Abstract
Epithelial ovarian cancer (EOC) remains the most lethal gynecological malignancy in the United States. EZH2 silences gene expression through trimethylating lysine 27 on histone H3 (H3K27Me3). EZH2 is often overexpressed in EOC and has been suggested as a target for EOC intervention. However, EZH2 target genes in EOC remain poorly understood. Here, we mapped the genomic loci occupied by EZH2/H3K27Me3 using chromatin immunoprecipitation followed by next generation sequencing (ChIP-seq) and globally profiled gene expression in EZH2 knockdown EOC cells. Cross-examination of gene expression and ChIP-seq revealed a list of 60 EZH2 direct target genes whose expression was upregulated >1.5-fold upon EZH2 knockdown. For three selected genes (ALDH1A1, SSTR1 and DACT3), we validated their upregulation upon EZH2 knockdown and confirmed the binding of EZH2/H3K27Me3 to their genomic loci. Further, the presence of H3K27Me3 at the genomic loci of these EZH2 target genes was dependent upon EZH2. Interestingly, expression of ALDH1A1, a putative marker for EOC stem cells, was significantly downregulated in high-grade serous EOC (n=53) compared with ovarian surface epithelial cells (n=10, p<0.001). Notably, expression of ALDH1A1 negatively correlated with expression of EZH2 (n=63, Spearman r=−0.41 and p<0.001). Thus, we identified a list of 60 EZH2 target genes and established that ALDH1A1 is a novel EZH2 target gene in EOC cells. Our results suggest a role for EZH2 in regulating EOC stem cell equilibrium via regulation of ALDH1A1 expression.
Keywords: Ovarian Cancer, EZH2, ALDH1A1
Introduction
Epithelial ovarian cancer (EOC) accounts for more deaths than any other gynecological malignancy in the United States. EOCs are classified into distinct histological types including serous, mucinous, endometrioid and clear cell (1). The most common histology of EOC is serous (~60% of all cancers) and less common histologies include endometrioid, clear cell and mucinous (1). Recently, an alternative classification has been proposed, in which EOC is broadly divided into two types (2). Type I EOC includes mucinous, low-grade serous, low-grade endometrioid and clear cell carcinomas, and type II EOC includes high-grade serous carcinomas, which is the most lethal histosubtype (2).
Enhancer of zeste homology 2 (EZH2) is a histone methyltransferase that mediates gene silencing by catalyzing the trimethylation on lysine 27 of histone H3 (H3K27Me3) (3). EZH2 is often expressed at higher levels in human EOC cells and its expression positively correlates with cell proliferation in these cells (4). Further underscoring the importance of EZH2 in EOC, EZH2 knockdown triggers apoptosis and inhibits the invasion of human EOC cells (4). In addition, EZH2 is overexpressed in ovarian tumor-associated endothelial cells, which promotes angiogenesis (5). Finally, there is evidence to suggest that EZH2 is overexpressed in ovarian cancer stem cell-like populations enriched by chemotherapy (6). Accordingly, EZH2 has been suggested as a putative target for developing EOC therapeutics. Thus, it is important to identify EZH2 target genes in EOC to gain insights into the biology of the disease and to facilitate translational EOC research related to EZH2. Although a number of EZH2 target genes have been characterized in a few cancer types, including prostate and breast, using chromatin immunoprecipitation (ChIP)-on-chip analysis (7, 8), studies that aim to globally identify EZH2 target genes in EOC cells have yet to be performed.
Here we report the identification of direct EZH2 target genes in human EOC cells using a combination of genome-wide approaches. Specifically, we identified the genomic loci occupied by EZH2/H3K27Me3 using ChIP followed by next generation sequencing (ChIP-seq). In addition, we discovered a list of genes whose expression was upregulated >1.5-fold in EZH2 knockdown EOC cells compared to controls using gene expression microarray analysis. Cross-examination of gene expression profiling and ChIP-seq analysis revealed a list of 60 genes that are direct EZH2/H3K27Me3 target genes, including 56 novel putative EZH2 target genes. For validation, we selected three genes that are implicated in regulating stem cells, apoptosis, cell growth or invasion. We validated their upregulation upon EZH2 knockdown in EOC cells and confirmed the binding of EZH2/H3K27Me3 by ChIP analysis. Interestingly, expression of ALDH1A1, a putative marker for EOC stem cells (9–11), was expressed at significantly lower levels in high-grade serous EOC compared with normal human ovarian surface epithelial cells and negatively correlated with expression of EZH2.
Materials and Methods
Cell culture, shRNA, lentivirus packaging and infection
The SKOV3 human EOC cell line was cultured according to the American Type Culture Collection and as previously described (4, 12). SKOV3 cell line identification was further confirmed by DNA Diagnostic Center. The sense sequences of two individual shRNAs to the human EZH2 genes are as we have previously published (4). Lentivirus packaging was performed using virapower system (Invitrogen) according to the manufacturer’s instruction and as previously described (4). Briefly, SKOV3 cells at 40–50% confluence were infected with lentivirus expressing shRNA to EZH2 or vector control. The infected cells were drug-selected with 3 µg/ml of puromycin to eliminate non-infected cells.
Antibodies, western blot analysis, RNA isolation and qRT-PCR
The following antibodies were used for western blot: mouse anti-EZH2 (BD Bioscience 1:2,500), rabbit anti-H3K27Me3 (Cell Signaling 1:1,000) and mouse anti-GAPDH (Millipore 1: 10,000). RNA from cultured human SKOV3 EOC cells was isolated using Trizol (Invitrogen) according to the manufacturer’s instruction. For qRT-PCR, Trizol-isolated RNA was further purified using an RNeasy kit (Qiagen) following the manufacturer’s instruction. The primers for ALDH1A1, SSTR1 and DACT3 genes used for qRT-PCR were purchased from Applied Biosystems. Expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression.
ChIP-seq analysis and ChIP validation for selected EZH2 target genes
Briefly, SKOV3 cells were fixed with 1% formaldehyde for 15 min and quenched with 0.125 M glycine. Chromatin was isolated by adding lysis buffer (1% SDS, 10mM EDTA, 50mM Tris-HCl, pH 8.1, 1mM PMSF) followed by disruption with a Dounce homogenizer. Lysates were sonicated using a Misonix Sonicator 3000 to shear the DNA to an average length of 300–500 bp. Lysates were cleared by centrifugation to collect chromatin suspensions. Prior to their use in the ChIP protocol, protein A agarose beads (Invitrogen) were preblocked using blocking proteins and nucleic acids for 3 hr. For each ChIP reaction, an aliquot of chromatin (20–30 µg) was precleared with 30 µl preblocked protein A agarose beads for 1–2 hr. ChIP reactions were set up using precleared chromatin and antibody (anti-H3K27Me3, Millipore 07-449; anti-EZH2, Millipore 07-689) and incubated overnight at 4 °C. Preblocked protein A agarose beads were added and incubation at 4 °C was continued for another 3 hr. Agarose beads containing the immune complexes were washed, and the immune complexes eluted from the beads were subjected to RNase treatment at 37 °C for 20 min and proteinase K treatment at 37 °C for 3 hr. Crosslinks were reversed, and ChIP DNAs were purified by phenol-chloroform extraction and ethanol precipitation.
ChIP DNA was amplified using the Illumina ChIP-Seq DNA Sample Prep Kit. In brief, DNA was re-sonicated and ends were polished and 5’-phosphorylated using T4 DNA polymerase, Klenow polymerase and T4 polynucleotide kinase. After addition of 3’-A to the ends using Klenow fragment (3’–5’ exo minus), Illumina genomic adapters were ligated and the sample was size-fractionated (300–400 bp) on a 2% agarose gel. After a final PCR amplification step (18 cycles, Phusion polymerase), the resulting DNA libraries were quantified and tested by qPCR at the same specific genomic regions as the original ChIP DNA to assess quality of the amplification reactions. DNA libraries were sequenced on a Genome Analyzer II. Sequences (36 nt reads) were aligned to the human genome (NCBI Build 37.1/hg19) using Eland software (Illumina). Aligned sequences were extended in silico at their 3’-ends to a length of 240 bp, which is the average genomic fragment length in the size-selected library, and assigned to 32 nt bins along the genome. The resulting histograms were stored in BAR (Binary Analysis Results) files. Peak locations were determined using the MACS algorithm.
For validation of binding of EZH2/H3K27Me3 to the genomic loci of the selected EZH2/H3K27Me3 target genes, SKOV3 EOC cells were transduced with lentivirus encoding control or shEZH2. Drug-selected cells were subjected to ChIP analysis as previously described (13, 14). The following antibodies were used to perform ChIP: anti-EZH2 (C11, BD Biosciences), anti-H3K27Me3 (C36B11, Cell signaling) and anti-histone H3 (05-928, Millipore). An isotype matched IgG was used a negative control. Immunoprecipitated DNA was analyzed with PCR against the genomic regions of ALDH1A1 (Forward: 5’-TGGCACTGGTTATTCAACGTGGTC-3’ and Reverse: 5’-GAGGGTGGAAGCTCTTGTAGGTTT-3’), DACT3 (Forward: 5’-CACACACACACACAAACAGTGCCT-3’ and Reverse: 5’-TTCCTCCAACTAGGCTGGCAGTTT-3’) and SSTR1 (Forward: 5’-TAGCCTAAGCTGCCTGCTGTGTTA-3’ and Reverse: 5’-AAAGTGCATGTGCGGTCTGTTAGC-3’). PCR products were visualized on a 2% agarose gel.
Gene expression microarray analysis
For gene expression microarray analysis in SKOV3 cells, 500 ng total RNA was amplified and labeled using Agilent QuickAmp labeling kit following the manufacturer’s protocol. A total of 1.65 µg of Cy-3 labeled cRNA targets were hybridized onto Agilent 4 × 44k whole genome arrays for 17 hours at 65 °C and washed according to Agilent’s procedure. The hybridized slides were scanned at 5-µm resolution on an Agilent scanner (Agilent) and fluorescent intensities of hybridization signals are extracted using Agilent Feature Extraction software.
Data Sets
Gene expression microarray data sets for 53 cases of laser capture and microdissected high-grade serous EOC and 10 individual isolations of normal HOSE cells were obtained from Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) (GEO accession number: GSE18521).
Statistical analysis
Quantitative data are expressed as mean ± SD unless otherwise stated. Analysis of variance (ANOVA) with Fisher's Least Significant Difference (LSD) was used to identify significant differences in multiple comparisons. Spearman’s test was used to measure statistical dependence between EZH2 mRNA levels and ALDH1A1 mRNA levels. For all statistical analyses, the level of significance was set at 0.05.
Results and Discussion
Genome-wide mapping of EZH2/H3K27Me3 direct target genes in human EOC cells
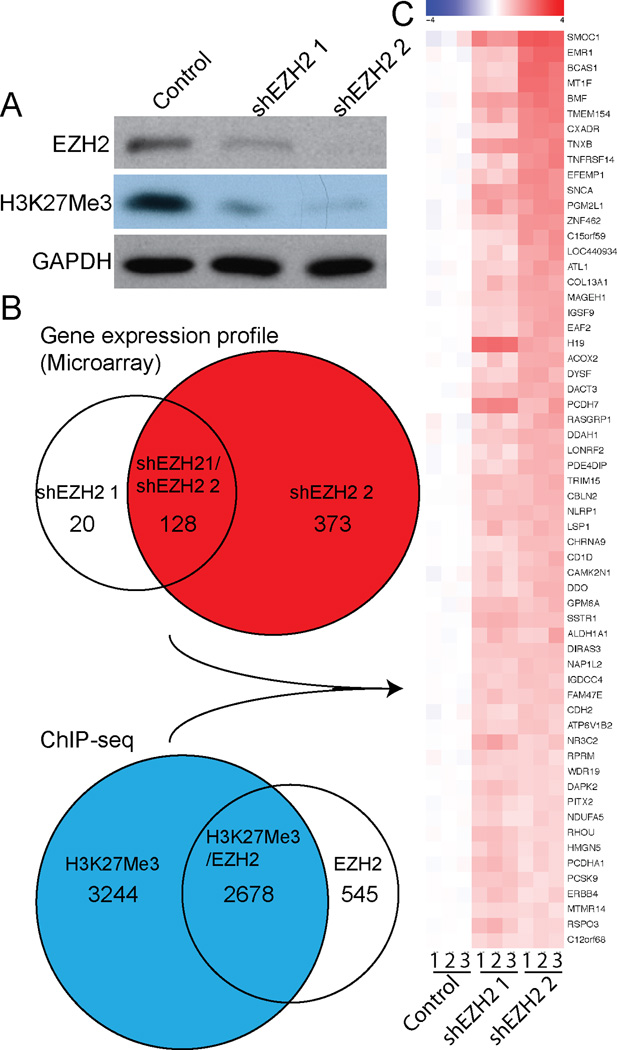
To identify genes whose expression was suppressed by EZH2, we performed gene expression microarray analysis in control and EZH2 knockdown SKOV3 human EOC cells. Two individual short hairpin RNAs to the human EZH2 gene (shEZH2) were used to limit potential off-target effects. Knockdown efficacy was confirmed by immunoblotting analysis (Figure 1A). EZH2 knockdown notably decreased the levels of H3K27Me3, which is consistent with the idea that EZH2 plays a major role in regulating the levels of H3K27Me3 in human EOC cells (Figure 1A). A list of 148 genes and 501 genes were upregulated >1.5-fold by shEZH2 #1 and shEZH2 #2, respectively, while 128 genes overlapped between the two different shEZH2s (Figure 1B). Further data can be found at Gene Expression Omnibus (GEO) database upon publication (GEO access number: GSE31433). The difference in the number of the genes altered by two individual shEZH2s may be due to different degrees of EZH2 knockdown. Consistent with this possibility, shEZH2 #2, which decreases EZH2 levels with better efficacy than shEZH2 #1, and resulted in a greater number of upregulated genes (Figure 1A–B). Alternatively, the differences in gene upregulation observed with individual EZH2 shRNAs may be due to off-target effects. To avoid this potential issue, we chose to analyze the genes that are upregulated by both shEZH2s. Of note, some of the known EZH2 target genes were approaching the 1.5-fold upregulation cutoff point but were not included in further analysis, including VASH1 (5) and E-cadherin (15) (data not shown). Although the conservative approach we implemented may lead to missing certain EZH2 target genes, we felt these rigorous methods allowed us to minimize false-positive EZH2 target genes in human EOCs.
Figure 1. Identification of EZH2 target genes in human SKOV3 EOC cells.
(A) SKOV3 cells were infected with indicated lentivirus encoding shEZH2 or control. Drug-selected cells were examined for expression of EZH2, H3K27Me3 and GAPDH by immunoblotting analysis using indicated antibodies. (B) Schematic of experimental strategies used to identify EZH2 target genes. Genes whose expression was upregulated >1.5-fold upon EZH2 knockdown by two individual shEZH2 were identified by global gene expression microarray analysis. Genomic loci occupied by EZH2/H3K27Me3 were profiled by ChIP-seq analysis. (C) Cross-examination of gene expression profiling and ChIP-seq analysis as illustrated in (B) revealed a list of 60 putative EZH2 target genes in human SKOV3 EOC cells.
We next sought to identify genomic loci that are directly bound by EZH2/H3K27Me3. Towards this goal, we performed ChIP-seq analysis in SKOV3 human EOC cells using antibodies specific to EZH2 or H3K27Me3. EZH2 and H3K27Me3 occupancy was mapped to the genomic loci of 3,223 and 5,922 genes, respectively, and 2,678 genes were associated with both EZH2 and H3K27Me3 (Figure 1B and Table S1). The difference between the number of genes whose locus was occupied by EZH2 and H3K27Me3 may reflect the difference in affinity of the antibodies used for ChIP. Alternatively, for methylated sites not bound by EZH2, it is possible that other epigenetic regulators in addition to EZH2 can also generate H3K27Me3. Consistently, EZH1, a homolog of EZH2 in human cells, is also capable of catalyzing H3K27Me3 epigenetic modifications, albeit at a lower rate than EZH2 (16). Further, genes bound by EZH2 but not H3K27Me3 may reflect an H3K27Me3-independent function for EZH2 as previous reports have suggested (e.g., (17)).
To identify the genes that are directly silenced by EZH2, we cross-examined the gene expression and ChIP-seq data. As a result, we identified a list of 60 EZH2/H3K27Me3 target genes whose expression was upregulated >1.5-fold upon EZH2 knockdown in SKOV3 human EOC cells (Figure 1C). Further confirming our approach, 4 out of the 60 identified genes have previously been demonstrated as EZH2/H3K27Me3 target genes, namely SNAC (18), H19 (19), DIRAS3 (20) and DACT3 (21). Notably, Ingenuity networks analysis revealed that the networks enriched by the identified genes included: 1) cell death, growth and proliferation (e.g., BMF, DAPK2, NLRP1 and DIRAS3) and 2) reproductive system development and cancer (e.g., EAF2, ALDH1A1, SSTR1 and MAGEH1) (data not shown). This is consistent with the proliferation-promoting and apoptosis-suppressing function of EZH2, which we have previously reported in human EOC cells (4).
Interestingly, the number of genes upregulated >1.5-fold upon EZH2 knockdown is notably lower than the number of genes whose genomic loci are directly occupied by EZH2/H3K27Me3 (Figure 1B). This result suggests that additional mechanisms may cooperate with EZH2/H3K27Me3 in silencing or reactivating EZH2 target genes. Consistent with this idea, previous reports have demonstrated that EZH2 target genes are also subject to epigenetic silencing by H3K9Me3 (22) or histone deacetylase (23). This implies that in order to achieve maximum reactivation of EZH2/H3K27Me3 silenced target genes in human EOC cells, additional epigenetic gene silencing mechanisms may be considered for simultaneous targeting together with EZH2 inhibition. Alternatively, this result may be due to the bivalent modification (i.e., H3K27Me3and H3K4Me3) at the genomic loci of those upregulated genes, which primes those genes for activation while keeping them silenced (24). Further studies are warranted to differentiate these possibilities.
Validation of the selected EZH2 target genes in human EOC cells
The list of upregulated genes was prioritized for validation by examining their expression in the newly released the Cancer Genomics Atlas (TCGA) ovarian database (25). We first chose those genes whose expression was downregulated >2-fold in >75% of EOC cases in TCGA ovarian database. In addition, known imprinted genes such as H19 (19) and DIRAS3 (20) or poorly annotated genes were excluded. Given that EZH2 promotes proliferation and invasion, suppresses apoptosis and regulates stem cell-like population in human EOCs (4, 6), we selected three identified EZH2/H3K27Me3 target genes with one or more of these roles for validation studies. Those genes are ALDH1A1 (11), SSTR1 (26) and DACT3 (21) (Table 1).
Table 1.
Three putative EZH2 target genes identified by genome-wide approaches selected for further validation.
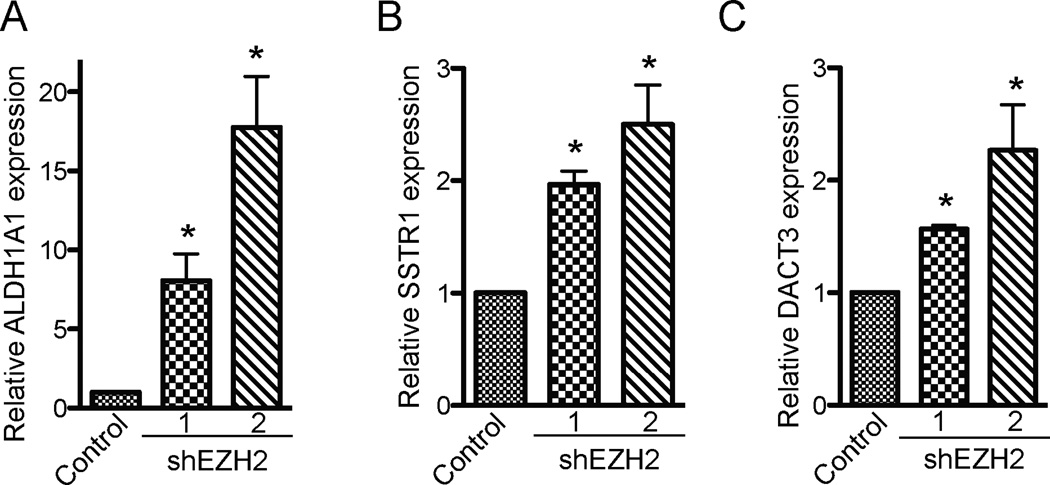
We first validated the upregulation of the selected three genes in EZH2 knockdown SKOV3 human EOC cells by quantitative reverse-transcriptase PCR (qRT-PCR). Indeed, all three selected genes were significantly upregulated in shEZH2-expressing SKOV3 cells compared with controls (Figure 2, p < 0.05 vs. controls.). In addition, all three genes were upregulated by both shEZH2s and there was a correlation between the degree of EZH2 knockdown and the levels of upregulation of these genes (Figure 1A and Figure 2). We conclude that EZH2 knockdown upregulates the expression of ALDH1A1, SSTR1 and DACT3 in SKOV3 human EOC cells.
Figure 2. Validation of upregulation of the selected EZH2 silenced target genes in SKOV3 human EOC cells upon EZH2 knockdown by qRT-PCR.
(A) SKOV3 cells were infected with lentivirus encoding the indicated shEZH2s or control. After drug selection, mRNA was extracted and examined for expression of ALDH1A1 mRNA by qRT-PCR. Expression of β-2-microglobulin was used to normalize the expression of ALDH1A1 mRNA. * p < 0.05 compared with controls. (B) Same as (A), but for SSTR1 mRNA expression. * p < 0.05 compared with controls. (C) Same as (A), but for DACT3 mRNA expression. * p < 0.05 compared with controls.
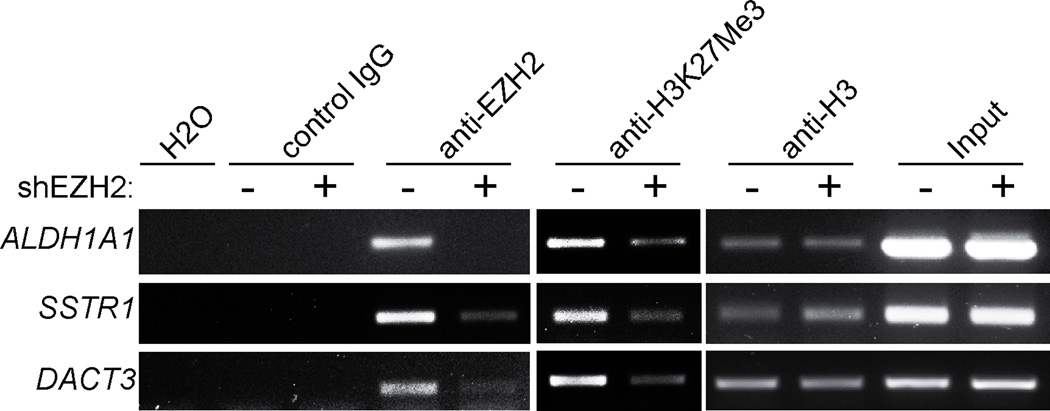
Next, we sought to validate the binding of EZH2/H3K27Me3 to the genomic loci of the selected genes. Towards this goal, we performed ChIP analysis using antibodies specific to EZH2 or H3K27Me3, respectively. An isotype matched IgG was used as a negative control, and an antibody to the core histone H3 was used as a positive control for ChIP analysis. Indeed, we observed the binding of both EZH2 and H3K27Me3 to the genomic loci of the selected EZH2 target genes in SKOV3 human EOC cells as determined by ChIP analysis (Figure 3).
Figure 3. Validation of occupancy of the genomic loci of the selected EZH2 target genes by EZH2 and H3K27Me3 in SKOV3 human EOC cells using ChIP analysis.
Control and shEZH2 #2 expressing SKOV3 cells were subjected to ChIP analysis using antibodies specific to EZH2 or H3K27Me3, respectively. An isotype matched IgG was used as a negative control, and an antibody specific to core histone H3 was used as a positive control. After ChIP analysis, the genomic loci of the indicated genes were subjected to PCR amplification using primers detailed in materials and methods. Please see Figure 1A for shEZH2 knockdown efficacy. Shown are representative images of 3 independent experiments. Note that for H3K27Me3 ChIP, a low number of PCR cycles were used compared with EZH2 or histone H3 ChIP to avoid over saturation of signals.
We next sought to determine whether the occupancy of H3K27Me3 on the genomic loci of EZH2 target genes depends upon EZH2. Towards this goal, we performed the ChIP analysis in EZH2 knockdown SKOV3 EOC cells. Indeed, knockdown of EZH2 severely weakened the association of both EZH2 and H3K27Me3 to the genomic loci of the selected EZH2 target genes (Figure 3). This result suggests that EZH2 plays a major role in regulating H3K27Me3 modification on the genomic loci of these genes in human EOC cells. This result also implies that the binding of EZH2/H3K27Me3 to the genomic loci of these genes we observed here is specific.
Expression of EZH2 inversely correlates with expression of ALDH1A1
We next sought to determine whether there is an inverse correlation between expression of EZH2 and expression of the EZH2 target genes that we have identified and validated in this study. In addition to EOC cells, EZH2 is upregulated in ovarian tumor-associated endothelial cells (5). To limit the confounding effects of EOC-associated stromal cells (including EOC-associated endothelial cells), we chose to analyze the correlation between expression of EZH2 and its target genes in specimens from laser capture and microdissected (LCM) high-grade serous subtype EOC, which accounts for the majority of EOC-associated mortalities (27).
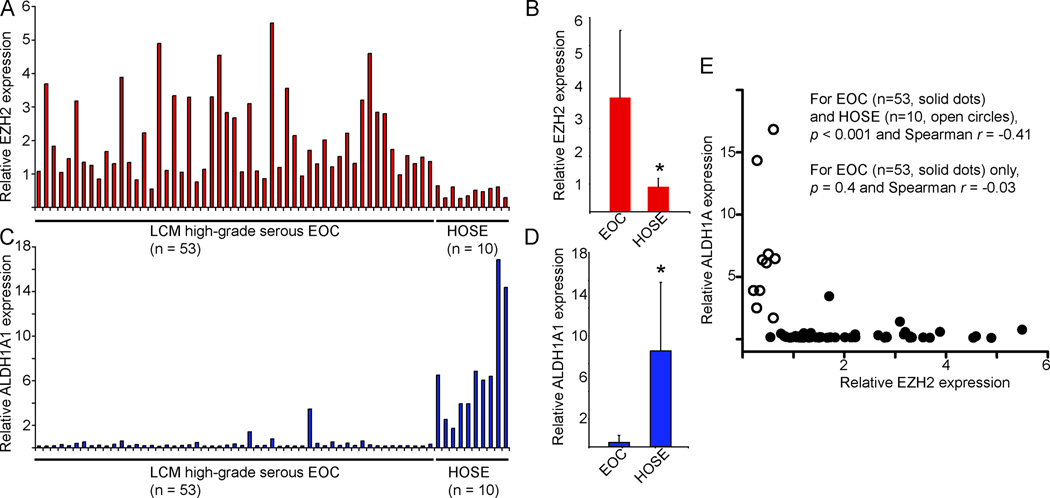
EZH2 is expressed at higher levels in human EOC cells compared with normal human ovarian surface epithelial (HOSE) cells (4). Therefore, we hypothesized that EZH2 target genes that are silenced by EZH2 would be expressed at lower levels in human EOC cells. Towards testing this hypothesis, we examined the expression of EZH2 and the three validated EZH2 target genes in a published microarray database, which compares the gene expression profile in 53 cases of LCM high-grade serous EOCs and 10 individual isolations of primary HOSE cells (28). Consistent with our previous report (4), EZH2 was expressed at significantly higher levels in human EOCs compared with primary HOSE cells (p<0.001) (Figure 4A–B). Notably, the EZH2 target gene ALDH1A1 was expressed at significantly lower levels in human EOCs compared with either normal HOSE cells (p<0.001) (Figure 4C–D). Indeed, there was a negative correlation between expression of EZH2 and its target gene ALDH1A1 in a Spearman statistical analysis of the cases including both EOC and primary HOSE cells (p<0.001 and r = −0.41) (Figure 4E, including both open circles and solid dots). However, the co-efficiency Spearman r is 0.41. This result indicates that other factors may also play a role in the expression relation. Consistently, there is evidence to suggest that notch signaling also regulates ALDH1A1 expression (29). In addition, the correlation between expression of EZH2 and ALDH1A1 is not significant among EOC cases (p=0.81) (Figure 4E, solid dots only). This may be due to the fact the ALDH1A1 is expressed at very low levels in the vast majority of EOC cases and, thus, the variation in expression may simply be a reflection of experimental variations.
Figure 4. EZH2 targets ALDH1A1 in high-grade serous subtype EOC.
(A) Relative expression of EZH2 mRNA in 53 cases of laser capture and microdissected (LCM) high-grade serous EOC and 10 individual isolations of normal human ovarian surface epithelial (HOSE) cells. (B) Quantitation of (A), * p < 0.001 compared with high-grade serous EOC. (C) Same as (A), but for relative expression of ALDH1A1 mRNA. (D) Quantitation of (C), * p < 0.001 compared with high-grade serous EOC. (E) Correlation between expression of ALDH1A1 and EZH2 as determined by Spearman’s statistical analysis using GraphPad Prism version 5.0 software.
Comparing EOC to normal HOSE cells, ALDH1A1 showed a high fold-change in expression (>8-fold), whereas SSTR1 (~3.5-fold) or DACT3 (<1.5-fold) only showed a moderate to minimal fold-change in expression (Figure 4D and S1). It is possible that EZH2 is the major epigenetic regulator of ALDH1A1 while additional epigenetic silencing mechanisms may contribute to suppression of the other two validated EZH2 target genes. In support of this possibility, EZH2 knockdown induced much greater levels of upregulation for ALDH1A1 (up to 22-fold) compared with the other two EZH2 target genes (Figure 2).
Next, we sought to examine the correlation between expression of EZH2 or ALDH1A1 and survival of high-grade serous EOC patients. Consistent with our previous report (4), the difference in overall survival between high and low EZH2 expression in high-grade serous EOC patients was not significant (p = 0.1684) (Figure S2). In addition, the difference in survival between low ALDH1A1 expression group and high ALDH1A1 group was not significant (p = 0.7789) (Figure S2). In contrast to our results, high ALDH1A has previously been reported to be associated with poor prognosis in EOC patients (10, 30). The basis for this discrepancy remains to be determined. However, the discrepancy could be due to different methods that were employed in this study (microarray) vs. the other two studies (immunohistochemical staining).
ALDH1A1 has been reported as a marker of cancer stem cells in certain types of cancers including breast and ovarian (10, 29, 31–33). Likewise, EZH2 plays an important role in stem cell biology (34). Our data suggest that EZH2 directly regulates the levels of ALDH1A1 in EOC cells, implying that EZH2 may regulate the EOC stem cell population by controlling the levels of ALDH1A1 expression. Similarly, it has been demonstrated that EZH2 directly regulates the epigenetic status of Nanog, an important factor for both embryonic stem cell and induced pluripotent stem cells, to balance the equilibrium between self-renewal and differentiation of stem cells (35). Similarly, other putative markers of cancer stem cells (such as CD133 (36) and TACSTD2 (37)) have been also reported to be hypermethylated in cancerous cells. While the expression in stem cells will be masked by the vastly more abundant non-stem cell population in our or any similar analysis, it is nevertheless intriguing that differential expression of ALDH1A1 has been described as a marker of stemness in cancer, including in EOC (10, 33). Together, our data suggest that EZH2 may regulate the EOC stem cell population by controlling the levels of ALDH1A1 expression. Our future work will test this hypothesis.
In the present study, using a combination of global gene expression profiling and genome-wide ChIP-seq analysis, we identified a list of 60 EZH2 direct target genes, whose expression was upregulated >1.5-fold upon EZH2 knockdown in human EOC cells. These genes include 56 novel putative EZH2 target genes and 4 known EZH2 target genes. We validated three selected EZH2 target genes that are implicated in regulating cancer stem cells, cell proliferation, apoptosis and cell invasion. We showed that ALDH1A1, a putative marker of EOC stem cells (11), was expressed at lower levels in high-grade serous EOCs compared with normal HOSE cells, and there was a negative correlation between expression of EZH2 and expression of ALDH1A1. Further studies are warranted to mine the data presented here as well as functional characterization of the identified EZH2 target genes. These studies should provide important insights into the biology of EOC development and the identification of potential candidate targets for prevention and intervention of EOC.
Supplementary Material
Acknowledgements
Grant support: R.Z. is an Ovarian Cancer Research Fund (OCRF) Liz Tilberis Scholar. This work was supported in part by a NCI FCCC-UPenn ovarian cancer SPORE (P50 CA083638) pilot project and SPORE career development award (to R.Z.), and a DOD ovarian cancer academy award (OC093420 to R.Z.). B.G.B is supported by a NCI postdoctoral training grant (CA-009035-35).
We thank Dr. Katherine Aird for critical reading of the manuscript, members of Dr. Rugang Zhang’s lab for discussion, and Dr. Yue-Sheng Li at the genomics facility of Fox Chase Cancer Center for technical assistance.
Footnotes
Disclosure of potential conflicts of interest: CLC and PJT are employees and stockholders of GlaxoSmithKline Pharmaceuticals.
References
- 1.Farley J, Ozbun LL, Birrer MJ. Genomic analysis of epithelial ovarian cancer. Cell Res. 2008;18:538–548. doi: 10.1038/cr.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Cai Q, Godwin AK, Zhang R. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res. 2010;8:1610–1618. doi: 10.1158/1541-7786.MCR-10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, et al. Ovarian cancer stem cell like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325–335. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryczek I, Liu S, Roh M, Vatan L, Szeliga W, Wei S, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2011 doi: 10.1002/ijc.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitler BG, Nicodemus JP, Li H, Cai Q, Wu H, Hua X, et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 2011;71:6184–6194. doi: 10.1158/0008-5472.CAN-11-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev Cell. 2011 doi: 10.1016/j.devcel.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27:5105–5119. doi: 10.1128/MCB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007;67:10657–10663. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 19.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Tan J, Li J, Kivimae S, Yang X, Zhuang L, et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6:479–491. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–237. doi: 10.1016/j.mce.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–532. doi: 10.1016/j.ccr.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner B, Hengstler JG, Dietrich B, Henrich M, Steinberg P, Weikel W, et al. Glutathione, glutathione S-transferase alpha and pi, aldehyde dehydrogenase content in relationship to drug resistance in ovarian cancer. Gynecol Oncol. 1997;65:54–62. doi: 10.1006/gyno.1996.4593. [DOI] [PubMed] [Google Scholar]
- 32.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Lin X, Zhong X, Kaur S, Li N, Liang S, et al. Double-negative feedback loop between reprogramming factor LIN28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010;70:9463–9472. doi: 10.1158/0008-5472.CAN-10-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villasante A, Piazzolla D, Li H, Gomez-Lopez G, Djabali M, Serrano M. Epigenetic regulation of Nanog expression by Ezh2 in pluripotent stem cells. Cell Cycle. 2011;10:1488–1498. doi: 10.4161/cc.10.9.15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi JM, Tsai HC, Glockner SC, Lin S, Ohm JE, Easwaran H, et al. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68:8094–8103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibragimova I, Ibanez de Caceres I, Hoffman AM, Potapova A, Dulaimi E, Al-Saleem T, et al. Global reactivation of epigenetically silenced genes in prostate cancer. Cancer Prev Res (Phila) 2010;3:1084–1092. doi: 10.1158/1940-6207.CAPR-10-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.