Abstract
Early recognition of SCID is a pediatric emergency, because a diagnosis before live vaccines or non-irradiated blood products are given and before development of infections permits lifesaving unfractionated HLA-identical or T-cell-depleted haploidentical hematopoietic stem cell transplantation, enzyme replacement therapy or gene therapy. The need for newborn screening for this condition has been recognized for the past 15 years. However, implementation of screening required development of an assay for T cell lymphopenia that could be performed on dried blood spots routinely collected from newborn infants for the past 48 years. This was accomplished 6 years ago and there have already been 7 successful pilot studies. A recommendation to add SCID to the routine newborn screening panel was approved by the Secretary’s AdvisoryCommittee on Heritable Disorders of Newborns and Children in 2010 and was soon after approved by the Secretary of Health and Human Services. It is important for allergists, immunologists and other health care providers to take an active role in promoting newborn screening for SCID and other T lymphocyte abnormalities in their states. Even more important will be their roles in establishing accurate diagnoses for screen positive infants and in ensuring that they are given the best possible treatment.
Keywords: TRECS, Dried bloodspots, SCID, T cell lymphopenia, T cell function studies, Early treatment, Molecular testing
Introduction
Severe combined immunodeficiency (SCID) is a syndrome of diverse genetic cause characterized by profound deficiencies of T and B cell function and, in some types, also of NK cells and function. Mutations in thirteen different genes1-11 have been found to cause this condition, which is uniformly fatal in the first two years of life unless immune reconstitution can be accomplished. Exposure to non-irradiated blood product transfusions, live vaccines12-14 and common infections can be life threatening. Early recognition of SCID should be considered a pediatric emergency,15 because a diagnosis before live vaccines or non-irradiated blood products are given and before the development of infections permits lifesaving unfractionated HLA-identical or T-cell-depleted haploidentical non-ablative hematopoietic stem cell transplantation,16-19 enzyme replacement therapy20 or gene therapy.21-23 However, unlike infants with DiGeorge syndrome, most SCID infants appear physically normal at birth and until they begin to develop infections, then failure to thrive begins. In most cases, there is no family history of SCID. If SCID is not detected until the infant is older, there is a much higher likelihood that death from live vaccines, graft-versus-host disease (GVHD) from non-irradiated blood products or infection will occur before successful definitive therapy can be achieved.16;18;19 In this article the history, challenges, and future direction of newborn screening for SCID are discussed.
Steps Toward Implementation of Newborn Screening for SCID
With advances in technology, screening for many disorders that are best treated before the infant becomes symptomatic will become technologically possible. All of the conditions currently included in newborn screening require treatment before the baby has any symptoms. It is important for physicians to be aware of the steps necessary to achieve screening status for a given condition. In order for a specific disorder to be considered for recommendation by the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) for routine newborn screening, the Committee developed a list of criteria that need to be met: 1) the disorder has to be considered medically serious; 2) there should be prospective pilot data from population-based screening; 3) the spectrum of the disorder should be well described in the medical literature; 4) the screening test characteristics should be reasonable, including having a low rate of false negatives; 5) if the spectrum of the disorder is broad, those most likely to benefit from treatment should be identifiable; and 6) there has be an effective treatment that is given before the infant becomes symptomatic.
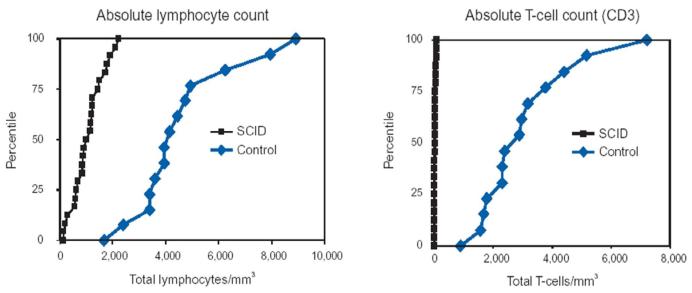
Since SCID is not apparent at birth and early recognition is essential for lifesaving treatment, SCID has been recognized as a candidate for newborn screening for many years.15;24-26 Because most SCID infants lack T cells, the absolute lymphocyte count (ALC) could be used as a screening test for this syndrome. T cells comprise 70 percent of circulating lymphocytes in normal humans throughout life. Therefore, lymphopenia will be present in nearly all infants with SCID from the time of birth as a result of their missing 70 percent of circulating lymphocytes (Figure 1).27 However, many infants do not have blood counts performed. Additionally, many physicians are not aware of the usefulness of the ALC, even though they are taught throughout medical school about the importance of the absolute neutrophil count (ANC). SCID patients referred to our Center often had had numerous prior blood counts clearly showing lymphopenia, but the physicians caring for the infants did not appreciate the significance of this. Knowledge that normal ranges for ALCs are much higher in infancy than in older children is essential to the recognition of this syndrome (Table 1).28;29;29 For example, the lower limit of normal for an ALC at 6 months of age is 3900/mm3 and the mean is 6300/mm3; the mean normal cord blood lymphocyte count is 5500/mm 3 and the lower limit of normal is 2000/mm3.
Figure 1.
Absolute lymphocyte count distributions in 25 SCID and 14 Healthy Newborns at Birth. Modified from Kalman et al.(Reference 25).
Table 1.
| Subset | N | 0-3 mo | 3-6 mo | 6-12mo | 1-2 yr |
|---|---|---|---|---|---|
| WBC | 800 | 10.6 (7.20-18.00) |
9.2 (6.70- 14.00) |
9.1 (6.40-13.00) |
8.80 (6.40- 12.00) |
| Lymphocytes | 800 | 5.40 (3.40-7.60) |
6.30 (3.90-9.00) |
5.90 (3.40-9.00) |
5.50 (3.60- 8.90) |
| CD3 | 699 | 3.68 (2.50-5.50) |
3.93 (2.50-5.60) |
3.93 (1.90-5.90) |
3.55 (2.10- 6.20) |
| CD19 | 699 | 0.73 (0.30-2.00) |
1.55 (0.43-3.00) |
1.52 (0.61-2.60) |
1.31 (0.72- 2.60) |
| CD16/56 | 770 | 0.42 (0.17-1.10) |
0.42 (0.17-0.83) |
0.40 (0.16-0.95) |
0.36 (0.18- 0.92) |
| CD4 | 699 | 2.61 (1.60-4.00) |
2.85 (1.80-4.00) |
2.67 (1.40-4.30) |
2.16 (1.30- 3.40) |
| CD8 | 699 | 0.98 (0.56-1.70) |
1.05 (0.59-1.60) |
1.04 (0.50-1.70) |
1.04 (0.62- 2.00) |
| CD4/CD45RA/CD62L | 694 | 2.25 (1.20-3.60) |
2.23 (1.30-3.60) |
2.10 (1.10-3.60) |
1.64 (0.95- 2.80) |
| CD8/CD45RA/CD62L | 696 | 0.73 (0.38-1.30) |
0.74 (0.45-1.20) |
0.70 (0.33-1.20) |
0.76 (0.40- 1.40) |
| CD4/CD45RA | 694 | 2.27 (1.20-3.70) |
2.32 (1.30-3.70) |
2.21 (1.10-3.70) |
1.65 (1.00- 2.90) |
| CD8/CD45RA | 696 | 0.87 (0.45-1.50) |
0.91 (0.55-1.40) |
0.87 (0.48-1.50) |
0.94 (0.49- 1.70) |
Adapted from Shearer et al: Journal of Allergy and Clinical Immunology 112:973-980, 2003, Table III, page 977.
The Secretary’s Advisory Committee, however, had no interest in the ALC as a likely candidate for a screening test for SCID, because ALCs cannot be obtained from dried bloodspots. Newborn bloodspot screening (NBS) was established by Guthrie in 1963 by the finding of elevated levels of phenylalanine in dried bloodspots collected on filter paper from infants with phenylketonuria.30 Since that time, all newborn screening has been performed on dried bloodspots with the exception of the hearing screen. No laboratory test for detecting SCID on newborn bloodspots was available to identify T-cell lymphopenia in infants with SCID until the current DNA-based technology was developed and validated for population-based screening in 2005.31 This screening test detects SCID through discovery of the absence of T cell receptor recombination excision circles (TRECs).32 TRECS are pieces of DNA cut out during intrathymic T cell receptor gene rearrangement and they can be detected and quantified by real time PCR analysis of DNA isolated from dried blood spots. Since patients with SCID have few or no T cells, the absence of TRECs, like lymphopenia, identifies SCID regardless of the underlying molecular defect.32 In 2005, the TREC test was brought to the attention of SACHDNC, and the Committee followed its development and testing subsequently.
In the Spring of 2007, an informal Working Group for Newborn Screening for SCID was formed and met in San Francisco in May of 2007. This meeting brought together experts in newborn screening, immunology and transplantation as well as federal, state, and nongovernmental agencies to consider obstacles to and implications of developing newborn screening for SCID. A consensus was reached that SCID newborn screening should be pursued with pilot studies, that test methodologies needed to be optimized and that screening programs must be integrated with plans for definitive diagnosis and management.33
In September 2007, SCID was nominated to SACHDNC for addition to the Recommended Uniform Screening Panel (RUSP). The latter panel, consisting of 29 conditions that are primarily metabolic and endocrine diseases plus sickle cell disease, hearing and cystic fibrosis, had been recommended by the American College of Medical Genetics and was adapted by SACHDNC without evidence review. In 2007, for the first time, SACHDNC recommended that a nominated condition (SCID) be investigated by an evidence review panel. This was undertaken over the following year, and the evidence report, which included one year’s experience with the first statewide routine NBS for SCID, was discussed by SACHDNC in February 2009.34 At that time, SACHDNC voted not to add SCID to the RUSP, noting specific gaps in evidence that should be addressed before SCID could be added to the RUSP: (1) prospective identification of at least one confirmed case of SCID through a population-based newborn screening program, (2) demonstrated willingness and capacity of additional states to implement newborn screening for SCID, (3) reproducibility of the screening test and continuance of a false positive rate of less than 0.1 percent, and (4) creation of a laboratory proficiency testing program through the Centers for Disease Control & Prevention’s (CDC) National Quality Assurance Program.
In January 2010, the nomination of SCID to the RUSP was again brought to SACHDNC for reconsideration. At that time, SACHDNC reviewed the activities successfully undertaken to address the evidence gaps and voted unanimously to recommend to Dr. Kathleen Sibelius, the Secretary of the Department of Health and Human Services (HHS), the addition of SCID to the RUSP and that related T cell deficiencies also be added to the list of secondary targets. This recommendation was made with the understanding that 1) the National Institutes of Health (NIH) would fund surveillance activities to determine health outcomes of affected newborns with any T cell deficiency receiving treatment as a result of prospective newborn screening, 2) the Health Resources and Services Administration (HRSA) would fund the development of appropriate education and training materials for families and public health and health care professionals relevant to the screening and treatment of SCID and related T cell deficiencies and 3) the CDC would develop and distribute to performing laboratories suitable dried blood spot specimens for quality control and quality assurance purposes. In May 2010, the Secretary approved the recommendation to add SCID as a core condition to the RUSP and to also add related T cell deficiencies to the list of secondary targets. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reportsrecommendations/reports/sachdnc2011report.pdf. She requested that SACHDNC submit a report in May 2011 on the status of states’ implementation of this recommendation, including surveillance activities conducted through the Newborn Screening Translational Research Network (NBSTRN). This was done and the report indicated that, as of May 2011, six states and one territory were performing newborn screening for SCID using the TREC assay (Table 2) and that they had identified 14 cases of classic SCID as well as 40 cases of T lymphopenia that were not SCID out of a total of 961,925 newborns creened. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. All cases had received appropriate referral to an immunologist and all had received appropriate therapy.
Table 2.
| State | Date Screening Started |
Number of Months Screening |
Annual Births or Number Studied |
Number Screened as of April 30, 2011 |
SCIDa | SCID Variantb |
Non SCIDc |
|---|---|---|---|---|---|---|---|
| WI | 1/1/2008 | 40 | 69,232 | 243,707 | 4 | 0 | 7 |
| MA | 2/1/2009 | 27 | 77,022 | 161,707 | 1 | 0 | 14 |
| Navajo Nation |
2/1/2009 | 27 | 2,000 | 1,297 | 0 | 0 | 0 |
| N | 9/30/2010 | 7 | 236,656 | 136,635 | 4 | 0 | 12 |
| CA | 8/1/2010 | 9 | 510,000 | 358,000 | 5 | 6 | 3 |
| PR | 8/1/2010 | 9 | 45,620 | 29,115 | 0* | 0 | 3 |
| LA | 10/1/2010 | 7 | 65,268 | 31,464 | 0 | 0 | 1 |
| Total | 126 | 1,005,798 | 961,925 | 14 | 6 | 40 |
Outcomes of Pilot Studies to Date
Wisconsin
This state has been screening the longest (44 months) and has reportedly http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. discovered 4 cases of SCID and 7 cases of T lymphopenia that were not SCID out of a total of 243,707 newborns screened. The initial results of screening for 1 year have been published.35 All discovered cases have received either hematopoietic stem cell transplants or enzyme replacement therapy and are surviving.
Massachusetts
This state was the second to initiate a pilot study and has been screening for 31 months, during which time it has reportedly http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. discovered 1 case of SCID and 14 cases of T lymphopenia that were not SCID out of a total of 161,707 newborns screened.36;37 The infant with SCID has been transplanted and is surviving.38
New York
This state has been screening for 11 months and has reportedly http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. discovered 4 cases of SCID and 12 cases of T lymphopenia that were not SCID out of a total of 136,635 newborns screened. All 4 cases of SCID have been treated appropriately and are surviving.
California
This state has been screening for 13 months and has reportedly http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. discovered 5 cases of SCID, 6 cases of variant SCID and 3 cases of T lymphopenia that were not SCID out of a total of 358,000 newborns screened. All 5 of the SCID infants have received appropriate therapy and are surviving.
Puerto Rico, Louisiana and the Navajo Nation
These three pilots have reportedly http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf. not yet discovered any cases of SCID and only 4 cases of T lymphopenia that were not SCID out of an aggregate total of just over 60,000 newborns screened.
Thus far, newborn screening for SCID using the TREC assay appears to be100% sensitive, as no other cases of SCID have been discovered in the states performing the pilot studies during the time period that their screening has been conducted. However, it is apparent that many other conditions with T cell lymphopenia that need medical attention are being detected by this screening method. For the 40 non-SCIDs that were detected in aggregate, approximately 30% had DiGeorge syndrome, 35% had idiopathic T lymphopenia and are being followed closely, 5% had Trisomy 21 and 30% had T lymphopenia associated with other genetic diseases or conditions. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reports/CommitteeSCIDReport.pdf.
Challenges
Technology Development
Until recently, most state newborn screening laboratories were not using DNA-based screening methods and were using tandem mass spectrometry for most of their testing. This changed when cystic fibrosis screening was widely adapted. However, there are still many state laboratories without significant capability to perform DNA-based screening. All but one of the states currently conducting SCID screening pilot studies are using in house modifications of the TREC assay.31;36;37;39 The one exception is California, which is using a TREC assay under development by Perkin-Elmer. Many state laboratories are waiting on a commercially available kit for performing TREC assays before beginning SCID screening. DNA-based screening assays are currently being evaluated to identify infants with other serious diseases, so it is likely that most state laboratories will be performing these types of assays in the future. It is also possible that other screening methods for detecting SCID will be developed, as a tandem mass spectrometry method has recently been developed to detect adenosine-deaminase deficient SCID.40
What happens when there is a positive screen?
The success of newborn screening for SCID or other T-cell deficiencies will be dependent upon the development in each state of a strong program to ensure rapid follow-up of all identified infants and to make certain that the infant is referred to an immunologist in order to ascertain the correct diagnoses in those who are screen positive. The patient should be seen initially by the primary care physician and examined for abnormal physical features that could indicate the infant has DiGeorge syndrome, because not all T lymphopenic patients will have SCID.35;36 Some could have the complete DiGeorge syndrome and would need a thymus transplant.41. However, many more will have partial DiGeorge syndrome and will not need any type of transplant. If there is any suspicion for DiGeorge syndrome, testing for 22q11 should be sent immediately. The primary care physician could also give initial counseling to the parents about avoiding exposures and potential implications for the finding but, most importantly, should refer the infant to a pediatric immunologist urgently as SCID is a pediatric emergency. Performance of a complete immune evaluation is imperative, including tests to quantify naïve (CD45RA+) and memory (CD45RO+) T cells and tests of T cell function, the latter of which is of utmost importance. Patients who have partial DiGeorge syndrome and low T cell numbers will have a significant number of naïve T cells and normal or near-normal T cell function. Not all will be 22q11 positive. On the other hand, patients who have SCID, variant SCID or complete DiGeorge syndrome will have no or almost no T cell function and will likely need a hematopoietic stem cell or thymus transplant or gene therapy. In addition, there are multiple other causes of T cell lymphopenia that have been uncovered in the pilot studies performed to date.35;36 Thus, the screen-positive infant should be referred immediately to a qualified immunologist for a complete immune evaluation. If a defect requiring a transplant is found, the infant should then be referred as quickly as possible to a center that has had considerable experience in transplanting SCID or complete DiGeorge patients.
In addition to close collaborations between public health personnel, pediatricians, immunologists and transplanters, there should also be close collaborations of these individuals with geneticists and genetic counselors. It is not necessary to wait for a molecular diagnosis before a SCID infant is transplanted, but it is important for molecular testing to be performed. Identification of the abnormal gene would allow for family counseling regarding future pregnancies and identification of family members who may carriers of the defect. The identification by newborn screening of other family members who are at risk for having affected children is another important public health benefit that needs to be factored in in considering the cost for implementation of newborn screening.
It should be kept in mind that there is the possibility of false negative results with this assay. Thus far, there has been no evidence that any SCID infants have been missed in the states where pilot studies have been conducted during the time that screening has been conducted. However, in the case of other types of immunodeficiency, that may not be true. For example, in patients with mutations in the gene encoding ZAP70, there are numerous CD45RA positive cells and they do have TRECS (author’s personal observations). It is also possible that other types of immunodeficiency diseases, such as MHC Class II deficiency, may also be missed by TREC screening. Therefore, physicians seeing patients who have had normal TREC screening but who have clinical pictures that lead to suspicion of immunodeficiency will still need to perform thorough immune evaluations in them.
What are the modes of therapy?
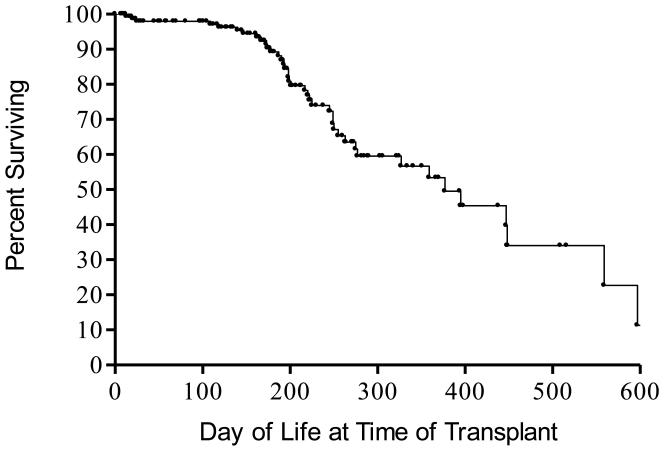
In the 43 years since the first hematopoietic stem cell transplant was given in 1968, the standard treatment for all forms of SCID has been allogeneic hematopoietic stem cell transplantation. Both HLA identical unfractionated and T cell depleted HLA haploidentical hematopoietic stem cell transplants have been very successful in effecting immune reconstitution,19 especially if performed in the first 3.5 months of life and without pre-transplant chemotherapy or post-transplantation immunosuppressive drugs for GVHD prophylaxis (Figure 2).18,42
Figure 2.
Effect of age at time of transplantation on survival in 169 SCIDs transplanted at Duke University Medical Center from 1982-2011. Forty-nine of the infants were less than 3.5 months of age when they were given non-chemoablated transplants at Duke University Medical Center from 1982-2011.Currently all but three of the 49 (94%) are surviving for up to 29.5 years post-transplantation.
There are many sources of hematopoietic stem cells and many different types of transplants that could be performed in newly discovered SCIDs (Table 3). However, many of the transplant types (such as matched unrelated donor transplants [MUD] adult or cord blood transplants) routinely use pre-transplant chemotherapy and post-transplantation immunosuppressive drugs for GVHD prophylaxis. In addition, pre-transplant conditioning is frequently recommended by some transplanters who feel that it is necessary to achieve B cell function. However, a recent review of reports of longterm outcomes of hematopoietic stem cell transplants in Europe and in the United States over the past two decades revealed that, while there were fewer patients requiring IG replacement at centers that routinely used chemotherapy as compared to centers that did not, there were still significant numbers who required IG therapy.43 There are many side effects of chemotherapy and these are even more deleterious for very young infants. For MUD transplants, whether adult or cord blood donors, there is also a certain amount of time required to identify and confirm the suitability of the donor. Sometimes this takes several months and the infant is at risk for infection during that waiting time. There are many advantages of non-ablated, rigorously T-cell-depleted haploidentical parental marrow transplants when compared to transplants of other types, including the facts that 1) a donor is usually always immediately available, 2) one does not have to wait for patient to get over infections or become stable, 3) such transplants can be done in neonates, 4) they can essentially be done as an outpatient procedure if the infant is well and 5) most importantly, they avoid the side effects of chemotherapy and post-transplant immunosuppressive drugs used to prophylax against GVHD in the other types of transplants.
Table 3. Sources of Stem Cells for Transplantation.
In most protocols pre-transplant chemotherapy and post-transplant GVHD immunosuppressive drugs are given.
Requires time for a registry search.
Another type of treatment that could be given is enzyme replacement therapy with polyethylene glycol modified bovine adenosine deaminase (PEGADA) for adenosine deaminase-deficient SCID.20 However, if a transplant is possible, it should be performed before PEGADA therapy is started, because the enzyme replacement therapy will confer upon the patient the ability to reject the transplant. If PEGADA therapy has already been initiated, chemoablation will be needed prior to the transplant and there is an increased risk of toxicity from chemablation in ADA-deficient SCIDs.
The other type of therapy that is possible is gene therapy. This has been successfully accomplished in ADA-deficient SCIDs in Italy by avoiding the use of PEGADA prior to the gene therapy.21 There are currently ongoing gene therapy trials in the United States in ADA-deficient patients. Finally, there have been gene therapy trials in X-linked SCID in Europe that were successful but that were, unfortunately, confounded by the development of leukemia/lymphoma in 25% of the recipients.44;45 Recent long-term follow-up studies of the survivors have shown that they have good T cell function and diversity but that there has been no B cell gene transduction.22;23 Thus, gene therapy does not appear to offer an advantage over non-ablated hematopoietic stem cell transplantation for correcting the B cell defects in X-linked SCID patients post-transplantation. Those original trials were halted but new trials are beginning in X-linked SCID in Europe and in the United States using a modified vector.
What are the barriers to implementation?
The principal barriers to full implementation of all aspects of SCID newborn screening, follow-up and treatment are 1) the costs of establishing the screening test in the state newborn screening laboratory, 2) lack of familiarity of primary care physicians with SCID or other types of T cell deficiency, 3) an inadequate number or lack of immunologists in some states, 4) lack of the ability to transplant a SCID in some states or the presence of only transplant centers that primarily treat cancer patients and are inexperienced in treating SCID, and 5) unwillingness of state Medicaid offices to allow SCID infants eligible for Medicaid to be evaluated or treated out of state.
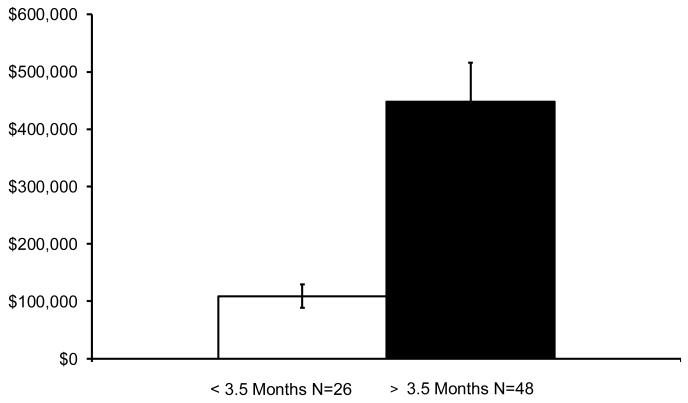
The cost barrier to setting up newborn screening is really not there, since the states that have implemented SCID screening have been able to do this at costs of under 1 million dollars. Once the initial costs for establishment of the procedure have been paid, the annual costs are far less. The Medicaid cost of treating one SCID baby not diagnosed until he or she develops a serious infection can easlly exceed 2 million dollars. A comparison of costs for transplanting SCID infants who were less than 3.5 months of age with the costs for those who were older than 3.5 months at the author’s institution revealed a striking difference (Figure 3). The much lower costs for those transplanted early were due to the facts that 1) they were outpatients for the most part because they were not chemoablated and 2) those treated late were often very sick when they presented, necessitating intensive care unit admissions as well as prolonged hospitalizations. This important cost differential was also noted in the SACHDNC evidence review report34 and in a recent published description of a Markov model to analyze cost-effectiveness of screening for SCID.46
Figure 3.
Cost of treatment for 74 SCID infants transplanted at Duke University Medical Center from 1998-2006, comparing costs for those who were transplanted before 3.5 months of life with the costs for those transplanted after age 3.5 months of life.
The lack of familiarity of primary care physicians with SCID or other types of T cell deficiency can easily be addressed by developing standard literature that spells out the features of these infants and the urgent next steps to take. The lack of immunologists within states can be overcome by the compilation of a list of immunologists in the surrounding region to whom the infant can be referred. The same is true for transplant centers experienced in transplanting SCID infants. In 2009, the National Institute of Allergy and Infectious Diseases funded a nationwide consortium of centers that treat patients with primary immunodeficiency diseases. This is called the Primary Immunodeficiency Treatment Consortium (PIDTC) and includes 14 major transplant centers that have experience in transplanting SCID infants. There are also more than 30 other smaller centers with some experience of this type. A list of these centers can be found online at: http://rarediseasesnetwork.epi.usf.edu/.
There is a great need for working and flexible partnerships with 3rd party payors (including Medicaid) to ensure rapid implementation of appropriate referrals and treatment for newborns identified as screen positive in underserved areas, even if this requires sending the infant out of state. Importantly, to accomplish this, local pediatricians as well as allergists and immunologists should work with public health staff and legislators to insure that this implementation is successful. Failure to do this can result in 1) failure to establish an accurate diagnosis of the infant’s condition, 2) a transplant performed by a hospital that has never transplanted a SCID before or, worse still, 3) a transplant performed in an infant who does not need a transplant or does not need that particular type of transplant.
Summary
Newborn screening for SCID and other T-cell deficiencies is a first for primary immunodeficiency diseases but, with modern molecular technology, screening for many more genetic defects in the immune system will be possible in the future. Even with the TREC assay alone, it is already clear that new causes of T lymphopenia have been and will continue to be identified. It is important for all allergists and immunologists to take an active role in promoting newborn screening for SCID and other T lymphocyte abnormalities in their states if it has not already been implemented. This could be done by 1) working closely with public health officials and newborn screening committees in their states and attending meetings of these groups to develop algorithms for care of screen positive infants and 2) volunteering to be available for consultation with the newborn screening laboratories. Even more important will be their roles in establishing accurate diagnoses for the infants identified as screen positive by the TREC assay and in ensuring that they are given the appropriate and best possible treatment.
Abbreviations
- SCID
Severe combined immunodeficiency
- ALC
Absolute lymphocyte count
- ANC
Absolute neutrophil count
- NBS
Newborn bloodspot screening
- SACHDNC
Secretary’s Advisory Committee for Heritable Disorders in Newborns and Children
- TRECs
T cell receptor recombination excision circles
- RUSP
Recommended Uniform Screening Panel
- NIH
National Institutes of Health
- HRSA
Health Resources and Services Administration
- ADA deficiency
Adenosine deaminase deficiency
- Jak3 deficiency
Janus kinase 3 deficiency
- RAG1, RAG2 deficiencies
Recombinase activating genes 1 and 2 deficiencies
- IL7Rα deficiency
IL-7 receptor alpha chain deficiency
- HST
Hematopoietic stem cell transplantation
- MUDs
Matched unrelated donors
- GVHD
Graft-versus-host disease
- PEGADA
Polyethylene-modified bovine adenosine deaminase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- (2).Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Sci. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- (3).Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- (4).Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Sci. 1996;274:97–9. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]
- (5).Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105(2):177–86. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- (6).Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, Yoo LI, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6(3):343–5. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- (7).Buck D, Moshous D, de Chasseval R, Ma Y, Le Deist F, Cavazzana-Calvo M, et al. Severe combined immunodeficiency and microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur J Immunol. 2006;36(1):224–35. doi: 10.1002/eji.200535401. [DOI] [PubMed] [Google Scholar]
- (8).van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, et al. A DNA-PKcs mutation in a radiosensitive T-B-SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest. 2009;119(1):91–8. doi: 10.1172/JCI37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N Engl J Med. 2003;349(19):1821–8. doi: 10.1056/NEJMoa031178. [DOI] [PubMed] [Google Scholar]
- (10).de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe combined immunodeficiency caused by deficiency in either the delta or the epsilon subunit of CD3. J Clin Invest. 2004;114(10):1512–7. doi: 10.1172/JCI22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Roberts JL, Lauritsen JHP, Cooney M, Parrott RE, Sajaroff EO, Win CM, et al. T-B+NK+ severe combined immunodeficiency caused by complete deficiency of the CD3 zeta subunit of the T cell antigen receptor complex. Blood. 2007;109:3198–206. doi: 10.1182/blood-2006-08-043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Patel NC, Hertel PM, Estes MK, De La MM, Petru AM, Noroski LM, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med. 2010;362(4):314–9. doi: 10.1056/NEJMoa0904485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Werther RL, Crawford NW, Boniface K, Kirkwood CD, Smart JM. Rotavirus vaccine induced diarrhea in a child with severe combined immune deficiency. J Allergy Clin Immunol. 2009;124(3):600. doi: 10.1016/j.jaci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- (14).Uygungil B, Bleesing JJ, Risma KA, McNeal MM, Rothenberg ME. Persistent rotavirus vaccine shedding in a new case of severe combined immunodeficiency: A reason to screen. J Allergy Clin Immunol. 2010;125(1):270–1. doi: 10.1016/j.jaci.2009.10.029. [DOI] [PubMed] [Google Scholar]
- (15).Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–87. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- (16).Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- (17).Kane L, Gennery AR, Crooks BN, Flood TJ, Abinun M, Cant AJ. Neonatal bone marrow transplantation for severe combined immunodeficiency. Arch Dis Child Fetal Neonatal Ed. 2001;85(2):F110–F113. doi: 10.1136/fn.85.2.F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- (19).Buckley RH. Transplantation of Hematopoietic Stem Cells in Human Severe Combined Immunodeficiency: Longterm Outcomes. Immunologic Research. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hershfield MS, Buckley RH, Greenberg ML, Melton AL, Schiff RI, Hatem C, et al. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase (PEG-ADA) N Engl J Med. 1987;316:589–96. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- (21).Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- (22).Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–64. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gaspar HB, Cooray S, Gilmour KC, Parsley KL, Adams S, Howe SJ, et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for x-linked severe combined immunodeficiency. Sci Transl Med. 2011;3(97):97ra79. doi: 10.1126/scitranslmed.3002715. [DOI] [PubMed] [Google Scholar]
- (24).Lindegren ML, Kobrynski L, Rasmussen SA, Moore CA, Grosse SD, Vanderford ML, et al. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Recomm Rep. 2004;53(RR-1):1–29. [PubMed] [Google Scholar]
- (25).McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr. 2005;147(5):603–8. doi: 10.1016/j.jpeds.2005.06.001. [DOI] [PubMed] [Google Scholar]
- (26).McGhee SA, Stiehm ER, Cowan M, Krogstad P, McCabe ER. Two-tiered universal newborn screening strategy for severe combined immunodeficiency. Mol Genet Metab. 2005;86(4):427–30. doi: 10.1016/j.ymgme.2005.09.005. [DOI] [PubMed] [Google Scholar]
- (27).Kalman L, Lindegren ML, Kobrynski L, Vogt R, Hannon H, Howard JT, et al. Mutations in selected genes required for T cell development: IL7R, CD45, IL2R gamma chain, JAK3, RAG1, RAG2, ARTEMIS and ADA and Severe Combined Immunodeficiency. Genetics in Medicine. 2004;6:16–26. doi: 10.1097/01.GIM.0000105752.80592.A3. [DOI] [PubMed] [Google Scholar]
- (28).Altman PL. Blood leukocyte values: man. In: Dittmer DS, editor. Blood and other body fluids. Federation of American Societies for Experimental Biology; Washington,D.C.: 1961. pp. 125–6. [Google Scholar]
- (29).Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- (30).Guthrie R, Susi A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- (31).Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–8. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- (32).Patel DD, Gooding ME, Parrott RE, Curtis KM, Haynes BF, Buckley RH. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342(18):1325–32. doi: 10.1056/NEJM200005043421804. [DOI] [PubMed] [Google Scholar]
- (33).Puck JM. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120(4):760–8. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- (34).Lipstein EA, Vorono S, Browning MF, Green NS, Kemper AR, Knapp AA, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125(5):e1226–e1235. doi: 10.1542/peds.2009-1567. [DOI] [PubMed] [Google Scholar]
- (35).Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302(22):2465–70. doi: 10.1001/jama.2009.1806. [DOI] [PubMed] [Google Scholar]
- (36).Comeau AM, Hale JE, Pai SY, Bonilla FA, Notarangelo LD, Pasternack MS, et al. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9103-9. [DOI] [PubMed] [Google Scholar]
- (37).Gerstel-Thompson JL, Wilkey JF, Baptiste JC, Navas JS, Pai SY, Pass KA, et al. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem. 2010;56(9):1466–74. doi: 10.1373/clinchem.2010.144915. [DOI] [PubMed] [Google Scholar]
- (38).Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, et al. Identification of an infant with severe combined immunodeficiency by newborn screening. J Allergy Clin Immunol. 2010;126(5):1073–4. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- (39).Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, et al. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:522–7. doi: 10.1016/j.jaci.2009.04.007. [DOI] [PubMed] [Google Scholar]
- (40).Azzari C, la MG, Resti M. Neonatal screening for severe combined immunodeficiency caused by an adenosine deaminase defect: a reliable and inexpensive method using tandem mass spectrometry. J Allergy Clin Immunol. 2011;127(6):1394–9. doi: 10.1016/j.jaci.2011.03.040. [DOI] [PubMed] [Google Scholar]
- (41).Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135(2):236–46. doi: 10.1016/j.clim.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117(11):3243–6. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- (43).Buckley RH. B cell function in severe combined immunodeficiency after stem cell or gene therapy: A review. J Allergy Clin Immunol. 2010;125 doi: 10.1016/j.jaci.2010.02.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–50. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) Mol Genet Metab. 2011 doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]