Abstract
Neuroblastomas arise from the neural crest cells and represent the most common solid tumors outside the nervous system in children. The amplification of N-Myc plays a primary role in the pathogenesis of neuroblastomas whereas acquired mutations of p53 lead to refractory and relapsed cases of neuroblastomas. In this regard, dietary compounds which can target N-Myc and exert anti-cancer effects independent of p53 status acquire significance in the management of neuroblastomas. Hence, we investigated the anti-cancer properties of the flavonoid didymin in neuroblastomas. Didymin effectively inhibited proliferation and induced apoptosis irrespective of p53 status in neuroblastomas. Didymin down regulated PI3K, pAkt, Akt, vimentin and up regulated RKIP levels. Didymin induced G2/M arrest along with decreasing the levels of cyclin D1, CDK4 and cyclin B1. Importantly, didymin inhibited NMyc as confirmed at protein, mRNA and transcriptional level by promoter-reporter assays. HPLC analysis of didymin (2 mg/kg b.w.) treated mice serum revealed effective oral absorption with free didymin concentration of 2.1 μM. Further in vivo mice xenograft studies revealed that didymin (2 mg/kg b.w.) treated animals had significant reductions in tumors size compared to controls. Didymin strongly inhibited the proliferation (Ki67) and angiogenesis (CD31) markers as well as N-Myc expression as revealed by the histopathological examination of paraffin embedded section of resected tumors. Collectively, our in vitro and in vivo studies elucidated the anti-cancer properties and mechanisms of action of a novel, orally active and palatable flavonoid didymin which makes it a potential new approach for neuroblastoma therapy (NANT) to target pediatric neuroblastomas.
Keywords: Neuroblastoma, Didymin, N-Myc, RKIP, tumor xenografts
Introduction
Neuroblastomas are the neural crest derived malignant tumors which constitute most common solid tumors in infants outside the central nervous system (1). More than 90% of the neuroblastomas are diagnosed before 5 years of age with greater occurrence of refractory and relapsed neuroblastomas in pediatric population over 18 months. Neuroblastomas metastasize to various sites like skull, bones, spine and retro-orbital tissues which lead to challenging clinical presentations like proptosis and retro-orbital ecchymosis in children (2–4). The major genetic causes for the incidence and therapeutic refractoriness of neuroblastomas include amplification of the oncogene N-Myc and loss of tumor suppressor p53 (5, 6). Raf-kinase inhibitory protein (RKIP) is a novel protein that interacts with MAP/ERK kinases and acts as an inhibitor of MAPK pathway (7). RKIP is an established metastasis suppressor protein and given the role of MAPK pathway in regulating the survival and metastatic potential, RKIP has been the focus of recent investigations in assessing the effects of novel anti-cancer agents (8, 9). Loss of p53 leads to increased activity of multifunctional proteins like RLIP76 which mediate enhanced proliferation, invasion and drug/radiation resistance in neuroblastoma (10, 11). In this regard, effective strategy for targeting the incidence of neuroblastoma in genetically identified risk groups should decrease the incidence of neuroblastoma as well as improve the survival following initial diagnosis.
Given the limited three year event free survival of 15% in the incident population and the nature of symptoms in affected children, validation of novel compounds becomes a vital focus of translational research in order to effectively target neuroblastomas. Also, due to the developing nervous and organ systems in children, the interventional choices will naturally favor active anti-cancer ingredients of safe dietary components, such as fruits, vegetables and the compounds derived from them. Didymin is a flavonoid that is richly expressed in citrus fruits like oranges, lemons as well as other dietary compounds like mandarin and bergamot. Recently, it was reported that didymin can cause cell death in non-small cell lung cancer (NSCLC) in a p53 independent manner (12). The use of chemotherapeutic drugs like cisplatin, doxorubicin and vincristine is highly limited in refractory and relapsed neuroblastomas due to frequent loss-of-function mutations in the tumor suppressor p53 (13, 14). Hence, the elucidation of signaling pathways through which didymin acts in neuroblastomas would have potential implications towards the effective management of neuroblastomas. In this regard, we investigated the efficacy and mechanisms of action of didymin in vitro cultures and in vivo mice xenograft models of neuroblastomas.
Our studies first focused on testing the effect of didymin on the survival and clonogenic potential of both p53-wild type and p53-mutant neuroblastomas. Next, we investigated the induction of apoptosis followed by analyzing the impact on key nodes of proliferation in neuroblastomas. Cell cycle analysis revealed a mechanism of action of didymin that includes its ability to regulate cyclin signaling down-stream of p53. Importantly, we investigated the effect of didymin on N-Myc by Western blot, mRNA and N-Myc luciferase promoter-reporter assays followed by in vivo mice xenograft studies. Our studies revealed the ability of didymin to regulate important nodes of signaling along with being effective irrespective of p53 status in neuroblastomas.
Materials and Methods
Reagents
Didymin, hesperidin and 2'-hydroxyflavanone (2HF) (purity >98%) were purchased from Indofine Chemical Company, Hillsborough, NJ. Poly-ADP ribose polymerase (PARP), cyclin B1, cyclin D1, CDK4, Akt, pAkt (S473), GAPDH, N-Myc, Ki67, CD31 and RKIP antibodies were purchased from Santa Cruz Biotechnology (Columbus, OH), Upstate Cell Signaling (Lake Placid, NY), and Cell Signaling Technologies (Danvers, MA). The siGENOME smart pool siRNA for RKIP and control scrambled siRNA were purchased from Dharmacon (Lafayette, CO). APO LOGIX™ carboxyfluorescein (FAM) caspase detection kit was purchased from Cell Technology (Minneapolis, MN). Lipofectamine 2000 and FITC-labeled annexin V conjugate detection kit were purchased from Invitrogen (Carlsbad, CA). The thymidine-kinase promoter driven Renilla luciferase (pTK-RL) and TUNEL fluorescence kit were obtained from Promega (Madison, WI). Avidin/biotin complex (ABC) detection kit was purchased from Vector (Burlingame, CA).
Cell Lines and Cultures
CHLA-90 and SK-N-BE2 (p53-mutant) as well as SMS-KCNR and LAN-5 (p53 wild-type) human neuroblastoma cell lines were kindly authenticated and provided in May 2010 by Dr. Patrick Reynolds, MD, PhD, Children's Neuroblastoma Cancer Foundation/Children Oncology group, Texas Tech University, School of Medicine, Lubbock, TX. The authentication of cell lines was done by analyzing fifteen different human short tandem repeat (STR) (performed by Center for Investigative Genetics core facility at University of North Texas Health Science Center, Fort Worth, TX) to test for interspecies contamination. The cell lines were last tested in April 2011. Cells were grown in complete medium consisting of Iscove's modified Dulbecco's medium supplemented with 3 mmol/L L-glutamine, 5 μg/mL each of insulin and transferrin, 5 ng/mL of selenous acid (ITS culture supplement), and 20% FBS at 37 °C in a humidified 5% CO2 atmosphere. Cell lines were subcultured by detaching without trypsin from culture plates by using a modified Puck's Solution A plus EDTA (Puck's EDTA) which contain 140 mmol/L NaCl, 5 mmol/L KCl, 5.5 mmol/L glucose, 4 mmol/L NaHCO3, 0.8 mmol/L EDTA, 13 mmol/L phenol red, and 9 mmol/L HEPES buffer (pH 7.3). All the cells were also tested for Mycoplasma once every 3 months.
Drug sensitivity (MTT) assay
Cell density measurements were done by a hemacytometer to count dye-excluding cells resistant to staining with trypan blue. Approximately 2 × 104 cells were plated into each well of a 96-well flat-bottomed micro-titer plate 24 h prior to addition of medium containing varying concentrations of indicated flavonoids. After 12 h of incubation, indicated flavonoids (didymin, hesperidin, 2HF; ranging from 0–200 μM each) were added to 8 replicate wells. After 48 h of incubation, 20 μL (5mg/mL stock) MTT was added to each well and incubated for 2 h at 37 °C. The plates were centrifuged and medium was removed. Formazan dye trapped in cells was dissolved by addition of 100 μL DMSO with gentle shaking for 2 h at room temperature, followed by measurement of absorbance at 570 nm (15–17).
siRNA mediated knock-down of RKIP
The desalted RKIP siRNA (Dharmacon, Lafayette, CO) was resuspended in 1× universal buffer provided by Dharmacon Research laboratory. Cells were transfected with scrambled and RKIP siRNA at the concentration of 1 μg / ml in serum free medium, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 3 h, according to the manufacturer's instructions. Excess siRNA was washed off with PBS and complete medium (i.e. with FBS) was added. After 24 h silencing, cells were exposed with 50 μM didymin. After 48 h incubation, MTT assays as well as Western blot analyses for RKIP and N-Myc expression were performed as described previously (18–20).
Colony forming assay
Cell survival was evaluated using a standard colony-forming assay. 1×105 cells / ml were incubated with one of the three drugs (didymin, hesperidin, 2HF-50 μM each) for 48 h, and aliquots of 50 or 100 μl were added to 60-mm size Petri dishes containing 4 ml culture medium. After 10 days, adherent colonies were fixed, stained with 0.5% methylene blue for 30 min and counted using the Innotech Alpha Imager HP (21).
In situ caspase-3 cleavage assay
Detection of active caspase-3 in live cell cultures was performed using an APO LOGIX™ carboxyfluorescein (FAM) caspase detection kit (Cell Technology). The kit detects active caspase-3 in living cells through FAM-labeled DEVD fluoromethyl ketone (FMK), which irreversibly binds to active caspase-3. The inhibitor is cell permeable and non-cytotoxic. The assay was performed according to manufacturer's instructions (22).
TUNEL assay
For measurement of apoptosis by TUNEL assay, 1×105 cells were grown on the cover-slips for ~12 h followed by treatment with test compounds (didymin, hesperidin, 2HF-50 μM each) for 24 h. Apoptosis was determined by the labeling of DNA fragments with terminal deoxynucleotidyl-transferase dUTP nick-end labeling (TUNEL) using Promega fluorescence apoptosis detection system. Slides were analyzed under a fluorescence microscope using a standard fluorescein filter set to view the green fluorescence at 520 nm and red fluorescence of propidium iodide at >620 nm (17).
In vitro migration assay
Cell migration was determined using a wound healing assay (23). 2×104 CHLA-90 and SMS-KCNR cells were seeded in 6-well plates to reach 100% confluence within 24 h and then treated with 10 μM mitomycin C for 2 h. Subsequently, a similarly sized scratch was made with a 200 μL pipette tip across the center of each well and immediately imaged at baseline and then at 24 h in control and didymin treated groups by using an Olympus Provis AX70 microscope. The rate of cell migration was determined by comparing the sizes of scratch area using Image J software.
Western blot analysis
The control and 50 μM didymin-treated (at 24 h) cells were lysed and analyzed by Western-blot analyses for PARP cleavage, PI3K (Y458/199), pAkt (S473), AKT, cyclin D1, cyclin B1, CDK4, N-Myc, RKIP, and vimentin by using specific antibodies. Briefly, lysates containing ~ 50 μg of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were transferred onto nitrocellulose membrane. After blocking with 5% non-fat dry milk, the membrane was incubated overnight with the desired primary antibody (1:1000 dilution). Subsequently, the membrane was incubated with appropriate secondary antibody for 2 h, and the immune-reactive bands were visualized using the enhanced chemiluminescence kit from Perkin-Elmer (Waltham, MA) according to the manufacturer's instructions. The same membrane was re-probed with the antibody against GAPDH (1:5000 dilution) as an internal control for equal protein loading. The intensity of immune-reactive bands on Western blots was determined using a densitometer (Molecular Dynamics, Sunnyvale, CA) equipped with Image QuaNT software.
Flow cytometry analysis of cell cycle regulation
2 × 105 cells were treated with didymin (50 μM) for 18 h at 37 °C. After treatment, floating and adherent cells were collected, washed with PBS, and fixed with 70 % ethanol. On the day of flow analysis, cell suspensions were centrifuged; counted and same numbers of cells were resuspended in 500 μl PBS in flow cytometry tubes. Cells were then incubated with 2.5 μl of RNase (stock 20 mg/ml) at 37 °C for 30 min after which they were treated with 10 μl of propidium iodide (stock 1mg/ml) solution and then incubated at room temperature for 30 min in the dark. The stained cells were analyzed using the Beckman Coulter Cytomics FC500, Flow Cytometry Analyzer. Results were processed using CXP2.2 analysis software from Beckman Coulter (17).
RT-PCR analysis
Expression of N-Myc mRNA in neuroblastomas was evaluated by RT-PCR analysis. The RNA from control and experimental groups was prepared 24 h after didymin treatment using an Rneasy kit (Qiagen, Valencia, CA), was quantified and 2 μg RNA was subjected to 1% agarose gel electrophoresis in MOPS-formaldehyde buffer. N-Myc gene-specific primers were used for RT-PCR (Biosynthesis Inc., Lewisville, TX) (24). RT-PCR one step kit was used according to the manufacturer's instructions (Qiagen, Valencia, CA). Level of N-Myc protein in control and didymin treated cells were also measured by immunoassay using anti-NMyc IgG. Aliquots of crude cell extracts containing ~50 μg protein were applied to SDS-PAGE and Western-blot analyses were performed.
N-Myc promoter-reporter assay
CHLA-90 and SMS-KCNR cells were cultured as adherent monolayers in a 96-well plate at a density of 2 × 104 cells per well. The Firefly luciferase reporter construct (0.4 ug, pEB-Luc-N-Myc), kindly gifted by Prof William L Carroll, M.D., New York University Medical Center, NY, was co-transfected with 100 ng of Renilla luciferase construct (thymidine kinase promoter-driven Renilla luciferase; pTK-RL) by Lipofectamine Reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). After 24 h of incubation, transfected cells were treated with 50 μM of Didymin. At 24 h post-treatment, promoter activity was measured as luciferase activity by mixing cells with 50 μl of passive lysis buffer (Promega) followed by 15 min of shaking at room temperature. On a luminometer plate, 20 μl of the lysate was then mixed with 100 μl of luciferase assay reagent followed by 100 μl of Stop N' Glo Buffer (Promega). Luciferase activity was then determined as a ratio of Firefly to Renilla luciferase. Renilla luciferase activity was thus used as an internal control.
HPLC analysis of didymin in serum
Mice (n=3 each for control and didymin treatment) were administered either 0.1 ml corn oil or 50 μg didymin /0.1ml corn oil/mice (2 mg/kg b.w.) by oral gavage on alternate days for 8 weeks. On the last day, the blood was collected within 1 h after final dosage. The 100 μl of serum was subjected to protein precipitation using 200 μl of methanol. The sample was centrifuged at 13,300 rpm for 15 min at 4 °C and the clear supernatant was lyophilized. The resulting pellet was dissolved in 200 μl of methanol: 0.2% phosphoric acid buffer (58:42 v/v). The 25 μl of each sample was subjected to HPLC analysis using C18 reverse-phase column at wavelength of 295 nm on Agilent 1100 system with auto injector (Agilent Technologies, CA). The mobile phase consisting of acetonitrile (ACN) and water at a flow rate of 1.0 ml/min with following gradient: 0–1 min: ACN-10%, H2O-90%; 1–2 min: gradually increasing to ACN-20%, H2O-80%; 2–14 min: gradually changing to ACN-90%, H2O-10%; 14–16 min: ACN-10%, H2O-90%.
In vivo xenograft studies
Hsd: Athymic nude nu/nu mice were obtained from Harlan, Indianapolis, IN. All animal experiments were carried out in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC). The 11-weeks-old mice (n=10) were divided into two groups of 5 animals [(For treatment with (i) corn oil (vehicle), (ii) didymin (2 mg/kg b.w.)]. The animals were injected with 2 × 106 SMS-KCNR neuroblastoma cell suspensions in 100 μl of PBS, subcutaneously into one flank of each nu/nu nude mouse. At the same time, animals were randomized in to treatment groups as indicated in the figure. Treatment was started 10 days after the neuroblastoma cells implantation to see palpable tumor growth. Treatment consisted of didymin (2 mg/kg b.w.) in 100 μl corn oil by oral gavage alternate day. Control groups were treated with 100 μl corn oil by oral gavage alternate day. Animals were examined daily for signs of tumor growth. Tumors were measured in two dimensions using calipers. Photographs of animals were taken at day 1, day 10, day 20, day 40, and day 60 after subcutaneous injection, are shown for all groups. Photographs of tumors were also taken at day 60.
Histopathological examination of tumors for angiogenic, proliferative and differentiation markers
Tumors from SMS-KCNR neuroblastoma mice xenografts (control and didymin treated) were harvested on day 60. Tumor samples fixed in buffered formalin for 12 h were processed conventionally for paraffin-embedded tumor sections (5 μm thick). Histopathologic analyses with ki67, anti-CD31 and N-Myc were performed, using Universal ABC detection kit (Vector). The sections were examined under Olympus Provis AX70 microscope connected to a Nikon camera.
Statistical Analyses
All data were evaluated with a two-tailed unpaired student's t test are expressed as mean ± SD. The statistical significance of differences between control and treatment groups was determined by ANOVA followed by multiple comparison tests. Changes in tumor size and body weight during the course of the experiments were visualized by scatter plot. Differences were considered statistically significant when the P value was less than 0.05.
Results
Anti-proliferative effect of didymin in Neuroblastoma
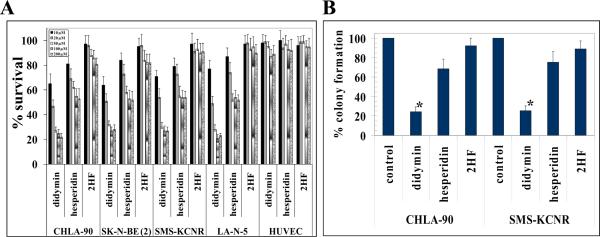
The cell survival study by MTT assay revealed that didymin is the most potent of the three flavonoids tested in four neuroblastomas cell lines [CHLA-90 and SK-N-BE2 (p53-mutant) as well as SMS-KCNR and LAN-5 (p53 wild-type)]. The other flavonoid hesperidin was less effective and 2-HF was not much effective against the four neuroblastoma cell lines tested. The 50 μM didymin caused ~75% cell death whereas hesperidin induced only ~35% cell death and 2-HF induced ~10% cell death in all tested neuroblastoma cell lines at similar concentrations. Didymin did not cause any cytotoxicity in normal HUVEC in MTT assay (Fig 1A). The ability of didymin to arrest the growth of neuroblastoma tumors was further evident in colony forming assay where 50 μM didymin caused ~70% growth inhibition as compared to hesperidin (~30% growth inhibition) and 2-HF (~10% growth inhibition) (Fig 1B). Thus, didymin decreased both survival and clonogenic potential to a greater extent than hesperidin and 2-HF in neuroblastomas as revealed by MTT and colony forming assays, respectively. The effect of didymin on apoptosis was further studied by TUNEL apoptosis assay. The didymin treatment induced enhanced cell death relative to hesperidin and 2-HF in neuroblastoma which was evident by increased green fluorescence, a measure of DNA strand breaks induced by apoptosis, in TUNEL apoptotic assay (Fig 2A) (25). Didymin also effectively inhibited the migration of neuroblastoma cells in vitro wound healing assay relative to hesperidin and 2-HF (Fig 2B). These findings collectively confirmed the enhanced cytotoxic potential of didymin in targeting neuroblastomas relative to hesperidin and 2-HF. Following initial assessment of the potent anti-cancer properties of didymin in neuroblastomas, we next performed detailed mechanistic studies in CHLA-90 (p53-mutant) and SMS-KCNR (p53 wild-type) neuroblastoma cells.
Figure 1. Anti-proliferative effect of didymin in neuroblastoma.
Drug-sensitivity assays were performed by MTT assay using didymin, hesperidin and 2HF at 48 h post treatment to determine IC50. Values are presented as mean ± s.d. from two separate determinations with eight replicates each (n =16) (panel A). Colony-forming assay was performed as described in methods. The colonies were counted using Innotech Alpha Imager HP (21). * p < 0.001 compared with control (panel B).
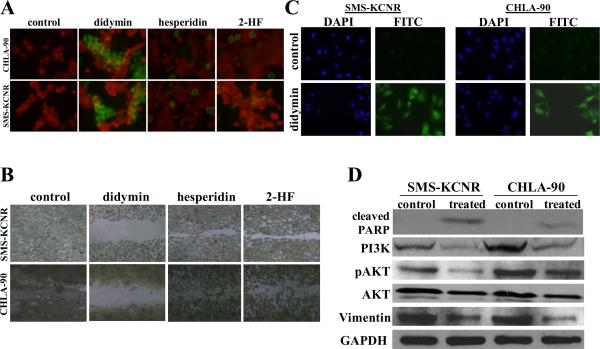
Figure 2. Pro-apoptotic and anti-migratory effects of didymin in neuroblastoma.
For TUNEL apoptosis assay, cells were grown on cover-slips and treated with 50 μM didymin for 24 h. TUNEL assay was performed using Promega fluorescence detection kit and examined using Zeiss LSM 510 META laser scanning fluorescence microscope with filters 520 and > 620 nm. Photographs taken at identical exposure at ×400 magnification are presented. Apoptotic cells showed green fluorescence (panel A). The in vitro migration assay shows that the didymin inhibits neuroblastoma cell migration in wound healing assay (panel B). For caspase activation detection, SMS-KCNR and CHLA-90 cells were plated on glass cover-slip in tissue culture treated 12-well plates and incubated with 50 μM of didymin for 24 h at 37 °C. Apoptotic cells were detected by staining with 5 μM Caspase FITC-VAD-FMK (Promega) in situ marker for 30 min (panel C). Analysis of the effect of didymin on PARP-cleavage, PI3K and Akt activation by Western-blot: The control and 50 μM didymin-treated cells were lysed and analyzed by Western-blot for PARP cleavage, PI3K (Y458/199), pAkt (S473), AKT and vimentin by using specific antibodies. Membranes were stripped and reprobed for GAPDH as a loading control (panel D).
Pro-apoptotic and anti-migratory effect of didymin in Neuroblastoma
The didymin treatment increased caspase-3 activation in in situ caspase-3 cleavage assay as revealed by enhanced green fluorescence in the treated group compared to controls (Fig 2C). Western blot revealed the enhanced PARP-cleavage consequent to didymin treatment in neuroblastomas. Didymin treatment also decreased the levels of PI3K, phosphorylation and protein levels of Akt. Didymin also effectively reduced the levels of vimentin, a major intermediate filament protein which regulates epithelial mesenchymal transition (EMT), an essential process during oncogenic transformation as well as metastasis (Fig 2D). These results provided the mechanistic rationale for the decreased survival and clonogenic potential induced by didymin as revealed by our studies by revealing the inhibition of PI3K and pAkt by didymin. The activation of caspase-3 and enhanced PARP-cleavage further validated the apoptosis observed by TUNEL apoptosis assay following didymin treatment. The expression of vimentin is associated with enhanced motility of tumor cells and hence inhibition of vimentin by didymin was significant in the context of anti-migratory effects of didymin in neuroblastomas as revealed by in vitro migration assay (26, 27). Thus, these results provided further corroborative evidence for the observed anti-proliferative and pro-apoptotic effects of didymin in initial cell survival, clonogenic and apoptosis studies.
Effect of didymin on cell cycle progression in Neuroblastoma
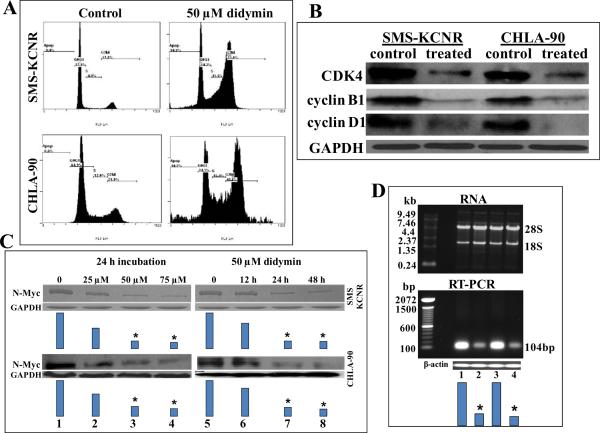
We next analyzed the effect of didymin on cell cycle progression by FACS analysis. Loss of p53 leads to decreased cell cycle checkpoint regulation which favors enhanced proliferation of cells (28–30). Didymin caused significant G2/M phase arrest in CHLA-90 (p53-mutant) and SMS-KCNR (p53 wild-type) neuroblastoma cells suggesting the inhibition of cell cycle as an additional mechanism for the anti-proliferative effects of didymin (Fig 3A). Western-blot of didymin treated neuroblastoma cells revealed decrease in cyclin B1, cyclin D1 and CDK4 (Fig 3B). Cyclin D1 is a key G2/M phase regulator whose increase during G2 phase leads to progression to mitosis and hence the decrease of cyclin D1 by didymin impacts G2/M phase progression (31–33). Another interesting finding was that we observed a decrease in the levels of CDK4, the kinase which is commonly associated with G1 transition along with G2/M phase arrest. CDK4 has multiple functions and has been also investigated for its role in G2/M transition. It has been shown that over-expression of dominant-negative CDK4 leads to arrest of G2 phase progression (33). Some of the anticancer compounds like apigenin and thimerosal also cause inhibition of CDK4 along with cyclin B1 while causing G2/M phase arrest (33–35). Cyclin B1, the regulatory subunit of cdc2 kinase is required for mitotic phase initiation. Cyclin B1 is localized to kinetochores during cell division and regulates the alignment of chromosomes during mitosis (36). The p53 activation leads to G2/M phase arrest by decreasing the levels of cyclin B1 levels as well as inhibiting the activity of cyclin B1 promoter while the activation of cyclin B1 leads to cell cycle progression even in the presence of p53 activation (37, 38). Thus, cyclin B1 is a critical requirement and mediator of G2/M phase progression downstream of p53. In this context, the ability of didymin to down regulate cyclin B1 in both p53-mutant and p53-wild type neuroblastomas represents a potentially significant mechanism of action for the observed anti-proliferative effects of didymin in neuroblastomas irrespective of p53 status.
Figure 3. Effect of didymin on cell cycle and N-Myc levels in Neuroblastoma.
Inhibitory effect of didymin on cell cycle distribution was determined by fluorescence activated cell sorting analysis. Experimental details are given in the Methods section. The stained cells were analyzed using the Beckman Coulter Cytomics FC500, Flow Cytometry Analyzer. The experiment was repeated three times and similar results were obtained (panel A). The 50 μM didymin-treated cells were processed for Western-blot analyses for cyclin D1, cyclin B1 and CDK4 expression by using specific antibodies. Membranes were stripped and reprobed for GAPDH as a loading control (panel B). The SMS-KCNR and CHLA-90 cells were treated with didymin and the effect on N-Myc protein level using anti-N-Myc IgG was determined in a concentration and time dependent study. Results were quantified by scanning densitometry (panel C). The mRNA level of N-Myc following 50 μM didymin treatment was assessed by RT-PCR using N-Myc gene-specific primers (Biosynthesis Inc.,). Total RNA after 50 μM didymin treatment (upper panel D) and N-Myc mRNA by RT-PCR (lower panel D): Lane 1, SMS-KCNR, Lane 2, SMS-KCNR didymin treated, Lane 3, CHLA-90, Lane 4, CHLA-90 didymin treated. Bars represents densitometry and * p-value < 0.001 compared to control.
Impact of didymin on N-Myc expression
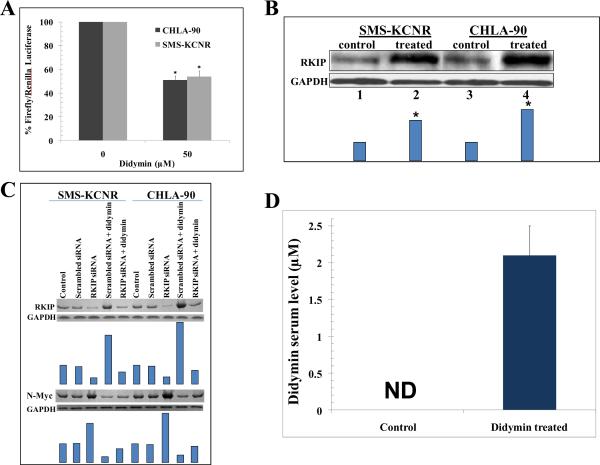
The amplification of N-Myc contributes both to pathogenesis and aggressive progression of neuroblastomas giving them a highly angiogenic phenotype (5, 39). Hence, we analyzed the impact of didymin on N-Myc expression. The didymin treatment decreased the levels of N-Myc protein in a time and concentration dependent manner as demonstrated by Western-blot analyses (Fig 3C). The 50 μM didymin treatment induced ~5 fold reduction in N-Myc mRNA levels as revealed by RT-PCR using N-Myc gene-specific primers (Fig 3D). We further explored whether didymin regulates N-Myc promoter activity by luciferase promoter-reporter assays. The Firefly luciferase reporter construct (0.4 μg pEB-Luc-N-Myc) was co-transfected with 100 ng of Renilla luciferase plasmid construct (thymidine kinase promoter-driven Renilla luciferase; pTK-RL) by Lipofectamine Reagent. After 24 h of incubation, the transfected cells were treated with 50 μM of didymin, further incubated for 24 h and the luciferase assay was performed. Didymin treatment induced significant decrease in the activity of N-Myc promoter compared to controls (Fig 4A).
Figure 4. Effect of didymin on N-Myc promoter activity and RKIP expression.
Down-regulation of luciferase-activity upon didymin treatment: Upon transfection with the N-Myc luciferase reporter construct or a control construct along with a Renilla luciferase vector and followed by 24 h treatment with 50 μM didymin, luciferase activities were measured. A significant decrease in the relative activity of the N-Myc luciferase reporter assay was observed in didymin treated cells as compared to that of controls. *p<0.001 compared to control (panel A). Western-blot for RKIP expression 24 h after 50 μM didymin treatment: Bars represents densitometry and * p-value < 0.005 compared to control (panel B). After knock-down of RKIP by siRNA, the levels of RKIP and N-Myc were detected by Western blot analyses. Membranes were stripped and reprobed for GAPDH as a loading control. Results were quantified by scanning densitometry (panel C), and the concentration of didymin in mice serum by HPLC analysis: Didymin concentration was determined after 1 h of final oral dosage (50 μg or 2 mg/kg b.w.) in mice serum. Bar represents the quantification (mean ± SD) of didymin in mice serum (n=3). ND, not detectable (panel D).
PI3K inhibition mediated down-regulation of N-Myc protein levels has been reported (40). Didymin caused significant inhibition of PI3K in neuroblastomas (Fig 2D). Raf-kinase inhibitory protein (RKIP) can inhibit MAPK and Myc signaling (41–43). Interestingly, RKIP over-expression is also known to positively induce normal neuronal differentiation in neuroblastoma (44). Hence, we examined the effect of didymin on RKIP expression. Didymin significantly enhanced the levels of RKIP in both p53-mutant (CHLA-90) and p53-wild type (SMS-KCNR) neuroblastomas (Fig 4B). Given the significant role of RKIP in tumor signaling, we further studied the impact of knock-down of RKIP in SMS-KCNR and CHLA-90 neuroblastoma cells. The MTT assay following knock-down of RKIP by siRNA treatment revealed partial reversal of the didymin induced inhibition of neuroblastoma cell survival (data not shown). The knock-down of RKIP was also associated with an increase in the expression of N-Myc in neuroblastoma cells. The knock-down of RKIP also caused a partial reversal in the didymin induced decrease of N-Myc expression as detected by Western blot analyses (Fig 4C). This observed inhibitory effect of RKIP on N-Myc expression is in accordance with previous studies implicating the role of RKIP in regulation of Myc expression (45). Hence, RKIP up-regulation, along with N-Myc repression, represents a potent mechanism of action of didymin of specific relevance in inducing its anti-cancer effects in neuroblastomas. The collective anti-proliferative, anti-migratory and pro-apoptotic effects of didymin in vitro along with its ability to inhibit N-Myc, PI3K/Akt and MAPK signaling pathways which play a significant mechanistic role in the incidence, progression and clinical refractoriness of neuroblastomas provided strong rationale for the further investigation of didymin in in vivo mice xenograft studies.
Anti-neoplastic effect of didymin in vivo
We first assessed the absorption of orally administered didymin in mice. HPLC analysis of didymin treated mice serum revealed that didymin is effectively absorbed after oral dosage and it reaches a serum concentration of 2.1 μM (Fig 4D). It is important to note that we quantified only didymin but not its metabolites to address whether didymin, a relatively new flavonoid in cancer research is absorbed following oral administration.
We next assessed the regression of neuroblastoma mice xenografts following oral administration of didymin. The mice harboring SMS-KCNR neuroblastoma xenografts were treated with 50 μg /mice (equivalent to 2 mg/kg b.w. or 0.0002% w/w) of didymin in 100 μl corn oil alternate day for sixty days by oral gavage. Control group was treated with 100 μl corn oil. Treatment was started 10 days post neuroblastoma cells implantation after a palpable tumor growth was observed. Photographs of animals were taken at day 1, day 10, day 20, and day 40 after subcutaneous injection, are shown for both groups. Tumors were dissected at day 60, photographed and weighed. Didymin treated animals had significant reductions in tumors as compared to control, whereas uncontrolled growth was observed in the control groups. The didymin treated mice were active and weight gain was comparable with non-tumor-bearing controls, and no overt toxicity was evident (Fig 5A). The final tumor weight on day 60 was 0.91 g in didymin treated group compared to 1.97 g in controls. Present studies for the first time demonstrated sustained regression of xenografts of neuroblastomas by didymin (Fig 5B and C).
Figure 5. Effect of oral administration of didymin in in vivo mice xenograft study.
Animals were examined daily for signs of tumor growth and body weights were recorded (panel A). Regression of SMS-KCNR neuroblastoma xenografts after didymin treatment in mice: Tumors were measured in two dimensions using calipers. Tumor cross-sectional revealing the regression of neuroblastoma tumors in vivo in didymin treated group relative to controls (panel B). Photographs of animals and tumor were taken during the course of study (panel C). Impact of didymin treatment on the proliferation marker Ki67, angiogenesis marker CD31, and neuroblastoma oncogenic marker N-Myc expression in in vivo mice xenografts: Control and didymin treated SMS-KCNR neuroblastoma bearing nude mice tumor sections were used for histopathologic analyses. IHC analyses for Ki67 expression (marker of cellular proliferation), CD31 (angiogenesis marker), and N-Myc (neuroblastoma oncogenic marker) from tumors in mice of control and didymin-treated groups were performed. Statistical significance of difference was determined by two-tailed Student's t test. p < 0.001, didymin-treated compared with control. Immunoreactivity is evident as a dark brown stain, whereas non-reactive areas display only the background color. Arrows represent the area for positive staining for an antigen. The intensity of antigen staining was quantified by digital image analysis. Bars represent mean ± S.E. (n = 5); * p<0.001 compared with control (panel D).
The effect of didymin treatment on the neuroblastoma progression markers in resected mice xenograft sections was further examined. The histo-pathological examination of paraffin embedded tumor xenograft sections revealed the decreased levels of proliferation marker, Ki67, angiogenesis marker, CD31, and N-Myc expression in SMS-KCNR neuroblastoma after didymin treatment which further supported our in vitro results (Fig 5D).
Discussion
Until today, there are no safe dietary choices to prevent the incidence and progression of neuroblastomas in children. The use of chemotherapeutic drugs, radiation and surgical approaches are often coupled with recurrent and diverse clinical challenges like limitations of young age as well as amplification of off-target cytotoxicity on developing nervous and skeletal systems over the course of time following initial therapy in children. In this regard, our current studies, for the first time, have revealed that the novel flavonoid didymin is effective against neuroblastomas both in vitro cell cultures and in vivo mice xenograft studies. Didymin effectively inhibited the proliferation of neuroblastomas and induced apoptosis by inhibiting PI3K, Akt and increasing RKIP levels. Didymin also decreased the levels of vimentin, a marker of epithelial mesenchymal transition, and decreased the migration of neuroblastoma cells in vitro. Importantly, didymin induced G2/M cell cycle arrest which was associated with decrease in the levels of cyclin B1, G2/M phase regulator down-stream of p53 and whose over-expression can override p53 induced G2/M phase arrest (35). The down-regulation of cyclin B1 explains one of the mechanisms responsible for the anti-proliferative effect of didymin in both clinically sensitive p53-wild type and refractory p53-mutant neuroblastomas. Didymin did not cause any cytotoxicity in normal HUVEC cells as well as any overt toxicity in mice. Didymin strongly inhibited the proliferation (Ki67) and angiogenesis (CD31) markers as well as N-Myc expression in vivo mice xenograft studies.
Mechanistically, the inhibition of N-Myc by didymin as elaborately confirmed at protein, mRNA and promoter-reporter assays was a hallmark of our studies. Amplification and over-expression of NMyc is associated with both the incidence and refractoriness of neuroblastomas (5, 36). Hence, the ability of didymin to inhibit N-Myc at both transcriptional and translational levels reflects the significance of didymin towards developing novel interventional strategies for neuroblastomas. The non-bitter nature of didymin saves the time and effort spent on the investigations of taste modifying additives for pediatric diets. As didymin is a highly palatable flavonoid, it can be possibly administered in milk or other liquid pediatric diets in the infants and adolescent children who are usually not compatible with derivatized preparations like pills and creams. Thus didymin has both fundamental mechanistic and clinical-translational significance due to its potential to regulate critical nodes of neuroblastoma signaling which reflects its relevance to effectively prevent the incidence and progression of neuroblastomas (Fig 6).
Figure 6. Effect of didymin signaling pathways in neuroblastoma.
The novel flavonoid didymin inhibits N-Myc transcription. Didymin down-regulated PI3K, Akt, vimentin and up regulated RKIP. Didymin down regulated cyclin D1, CDK4 and importantly cyclin B1, which is down-stream effector of p53 mediated cell-cycle regulation. Didymin decreased the angiogenic marker CD31, proliferation marker ki67 and N-Myc in vivo as revealed by histopathological examination of resected tumors. Dotted arrows -Normal signal transduction, normal arrows - Up regulation of signaling protein due to didymin, T-shaped lines -Inhibitory effect of didymin.
In summary, the present study provides strong evidence for the “N-Myc inhibitory”, “anti-proliferative” and “anti-angiogenic” properties of didymin with “no overt toxicity to normal cells and tissues” while characterizing potent anti-cancer effects in vitro cell cultures and in vivo mice xenograft models of neuroblastomas. This multi-specific anti-cancer potential of didymin which is of specific relevance to both the pathogenesis and progression of neuroblastomas makes didymin a novel and highly relevant dietary agent for the treatment of neuroblastomas. Our studies also indicate that didymin is absorbed effectively following oral administration. In this context, the further studies focused on detailed absorption, distribution, metabolism and excretion (ADME) kinetics of didymin should help to develop and test appropriate doses and formulations of didymin in milk and pediatric diets for use in children till 5 years, the high risk window for the incidence of neuroblastomas. Collectively, didymin represents a novel and highly promising flavonoid with potential clinical significance to effectively prevent the incidence of neuroblastomas and also as an innovative approach for strategies proposed by new approaches for neuroblastoma therapy (NANT) consortium.
Acknowledgements
We thank Dr. Sumihiro Suzuki, Department of Biostatistics, School of Public Health, UNTHSC, Fort Worth, TX, for his assistance in the statistical analyses of the data.
This work was supported in part by USPHS grant CA 77495, the Cancer Research Foundation of North Texas, and Institute for Cancer Research & the Joe & Jessie Crump Fund for Medical Education.
The abbreviations used are:
- PARP
Poly ADP ribose polymerase
- PI3K
Phosphatidylinositol-3-kinase
- RKIP
Raf kinase inhibitory protein
References
- 1.Smith SJ, Diehl N, Leavitt JA, Mohney BG. Incidence of pediatric Horner syndrome and the risk of Neuroblastoma: a population-based study. Arch Ophthalmol. 2010;128:324–9. doi: 10.1001/archophthalmol.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob ES, Varghese RG, Toi PC, Bhaskaran R, Rai R. Congenital neuroblastoma with liver metastasis presenting with Hashimoto Pritzker disease. Ind J Pathol Microbiol. 2009;52:374–6. doi: 10.4103/0377-4929.54998. [DOI] [PubMed] [Google Scholar]
- 3.DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–9. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Janardhanan R, Banik NL, Ray SK. N-Myc down regulation induced differentiation, early cell cycle exit, and apoptosis in human malignant neuroblastoma cells having wild type or mutant p53. Biochem Pharmacol. 2009;78:1105–14. doi: 10.1016/j.bcp.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–93. [PubMed] [Google Scholar]
- 7.Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by raf kinase inhibitory protein. Cell Res. 2005;15:19–23. doi: 10.1038/sj.cr.7290258. [DOI] [PubMed] [Google Scholar]
- 8.Fu Z, Smith PC, Zhang L, et al. Effects of Raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–89. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 9.Yeung K, Janosch P, McFerran B, et al. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the Raf kinase inhibitor protein. Mol Cell Biol. 2000;20:3079–85. doi: 10.1128/mcb.20.9.3079-3085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal J, Yadav S, Nagaprashantha L, Vatsyayan R, Singhal SS, Awasthi S. Targeting p53 null neuroblastomas through RLIP76. Cancer Prev Res. 2011;4:879–89. doi: 10.1158/1940-6207.CAPR-11-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal SS, Wickramarachchi D, Yadav S, Singhal J, Leake K, Vatsyayan R, et al. Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol Cancer Ther. 2011;10:16–28. doi: 10.1158/1535-7163.MCT-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung JY, Hsu YL, Ko YC, Tsai YM, Yang CJ, Huang MS, et al. Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer. 2010;68:366–74. doi: 10.1016/j.lungcan.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Marimpietri D, Brignole C, Nico B, Pastorino F, Pezzolo A, Piccardi F, et al. Combined therapeutic effects of vinblastine and rapamycin on human neuroblastoma growth, apoptosis, and angiogenesis. Clin Cancer Res. 2007;13:3977–88. doi: 10.1158/1078-0432.CCR-06-2757. [DOI] [PubMed] [Google Scholar]
- 14.Modak S, Cheung NK. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat Rev. 2010;36:307–17. doi: 10.1016/j.ctrv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Singhal SS, Sehrawat A, Sahu M, Singhal P, Vatsyayan R, Lelsani P, et al. Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int J Cancer. 2010;126:1327–38. doi: 10.1002/ijc.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, Awasthi S. RLIP76: A target for kidney cancer therapy. Cancer Res. 2009;69:4244–51. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug sensitivity and apoptosis by inhibiting Ralbp1. J Biol Chem. 2008;283:19714–29. doi: 10.1074/jbc.M708703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem Pharmacol. 2005;70:481–8. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208–17. doi: 10.1053/j.gastro.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos SD, Verveer PJ, Bastiaens PI. Growth factor-induced MAPK network topology shapes erk response determining PC-12 cell fate. Nat Cell Biol. 2007;9:324–30. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 21.Singhal J, Singhal SS, Yadav S, Suzuki S, Warnke MM, Yacoub A, et al. RLIP76 in defense of radiation poisoning. Int J Radiat Oncol Biol Phys. 2008;72:553–61. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskin DS, Ngo H, Didenko VV. Thimerosal induces DNA breaks, caspase-3 activation, membrane damage, and cell death in cultured human neurons and fibroblasts. Toxicol Sci. 2003;74:361–8. doi: 10.1093/toxsci/kfg126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg Zand RS, Jenkins DJ, Brown TJ, Diamandis EP. Flavonoids can block PSA production by breast and prostate cancer cell lines. Clin Chim Acta. 2002;317:17–26. doi: 10.1016/s0009-8981(01)00698-2. [DOI] [PubMed] [Google Scholar]
- 24.Lillington DM, Goff LK, Kingston JE, Onadim Z, Price E, Domizio P, et al. High level amplification of N-Myc is not associated with adverse histology or outcome in primary retinoblastoma tumors. Br J Cancer. 2002;87:779–82. doi: 10.1038/sj.bjc.6600532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, Otsuki Y. Localization of apoptotic cells in the human epidermis by an in situ DNA nick end-labeling method using confocal reflectant laser microscopy. J Histochem Cytochem. 1998;46:783–6. doi: 10.1177/002215549804600613. [DOI] [PubMed] [Google Scholar]
- 26.Tseng YH, Chang KW, Yang CC, Liu CJ, Kao SY, Liu TY, et al. Association between areca-stimulated vimentin expression and the progression of head and neck cancers. Head Neck. 2011 doi: 10.1002/hed.21726. (In press) [DOI] [PubMed] [Google Scholar]
- 27.McInroy L, Maatta A. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun. 2007;360:109–14. doi: 10.1016/j.bbrc.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Hede SM, Nazarenko I, Nister M, Lindstrom MS. Novel perspectives on p53 function in neural stem cells and brain tumors. J Oncol. 2011 doi: 10.1155/2011/852970. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paolella BR, Havrda MC, Mantani A, Wray CM, Zhang Z, Israel MA. p53 directly represses Id2 to inhibit the proliferation of neural progenitor cells. Stem Cells. 2011 doi: 10.1002/stem.660. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 31.Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15:158–63. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 32.Guo Y, Stacey DW, Hitomi M. Post-transcriptional regulation of cyclin D1 expression during G2 phase. Oncogene. 2002;21:7545–56. doi: 10.1038/sj.onc.1205907. [DOI] [PubMed] [Google Scholar]
- 33.Gabrielli BG, Sarcevic B, Sinnamon J, Walker G, Castellano M, Wang XQ, et al. A cyclin DCdk4 activity required for G2 phase cell cycle progression is inhibited in ultraviolet radiation-induced G2 phase delay. J Biol Chem. 1999;274:13961–9. doi: 10.1074/jbc.274.20.13961. [DOI] [PubMed] [Google Scholar]
- 34.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–20. [PubMed] [Google Scholar]
- 35.Woo KJ, Lee TJ, Bae JH, Jang BC, Song DK, Cho JW, et al. Thimerosal induces apoptosis and G2/M phase arrest in human leukemia cells. Mol Carcinog. 2006;45:657–66. doi: 10.1002/mc.20202. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Zhang X, Jiang Q, Clarke PR, Zhang C. Cyclin B1 is localized to unattached kinetochores and contributes to efficient microtubule attachment and proper chromosome alignment during mitosis. Cell Res. 2008;18:268–80. doi: 10.1038/cr.2008.11. [DOI] [PubMed] [Google Scholar]
- 37.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci U S A. 1999;96:2147–52. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park M, Chae HD, Yun J, Jung M, Kim YS, Kim SH, et al. Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res. 2000;60:542–5. [PubMed] [Google Scholar]
- 39.Kang J, Rychahou PG, Ishola TA, Mourot JM, Evers BM, Chung DH. N-myc is a novel regulator of PI3K-mediated VEGF expression in neuroblastomas. Oncogene. 2008;27:3999–4007. doi: 10.1038/onc.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chesler L, Schlieve C, Goldenberg DD, Kenney A, Kim G, McMillan A, et al. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66:8139–46. doi: 10.1158/0008-5472.CAN-05-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, et al. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem. 2004;279:17515–23. doi: 10.1074/jbc.M313816200. [DOI] [PubMed] [Google Scholar]
- 42.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–7. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 44.Hellmann J, Rommelspacher H, Muhlbauer E, Wernicke C. Raf kinase inhibitor protein enhances neuronal differentiation in human SH-SY5Y cells. Dev Neurosci. 2010;32:33–46. doi: 10.1159/000236595. [DOI] [PubMed] [Google Scholar]
- 45.Dangi-Garimella S, Yun J, Eves EM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]