interspersing their normal regular action potential bursts with occasional prolonged plateau potentials followed by massive hyperpolarizations mediated by Na+/K+-ATPase pump currents [13].These studies establish that spider t-toxins are effective tools for genetically targeted pharmacologically specific ion channel modulation in behavioral control circuits of transgenic Drosophila.
Generalization of Tethered Toxin System to Endogenous Peptide GPCR Ligands
The first indication for the likelihood of success of the t-toxin approach to produce t-peptides derived from the fact that constitutively active class B1 neuropeptide receptors can be generated by fusing their peptide ligands to the extracellular receptor N terminal domain [17]. Accordingly, GPI-tethered versions of the Drosophila class B1 neuropeptides PDF and DH31 (fly homolog of calcitonin) were generated for expression either in vitro in tissue culture cells or in vivo in transgenic flies [15].
Because all class B1 neuropeptide GPCRs signal through Galphas—and consequently via increased adenylate cyclase activity and cytoplasmic cAMP levels—their activation can be detected in transfected tissue culture cells by cotransfection of a CRE-luciferase reporter construct that responds to increased cAMP with increased production of luciferase detectable by bioluminescence. When t-PDF was co-expressed in tissue culture with PDF receptor (PDFR), it dose-dependently activated PDFR leading to increased cAMP [15]. Similarly, t-DH31 activated its co-expressed receptor. Importantly, however, neither t-ligand had any ability to activate other class B1 neuropeptide GPCRs, thus establishing that neuropeptides maintain their appropriate pharmacological specificity when expressed in membrane-tethered form [15]. To assess the cell autonomy of GPCR activation by t-peptides, tissue culture cells transfected only with PDFR and the CRE-luciferase reporter were mixed with cells transfected only with t-PDF. As expected for a cell-autonomous ligand, there was no detectable PDFR activation in these mixed cultures [15].
Class B1 t-peptide ligands were also tested as tools for cell-autonomous pharmacologically specific receptor activation in vivo in transgenic Drosophila [15]. Null-mutant flies completely lacking all endogenous PDF exhibit a severe circadian phenotype of nearly complete arrhythmicity of rest-activity cycles [18]. When expressed in approximately 150 circadian clock neurons of these PDF null-mutant flies, t-PDF induced strong rescue of rhythmicity of rest-activity cycles, restoring the usual pattern of activity concentrated around dawn and dusk with rest concentrated at night and midday [15]. Based on this in vitro and in vivo application for Drosophila class B1 neuropeptides, membrane-tethered versions of a number of mammalian class B1 peptides (including corticotropin releasing factor, parathyroid hormone, and glucagon-like peptide) were also generated and validated in vitro against their cognate receptors [10]. Taken together, these studies establish that class B1 t-neuropeptides can be effectively used in vivo in transgenic Drosophila for genetically targeted pharmacologically specific GPCR activation in neural circuits, and raise the possibility of similar utility in mammals.
Application of t-toxins to the dissection of mammalian circuits
As a first proof of principle of the validity of t-toxins to dissect the contribution of specific ionic currents to behaviors in the mouse, we performed transgenic studies to distinguish Nav1.8 (tetrodotoxin resistant, TTX-R) from Nav1.7 (TTX-sensitive) currents in pain transmission. These two channels are major targets for pain research because they control the excitability of nociceptive sensory neurons that innervate skin and muscle. However gene deletion of Nav1.8 leads to increased Nav1.7 channels and TTX-sensitive currents in nociceptors of Nav1.8 knockout mice [19] making it difficult to interpret the single contribution of each channel type to pain modalities (inflammatory, neuropathic acute pain or cold pain). To address this, we genetically delivered t-MrVIa μO-conotoxin (preferential blocker of Nav1.8) to nociceptors by mouse BAC transgenesis [12]. Electrophysiological analyses showed that the t-toxin inhibited TTX-R Na+ currents at the membrane, as it was released by enzymatic cleavage of the GPI anchor [12]. Behaviorally, t-toxin transgenic mice displayed reduced inflammatory and cold pain perception [12]. As research on venom peptide toxins progresses, it would be interesting to identify and test other antagonists of Nav channels expressed in central neurons for cell-specific silencing studies in the brain.
We have developed a second set of t-toxins against Cav channels, first to dissect the contribution of Cav channels to specific behaviors and secondly, as a universal strategy for cell-specific silencing of synaptic transmission. Studies in transgenic mice expressing t-conotoxin MVIIA (blocker of Cav2.2, medically used to treat severe pain) showed specific inhibition of these currents in nociceptive neurons and consequent alleviation of inflammatory and neuropathic pain [9]. These studies have led to the optimization of a t-toxin dual viral system that allows the inhibition of synaptic transmission from basically any given neuronal population. This system is based on expression of t-MVIIA and an additional toxin (t-Agatoxin IVa) for simultaneous block of Cav2.1 and Cav2.2, which together control presynaptic calcium influx necessary for neurotransmitter release and synaptic transmission in basically every neuron [9]. The capability to block one or both Cav channels, shown by paired-pulse electrophysiological recordings in neurons, confirmed that transduction of neurons with both t-toxin lentiviruses completely silences GABAergic and Glutamatergic transmission in the absence of detectable neurotoxicity [9]. In vivo studies in mice stereotactically injected with t-toxin lentiviruses revealed specific and robust expression of t-toxins at the cell-membrane of transduced neurons [9]. Functionally, we demonstrated that injection of t-toxins in the substantia nigra pars compacta resulted in strong circling motor behavior as a consequence of the unilateral inhibition of the dopaminergic nigro-striatal pathway by the action of t-toxins [9]. These studies demonstrate the validity and general applicability of virally encoded t-toxins in vivo as a straightforward method for long-term inactivation of Cav2.1 and Cav2.2 calcium currents, resulting in cell-specific and cell-autonomous silencing of neurotransmission. Importantly no signs of neurotoxin damage have been detected in mice several months after t-toxin viral injection.
The fact that t-toxins maintain their functionality in a long-term manner when expressed exogenously in mammalian neurons, causing no obvious signs of toxicity, has opened up the possibility to implement an important number of genetic strategies for studies of neurocircuitry in vivo. Thus besides mouse BAC transgenesis, and constitutive viral vectors [9,12] (Figure 1), these genetic tools are now being used in intersectional strategies that employ Cre driver mouse lines and Cre-dependent viral vectors to assess the contributions of specific cell types to specific behaviors by manipulation of neuronal activity within specific cell populations in the living mouse.
Comparison of tethered modulators to other approaches
As with any new technology, comparison to existing technologies is essential. The expression of ion channels and peptide GPCRs can be decreased via genetic manipulations such as gene knock-out or RNAi-mediated knock-down, or increased via transgenic overexpression. These manipulations are by their nature focused on particular ion channel or GPCR subunit genes, and thus cannot be used in any simple way to target particular heteromultimer functional isoforms, where gene deletion of one subunit might be accompanied by compensatory upregulations of closely related receptors that share common subunits or compete for the same binding partners [19,20]. In addition, effects of transgenic expression in a particular cell population do not distinguish overexpression of a native channel subtype from ectopic expression. Tethered modulators effectively overcome these limitations. Because each tethered modulator has defined pharmacological activity against a particular ion channel or receptor—such as specific bungarotoxins for specific nAChR heteromultimer isoforms [21]—the need to target specific isoforms genetically is obviated. Also, tethered modulators allow for modulatory actions other than just increasing or decreasing channel or receptor activity, such as the delta t-toxin that inhibits Na+ channel inactivation [13]. Finally, tethered modulators have the important property of being functionally inert when expressed in cells that lack their target ion channels or receptors.
Summary and future directions
Several extensions of the t-toxin/t-peptide strategy are of immediate interest for studies of neurocircuitry and cell networks. First, although reversible expression of t-toxins or t-peptides can be achieved using established methods, as we have shown with the DOX-inducible t-toxins [20], development of strategies for the rapid regulation of these activities for use in short-term experiments (e.g. light- or small-molecule-inducible methods) remains an important goal. Second, the cell-autonomous modulatory action of t-toxins/t-peptides and their selectivity for specific ion channels and receptors could be further exploited by directing t-peptide molecules to subcellular compartments within the neuron. Third, given the small size of most peptide toxins and peptide ligands, the creation of novel specificities by mutagenesis will extend the use of this approach to receptors and ion channels for which natural toxins have not been identified yet. Thus, we anticipate that t-toxins and other peptides will become critical instruments for the genetic dissection of CNS cells and circuits. [9]
Highlights.
Cell specific action of toxins and neuropeptides by tethering 2. Design principles of tethered peptide ligands
T-toxins and t-neuropeptides can be used in a wide variety of species
Targeted expression of t-toxins/t-peptides for dissection of CNS circuits
Acknowledgments
IIT thanks Sebastian Auer, Annika Stürzebecher, Julio Santos-Torres, Mande Holford, Marta A. Slimak, Branka Kampfrath, Silke Frahm, Martin Laqua, Beatriz Antolin-Fontes, Jessica Ables, Beate Liehl and Rene Juttner for their essential contribution to the development and research on t-toxins. MNN thanks Ying Wu and Charles Choi for pioneering work with t-ligands in his lab. Research in the laboratory of IIT was supported by the Helmholtz Association (31-002) and the Sonderforschungsbereich (SFB 665). Research in the laboratory of MNN is funded in part by National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH) grants R21NS058330, R01NS055035, and R01NS056443, and by National Institute of General Medical Sciences, NIH grant R01GM098931.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Inés Ibañez-Tallon, Email: ibanezi@mdc-berlin.de.
Michael N. Nitabach, Email: michael.nitabach@yale.edu.
References and recommended reading
** of outstanding interest
- 1.Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 3.Wu MN, Joiner WJ, Dean T, Yue Z, Smith CJ, Chen D, Hoshi T, Sehgal A, Koh K. SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nat Neurosci. 2010;13:69–75. doi: 10.1038/nn.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann SH, Leipold E. Conotoxins of the O-superfamily affecting voltage-gated sodium channels. Cell Mol Life Sci. 2007;64:1329–1340. doi: 10.1007/s00018-007-6565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker CS, Jensen S, Ellison M, Matta JA, Lee WY, Imperial JS, Duclos N, Brockie PJ, Madsen DM, Isaac JT, et al. A novel Conus snail polypeptide causes excitotoxicity by blocking desensitization of AMPA receptors. Curr Biol. 2009;19:900–908. doi: 10.1016/j.cub.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibanez-Tallon I, Wen H, Miwa JM, Xing J, Tekinay AB, Ono F, Brehm P, Heintz N. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron. 2004;43:305–311. doi: 10.1016/j.neuron.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Holford M, Auer S, Laqua M, Ibanez-Tallon I. Manipulating neuronal circuits with endogenous and recombinant cell-surface tethered modulators. Front Mol Neurosci. 2009;2:21. doi: 10.3389/neuro.02.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Auer S, Sturzebecher AS, Juttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibanez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. Development and application of general/universal strategy to silence neurotransmission in a cell-specific manner by inactivation of calcium channels with t-toxins. [DOI] [PubMed] [Google Scholar]
- 10.Fortin JP, Zhu Y, Choi C, Beinborn M, Nitabach MN, Kopin AS. Membrane-tethered ligands are effective probes for exploring class B1 G protein-coupled receptor function. Proc Natl Acad Sci U S A. 2009;106:8049–8054. doi: 10.1073/pnas.0900149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hruska M, Ibanez-Tallon I, Nishi R. Cell-autonomous inhibition of alpha 7-containing nicotinic acetylcholine receptors prevents death of parasympathetic neurons during development. J Neurosci. 2007;27:11501–11509. doi: 10.1523/JNEUROSCI.3057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Sturzebecher AS, Hu J, Smith ES, Frahm S, Santos-Torres J, Kampfrath B, Auer S, Lewin GR, Ibanez-Tallon I. An in vivo tethered toxin approach for the cell-autonomous inactivation of voltage-gated sodium channel currents in nociceptors. J Physiol. 2010;588:1695–1707. doi: 10.1113/jphysiol.2010.187112. This paper presents an elegant demonstration of the efficacy of t-toxins for inhibition of sodium channels in nociceptive neurons in BAC transgenic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Cao G, Pavlicek B, Luo X, Nitabach MN. Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin. PLoS Biol. 2008;6:e273. doi: 10.1371/journal.pbio.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auer S, Ibanez-Tallon I. “The King is dead”: Checkmating ion channels with tethered toxins. Toxicon. 2010;56:1293–1298. doi: 10.1016/j.toxicon.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 15**.Choi C, Fortin JP, McCarthy E, Oksman L, Kopin AS, Nitabach MN. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. The first demonstration of the efficacy of GPI-tethered endogenous class B1 bioactive peptides when expressed in a transgenic animal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen SM, Nielsen LZ, Hjorth SA, Perrin MH, Vale WW. Constitutive activation of tethered-peptide/corticotropin-releasing factor receptor chimeras. Proc Natl Acad Sci U S A. 2000;97:10277–10281. doi: 10.1073/pnas.97.18.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 19.Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 20.Drago J, McColl CD, Horne MK, Finkelstein DI, Ross SA. Neuronal nicotinic receptors: insights gained from gene knockout and knockin mutant mice. Cell Mol Life Sci. 2003;60:1267–1280. doi: 10.1007/s00018-003-2259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirthanan S, Gwee MC. Three-finger alpha-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J Pharmacol Sci. 2004;94:1–17. doi: 10.1254/jphs.94.1. [DOI] [PubMed] [Google Scholar]
- 22.Chang CC, Lee CY. Isolation of Neurotoxins from the Venom of Bungarus Multicinctus and Their Modes of Neuromuscular Blocking Action. Arch Int Pharmacodyn Ther. 1963;144:241–257. [PubMed] [Google Scholar]
- 23.Chiappinelli VA. Kappa-bungarotoxin: a probe for the neuronal nicotinic receptor in the avian ciliary ganglion. Brain Res. 1983;277:9–22. doi: 10.1016/0006-8993(83)90902-2. [DOI] [PubMed] [Google Scholar]
- 24.Dessaud E, Salaun D, Gayet O, Chabbert M, deLapeyriere O. Identification of lynx2, a novel member of the ly-6/neurotoxin superfamily, expressed in neuronal subpopulations during mouse development. Mol Cell Neurosci. 2006;31:232–242. doi: 10.1016/j.mcn.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Tekinay AB, Nong Y, Miwa JM, Lieberam I, Ibanez-Tallon I, Greengard P, Heintz N. A role for LYNX2 in anxiety-related behavior. Proc Natl Acad Sci U S A. 2009;106:4477–4482. doi: 10.1073/pnas.0813109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 27.Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208:238–245. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- 28.Choo YM, Lee BH, Lee KS, Kim BY, Li J, Kim JG, Lee JH, Sohn HD, Nah SY, Jin BR. Pr-lynx1, a modulator of nicotinic acetylcholine receptors in the insect. Mol Cell Neurosci. 2008;38:224–235. doi: 10.1016/j.mcn.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Yao X, Gu S, Zhang Y, Liu Z. Selectivity of lynx proteins on insect nicotinic acetylcholine receptors in the brown planthopper, Nilaparvata lugens. Insect Mol Biol. 2010;19:283–289. doi: 10.1111/j.1365-2583.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Cao G, Li J, Bao H, Zhang Y. Identification of two Lynx proteins in Nilaparvata lugens and the modulation on insect nicotinic acetylcholine receptors. J Neurochem. 2009;110:1707–1714. doi: 10.1111/j.1471-4159.2009.06274.x. [DOI] [PubMed] [Google Scholar]
- 31.Hruska M, Keefe J, Wert D, Tekinay AB, Hulce JJ, Ibanez-Tallon I, Nishi R. Prostate stem cell antigen is an endogenous lynx1-like prototoxin that antagonizes alpha7-containing nicotinic receptors and prevents programmed cell death of parasympathetic neurons. J Neurosci. 2009;29:14847–14854. doi: 10.1523/JNEUROSCI.2271-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]