Abstract
Mescaline and phencyclidine (PCP) are potent hallucinogenic agents affecting human and animal behavior. As their psychotropic effects remain poorly understood, further research is necessary to characterize phenotypes they evoke in various animal models. Zebrafish (Danio rerio) are rapidly emerging as a new model organism for neuroscience research. Here, we examine the effects of mescaline (5–20 mg/l) and PCP (0.5–3 mg/l) in several zebrafish paradigms, including the novel tank, open field and shoaling tests. Mescaline and PCP dose-dependently increased top activity in the novel tank test, also reducing immobility and disrupting the patterning of zebrafish swimming, as assessed by ethograms. PCP, but not mescaline, evoked circling behavior in the open field test. At the highest doses tested, mescaline increased, while PCP did not affect, zebrafish shoaling behavior. Finally, 20 mg/l mescaline did not alter, and 3 mg/l PCP elevated, whole-body cortisol levels. Overall, our studies indicate high sensitivity of zebrafish models to hallucinogenic compounds with complex behavioral and physiological effects.
Keywords: mescaline, phencyclidine, zebrafish, anxiety, social behavior
1. Introduction
Mescaline (3,4,5-trimethoxyphenethylamine) is one of the oldest hallucinogens known to man (Kapadia and Fayez, 1970). Produced naturally in the peyote cacti, this phenethylamine agent acts primarily on the serotonergic 5HT1A/2A/B/C receptors (Glennon et al., 1984; Halberstadt et al., 2009; Monte et al., 1997; Palenicek et al., 2008; Shannon et al., 1984), also showing dopaminergic activity (Trulson et al., 1983). The clinical effects of mescaline, similar to the psychedelic action of lysergic acid diethylamide (LSD) and 3,4-methylenedioxymethamphetamine (MDMA), include euphoria, hallucinations, depersonalization and psychoses (Gouzoulis-Mayfrank et al., 1998; Hermle et al., 1992; Schwarz et al., 1956; Wolbach et al., 1962). In rodent models, mescaline modulates locomotion, exploration, cognitive function (Geyer et al., 1979; Koupilova et al., 1999; Palenicek et al., 2008; Sykes, 1986), aggression, startle (Palenicek et al., 2008; Sbordone and Carder, 1974; Sbordone et al., 1979) and motor responses (Canal et al., 2010; Silva and Calil, 1975; Yamamoto et al., 1992).
Phencyclidine (PCP) is another potent hallucinogenic drug, representing a synthetic arylcyclohexylamine originally developed as an anesthetic (Bristow et al., 1993; Dimpfel and Spuler, 1990; Johnson and Jones, 1990). Similar to ketamine, PCP acts as a glutamatergic N-methyl-D-aspartate (NMDA) antagonist (Bristow et al., 1993; Dimpfel and Spuler, 1990; Johnson and Jones, 1990), also showing cholinergic (Haring and Kloog, 1984) and monoaminergic activity (Choi et al., 2009; Nagai et al., 2009; Pozzi et al., 2010; Seeman et al., 2009). In humans, PCP evokes analgesia, ataxia, euphoria, anxiety, hallucinations and psychoses (Gorelick et al., 1986; Gorelick and Wilkins, 1989; Gorelick et al., 1989; Pearlson, 1981). Rodent models demonstrate similar behavioral effects of PCP, including hyperlocomotion, stereotypies (Sturgeon et al., 1982), context-specific alteration of anxiety (Turgeon et al., 2011) and learning/memory deficits (Willetts et al., 1990).
Mounting evidence indicates the growing significance of hallucinogenic drugs in biopsychiatry research (Check, 2004; Friedman, 2006; Halpern, 1996; Sessa, 2008). The resurgence of interest in psychedelic research requires new approaches and novel experimental models to study hallucinogenic drugs (Marona-Lewicka et al., 2011; Vollenweider and Kometer, 2010). Complementing rodent models, aquatic species can be effectively used to study various hallucinogenic drugs (Abramson et al., 1979; Braida et al., 2007; Gettner et al., 1965; Saxena et al., 1962; Webb and Farquharson, 1971).
Zebrafish (Danio rerio) possess a complex behavioral repertoire and fully characterized genome and are rapidly becoming a popular model in biomedical research (He et al., 2006; Hogan et al., 2008). As a vertebrate species, they exhibit substantial physiological and morphological homology to humans, including the expression of all major brain structures, neurotransmitters, receptors, and hormones (Alsop and Vijayan, 2009; Panula et al., 2006). Adult zebrafish have already been established as a model sensitive to various hallucinogens, including both serotonergic (LSD, MDMA (Grossman et al., 2010; Stewart et al., 2011a)) and glutamatergic (ketamine (Riehl et al., 2011a; Zakhary et al., 2011)) agents. Based on the developing utility of zebrafish for neurobehavioral research, and recent advances in video-tracking and neuroendocrine assays (Cachat et al., 2011a; Egan et al., 2009), the present study aimed to characterize the effects of mescaline and PCP on zebrafish behavior and physiology.
2. Methods
2.1. Animals and housing
A total of 267 adult (5–8 month-old) “wild type” short fin zebrafish (~50:50 male:female ratio) were obtained from a commercial distributor (50 Fathoms, Metairie, LA). All fish were given at least 14 days to acclimate to the laboratory environment and housed in groups of 20–30 fish per 40-L tank. Tanks were filled with filtered system water and maintained at 25–27 °C. Illumination (1000–1100 lux) was provided by ceiling-mounted fluorescent lights on a 12-h cycle (on: 6.00 h, off: 18.00 h) according to the standards of zebrafish care (Westerfield, 2007). All fish used in this study were experimentally naïve and fed Tetramin Tropical Flakes (Tetra USA, Blacksburg, VA) twice a day. Following behavioral testing, the animals were euthanized in 500 mg/l Tricaine (Sigma–Aldrich, St. Louis, MO) and dissected on ice for further analysis.
2.1. Behavioral testing
Behavioral testing was performed between 11.00 and 15.00 h using tanks with water adjusted to the holding room temperature. The present study used several different behavioral tests, including the novel tank, open field, and shoaling tests, as described in (Grossman et al., 2010). To avoid the test battery effect, each test was performed on a separate cohort of naïve fish. Prior to testing, fish were pre-exposed in a 1-L plastic beaker for 20 min to either drug-treated or drug-free vehicle solution. During testing, zebrafish behavior was recorded by trained observers, manually scoring different behavioral endpoints (inter-rater reliability >0.85) with subsequent automated analysis of traces by Ethovision XT7 software (Noldus IT, Wageningen Netherlands).
The novel tank test, used to assess zebrafish anxiety and locomotion (Cachat et al., 2010; Levin et al., 2007; Stewart et al., 2011a; Stewart et al., 2011c), was a 1.5-L trapezoidal tank (15 cm height × 28 cm top × 23 cm bottom × 7 cm width; Aquatic Habitats, Apopka, FL) maximally filled with water and divided into two equal virtual horizontal portions, by a line marking the outside walls. In Experiment 1, fish were individually pre-exposed to mescaline (n = 20) or PCP (n = 13) for 20 min (see details further), and tested in the standard 6-min novel tank test. Zebrafish behavior was recorded by trained observers, scoring the latency to reach the top half of the tank (s), time spent in top (s), number of transitions to top, as well as the number and duration (s) of freezing bouts. Freezing was defined as a total absence of movement, except for the gills and eyes, for >2 s. Trials were also recorded to a computer using a USB webcam (2.0-Megapixel, Gigaware, UK) and subsequently analyzed by Ethovision XT7, assessing distance travelled (m), velocity (m/s), high mobility and immobility duration (s) (Grossman et al., 2010). Ethograms of zebrafish behavior in this test were also constructed by manually scoring episodes of bottom swimming, top swimming, bottom freezing and erratic movements, in order to visualize the occurrence of behaviors and the transitions between them. Ethograms were generated for both drug-treated and control fish, with the diameter of each circle reflecting the frequency of the behavioral activity, and the width and direction of each arrow representing the frequency of transitions between behaviors (Grossman et al., 2010).
The open field test (Experiment 2) consisted of a white plastic cylinder (21 cm diameter, 24 cm height) filled with water to a height of 12 cm. Following drug pre-treatment, the animals (n = 12–14 in each group) were individually placed in the center of the tank, and video-recorded from the top for 6 min, using Ethovision XT7 to calculate the distance travelled (m), average velocity (m/s), meandering (°/m), moving duration (s), highly mobile duration (s) and immobile duration (s), as defined in (Cachat et al., 2011a). Since several psychotropic drugs, including NMDA antagonists MK-801 (Ali et al., 1994; Burket et al., 2010; Swain et al., 2004) and ketamine (Becker et al., 2003; Byrd, 1982; Riehl et al., 2011a; Zakhary et al., 2011), induce prominent circling behavior in both rodent and zebrafish open field tests, we have examined the ability of mescaline and PCP to evoke rotations in our study. Video-tracking data generated by Noldus Ethovision XT7 was replayed in slow motion (~0.5 fps) and manually analyzed by two trained observers to assess zebrafish circling behavior. Rotation was defined as a full 360° circle of 5 cm (~2 fish lengths) in diameter, and scored as the number of left, right and total (left + right) rotations (Riehl et al., 2011a). In addition, we quantified left:right rotation ratios and the number (%) of fish demonstrating `high rotation' behavior, defined as 5 or more full rotations per a 6-min trial (Riehl et al., 2011a).
The shoaling test (Experiment 3), assessing the effects of drugs on social/group behavior, was chosen based on modulation of rodent social behavioral by mescaline (Poshivalov, 1980) and PCP (Sams-Dodd, 1997; Savage et al., 2011; Snigdha and Neill, 2008), and the sensitivity of zebrafish shoaling to various psychotropic drugs (Grossman et al., 2010; Riehl et al., 2011a; Saverino and Gerlai, 2008; Speedie and Gerlai, 2008). Three groups of 8 zebrafish were pre-exposed in a 1-L plastic beaker for 20 min to either drug-treated water or drug-free water, and group-tested (8 fish per trial) in the novel tank. Zebrafish shoaling behavior was video-recorded for 6 min, and analyzed using 8 screenshots made every 20 s during the last half of the observation period. Total 24 screenshots (8 per each 8-fish shoal) per drug were used for analyses in this study, similar to (Grossman et al., 2010; Pham et al., 2011). Each screenshot was calibrated to the size of the tank and analyzed by trained observers, measuring the distances (cm) between each fish in the group using ImageTool software (University of Texas Health Sciences Center, San Antonio, TX), averaging this data to obtain an average inter-fish distance per screenshot. The number of fish in top (vs. bottom), the distance to the nearest and to the farthest neighbors was measured for each fish, and averaged over all screenshots per group (final data represented averaged results for 24 screenshots per a 24-fish cohort), similar to (Grossman et al., 2010).
2.3. Video-tracking and track reconstruction
Recorded videos were analyzed with Ethovision XT7 software, as described previously (Cachat et al., 2011a). All arenas were calibrated across the bottom wall of the tanks, and the calibration axes were placed to designate the origin (0,0) at the tank center. Behavioral data were exported to Excel to generate total and per-minute plots for each endpoint. The exported traces were independently rated from 1 to n (based on similarity to each other) by three trained observers (inter-rater reliability >0.85), on a consensus basis. The middle trace was selected as representative for the group, to illustrate the pattern of exploration.
2.4. Pharmacological manipulations
Based on our previous studies with LSD, MDMA (Grossman et al., 2010; Stewart et al., 2011a) and ketamine (Riehl et al., 2011a), similar in action to the serotonergic agonist mescaline and glutamatergic antagonist PCP, respectively, a relatively wide dose ranges (mescaline: 5, 10 and 20 mg/l; PCP: 0.5, 1 and 3 mg/l) were selected for testing in the novel tank test (Experiment 1). This paradigm was chosen for the initial screening for its high construct, face and predictive validity, and as one of the most sensitive and commonly used zebrafish behavioral tests (Cachat et al., 2011b; Egan et al., 2009; Levin et al., 2007). Since the pilot assays have established highest doses as most effective, subsequent behavioral testing were conducted using 20 mg/l mescaline and 3 mg/l PCP (Experiments 2–3). To ensure drug solubility, each agent was dissolved in 0.1% vol/vol dimethyl sulfoxide (DMSO), which does not affect zebrafish swimming alone, based on published literature (Sackerman et al., 2010), and our own systematic observations (accordingly, control zebrafish were also exposed to 0.1% vehicle). The 20 min pre-treatment time was chosen given the time course of mescaline (Sbordone et al., 1979) and PCP (Sams-Dodd, 1995) in rodents, and based on our prior experience screening various psychotropic compounds in zebrafish models (Grossman et al., 2010; Riehl et al., 2011a; Stewart et al., 2011a).
2.5. Whole-body cortisol assay
Whole-body samples were taken from fish used in Experiments 1 and 3. Only 16 fish per group were used from Experiment 3. Individual body samples obtained from experimental and control cohorts were homogenized in 500 μl of ice-cold 1× PBS buffer, as described in (Cachat et al., 2010). The homogenizing rotor blade was washed with an additional 500 μL of PBS and collected in a 2-ml tube containing the homogenate. Samples were transferred to glass extract-O tubes, and cortisol was extracted twice with 5 ml of diethyl ether (Fisher Scientific, Pittsburgh, PA). After ether evaporation, cortisol was reconstituted in 1 ml of 1×PBS. To quantify cortisol concentrations, ELISA was performed using a human salivary cortisol assay kit (Salimetrics LLC, State College, PA) (Cachat et al., 2010). ELISA plates were measured in a VICTOR-WALLAC plate reader using the manufacturer's software package. Whole-body cortisol levels were determined using a 4-parameter sigmoid minus curve fit based on the absorbencies of standardized concentrations, and presented as relative concentrations per gram of body weight for each fish, as described in (Cachat et al., 2010).
2.6. Statistical analysis
The novel tank test data (Experiment 1) was analyzed in SPSS for each drug using one-way ANOVA (factor: dose), followed by a post-hoc Tukey test for significant ANOVA results. The open field and shoaling test data (Experiments 2–3) were analyzed using one-way ANOVA (factor: drug) followed by a post-hoc Tukey test for significant ANOVA results. The Mann-Whitney U-test was used to compare drug-treated groups with their respective controls in the ethograms and cortisol assays. Data were expressed as mean ± SEM. Significance was set at p < 0.05 in all experiments
3. Results
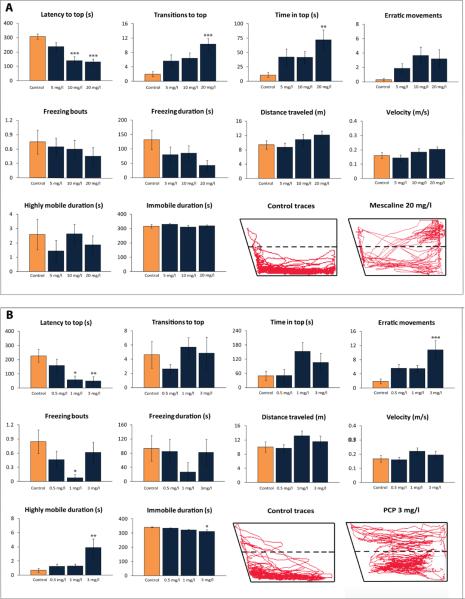
In the novel tank test (Experiment 1), mescaline significantly altered the latency to top (F(3,76) = 11.9, p<0.0005), transitions to top (F(3,76) = 5.8, p<0.001) and time in top (F(3,76) = 3.8, p<0.05). While the latency to top was decreased in fish treated with 10 or 20 mg/l, transitions to top and time in top were significantly elevated by 20 mg/l mescaline, with no effects on freezing activity, distance traveled, velocity and high/low mobility duration (Fig. 1A).
Figure 1. Behavioral effects of 20-min acute exposure to mescaline and phencyclidine (PCP) on zebrafish tested in the novel tank (Experiment 1).
Behavioral endpoints were obtained in the standard 6-min novel tank test for 5–20 mg/l mescaline (A; n = 20 per group) and 0.5–3 mg/l PCP (B; n = 13 per group). Representative 2D traces were generated by Noldus Ethovision XT7 software using the side view video-recording (the traces were examined for each experimental cohort, rated from 1 to n based on similarity, and the middle trace was selected as representative, to illustrate the patterns of zebrafish locomotion). Panel C shows the effects of drugs on the patterning of zebrafish novel tank test behavior, assessed by ethograms generated based on frequencies and transitions between each individual behavioral activity. The diameter of each circle corresponds to the frequency of each individual behavioral activity; the arrow width and direction reflect the frequency of transitions between these behaviors (asterisks next to the circles denote significant differences vs. the respective control fish behaviors; asterisks placed on top of arrows indicate significant differences in the respective transitions, compared to the respective controls). *P<0.05, **P<0.01, ***P<0.001 vs. control; post-hoc Tukey test for significant ANOVA data.
PCP significantly affected the latency to top (F(3,48) = 5.0, p<0.005), erratic movements (F(3,48) = 5.6, p<0.005) and freezing bouts (F(3,48) = 2.8, p<0.05) in the novel tank test. While 1 and 3 mg/l PCP reduced latency to top, 3 mg/l increased erratic movements, and 1 mg/l significantly decreased freezing bouts (Fig. 1B). PCP had no significant effects on transitions to top, time in top, freezing duration, distance travelled and velocity. Ethovision XT7-based analysis also revealed significant effects of PCP on highly mobile duration (F(3,48) = 4.8, p<0.005) and immobile duration (F(3,48) = 2.9, p<0.05), with the 3 mg/l dose reducing immobility duration and increasing highly mobile duration (Fig 1B).
Representative traces generated by Ethovision XT7 software generally support these observations (Fig. 1). Analyzing ethograms of zebrafish novel tank behavior, we also found that both agents altered behavioral patterning of fish swimming. Mescaline (20 mg/l) increased top swimming episodes, transitions from bottom to top swimming, and transitions from top swimming to erratic movements, whereas PCP (3 mg/l) reduced bottom swimming episodes and transitions from bottom freezing to bottom swimming (Fig. 1C).
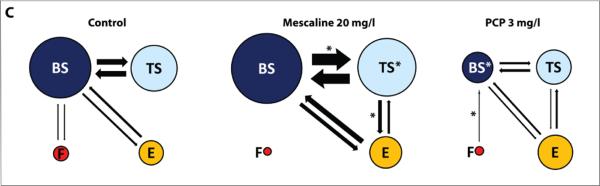
Experiment 2 assessed zebrafish behavior in the open field test, where many behavioral endpoints remained unaltered by 20 mg/l mescaline (Table 1, Fig. 2). PCP (3 mg/l) also had no effect on these endpoints, but evoked prominent circling behavior, with significantly higher left, right and total rotations, as well as more animals with high rotation phenotype in 3 mg/l PCP vs. both the control and mescaline-treated groups (Table 1, Fig. 2). Representative traces, showing robust circling in PCP-treated fish, strongly support these observations (Fig. 2), with no preference of left vs. right rotations (Table 1).
Table 1. Behavioral effects of 20-min acute exposure to mescaline and phencyclidine (PCP) on zebrafish tested in the open field test (Experiment 2).
Behavioral data were generated by Noldus Ethovision XT7 software using the top view video-recording. Rotational data (also see Fig. 2 for details) were obtained during manual observation of the recorded videos, using a slow-mode replay function.
| Endpoints | Control | Mescaline 20 mg/l | PCP 3 mg/l | ANOVA Data |
|---|---|---|---|---|
| Distance traveled (m) | 15±1.3 | 11±1.5 | 12±1.3 | F(2,37) = 2.3, NS |
| Velocity (m/s) | 0.26±0.02 | 0.19±0.03 | 0.25±0.03 | F(2,37) = 2.1, NS |
| Moving duration (s) | 282± 12 | 204±29* | 253±16 | F(2,37) = 3.4, p<0.05 |
| Highly mobile duration (s) | 4.4±0.9 | 3.5±0.7 | 7.2±3.7 | F(2,37) = 0.8, NS |
| Immobile duration (s) | 223±14 | 261±18 | 236±12 | F(2,37) = 1.4, NS |
| Meandering (1000°/m) | 425±153 | 1370±434 | 571±129 | F(2,37)=3.2, NS |
| Left rotations | 2±0.9 | 1.1±0.5 | 5.8±1.5*& | F(2,38) = 5.4, p<0.01 |
| Right rotations | 1.2±0.4 | 3.1±1.2 | 8.9±2.1**& | F(2,38) = 7.8, p<0.001 |
| Total rotations | 2.5±0.8 | 4.2±1.6 | 15±3.4***& | F(2,38) = 8.6, p<0.001 |
P<0.05,
P<0.01,
P<0.001 vs. control, &P<0.05 vs. mescaline-treated group (n = 12–14 per group); post-hoc Tukey test for significant ANOVA data; NS – no significant difference by ANOVA.
Figure 2. Behavioral effects of 20-min acute exposure to mescaline and phencyclidine (PCP) on zebrafish rotational behavior in the open field test (Experiment 2).
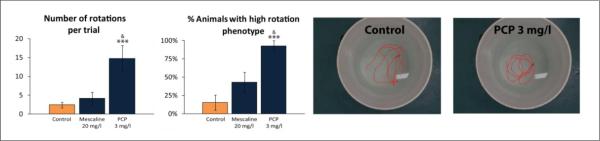
Rotation data represent the number of total (left + right) rotations and the percentage of animals per group displaying `high rotation' behaviors (5 or more `full' rotations) during the 6-min test (n = 13–14 per group). ANOVA results show a significant drug effect on the number of total rotation (F(2,38) = 8.6, p<0.001) and the percentage of animals showing `high rotation' phenotype (F(2,38) = 13.2, p<0.001). Representative traces showing rotation behavior in PCP-treated fish (vs. control) were generated by Noldus Ethovision XT7 software using the top view video-recording (only a 1-min segment of a 6-min test if presented, for clarity). In all experiments, the traces were examined for each experimental cohort, rated from 1 to n (based on similarity to each other), and the middle trace was selected as representative, to illustrate the patterns of zebrafish locomotion (see Table 1 for details of other behaviors observed in the open field test). ***P<0.001 vs. control, &P<0.05 vs. mescaline group; post-hoc Tukey test for significant ANOVA data.
Experiment 3 assessed the effects of mescaline and PCP on zebrafish shoaling behavior. Mescaline significantly affected average inter-fish distance (F(2,61) = 11.1, p<0.0005), distance to nearest neighbor (F(2,61) = 5.2, p<0.01) and distance to farthest neighbor (F(2,61) = 13.1, p<0.0005). As shown in Fig. 3, all three endpoints were decreased by 20 mg/l mescaline. While the control shoals were fairly tight, mescaline caused fish to remain markedly closer together while swimming in a group. In contrast, PCP (3 mg/l) had no effect on any of the endpoints in this test (Fig. 3).
Figure 3. Behavioral effects of 20-min acute exposure to mescaline (A) and phencyclidine (PCP; B) on zebrafish tested in the shoaling tests (Experiment 3).
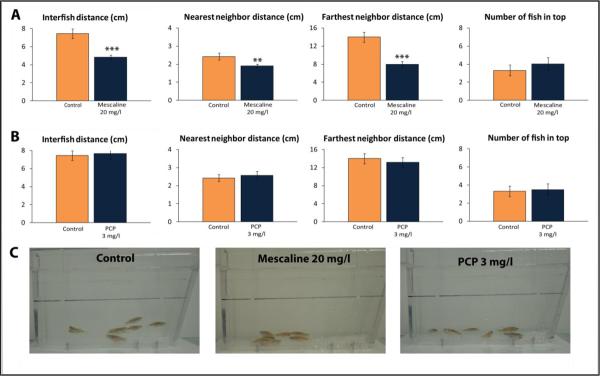
Panel C shows typical patterns of zebrafish shoaling evoked by these drugs (representative photographs for each cohort were rated from 1 to n, based on similarity to each other, and the middle image was selected as representative, to illustrate the patterns of zebrafish shoaling). **P<0.01, ***P<0.001 vs. control (n = 24 per group); post-hoc Tukey test for significant ANOVA data.
In the novel tank test, whole-body cortisol levels did not significantly differ in mescaline-treated fish (4.1±1 pg/g, 20 mg/l) vs. control (4.3±1 pg/g, NS). In the shoaling test, cortisol levels were 4.8±1 and 8.8±2 pg/g, respectively (NS). In contrast, PCP (3 mg/l) significantly elevated cortisol in the novel tank test (8.2±1 vs. 4.3±1 pg/g in controls, p<0.01, U-test), with a similar effect in the shoaling test (24±1 vs. 0.5±1 pg/g, respectively, p<0.0001, U-test).
4. Discussion
As zebrafish are rapidly becoming popular animal models for neuropsychiatric disorders (Airhart et al., 2007; Baraban et al., 2007; Bencan et al., 2009; Chakraborty et al., 2009; Gonzalez-Nunez and Rodriguez, 2009), studies of hallucinogenic drug action may benefit from utilizing novel fish-based paradigms (Grossman et al., 2010; Stewart et al., 2011a). Although the hallucinogenic effects of mescaline and PCP have been studied in several species (Aanonsen and Wilcox, 1987; Audet et al., 2006; Hardman et al., 1973; Jentsch et al., 1998; Palenicek et al., 2008; Savage et al., 2011; Sbordone et al., 1979), little is known about the effects of these drugs in aquatic models. This study is the first report assessing the effects of mescaline and PCP on zebrafish behavior and physiology.
Overall, mescaline dose-dependently increased top activity and reduced immobility in the novel tank test (Fig. 1A), strikingly resembling the effects of LSD (Grossman et al., 2010), MDMA (Stewart et al., 2011a) and ketamine (Riehl et al., 2011a) in zebrafish. While early reports showed hyperactivity in mice treated with mescaline (Shah, 1973; Shah, 1976), mescaline produced both anxiolytic and hyperactivating effects in zebrafish (Fig. 1–2). The anxiolytic-like top swimming profile evoked by mescaline in the novel tank (Fig. 1A) is consistent with its ability to decrease emotionality in mice (Lush, 1975) and increase top swimming in goldfish (Abramson et al., 1979). The drug also affected the patterning of zebrafish swimming (Fig. 1C), in line with its ability to disrupt exploration in rodents (Gorelick and Bridger, 1975), which may be part of its hallucinogenic effects. PCP also evoked top dwelling, decreased latency to top and increased erratic movements in the novel tank test (Fig. 1B). Taken together, this suggests that PCP evokes hyperactivity in zebrafish, paralleling recent rodent data (McLean et al., 2010; Turgeon et al., 2011). Although shorter latency to top is generally considered anxiolytic, the parallel elevation in erratic movements suggests increased mobility.
While mescaline increases behavioral activity in rodents (Lush, 1975; Nakama et al., 1972; Shah, 1973; Shah, 1976), we found no difference in distance traveled or velocity between control and mescaline-treated fish in the open field test. PCP also did not affect open field behavior, in agreement with past rodent studies at similar doses (Lee et al., 2005; McLean et al., 2010). However, the 3 mg/l PCP group displayed longer `highly mobile' duration and frequency, paralleling manual data showing increased erratic movements and decreased immobility duration. This is in line with the complex effects of PCP on activity in various animal (Boren and Consroe, 1981; Byrd, 1982; Castellani and Adams, 1981; Sturgeon et al., 1979) and clinical (Domino et al., 1982; Pradhan, 1984) studies.
Prominent circling behavior induced by PCP (Fig 2) is consistent with rotations reported in zebrafish exposed to ketamine (Riehl et al., 2011a; Zakhary et al., 2011) and MK-801 (Chen et al., 2010; Seibt et al., 2010; Swain et al., 2004), and in rodents injected with PCP and other NMDA antagonists (Ali et al., 1994; Ali et al., 1995; Burket et al., 2010; Eshel and Korczyn, 1985; Haggerty et al., 1984; Van Ree and Leys, 1985). No left or right preference was found in the present study (Table 1), in agreement with our earlier zebrafish data on ketamine (Riehl et al., 2011a). Interestingly, NMDA antagonists ketamine (Becker et al., 2003) and PCP (Neill et al., 2010b) both trigger clinical and experimental psychoses, raising the possibility that zebrafish circling mimics psychosis-like states, or is due to the known hallucinogenic profile of PCP. However, since other hallucinogenic drugs (e.g., LSD (Grossman et al., 2010), MDMA (Stewart et al., 2011a), salvinorin A (Braida et al., 2007) and mescaline; Fig. 2) did not evoke circling in zebrafish, PCP-induced rotations observed here are likely to be associated with NMDA antagonism. The reversal of glutamatergically-induced circling by antipsychotic drugs (Seibt et al., 2010) suggests a potential pro-psychotic nature of this behavior in zebrafish, which may also contribute to effects of PCP observed here (Fig. 2)
As shown in Fig. 3, mescaline strongly affects zebrafish shoaling behavior, representing one of the most robust effects observed in this lab after screening 30 psychotropic compounds over the last several years. While increased shoaling may be due to fear-like shoal cohesion or increased sociability, the lack of anxiogenic effects of mescaline in other tests (discussed above) implicates higher sociability, rather than anxiety-like states, in this response. Our results on increased aggregation of zebrafish support the notion that social behaviors are modulated by mescaline, exerting robust but poorly understood effects (e.g., increasing (Sbordone and Carder, 1974; Sbordone et al., 1978; Sbordone et al., 1979) or reducing aggression in animals (Poshivalov, 1980; Rewerski et al., 1971)). However, all these studies agree that mescaline is a potent modulator of social behavior, and our results support this conclusion. It is also likely that mescaline distorts normal perception of other animals, thereby leading to various forms of aberrant social behaviors (Elliott and Sbordone, 1982; Poshivalov, 1980) – a factor that may have contributed to increased zebrafish shoaling (Fig. 3). Earlier reports on reduced aggression in Siamese fighting fish (Saxena et al., 1962) further support the possibility of altered social behavior by mescaline in fish (Fig. 3C). In contrast, while PCP can disrupt social behavior in several species (Brigman et al., 2009; Cleary et al., 1981; Newman et al., 2007; Russell et al., 1984; Sams-Dodd, 1996; Wilmot et al., 1987), its behaviorally active dose (3 mg/l) did not affect zebrafish shoaling (Fig. 3), in line with some rodent studies showing no PCP effects on social/aggressive behavior (Miczek and Haney, 1994; Tyler and Miczek, 1982). Since we only explored the effects of acute drug exposure, further studies using longer-term and/or repeated treatment, as well as applying various agonists and antagonists, may provide further insights into the social effects of mescaline and PCP in zebrafish.
The search for novel biomarkers is becoming an important direction in drug abuse research. Endocrine phenotypes, such as cortisol released by the hypothalamic-pituitary-interrenal (HPI) system may represent an interesting aspect to assess. Analyzing whole-body cortisol data, we noted an increase produced by PCP (3 mg/l) in both the novel tank and shoaling tests. This profile is consistent with the possibility of hallucinogenic/psychotogenic action (Gorelick and Balster, 1995), since such states are commonly associated with elevated glucocorticoids in non-human primates (Elvidge et al., 1976; Setchell et al., 1975) and rodents (Deutsch et al., 1983; Pechnick et al., 1986; Pechnick et al., 1989) following PCP administration. Interestingly, a related compound, ketamine, lowered cortisol levels in zebrafish (Riehl et al., 2011b), which may be due to varying psychopharmacological profiles, with ketamine exerting anxiolytic-like clinical and preclinical effects (Annetta et al., 2005; Engin et al., 2009), and PCP showing much less robust responses (Gonzalez-Maeso and Sealfon, 2009; Gorelick and Balster, 1995). In contrast, the behaviorally active dose of mescaline (20 mg/l) did not significantly alter cortisol in the novel tank and shoaling tests. Although relatively little is known about the effect of mescaline on glucocorticoids (Check, 2004; Sackler et al., 1971), the lack of cortisol elevation observed in this study may be due to a combination of the anxiolytic and psychotomimetic activities of mescaline. While LSD increased cortisol levels in zebrafish (Grossman et al., 2010), another serotonergic hallucinogen, MDMA, did not produce consistent effects on cortisol (own unpublished observations), indirectly supporting this notion. Clearly, further studies are needed to assess endocrine correlates of various hallucinogenic drugs in zebrafish. There were several other limitations of this study. First, it did not include repeated or chronic treatment, which may elucidate further some of the complex neural effects of mescaline and PCP (Neill et al., 2010a; Poshivalov, 1980). Also, drug administration via other routes (vs. immersion), and measuring the drug concentrations in the brain, may be necessary.
Finally, comparison of mescaline and PCP with other hallucinogenic drugs reveals interesting parallels between their relative efficacies. Since psychedelic doses in humans are approximately 200 mg for mescaline and MDMA, >10 mg for PCP, 125 mg for ketamine and <1 mg for LSD, mescaline is equally potent as MDMA, >200-fold less potent than LSD, 10–20 fold less potent than PCP, and twice less potent than ketamine. In zebrafish, the effective dose of mescaline (20 mg/l) was 15 times less potent than PCP (3 mg/l; Fig. 1), 200-fold less potent than LSD (0.1 mg/l (Stewart et al., 2011b)), 4 times less potent than MDMA (80 mg/l (Stewart et al., 2011a)), and twice less potent than ketamine (40 mg/l (Riehl et al., 2011a)). Human `psychedelic' doses of PCP (>10 mg) are ~20 times stronger than mescaline and MDMA, 10–30 times less potent than LSD, and ~10 times more potent than ketamine. In the present study, the dose of PCP (3 mg/l) active in zebrafish was ~30-fold weaker than LSD (0.1 mg/l (Stewart et al., 2011b)), ~30-fold stronger than MDMA (80 mg/l (Stewart et al., 2011a)), and ~10-fold stronger than ketamine (40 mg/l (Riehl et al., 2011a)). Collectively, this shows that relative efficacy of mescaline, PCP and other common hallucinogens in fish strikingly resemble that in humans, further supporting the translational value of zebrafish models for psychedelic drug research.
5. Conclusion
Overall, zebrafish paradigms are highly sensitive to various drugs of abuse (Bencan et al., 2009; Fernandes and Gerlai, 2009; Gerlai et al., 2006; Gerlai et al., 2008; Levin et al., 2007), providing a useful animal model to study hallucinogenic drugs, which will likely increase our understanding of the neurobiology of drug-induced neurobehavioral disorders. In line with this, the behavioral and physiological changes elicited in adult zebrafish by mescaline and PCP (Fig. 1–3) parallel drug-evoked responses observed in clinical patients and other animal models.
The similarity of effects evoked in zebrafish by two psychedelic drugs (Fig. 1) sharing some clinical and pre-clinical hallucinogenic effects, but acting via different pharmacological mechanisms, raises another important question: Can aquatic zebrafish-based tests serve as a useful specific screen for testing various hallucinogenic drugs? Recent zebrafish data on LSD, MDMA, ketamine (Grossman et al., 2010; Riehl et al., 2011a; Stewart et al., 2011a) and our present findings with mescaline and PCP (Figs. 1–3) seem to support this notion. The possibility of developing such high-throughput zebrafish-based screens not only offer an evolutionary perspective on drug-induced states, but also foster further searches for new compounds with potential pro- and anti-hallucinogenic properties.
Research Highlights
Mescaline and phencyclidine are potent hallucinogenic drugs.
Zebrafish is becoming a promising model for investigating hallucinogenic compounds.
Mescaline (20 mg/l) increases top swimming and shoaling behavior in zebrafish.
Phencyclidine (3 mg/l) induces circling and elevated cortisol levels in zebrafish.
Acknowledgements
This study is supported by Tulane University, Tulane Medical School Intramural and Pilot funds, LA Board of Regents P-Fund, Zebrafish Neurophenome Project and NIH/NIDA DA030900-02 grant to AVK.
List of Abbreviations
- ANOVA
analysis of variance
- DMSO
dimethyl sulfoxide
- ELISA
Enzyme-linked immonosorbent assay
- Fps
frames per second
- HPI
Hypothalamic-pituitary-interrenal
- LSD
lysergic acid diethylamide
- MDMA
3,4-methylenedioxymethamphetamine
- NMDA
N-methyl-D-aspartate
- PBS
Phosphate buffered saline
- PCP
Phencyclidine
- SPSS
Statistical Package for the Social Sciences
- 5HT
Serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanonsen LM, Wilcox GL. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharmacol Exp Ther. 1987;243:9–19. [PubMed] [Google Scholar]
- Abramson HA, Gettner HH, Carone PA, Rolo A, Krinsky L. The intracranial injection of drug in goldfish. I: Hallucinogens and their antagonism to smooth muscle activity. J Asthma Res. 1979;16:55–61. doi: 10.3109/02770907909106614. [DOI] [PubMed] [Google Scholar]
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ali SF, Newport GD, Bracha HS. Phencyclidine and (+)-MK-801-induced circling preference: correlation with monoamine levels in striatum of the rat brain. Neurotoxicol Teratol. 1994;16:335–42. doi: 10.1016/0892-0362(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Ali SF, Kordsmeier KJ, Gough B. Drug-induced circling preference in rats. Correlation with monoamine levels. Mol Neurobiol. 1995;11:145–54. doi: 10.1007/BF02740691. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan M. The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen Comp Endocrinol. 2009;161:62–6. doi: 10.1016/j.ygcen.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr Drug Targets. 2005;6:789–94. doi: 10.2174/138945005774574533. [DOI] [PubMed] [Google Scholar]
- Audet MC, Goulet S, Dore FY. Repeated subchronic exposure to phencyclidine elicits excessive atypical grooming in rats. Behav Brain Res. 2006;167:103–10. doi: 10.1016/j.bbr.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Castro PA, Chege S, Guyenet S, Taylor MR. A large-scale mutagenesis screen to identify seizure-resistant zebrafish. Epilepsia. 2007;48:1151–7. doi: 10.1111/j.1528-1167.2007.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren JL, Consroe PF. Behavioral effects of phencyclidine (PCP) in the dog: a possible animal model of PCP toxicity in humans. Life Sci. 1981;28:1245–51. doi: 10.1016/0024-3205(81)90450-1. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 2007;190:441–8. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A. Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LJ, Hutson PH, Thorn L, Tricklebank MD. The glycine/NMDA receptor antagonist, R-(+)-HA-966, blocks activation of the mesolimbic dopaminergic system induced by phencyclidine and dizocilpine (MK-801) in rodents. Br J Pharmacol. 1993;108:1156–63. doi: 10.1111/j.1476-5381.1993.tb13520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Cannon WR, Jacome LF, Deutsch SI. MK-801, a noncompetitive NMDA receptor antagonist, elicits circling behavior in the genetically inbred Balb/c mouse strain. Brain Res Bull. 2010;83:337–9. doi: 10.1016/j.brainresbull.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Byrd LD. Comparison of the behavioral effects of phencyclidine, ketamine, d-amphetamine and morphine in the squirrel monkey. J Pharmacol Exp Ther. 1982;220:139–44. [PubMed] [Google Scholar]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Min Chung K, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, DiLeo J, Frank K, Chang K, Utterback E, Hart P, Kalueff A. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protocols. 2010;5:1786–99. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Utterback E, Hart P, Gaikwad S, Wong K, Kyzar E, Wu N, Kalueff A. Three-dimension neurophenotyping of adult zebrafish behavior. PLoS One. 2011a;6:e17597. doi: 10.1371/journal.pone.0017597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Utterback E, Hart P, Gaikwad S, Wong K, Kyzar E, Wu N, Kalueff AV. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One. 2011b;6:e17597. doi: 10.1371/journal.pone.0017597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–74. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani S, Adams PM. Acute and chronic phencyclidine effects on locomotor activity, stereotypy and ataxia in rats. Eur J Pharmacol. 1981;73:143–54. doi: 10.1016/0014-2999(81)90086-8. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10:116–24. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- Check E. Psychedelic drugs: the ups and downs of ecstasy. Nature. 2004;429:126–8. doi: 10.1038/429126a. [DOI] [PubMed] [Google Scholar]
- Chen J, Patel R, Friedman TC, Jones KS. The Behavioral and Pharmacological Actions of NMDA Receptor Antagonism are Conserved in Zebrafish Larvae. Int J Comp Psychol. 2010;23:82–90. [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI. Subchronic effects of phencyclidine on dopamine and serotonin receptors: implications for schizophrenia. J Mol Neurosci. 2009;38:227–35. doi: 10.1007/s12031-009-9204-9. [DOI] [PubMed] [Google Scholar]
- Cleary J, Herakovic J, Poling A. Effects of phencyclidine on shock-induced aggression in rats. Pharmacol Biochem Behav. 1981;15:813–8. doi: 10.1016/0091-3057(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Kineiko R, Garcia-Soto M. Effect of chronic phencyclidine administration upon blood biochemical profiles in rats. Subst Alcohol Actions Misuse. 1983;4:401–3. [PubMed] [Google Scholar]
- Dimpfel W, Spuler M. Dizocilpine (MK-801), ketamine and phencyclidine: low doses affect brain field potentials in the freely moving rat in the same way as activation of dopaminergic transmission. Psychopharmacology (Berl) 1990;101:317–23. doi: 10.1007/BF02244048. [DOI] [PubMed] [Google Scholar]
- Domino SE, Domino LE, Domino EF. Comparison of two and three compartment models of phencyclidine in man. Subst Alcohol Actions Misuse. 1982;3:205–11. [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Sbordone RJ. Drug-induced ataxia in opponents elicits “pathological” fighting in undrugged rats exposed to footshock. Pharmacol Biochem Behav. 1982;16:63–6. doi: 10.1016/0091-3057(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Elvidge H, Challis JR, Robinson JS, Roper C, Thorburn GD. Influence of handling and sedation on plasma cortisol in rhesus monkeys (Macaca mulatta) J Endocrinol. 1976;70:325–6. doi: 10.1677/joe.0.0700325. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–69. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Eshel Y, Korczyn AM. Circling behavior induced by phencyclidine in mice and its inhibition by naloxone. Experientia. 1985;41:73–4. doi: 10.1007/BF02005882. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–9. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. The Renewal of Psychedelic Research: Implications for Humanistic and Transpersonal Psychology. The Humanistic Psychologist. 2006;34:39–58. [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–61. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Ahmad F, Prajapati S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–73. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettner HH, Rolo A, Abramson HA. Lysergic acid diethylamide (LSD 25). 36. Comparison of effect of methysergide (UML 491) on goldfish and Siamese fighting fish. J Psychol. 1965;61:87–92. doi: 10.1080/00223980.1965.10544800. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Light RK, Rose GJ, Petersen LR, Horwitt DD, Adams LM, Hawkins RL. A characteristic effect of hallucinogens on investigatory responding in rats. Psychopharmacology (Berl) 1979;65:35–40. doi: 10.1007/BF00491975. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–11. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–32. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nunez V, Rodriguez RE. The zebrafish: a model to study the endogenous mechanisms of pain. ILAR J. 2009;50:373–86. doi: 10.1093/ilar.50.4.373. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Bridger WH. Does increasing stress change the behavioral action of mescaline from disruption to facilitation? Psychopharmacology. 1975;44:307–309. doi: 10.1007/BF00428913. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Wilkins JN, Wong C. Diagnosis and treatment of chronic phencyclidine (PCP) abuse. NIDA Res Monogr. 1986;64:218–28. [PubMed] [Google Scholar]
- Gorelick DA, Wilkins JN. Inpatient treatment of PCP abusers and users. Am J Drug Alcohol Abuse. 1989;15:1–12. doi: 10.3109/00952998908993395. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Wilkins JN, Wong C. Outpatient treatment of PCP abusers. Am J Drug Alcohol Abuse. 1989;15:367–74. doi: 10.3109/00952998908992797. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Balster RL. Phencyclidine (PCP) In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1767–1776. [Google Scholar]
- Gouzoulis-Mayfrank E, Hermle L, Thelen B, Sass H. History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry. 1998;31(Suppl 2):63–8. doi: 10.1055/s-2007-979348. [DOI] [PubMed] [Google Scholar]
- Grossman L, Utterback U, Stewart A, Gaikwad S, Wong K, Elegante M, Tan J, Gilder T, Wu N, DiLeo J, Cachat J, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214:277–84. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Haggerty GC, Forney RB, Johnson JM. The effect of a single administration of phencyclidine on behavior in the rat over a 21-day period. Toxicol Appl Pharmacol. 1984;75:444–53. doi: 10.1016/0041-008x(84)90181-9. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern JH. The Use of Hallucinogens in the Treatment of Addiction. Addiction Research & Theory. 1996;4:177–189. [Google Scholar]
- Hardman HF, Haavik CO, Seevers MH. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicol Appl Pharmacol. 1973;25:299–309. doi: 10.1016/s0041-008x(73)80016-x. [DOI] [PubMed] [Google Scholar]
- Haring R, Kloog Y. Multiple binding sites for phencyclidine on the nicotinic acetylcholine receptor from Torpedo ocellata electric organ. Life Sci. 1984;34:1047–55. doi: 10.1016/0024-3205(84)90018-3. [DOI] [PubMed] [Google Scholar]
- He S, Salas-Vidal E, Rueb S, Krens SF, Meijer AH, Snaar-Jagalska BE, Spaink HP. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish. 2006;3:441–53. doi: 10.1089/zeb.2006.3.441. [DOI] [PubMed] [Google Scholar]
- Hermle L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–91. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Verkade H, Lieschke GJ, Heath JK. Manipulation of gene expression during zebrafish embryonic development using transient approaches. Methods Mol Biol. 2008;469:273–300. doi: 10.1007/978-1-60327-469-2_19. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Elsworth JD, Taylor JR, Redmond DE, Jr., Roth RH. Dysregulation of mesoprefrontal dopamine neurons induced by acute and repeated phencyclidine administration in the nonhuman primate: implications for schizophrenia. Adv Pharmacol. 1998;42:810–4. doi: 10.1016/s1054-3589(08)60870-4. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Jones SM. Neuropharmacology of phencyclidine: basic mechanisms and therapeutic potential. Annu Rev Pharmacol Toxicol. 1990;30:707–50. doi: 10.1146/annurev.pa.30.040190.003423. [DOI] [PubMed] [Google Scholar]
- Kapadia GJ, Fayez MB. Peyote constituents: chemistry, biogenesis, and biological effects. J Pharm Sci. 1970;59:1699–727. doi: 10.1002/jps.2600591202. [DOI] [PubMed] [Google Scholar]
- Koupilova M, Herink J, Krs O. Influencing of spatial memory in rats by DSP-4 and mescaline. Acta Medica (Hradec Kralove) 1999;42:69–72. [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social Interaction Deficits Caused by Chronic Phencyclidine Administration are Reversed by Oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–8. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Lush IE. A comparison of the effect of mescaline on activity and emotional defaecation in seven strains of mice. Br J Pharmacol. 1975;55:133–9. doi: 10.1111/j.1476-5381.1975.tb07621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols CD, Nichols DE. An animal model of schizophrenia based on chronic LSD administration: Old idea, new results. Neuropharmacology. 2011;61:503–512. doi: 10.1016/j.neuropharm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Woolley ML, Neill JC. Effects of subchronic phencyclidine on behaviour of female rats on the elevated plus maze and open field. J Psychopharmacol. 2010;24:787–90. doi: 10.1177/0269881109103112. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Haney M. Psychomotor stimulant effects of d-amphetamine, MDMA and PCP: aggressive and schedule-controlled behavior in mice. Psychopharmacology (Berl) 1994;115:358–65. doi: 10.1007/BF02245077. [DOI] [PubMed] [Google Scholar]
- Monte AP, Waldman SR, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, Nichols DE. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J Med Chem. 1997;40:2997–3008. doi: 10.1021/jm970219x. [DOI] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology (Berl) 2009;202:315–28. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Nakama M, Ochiai T, Kowa Y. Effects of psychotropic drugs on emotional behavior: exploratory behavior of naive rats in holed open field. Jpn J Pharmacol. 1972;22:767–75. doi: 10.1254/jjp.22.767. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther. 2010a doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010b;128:419–32. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87:280–8. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenicek T, Balikova M, Bubenikova-Valesova V, Horacek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology (Berl) 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, Moshnyakov M, Podlasz P. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–47. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- Pearlson GD. Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J. 1981;148:25–33. [PubMed] [Google Scholar]
- Pechnick RN, George R, Lee RJ, Poland RE. The effects of the acute administration of phencyclidine hydrochloride (PCP) on the release of corticosterone, growth hormone and prolactin in the rat. Life Sci. 1986;38:291–6. doi: 10.1016/0024-3205(86)90315-2. [DOI] [PubMed] [Google Scholar]
- Pechnick RN, George R, Poland RE, Hiramatsu M, Cho AK. Characterization of the effects of the acute and chronic administration of phencyclidine on the release of adrenocorticotropin, corticosterone and prolactin in the rat: evidence for the differential development of tolerance. J Pharmacol Exp Ther. 1989;250:534–40. [PubMed] [Google Scholar]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, Fryar C, Chanin S, Enriquez J, Bagawandoss S, Zapolsky I, Green J, Stewart A, Robison B, Kalueff A. Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference and mirror biting tests. In: Kalueff A, Stewart A, editors. Zebrafish Protocols for Neurobehavioral Research. Humana Press; New York: 2011. [Google Scholar]
- Poshivalov VP. The integrity of the social hierarchy in mice following administration of psychotropic drugs. Br J Pharmacol. 1980;70:367–73. doi: 10.1111/j.1476-5381.1980.tb08712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Greco B, Sacchetti G, Leoni G, Invernizzi RW, Carli M. Blockade of serotonin 2A receptors prevents PCP-induced attentional performance deficit and CREB phosphorylation in the dorsal striatum of DBA/2 mice. Psychopharmacology (Berl) 2010;208:387–99. doi: 10.1007/s00213-009-1738-6. [DOI] [PubMed] [Google Scholar]
- Pradhan SN. Phencyclidine (PCP): some human studies. Neurosci Biobehav Rev. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Rewerski W, Kostowski W, Piechocki T, Rylski M. The effects of some hallucinogens on aggressiveness of mice and rats. I. Pharmacology. 1971;5:314–20. doi: 10.1159/000136205. [DOI] [PubMed] [Google Scholar]
- Riehl R, Kyzar E, Allain A, Green J, Hook M, Monnig L, Rhymes K, Roth A, Pham M, Razavi R, DiLeo J, Gaikwad S, Hart P, Kalueff AV. Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol Teratol. 2011a;33:658–667. doi: 10.1016/j.ntt.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Riehl R, Kyzar E, Allain A, Green J, Hook M, Monnig L, Rhymes K, Roth A, Pham M, Razavi R, Dileo J, Gaikwad S, Hart P, Kalueff AV. Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol Teratol. 2011b doi: 10.1016/j.ntt.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Russell JW, Greenberg BD, Segal DS. The effects of phencyclidine on spontaneous aggressive behavior in the rat. Biol Psychiatry. 1984;19:195–202. doi: 10.1016/0304-3959(84)90863-7. [DOI] [PubMed] [Google Scholar]
- Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- Sackler AM, Weltman AS, Johnson L. Acute effects of mescaline HCl on behavior, resistance, and endocrine function of male mice. Exp Med Surg. 1971;29:118–27. [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol. 1997;8:196–215. [PubMed] [Google Scholar]
- Savage S, Kehr J, Olson L, Mattsson A. Impaired social interaction and enhanced sensitivity to phencyclidine-induced deficits in novel object recognition in rats with cortical cholinergic denervation. Neuroscience. 2011;195:60–9. doi: 10.1016/j.neuroscience.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Bhattacharya BK, Mukerji B. Behavioural studies in fish with mescaline, LSD, and thiopropasate and their interactions with serotonin and dopa. Arch. int. Pharmacodyn. 1962;140:327–335. [PubMed] [Google Scholar]
- Sbordone RJ, Carder B. Mescaline and shock induced aggression in rats. Pharmacol Biochem Behav. 1974;2:777–82. doi: 10.1016/0091-3057(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Sbordone RJ, Wingard JA, Elliott MK, Jervey J. Mescaline produces pathological aggression in rats regardless of age or strain. Pharmacol Biochem Behav. 1978;8:543–6. doi: 10.1016/0091-3057(78)90385-4. [DOI] [PubMed] [Google Scholar]
- Sbordone RJ, Wingard JA, Gorelick DA, Elliott ML. Severe aggression in rats induced by mescaline but not other hallucinogens. Psychopharmacology (Berl) 1979;66:275–80. doi: 10.1007/BF00428319. [DOI] [PubMed] [Google Scholar]
- Schwarz BE, Bickford RG, Mulder DW, Rome HP. Mescaline and LSD-25 in activation of temporal lobe epilepsy. Neurology. 1956;6:275–80. doi: 10.1212/wnl.6.4.275. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Hirbec H. Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil. Synapse. 2009;63:698–704. doi: 10.1002/syn.20647. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, Oliveira Rda L, Zimmermann FF, Capiotti KM, Bogo MR, Ghisleni G, Bonan CD. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2010;214:417–22. doi: 10.1016/j.bbr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Sessa B. Is it time to revisit the role of psychedelic drugs in enhancing human creativity? J Psychopharmacol. 2008;22:821–7. doi: 10.1177/0269881108091597. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Shackleton CH, Himsworth RL. Studies on plasma corticosteroids in the rhesus monkey (Macaca mulatta) J Endocrinol. 1975;67:241–50. doi: 10.1677/joe.0.0670241. [DOI] [PubMed] [Google Scholar]
- Shah NS. Brain levels of serotonin (5-HT) and norepinephrine (NE) during mescaline-induced behavior in mice. Res Commun Chem Pathol Pharmacol. 1973;5:201–4. [PubMed] [Google Scholar]
- Shah NS. Influence of psychotropic drugs and beta-diethylaminoethyl-diphenylpropylacetate (SKF 525-A) on mescaline-induced behavior and on tissue levels of mescaline in mice. Biochem Pharmacol. 1976;25:591–7. doi: 10.1016/0006-2952(76)90393-2. [DOI] [PubMed] [Google Scholar]
- Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA) Eur J Pharmacol. 1984;102:23–9. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- Silva MT, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–71. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC. Efficacy of antipsychotics to reverse phencyclidine-induced social interaction deficits in female rats--a preliminary investigation. Behav Brain Res. 2008;187:489–94. doi: 10.1016/j.bbr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–77. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Riehl R, Wong K, Green J, Cosgrove J, Vollmer K, Kyzar E, Hart P, Allain A, Cachat J, Gaikwad S, Hook M, Rhymes K, Newman A, Utterback E, Chang K, Kalueff AV. Behavioral effects of MDMA (`ecstasy') on adult zebrafish. Behav Pharmacol. 2011a;22:275–80. doi: 10.1097/FBP.0b013e328345f758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff AV. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci. 2011b;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- Stewart A, Wu N, Cachat J, Hart P, Gaikwad S, Wong K, Utterback E, Gilder T, Kyzar E, Newman A, Carlos D, Chang K, Hook M, Rhymes C, Caffery M, Greenberg M, Zadina J, Kalueff AV. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuropsychopharmacol Biol Psychiatry. 2011c;35:1421–31. doi: 10.1016/j.pnpbp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, Meltzer HY. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol. 1979;59:169–79. doi: 10.1016/0014-2999(79)90279-6. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, London SF, Meltzer HY. Behavioral effects of chronic phencyclidine administration in rats. Psychopharmacology (Berl) 1982;76:52–6. doi: 10.1007/BF00430755. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–9. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Sykes EA. Mescaline-induced motor impairment in rats, assessed by two different methods. Life Sci. 1986;39:1051–8. doi: 10.1016/0024-3205(86)90196-7. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Crisp T, Henderson LJ. Mescaline elicits behavioral effects in cats by an action at both serotonin and dopamine receptors. Eur J Pharmacol. 1983;96:151–4. doi: 10.1016/0014-2999(83)90544-7. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Kim D, Pritchard M, Salgado S, Thaler A. The effects of phencyclidine (PCP) on anxiety-like behavior in the elevated plus maze and the light-dark exploration test are age dependent, sexually dimorphic, and task dependent. Pharmacol Biochem Behav. 2011;100:191–8. doi: 10.1016/j.pbb.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Tyler CB, Miczek KA. Effects of phencyclidine on aggressive behavior in mice. Pharmacol Biochem Behav. 1982;17:503–10. doi: 10.1016/0091-3057(82)90311-2. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Leys A. Behavioral effects of morphine and phencyclidine in rats: the influence of repeated testing before and after single treatment. Eur J Pharmacol. 1985;113:353–62. doi: 10.1016/0014-2999(85)90083-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Webb GD, Farquharson DA. Effects of LSD-25 and mescaline on the electroplax of the electric eel. Am J Physiol. 1971;221:1802–8. doi: 10.1152/ajplegacy.1971.221.6.1802. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press; Eugene: 2007. The zebrafish book. [Google Scholar]
- Willetts J, Balster RL, Leander JD. The behavioral pharmacology of NMDA receptor antagonists. Trends Pharmacol Sci. 1990;11:423–8. doi: 10.1016/0165-6147(90)90150-7. [DOI] [PubMed] [Google Scholar]
- Wilmot CA, Vanderwende C, Spoerlein MT. The effects of phencyclidine on fighting in differentially housed mice. Pharmacol Biochem Behav. 1987;28:341–6. doi: 10.1016/0091-3057(87)90450-3. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Jr., Miner EJ, Isbell H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia. 1962;3:219–23. doi: 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ohno M, Yatsugi S, Fujikawa Y, Ueki S. Nootropic candidates inhibit head-twitches induced by mescaline in mice. Jpn J Pharmacol. 1992;59:419–21. doi: 10.1254/jjp.59.419. [DOI] [PubMed] [Google Scholar]
- Zakhary SM, Ayubcha D, Ansari F, Kamran K, Karim M, Leheste JR, Horowitz JM, Torres G. A behavioral and molecular analysis of ketamine in zebrafish. Synapse. 2011;65:160–7. doi: 10.1002/syn.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]