Abstract
Coordinated cell movements are crucial for vertebrate gastrulation and are controlled by multiple signals. Although many factors are shown to mediate non-canonical Wnt pathways to regulate cell polarity and intercalation during gastrulation, signaling molecules acting in other pathways are less investigated and the connections between various signals and cytoskeleton are not well understood. In this study, we show that the cytoplasmic tyrosine kinase Arg modulates gastrulation movements through control of actin remodeling. Arg is expressed in the dorsal mesoderm at the onset of gastrulation, and both gain- and loss-of-function of Arg disrupted axial development in Xenopus embryos. Arg controlled migration of anterior mesendoderm, influenced cell decision on individual versus collective migration, and modulated spreading and protrusive activities of anterior mesendodermal cells. Arg also regulated convergent extension of the trunk mesoderm by influencing cell intercalation behaviors. Arg modulated actin organization to control dynamic F-actin distribution at the cell-cell contact or in membrane protrusions. The functions of Arg required an intact tyrosine kinase domain but not the actin-binding motifs in its carboxyl terminus. Arg acted downstream of receptor tyrosine kinases to regulate phosphorylation of endogenous CrkII and paxillin, adaptor proteins involved in activation of Rho family GTPases and actin reorganization. Our data demonstrate that Arg is a crucial cytoplasmic signaling molecule that controls dynamic actin remodeling and mesodermal cell behaviors during Xenopus gastrulation.
Keywords: cytoplasmic tyrosine kinase Arg, gastrulation, actin remodeling, protrusive activity
Introduction
Coordinated cell movements are a recurrent theme during vertebrate embryogenesis. They are vital for establishment of body axes and creation of distinct organ shapes and help ensure that structure and function co-evolve harmoniously in developing individuals. Among various morphogenetic processes, gastrulation is the earliest and involves extensive cell movements in all three germ layers. In the ectoderm, cells intercalate radially and flatten during the epiboly movement to form a thinner tissue that spreads to cover mesendoderm. Simultaneously, mesodermal and endodermal cells move inside the embryos by different strategies, such as ingression, involution, and intercalation, to form internal tissues and organs. Coordination of cell movements in different parts of the embryos are controlled by conserved extracellular signals and intracellular machineries that modulate cytoskeleton organization. As these signals and regulators of cytoskeleton are used reiteratively in cell movements in multiple contexts, including organogenesis and cancer metastasis, study of gastrulation can serve as a paradigm to understand detailed molecular mechanisms controlling cell motility.
One best investigated system illustrating molecular control of gastrulation is that of the mesendodermal cell movements during Xenopus embryogenesis (reviewed in Winklbauer et al., 1996; Wallingford et al., 2002; Keller et al., 2003; Keller and Shook, 2004). Following involution, anterior mesendoderm migrates actively as a continuous sheet on the extracellular matrix (ECM) of the blastocoel roof toward the animal pole. In the more posterior region, trunk mesodermal cells do not migrate, but instead undergo mediolateral and radial intercalation to form narrow and elongated tissue. These cell movements are controlled by overlapping and distinct signals, including those of growth factors, ECM, and adhesion molecules. For example, directed migration of anterior mesendoderm is regulated by the platelet-derived growth factor (PDGF) signal and involves the matrix protein fibronectin (Ataliotis et al., 1995; Nagel et al., 2004; Smith et al., 2009). In contrast, convergent extension movements in the trunk mesoderm are influenced by fibroblast growth factor (FGF) and non-canonical Wnt signals (Conlon and Smith, 1999; Nutt et al., 2001; Wallingford et al., 2002; Sivak et al., 2005). The ErbB pathway, unlike the other signals, seems to modulate both head and trunk mesodermal cell movements (Nie and Chang, 2007a). Downstream of these signals, Rho, Rac and Cdc42 GTPases, protein kinase A and protein kinase C pathways, Src family kinases, Jun N-terminal kinase (JNK), phosphoinositol-3-kinase (PI3K) and Erk MAP kinase pathways have all been implicated to play different roles in regulating convergent extension and/or migratory cell behaviors (Symes and Mercola, 1996; Denoyelle et al., 2001; Habas et al., 2001, 2003; Choi and Han, 2002; Yamanaka et al., 2002; Kinoshita et al., 2003; Penzo-Mendez et al., 2003; Song et al., 2003; Tahinci and Symes, 2003; Kim and Han, 2005; Ren et al., 2006; Nie and Chang, 2007b). However, how these signaling molecules control dynamic reorganization of cytoskeleton is not understood in detail.
In this study, we investigate the function of the cytoplasmic tyrosine kinase Arg in cell movements during Xenopus gastrulation. Arg and its related kinase Abl are unique among cytoplasmic tyrosine kinases in that they contain not only the catalytic kinase domain, but also the actin-binding motifs in their carboxyl termini. In mammalian cell culture, Arg and Abl modulate a variety of F-actin-dependent processes, such as spreading and migration of fibroblasts and neurite extension of neuronal cells (Woodring et al., 2003; Hernandez et al., 2004; Backert et al., 2007). These kinases can be activated by PDGF, FGF and epidermal growth factor (EGF)/ErbB signals to control cell shape, membrane protrusions and cell motility (Zhu et al., 1993; Plattner et al., 1999, 2004; Schulze et al., 2005; Frasca et al., 2007; Yan et al., 2008). However, the functions of these proteins during early vertebrate development are not well comprehended. Since the receptor tyrosine kinases that potentially modulate Arg/Abl participate in gastrulation, we address here whether Arg also plays a role in this process. In this study, we show that Arg indeed regulates gastrulation in Xenopus. Arg controls phosphorylation of proteins involved in actin organization to modulate dynamic F-actin remodeling. In doing so, Arg influences cell movements in both head and trunk mesoderm.
Materials and Methods
Embryo manipulation
Embryo manipulation was performed as described (Chang et al., 1997). Full length Arg and Arg mutant constructs were obtained by RT-PCR. The sequences of Arg-MO and Arg-MO2 are 5′-GCCTACCTGCTGGCCCATCACCG -3′ (−5 to +18 nucleotide position relative to translation start site) and 5′-CTGGCCCATCAC CGGCCTTTAGTAA -3′ (−16 to +9 nucleotide position).
Anterior mesendoderm migration and trunk mesoderm convergent extension
The explant assays were performed as described (Nie and Chang, 2007a, b). Anterior mesendoderm taken from stage 10 embryos was allowed to migrate on fibronectin-coated dishes for 4–6 hours, while trunk mesoderm was cultured to the neurula stages. Each experiment was repeated at least four times, with 6 to 10 explants in each sample. Quantification was performed using NIH Image J program, and student t-test was used to evaluate the significance of the differences between the samples. For cell intercalation assay in the trunk mesoderm, RNAs encoding membrane-tethered fluorescent proteins were coinjected with Arg RNA or MO, and post-involution dorsal mesoderm was examined at mid-gastrula stages. For actin organization in the explants, fixed explants were stained with fluorescent dye – conjugated phalloidin, using the protocol described by Tao et al. (2007).
Cell spreading and membrane protrusion assays
The assays were performed as described (Nie and Chang, 2007a, b). The number of lamellipodia and membrane blebs was counted for each cell, and the distribution of cells with different numbers of protrusions was plotted. Time lapse movies were also made to record dynamic protrusive activities in individual cells, and the rate of changes was calculated as changes in protrusions per cell per minute. Excluding cells in small clusters, 25 control cells, 26 cells from Arg-expressing embryos, and 29 cells from Arg morphants were analyzed for changes in lamellipodia and blebs.
Dynamic actin remodeling
Utropin-GFP RNA (0.2–0.5ng) was coinjected with Arg RNA or Arg-MO. Anterior mesendodermal cells from injected embryos were isolated and plated on fibronectin-coated dishes as described above. F-actin reorganization in live cells was recorded by time-lapse video microscopy. Total 19 control cells, 11 Arg-overexpressing cells, and 38 cells from Arg morphants were analyzed.
Western blot
Western blot analyses were performed as described (Nie and Chang, 2007b), using the following antibodies: Arg (1:200, Santa Cruz Biotechnology); p-CrkII(Y221) and CrkII (1:250, Cell Signaling); p-paxillin(Tyr118) and paxillin (1:200, Santa Cruz Biotechnology); dpErk (1:2000 active MAPK pAb, Promega), and Erk (1:1000, BD Biosciences). To detect endogenous Arg, embryonic lysate equivalent of that from 10 to 20 embryos was precipitated with anti-Arg antibody before loaded on SDS-PAGE for Western blot analyses.
Results
Expression of Arg during early Xenopus development
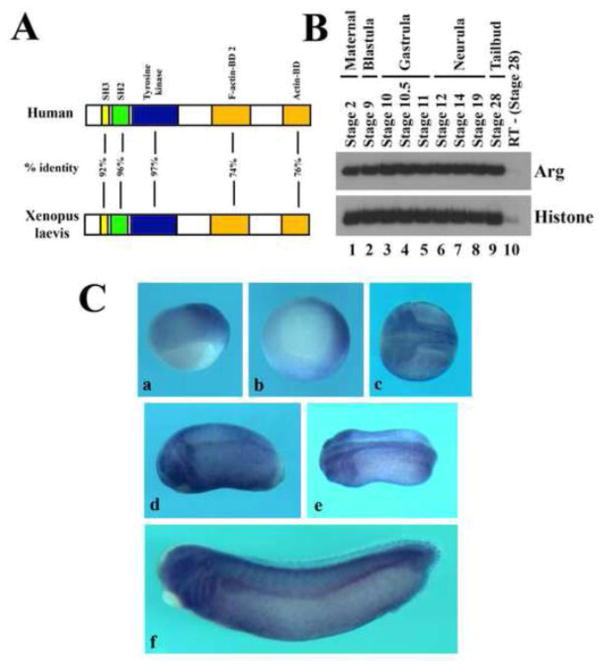
Arg is a cytoplasmic tyrosine kinase that contains Src homology 3 (SH3) and SH2 motifs in front of the tyrosine kinase domain. Unlike other cytoplasmic tyrosine kinases, Arg also contains F-actin and G-actin binding motifs in its carboxyl terminus. Xenopus Arg shares about 75% overall identity with mouse and human Arg at the amino acid level, with the most homologous sequences (over 92% identical) located in the SH3, SH2 and the tyrosine kinase domains (Fig. 1A and Suppl. Fig. S1). The high sequence homology of Arg in vertebrate species implies that functions of Arg are likely conserved.
Figure 1. Expression of Arg during early Xenopus development.
A) Schematic representation of sequence alignment between human and Xenopus Arg proteins. The SH2, SH3 and tyrosine kinase domains are over 92% identical, while the actin binding domains (BDs) are 74–76% identical between the two proteins. B) Temporal expression of Arg assayed by RT-PCR (25 cycles) showed that Arg was expressed maternally and its transcripts persisted till at least tailbud stages. C) Spatial expression of Arg assayed by in situ hybridization of gastrula (panels a and b), neurula (panel c), tailbud (panels d and e) and early tadpole (panel f) embryos. The orientations of the embryos are: panel a, side view with dorsal to the right; panel b, vegetal view with dorsal to the right; panels c and e, dorsal view with anterior to the left; panels d and f, side view with anterior to the left.
To understand the role of Arg in Xenopus embryogenesis, we first examined its expression during development. RT-PCR analysis showed that Arg was present maternally, and its zygotic transcripts persisted until at least tailbud stages (Fig. 1B). Whole mount in situ hybridization revealed that Arg was expressed in the dorsal region that encompassed both mesodermal and neural tissues at gastrula stages. As development proceeded, Arg was enriched in the neural and neural crest cells. At later stages, Arg was found in the central nervous system, eyes, otic vesicle, migrating neural crest cells, and the pronephric tubule and duct (Fig. 1C). The expression pattern suggests that Arg can potentially regulate multiple processes during Xenopus development.
Alteration of Arg levels in Xenopus embryos leads to gastrulation defects
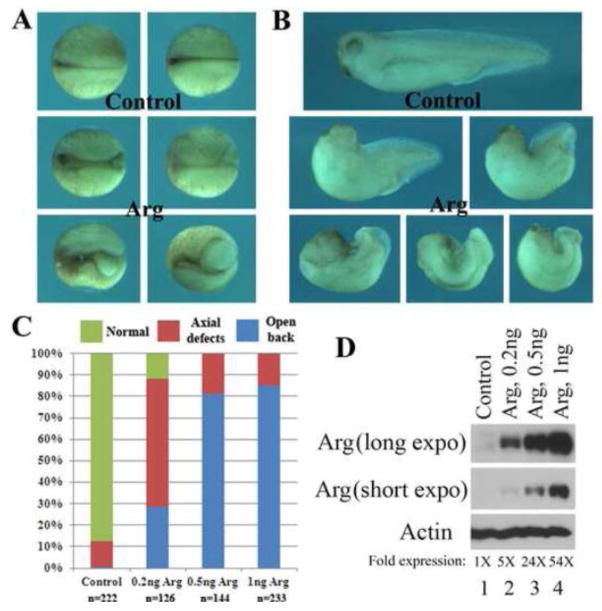
To assay for functions of Arg, we first performed gain-of-function studies by overexpression of Arg in early Xenopus embryos. Examination of injected embryos at neurula stages showed that many embryos did not complete blastopore closure, so that the yolk plugs were open, exposing endodermal cells (Fig. 2A). Subsequently, these embryos developed split axial structures, characterized as “open back” phenotype, at tadpole stages. In addition, embryos that succeeded in blastopore closure tended to have severely reduced head and much shortened body axis (Fig. 2B). The severity of the phenotypes was dose-dependent, so that more embryos displayed open back morphology when higher doses of Arg RNA were injected (Fig. 2C). Western blot analysis revealed that Arg level was more than 5 fold higher than that of the endogenous protein at the RNA doses we used (Fig. 2D). The phenotype of the embryos indicated that ectopic expression of Arg disrupted gastrulation movements.
Figure 2. Overexpression of Arg induced gastrulation defects in early Xenopus embryos.
Ectopic expression of Arg led to failure of blastopore closure, reduced head structure and shortened body axis at neurula (A, dorsal view) or tadpole (B, side view) stages. The effects were dose-dependent, so that more severe defects (open back) were observed in a larger portion of the embryos when the doses of Arg RNA were increased (C). The expression level of Arg was shown in panel D.
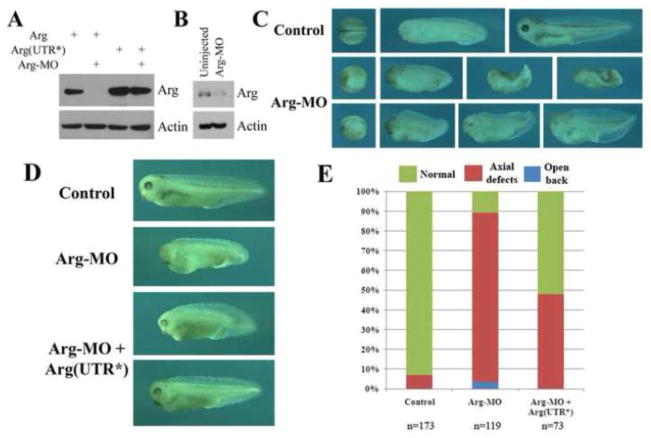
To complement the overexpression studies, we next performed loss-of-function analyses, using a translational inhibitory antisense morpholino oligo (MO). When coinjected into early frog embryos and examined at gastrula stages, Arg-MO efficiently blocked translation of Arg RNA that contained the MO target sequence, but it had no effect on a modified RNA with altered sequences around its translation start site [Arg(UTR*), Fig. 3A]. Arg-MO also reduced endogenous Arg protein to about 35% of its original level at gastrula stages (Fig. 3B). Injection of Arg-MO into the dorsal region of early Xenopus embryos led to delay in blastopore closure and neurulation. At the tailbud and tadpole stages, many morphant embryos showed severely reduced head and/or bent and shortened body axis. A minority of the embryos also had the open back phenotype (Fig. 3C). The defects in the head structures and the body axis could be partially rescued when Arg-MO was coinjected with low doses of modified Arg RNA (5–10pg), so that 52% rather than 11% of the embryos developed normally (Fig. 3D and 3E). The rescue suggested that the morphant phenotype was specific. To further illustrate that the defects were associated with reduction of Arg, we used a second MO (Arg-MO2) that hybridized to a more upstream region of Arg 5′-UTR. Interestingly, Arg-MO2 was inefficient in reducing endogenous Arg protein. Accordingly, we did not observe gastrulation defects in embryos injected with Arg-MO2 (Suppl. Fig. S2). The results further supported the notion that the gastrulation phenotype was specific and sensitive to the level of Arg.
Figure 3. Knockdown of Arg protein production led to gastrulation defects.
A) Antisense Arg-MO specifically blocked translation of Arg from the RNA that contained its target sequence, but had no effect on protein translation from a RNA containing a modified 5′-UTR [Arg(UTR*)]. 1ng RNA and 20ng Arg-MO were used. B) Arg-MO (40ng) reduced endogenous Arg protein to about 35% of its original level. C) Expression of Arg-MO (40ng) in early frog embryos resulted in delayed blastopore closure and neurulation, severe reduction of head structures, shortened and bent body axis, and open back phenotypes. D, E) The defects induced by Arg-MO (40ng) were partially rescued by co-expression with the modified Arg(UTR*) RNA (5–10pg).
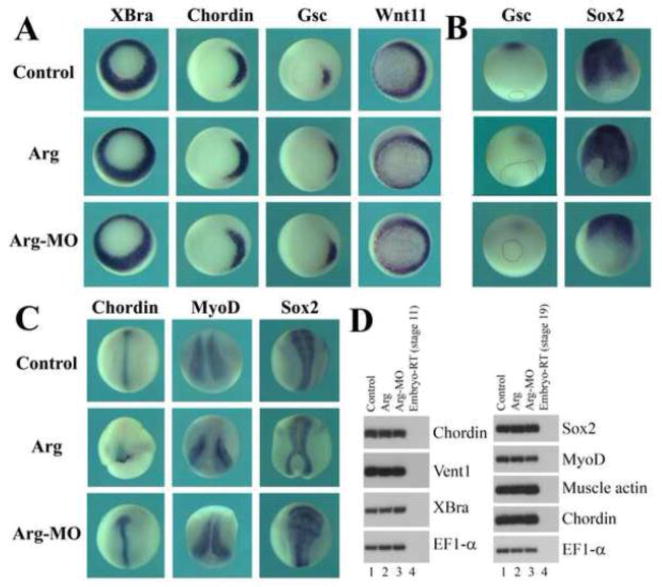
To exclude the possibility that alteration in Arg protein levels might change mesodermal cell fate, which then influenced gastrulation secondarily, we examined several mesodermal markers in injected embryos. At early gastrula stages, expression of the pan mesodermal gene Brachyury (XBra; Smith et al., 1991) and its direct target Wnt11 (Tada and Smith, 2000), the head mesoderm marker goosecoid (Gsc; Cho et al., 1991) and the dorsal trunk mesoderm marker chordin (Chd; Sasai et al., 1994) was all normal (Fig. 4A). However, at neurula stages, while Gsc-expressing cells moved away from the closing blastopore toward the animal pole in uninjected embryos, the Gsc domain was seen to be near the blastopore in embryos that had ectopic or reduced levels of Arg (Fig. 4B). In addition, dorsal mesodermal (Chd and MyoD) and neural (Sox2; Mizuseki et al., 1998) markers were detected in either split domains surrounding the blastopore or shorter and wider regions compared with those in wild type embryos (Fig. 4B and C). Semi-quantitative RT-PCR analysis also showed that none of the mesodermal or neural markers were affected significantly by altered levels of Arg (Fig. 4D). Furthermore, examination of neural crest induction and anterior-posterior neural patterning by RT-PCR demonstrated that Arg did not directly modulate patterning of embryonic tissues to affect axial development (suppl. Fig. S3). Our data thus reveal that Arg does not regulate cell fate determination, but seems to control gastrulation movements directly.
Figure 4. Arg regulated gastrulation without affecting mesodermal cell fate.
A) Alteration of Arg levels did not affect expression of the mesodermal markers XBra, Chordin, Gsc and Wnt11 at gastrula stages. B) Arg modulated migration of Gsc-expressing anterior mesendoderm cells during neurulation. C) Arg regulated the shape of the expression domains of the mesodermal markers Chordin and MyoD as well as the neural marker Sox2. D) RT-PCR studies revealed that Arg did not influence mesodermal cell fate determination.
Arg modulates anterior mesendoderm migration
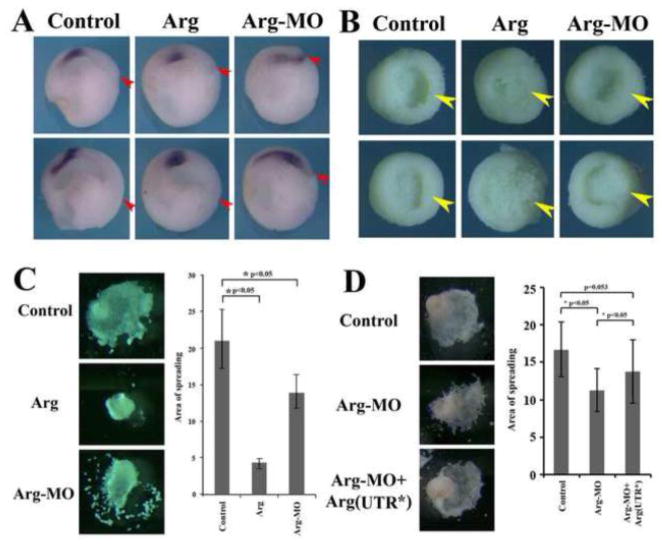
The failure of Gsc-expressing domain to effectively move away from the blastopore implied that anterior mesendoderm migration might be affected by the altered Arg levels. To address this in more detail, we examined migration of Gsc-expressing cells in embryos cut sagittally in half. As cells expressing Gsc moved away from the blastopore, they spread forward and form both anterior endoderm and head mesoderm. Embryos injected with Arg RNA showed aggregation of Gsc-expressing cells without further spreading, whereas in Arg morphant embryos Gsc-expressing cells were widely distributed but did not leave the blastopore efficiently. In both manipulated embryos, the expansion of archenteron was reduced (Fig. 5A). Removal of the ectodermal layer at mid-gastrula stages further revealed that while dorsal mesendodermal tissue had migrated toward the animal pole in control embryos (yellow arrowhead), reduced tissue elevation over the endodermal mantle of the blastocoel floor was observed on the dorsal side of the embryos with altered levels of Arg (Fig. 5B). To further analyze migratory behaviors of the anterior mesendodermal cells, we explanted the tissues surrounding the dorsal lip at early gastrula stages and placed them on fibronectin-coated dishes. The tissue spread on this matrix and migrated as a continuous cell sheet. When explants were taken from the embryos that overexpressed Arg, most remained as compact balls and did not spread substantially on the fibronectin substrate and showed drastically reduced migration. In contrast, explants from Arg morphant embryos adhered to fibronectin and migrated on this substrate. However, the cells had increased propensity to detach from the central core and move away as individual cells when compared with the explants from control embryos. The remaining core tissue displayed reduced migratory distances (Fig. 5C). Measurement of the continuous areas that anterior mesendoderm spread over fibronectin after 6 hours showed that there was a statically significant reduction of the area covered by anterior mesendoderm when Arg levels were altered in embryos (Fig. 5C). The defects in migration in Arg morphants were partially rescued by the modified Arg(UTR*) construct (Fig. 5D). Collectively, these data indicate that Arg regulates migration of anterior mesendoderm during Xenopus gastrulation.
Figure 5. Arg modulated migration of anterior mesendoderm.
A) Alteration of Arg levels interfered with spreading of Gsc-expressing cells anteriorly. The position of the dorsal blastopore is marked by the red arrowhead. B) Arg modulated elevation of dorsal mesendoderm (yellow arrowhead) over the endodermal mantle of the blastocoel floor. Dorsal side is on the right. C) Arg influenced migratory behaviors of anterior mesendoderm on fibronectin, and the continuous area covered by spreading mesendoderm was significantly reduced when Arg levels were changed. D) The defects in anterior mesendoderm migration in Arg morphants were partially rescued by the modified Arg(UTR*).
Arg does not directly regulate cadherin-mediated cell adhesion
The defects in collective cell migration in anterior mesendoderm implied that Arg might control cadherin-mediated cell adhesion during gastrulation. To examine this possibility, we dissociated dorsal mesendodermal cells at early gastrula stages and allowed them to reaggregate on agarose-coated dishes for 2 to 4 hours. Cells taken from control embryos clustered together to form tight balls. The formation of cell clusters was similar in samples with elevated or reduced levels of Arg (Suppl. Fig. S4). This was true regardless whether or not continuous slow swirling was used to bring the cells into contact and thereby facilitate cell interaction. The results therefore suggest that Arg does not directly regulate cadherin-mediated adhesion to affect cell motility, but may influence cell adhesion indirectly, e.g. through modulation of cell-matrix interaction and membrane protrusive activity.
Arg controls spreading and dynamic protrusive activities of anterior mesendodermal cells
Migration of anterior mesendoderm requires cells to adhere to ECM, spread and actively modify membrane protrusive activities. To understand how Arg regulates these cell behaviors, we plated dissociated anterior mesendodermal cells on fibronectin-coated dishes for an hour before gently removing the non-adherent cells. Examination of cell morphology indicated that about half of the cells from control embryos spread; but this number was significantly reduced or increased in embryos expressing Arg or Arg-MO, respectively (Fig. 6A, B). When RNA encoding membrane-tethered GFP was co-injected with Arg or Arg-MO to allow better observation of cell morphology and protrusive activities at high resolution, we found that cells from embryos injected with Arg had a dramatic decline in lamellipodia formation. Instead, a majority of these cells displayed membrane blebs (Fig. 6C, D). In contrast, cells from Arg morphants displayed enhanced ability to form lamellipodia, so that a shift in distribution of the cells toward the ones with multiple lamellipodia was observed (Fig. 6C, D). Most cells also extended multiple filopodia, which could number more than 20 per cell, and they underwent rapid remodeling (extension, retraction, folding back into the cell). However, since they were numerous in number and displayed very complex movements in three dimensions, we did not count or otherwise quantify the behaviors of this type of protrusion. When time lapse video microscopy was performed to follow each individual cell, an effect of Arg on dynamic changes in membrane protrusions was detected (examples shown in supplemental movies 1 to 9 and summarized in Fig. 6E). Elevation of Arg levels not only increased the number of cells with blebs (white arrowhead in Fig. 6F, 6D), but also enhanced the dynamic flowing and ebbing of the blebs (Fig. 6E, F). It should be noted that contrary to mammalian culture systems, where blebbing is often taken as a characteristic of sick or dying cells, blebbing in frog and fish embryonic cells is a common type of protrusive activity displayed by healthy cells in vivo and in culture (Holtfreter, 1943; Erickson and Trinkaus, 1976; Tickle and Trinkaus, 1976). Knockdown of Arg resulted in increased dynamics of lamellipodia, which included formation of new lamellipodia and split, expansion or withdrawal of existing lamellipodia (yellow, red, purple and blue arrowheads, respectively, in Fig. 6F). Statistical analyses suggested that there were significant differences in changes in lamellipodia per cell per minute in control versus injected samples (Fig. 6E). Our data thus reveal that Arg controls cell spreading and dynamic protrusive activities during anterior mesendoderm migration.
Figure 6. Arg regulated cell spreading and dynamic changes in protrusive activity.
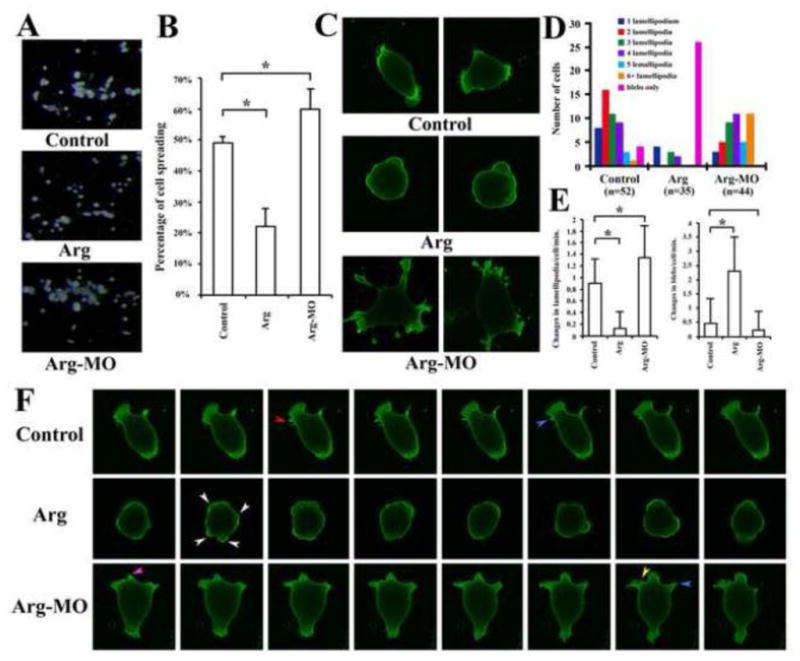
A, B) Arg reduced, while Arg-MO enhanced, significantly the spreading of anterior mesendodermal cells on fibronectin. C) Arg modulated cell morphology. D) Distribution of cells with different numbers of lamellipodia or blebs only suggested that overexpression of Arg promoted formation of blebs, whereas reduction of Arg favored formation of multiple lamellipodia. E) Arg regulated dynamic changes of protrusive activities. Changes in lamellipodia or blebs were counted from recorded time lapse videos and the average changes per cell per minute were calculated and plotted here. Reduction of Arg significantly increased lamellipodia dynamics, whereas elevation of Arg levels resulted in augmented changes in membrane blebs. F) Changes in lamellipodia and blebs in selected cells from time lapse movies. Equal time intervals between each frame were used for all samples. The arrowheads pointed to the following changes: white: blebs; red: split lamellipodia; blue: withdrawal of existing lamellipodia; purple, expansion of lamellipodia; yellow, formation of a new lamellipodium.
Arg regulates convergent extension movements of trunk mesoderm
In addition to modulate movements of anterior mesendoderm, the expression patterns of trunk mesodermal markers (Chd and MyoD) in embryos with altered levels of Arg (Fig. 4) indicated that Arg might also modulate convergent extension movements. To test this idea, we dissected tissues from dorsal marginal zone (DMZ) region at early gastrula stages and cultured them in vitro until neurula stages. These DMZ explants contained trunk mesodermal cells that underwent mediolateral cell intercalation to form narrower and more elongated tissues. When explants were taken from embryos injected with Arg RNA or Arg-MO, they showed reduction in elongation and increase in width of the tissues (Fig. 7A). Quantification of length over width ratio of these explants demonstrated that there was a statistically significant reduction of the ratio in explants with altered Arg levels (Fig. 7B). To visualize cell intercalation in trunk mesoderm, we co-injected Arg RNA or MO with membrane-tethered GFP in one dorsal cell and membrane-tethered Cherry in another dorsal cell of 4-cell stage embryos. Post-involution dorsal mesodermal tissues were then dissected at mid-gastrula stages and cell intercalation was examined with fluorescence microscopy. Convergent extension movements led to cell mixing across the midline, so that the red- and the green-labeled cells were seen to invade each other’s domain, resulting in salt-and-pepper distribution of the labeled cells around the midline (Fig. 7C). Tissues from embryos injected with Arg showed remarkable reduction in cell mixing, so that the border between red and green labeling was largely retained. Intriguingly, although we observed reduction in elongation of DMZ explant from Arg morphant embryos, cells could intercalate efficiently so that extensive mixing of the labeled cells across the midline was detected (Fig. 7C). This suggested that increase and decrease of Arg levels affected cell intercalation and tissue elongation in different manners (see Discussion). Taken together, our results support the notion that Arg modulates convergent extension movements during Xenopus gastrulation.
Figure 7. Arg controlled convergent extension cell movements.
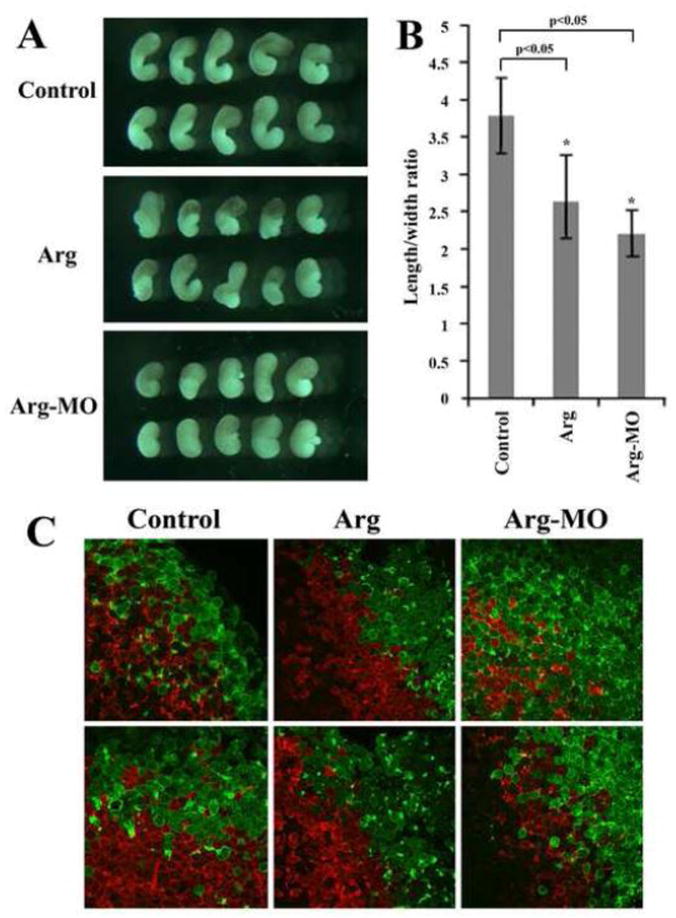
A) DMZ explants taken from embryos with altered levels of Arg did not extend as much as those from control embryos. B) Ectopic expression or reduction of Arg led to statistically significant decrease in length over width ratio of the DMZ explants. C) Ectopic expression of Arg reduced mediolateral cell intercalation, but Arg-MO did not prevent, and seemed to enhance, cell mixing in the trunk mesoderm. 0.5ng Arg RNA and 40ng Arg-MO were used in these experiments.
Arg controls actin organization during gastrulation
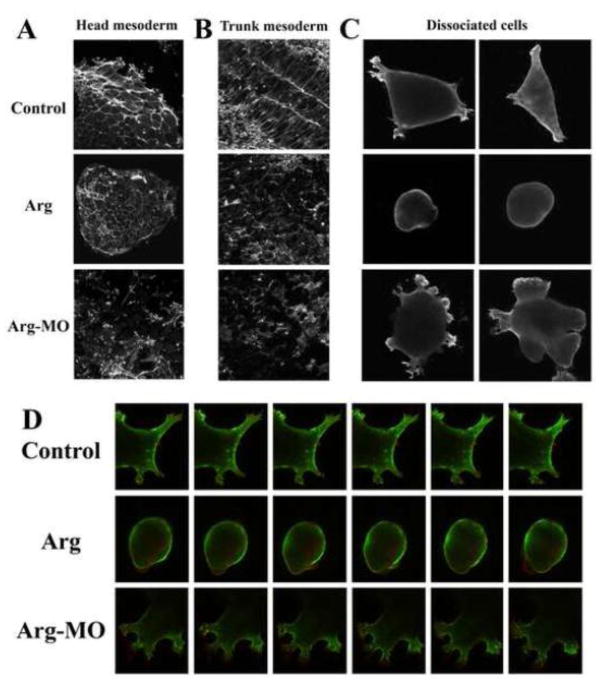
Arg is a cytoplasmic tyrosine kinase with actin-binding motifs, it may thus regulate gastrulation through modulation of actin cytoskeleton. To examine this hypothesis, we first stained anterior mesendodermal explants with phalloidin to show the organization of F-actin. In control explants, F-actin was concentrated at the cell-cell boundary in the central region and was seen in lamellipodia and filopodia at the outer edge of the explants. There was also staining in dorsal ruffles in cells close to the migratory front (Fig. 8A). Anterior mesendoderm taken from the embryos overexpressing Arg still contained F-actin at the cell-cell junctions, but there was a drastic reduction of lamellipodia and filopodia at the periphery (Fig. 8A). In contrast, explants from Arg morphant embryos showed a significant decrease of F-actin at the cell-cell borders. Instead, many cells had increased F-actin distribution in filopodia, lamellipodia and dorsal ruffles (Fig. 8A). Time-lapse movie revealed that the cells at the periphery of these explants displayed multiple types of protrusive activity, separated easily, and migrated actively, suggesting that these cells did not fall off the explants and undergo apoptosis (suppl. movie 10). The opposite effects of gain- and loss-of function of Arg on F-actin organization in anterior mesendoderm demonstrate that proper level of Arg is important for precise actin remodeling required for cell adhesion and motility.
Figure 8. Arg controlled actin organization.
A) Anterior mesendodermal explants stained with phalloidin revealed that Arg controlled F-actin distribution at the cell-cell contact and in membrane protrusions. B) Arg modulated F-actin distribution and cell polarity in trunk mesoderm. C) Localization of F-actin in dissociated anterior mesendodermal cells revealed by phalloidin staining. D) Dynamic changes in F-actin remodeling in individual cells. Selected frames with equal time intervals for all samples were taken from time lapse video (Green = Utropin-GFP-labeled F-actin, red = membrane-tethered Cherry fluorescent protein).
To see whether Arg also modulated actin organization in the trunk, we dissected trunk mesoderm from neurula stage embryos and stained the tissue with phalloidin. In control embryos, F-actin fibers aligned predominately along the medial-lateral axis in the notochord and at the cell-cell contacts in the paraxial mesoderm, with strong F-actin bundles also observed at the boundary between the notochord and the presomitic mesoderm (Fig. 8B). When Arg was overexpressed or depleted, cells did not assume elongated morphology and appeared round shaped. F-actin distribution remained at the cell-cell contacts, but it was not aligned along the mediolateral axis and its concentration at the border between the notochord and the somitic tissues was often lost (Fig. 8B). These data imply that Arg modulates F-actin distribution in the trunk mesoderm and is essential for intercalating cells to adopt proper polarity.
To further investigate how Arg controls actin remodeling in individual cells, we first stained dissociated cells with phalloidin (Fig. 8C). Unlike in mammalian cells, we did not observe formation of long stress fibers. Instead, F-actin was seen along the cell cortex and in membrane protrusions. In Arg-expressing cells, F-actin was located only at the cell cortex just inside the plasma membrane, but was absent inside the volume of the blebs. In cells from Arg morphants, F-actin was abundant in multiple protrusions (Fig. 8C). To follow dynamic changes in actin reorganization, we next labeled the cells with GFP-tagged Utropin, which binds to F-actin, together with membrane-tethered Cherry. In control cells, Utropin-GFP-labeled F-actin formed multiple star-like foci, which could resolve into actin fibers. Actin bundles perpendicular to the plasma membrane were seen in protrusions, while actin fibers parallel to the plasma membrane were located in between membrane protrusions. Dynamic reorganization of F-actin occurred continuously, so that actin foci moved around inside the cells, actin fibers flowed from the cortex into the protrusions and vice versa, and new fibers were assembled and existing fibers were dismantled (Fig. 8D, Suppl. movies 11 to 15). In cells expressing ectopic Arg, F-actin was seen as thick bundles in the cortex. As blebs formed, actin-free membrane first appeared; and with a delay, cortical actin flowed into the blebs underneath the plasma membrane. No actin fibers were observed internal of the bleb structures (Fig. 8D, Suppl. movies 16 to 19). In cells from Arg morphants, F-actin fibers tended to be shorter and oriented in different directions, which might be suggestive of branched instead of linear fiber formation. Dot-like, rather than star-like, foci were abundant, especially inside lamellipodia. These seemed to represent actin assembly perpendicular to the plane of matrix substratum, possibly in structures like dorsal ruffles. Dynamic F-actin remodeling was particularly active, so that stable actin bundles were rarely seen. This was in stark contrast with that in control cells (Fig. 8D, Suppl. movies 20 to 24). Our data therefore indicate that Arg modulates formation, stability and orientation of actin fibers to control cell movements during gastrulation.
Regulation of gastrulation by Arg requires its tyrosine kinase domain but not the actin-binding motifs
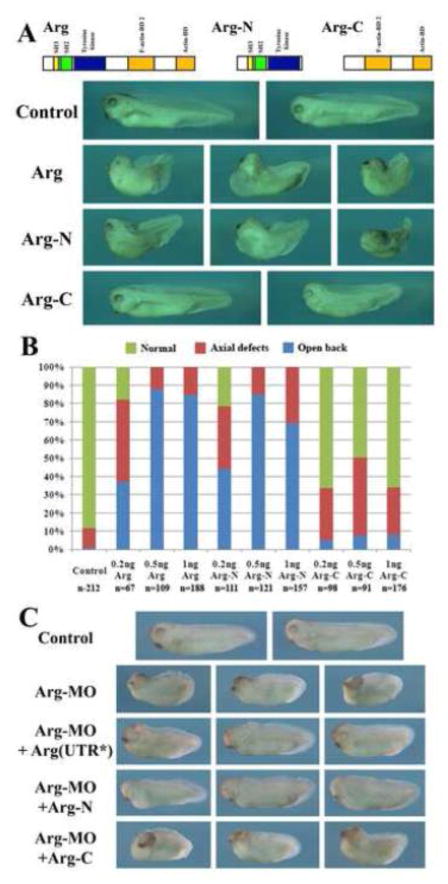
Since Arg can bind to actin directly and regulate actin-dependent cell shape changes and movements in cultured mammalian cells (Woodring et al., 2003; Hernandez et al., 2004), it is possible that Arg utilizes its actin-binding motifs to directly modulate actin remodeling. Alternatively, Arg may use its tyrosine kinase activity to regulate downstream factors involved in actin organization. To distinguish these two possibilities, we constructed two mutant Arg proteins. Arg-N included the intact kinase domain as well as the SH3 and SH2 motifs, but with its actin-binding domains removed. Arg-C contained only the C-terminal portion of the protein that consisted of the two actin-binding motifs (Fig. 9A). When the RNAs encoding these truncated Arg were injected into early frog embryos, Arg-N induced gastrulation defects at similar doses and in similar percentages of embryos as wild type Arg did, but Arg-C was ineffective (Fig. 9A, B). When used to rescue Arg morphants, Arg-N partially rescued the defects in embryos injected with Arg-MO, but Arg-C could not rescue the defects (Fig. 9C). Our results thus imply that the kinase activity is crucial for the function of Arg during gastrulation, whereas the actin-binding motifs are not essential.
Figure 9. The tyrosine kinase domain was required for efficient induction of gastrulation defects by Arg.
A, B) The mutant Arg-N induced gastrulation defects at similar doses in similar percentages of embryos as Arg, but Arg-C was ineffective. C) Arg-N, but not Arg-C, partially rescued gastrulation defects in Arg morphant embryos.
To further confirm that Arg required its kinase activities to regulate gastrulation, we made a point mutation in Arg that altered a conserved lysine in the kinase domain at the amino acid position 306 to arginine [Arg(KR), Suppl. Fig. S5A]. This mutation resulted in an inactive Arg protein; and at high doses [e.g. 2ng Arg(KR) to 0.25ng Arg with a ratio of 8:1], it could act as a weak dominant negative mutant (Suppl. Fig. S5B). When injected into early frog embryos, Arg(KR) did not induce substantial malformation of the embryos at the doses at which Arg induced gastrulation defects (0.2–1ng); only at high doses (2–4ng) when Arg(KR) acted to block endogenous Arg, it induced defects in head structures (Suppl. Fig. S5C). Since Arg(KR) contained intact actin-binding motifs but a defective kinase domain, the ineffectiveness of this mutant to interfere with gastrulation confirms that the kinase function is critical for Arg to control gastrulation movements.
Arg regulates phosphorylation of CrkII and Paxillin
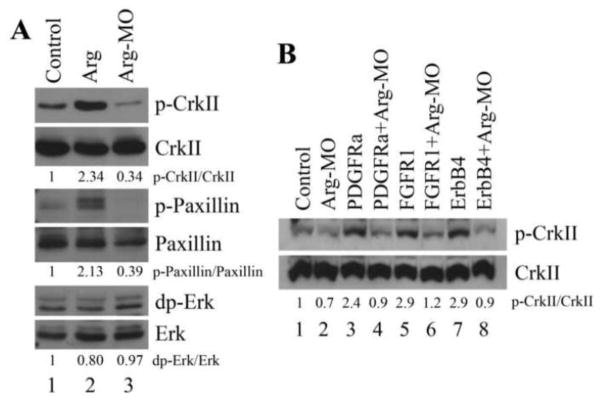
To identify downstream targets of Arg, we tested several proteins that have been shown in mammalian cells to either act as substrates for Arg or be capable of modulating cell adhesion and/or motility. CrkII is an adaptor protein that contains SH2 and SH3 domains. It can be phosphorylated by Arg in cell culture and modulate actin organization through Rho family GTPases (Feller 2001). Paxillin is a protein localized at the focal adhesion sites and is important for cell adhesion and migration (Deakin and Turner, 2008). Erk MAP kinase has been shown to affect cell adhesion and motility in a variety of contexts, including in Xenopus gastrulation (Huang et al., 2004; Nie and Chang, 2007b; Pullikuth and Catling, 2007). The phosphorylation status of these proteins was examined at gastrula stages by Western blot analyses. When Arg was overexpressed, phosphorylation of both CrkII(Y221) and Paxillin(Y118), but not that of Erk, was enhanced more than 2 fold over that of the control samples. Conversely, when Arg was knocked down by the antisense MO, phosphorylated CrkII(Y221) and Paxillin(Y118) were reduced to about 34% to 39% of their endogenous levels, but phosphorylation of Erk was not affected (Fig. 10A). Our results suggest that Arg modulates phosphorylation of actin regulators to control cell movements during gastrulation.
Figure 10. Arg regulated phosphorylation of CrkII and paxillin.
A) Arg enhanced, and Arg-MO reduced, phosphorylation of endogenous CrkII and paxillin, but neither significantly affected phosphorylation of Erk. B) Arg was required for activation of CrkII phosphorylation by RTKs. Protein phosphorylation was normalized with the total amount of that protein and the level in the control extracts was considered as 1.
In Xenopus, PDGF, FGF and ErbB signals have all been implicated in regulation of gastrulation (see Introduction). As these pathways are shown to activate Arg/Abl in mammalian cells, it is possible that Arg mediates some of the effects of RTK signals on cell movements during Xenopus gastrulation. To test this possibility, we examined the requirement for Arg in RTK-dependent activation of actin regulators. Overexpression of PDGFRa, FGFR1 and ErbB4 all stimulated phosphorylation of CrkII(Y221) in Xenopus gastrulae. However, these RTKs were not capable of activating CrkII phosphorylation when Arg was knocked down with the antisense MO (Fig. 10B). Our data reveal that Arg acts downstream of the RTKs to control phosphorylation of actin regulators.
Discussion
Formation of vertebrate embryonic body axes requires not only differential gene expression and asynchronous and asymmetrical cell division in different regions, but also massive cell rearrangements during development. Cells may move as individual units, such as in the case of cell ingression; or cells may never break contact with each other and move as a continuous sheet or shuffle among each other by changing their neighbors. Regardless of the mode of cell movements, cells need to continuously monitor their environment and modify their adhesive strength to cell matrix and other cells. Disparity in adhesion in different parts of a cell is a major force that leads to migration of cells on extracellular matrix or alteration in cell positions in a group of cells. Cytoskeleton organization underlies the adhesive properties of a cell and undergoes dynamic remodeling constantly. Not surprisingly, therefore, many factors are found to modulate cytoskeleton organization to coordinate cell movements and cell fates. One such factor is the cytoplasmic tyrosine kinase Arg. Arg contains actin binding motifs and can regulate cell shape and motility in mammalian cells downstream of growth factor signals. However, the function of Arg during vertebrate development is not well understood.
In this study, we show that Arg plays a critical role in regulating gastrulation movements during Xenopus development. Arg regulates F-actin organization to balance adhesive and migratory behaviors of cells during collective migration of anterior mesendodermal cells. The role of Arg here is best understood in the context of the integrated motile activities displayed by cells in the “shingled” arrangement in the migratory cell stream (Winklbauer and Nagel, 1991; Davidson et al., 2002). Normally, the migratory mesendodermal cells form a shingled array in which the leading cells at the front extend dynamic filo-lamelliform protrusions into a cell-free space, the blastocoel, where they attach to and actively crawl along the matrix of the blastocoel roof. The trailing edges of these cells are lifted off the blastocoel roof and are integrated into the mesendodermal mass. Submarginal cells likewise have their trailing edges raised and attached to the mesendodermal cell mass, but have their leading edges tucked underneath the cell bodies of the cells ahead of them. Thus both the leading and trailing cells have a free space at their leading edge in which to extend protrusions and migrate directionally while their trailing edges are integrated into the mesendodermal cell population that is not in contact with the substratum. This under-lapping, shingled array allows both leading edge cells and trailing cells to be polarized (Winklbauer and Nagel, 1991; Davidson et al., 2002) and to migrate coordinately and directionally toward the animal pole in response to the matrix-associated PDGFA signal (Nagel et al., 2004). This mechanism of collective directional migration of an integrated cell population requires stable cell-cell contacts at the trailing edges of multiple tiers of shingled cells, while leaving the under-lapping leading edges of the cells freely migrate on the blastocoel roof. By modulating actin organization and orientation of actin bundles, Arg appears to strengthen cell-cell adhesion and to prevent formation of arbitrary protrusions at these integrated trailing edges of the cells, so that synchronized cell movements of a contiguous, multi-tiered cell sheet can be achieved. In the absence of Arg function, trailing edges of both leading and trailing cells behave like the leading edges and form ectopic protrusive activity. As the result of increased dynamic protrusive activity at both ends of the cells, their polarized behavior and the cohesiveness of the migratory stream are lost.
Like the directed migration of the mesendodermal cell stream, convergent extension also depends on highly integrated cell behavior and regulation of protrusive activity; thus it is not surprising that Arg also controls convergent extension movements in the trunk. In wild type embryos, axial and paraxial mesodermal cells are polarized and appear spindle-shaped that align along the mediolateral axis. F-actin is assembled preferentially at the cell-cell contact along the mediolateral axis in these cells (Skoglund et al., 2008). Increased Arg levels tend to stimulate rounded cell morphology and a blebbing motility, a type of motility inconsistent with the balanced bipolar protrusive activity and elongated cell morphology that are characteristic of the mediolateral intercalation behavior driving convergent extension (Shih and Keller, 1992). Decrease in Arg expression does not block cell mixing but instead seems to increase it, but without producing as much convergent extension as in controls. This paradoxical result is actually not surprising, and is likely due to lack of suppression of lamelliform protrusive activity when Arg levels are lowered. F-actin at the cell-cell contacts in Arg morphants appears to be reduced, and this may reflect more dynamic protrusive activity, less stable and faster turnover of cell adhesions. For bipolar protrusive activity to produce efficient cell intercalation and maximum convergent extension, it must be balanced and produce similar levels of traction at both ends of the bipolar cell at any one time (Shih and Keller, 1992; Keller et al., 2008). Cells in early explants of Xenopus deep neural tissue, which lack a midline notoplate, display an abnormal mode of intercalation in which the protrusive activity is bipolar when averaged over time, but is inconsistently balanced at any one time, with individual cells first showing protrusive activity in one direction and then the other, moving first to the left along the mediolateral axis and then back again to the right (Elul and Keller, 2000). This behavior produces a promiscuous pattern of cell mixing instead of an organized cell intercalation, and it produces much weaker convergent extension than the balanced bipolar mode in the mesoderm (Elul and Keller, 2000; Keller et al., 2008). This promiscuous mixing without efficient convergent extension is very similar to the promiscuous mixing in the absence of effective convergent extension seen here at lowered levels of Arg, and suggests that Arg is essential for stabilizing the balanced bipolar protrusive activity essential for organized cell intercalation. Thus the function of Arg in suppressing ectopic protrusive activity through regulation of polarized actin organization may have a consistent role in both cell intercalation and in coordinated cell migration, two processes that are quite different in many ways, but have a common requirement of constraining and stabilizing protrusive activity. In a similar vein, integrin-fibronectin interactions are needed to suppress ectopic protrusive activity, which in turn is essential for the bipolarization of intercalating mesodermal cells (Davidson et al., 2006). These findings highlight the role of regulation of the level and stability of protrusive activity, and suggest that Arg is an important regulator of polarized actin organization in morphogenesis.
Although Arg may potentially regulate actin remodeling directly with its actin-binding domains, as it has been shown to use these domains to directly bundle F-actin in vitro (Wang et al., 2001), our data suggest that tyrosine phosphorylation, rather than direct binding to actin, is essential for the function of Arg in development. We identified CrkII and paxillin as two endogenous effectors of Arg. CrkII is an adaptor protein that contains SH2 and SH3 domains. It binds to p130CAS (Crk-associated substrate) upon activation by many growth factors or integrin engagement and dynamically localizes to membrane ruffles and adhesion sites. Crk/CAS complexes can stimulate activation of Rac1, a regulator of actin cytoskeleton and cell motility (Klemke et al., 1998; Feller, 2001; Chodniewicz and Klemke, 2004). Paxillin is a multi-domain scaffold protein that is associated with focal adhesion. It interacts with many signaling and structural proteins, including CrkII, to transmit adhesive and migratory messages from cell surface to dynamic changes in cytoskeleton (Brown and Turner, 2004; Deakin and Turner, 2008). In mammalian cells, CrkII and paxillin are both shown to be phosphorylated by Abl/Arg family members, which can influence complex formation between these proteins and their partners. Phosphorylation of CrkII by Abl/Arg leads to uncoupling of Crk/CAS complexes and reduction in cell migration (Kain and Klemke, 2001; Kain et al., 2003). Phosphorylation of tyrosine 118 (Y118) in paxillin by Abl/Arg, on the other hand, promotes its association with CrkII and stimulates Rac activation (Lewis and Schwartz, 1998; Deakin and Turner, 2008). In Xenopus, paxillin has been shown to act downstream of Wnt signal to control mesodermal cell movements during gastrulation (Iioka et al., 2007). Paxillin also binds to a PTP-PEST like protein phosphatase to regulate cell adhesion, spreading and head mesoderm migration (Cousin and Alfandari, 2004). Interestingly, though both CrkII and paxillin are traditionally considered to associate only with focal adhesion and membrane protrusions, data obtained from mammalian cells indicate that they may also modulate cell-cell adhesion. In HeLa cells depleted of paxillin, cells lose contact with each other and migrate individually rather than collectively during the wound healing response (Schaller, 2004; Yano et al., 2004). In mouse embryonic fibroblasts and epithelial cells, CrkII mediates the action of Abl/Arg to strengthen cell-cell adhesion (Zandy et al., 2007; Zandy and Pendergast, 2008). These results are similar to our observations on the effect of Arg on cell adhesion and migration during Xenopus gastrulation, and imply that Arg likely exerts its function on cell behaviors through modulation of CrkII and paxillin phosphorylation.
Though we show here that Arg is a critical signaling molecule that controls gastrulation movements, we do not exclude its involvement in other developmental processes. As Arg is expressed in neural, neural crest and pronephric cells, it is likely that it modulates development of these tissues. Indeed, targeted gene inactivation in mouse suggests that Arg/Abl regulates vertebrate neurulation (Koleske et al., 1998). Future investigation is needed to understand the full range of Arg function in vertebrate embryogenesis.
Supplementary Material
A) Sequence alignment of X. laevis, X. tropicalis, human, and mouse Arg proteins. B) The identity table for the above-mentioned vertebrate Arg proteins.
A) Unlike Arg-MO, Arg-MO2 did not effectively block Arg protein production in vivo. B) Arg-MO2 did not interfere with anterior mesendoderm migration on fibronectin. C) Arg-MO2 did not inhibit convergent extension in DMZ explants. D) Embryos injected with Arg-MO2 did not show gastrulation defects. 40ng Arg-MO2 was used in all the experiments.
Animal caps from noggin-injected embryos expressed the pan-neural marker Sox2 and the anterior neural marker Otx2. Wnt8 both caudalizes and dorsalizes the neural tissues, so that the neural crest marker Twist, the hindbrain marker Krox20, and the spinal cord marker HoxB9 were all expressed. Overexpression or knockdown of Arg did not change the expression of any of the markers. The doses of RNAs used were: 10pg Noggin, 20pg Wnt8, and 0.5ng Arg; and 40ng Arg-MO was used.
Dorsal mesodermal cells taken from early gastrula embryos were dissociated in calcium (Ca2+) - and magnesium (Mg2+) - free buffer. The cells were then transferred into the buffer containing Ca2+ and Mg2+ in tissue culture dishes coated with agarose, swirled to the center of the dishes, and either left alone without further disturbance (left column) or placed on the shaker with continuous slow swirling (right column) for 2 to 4 hours. Reaggregation of the cells resulted in formation of cell clusters. Formation of cell clusters was similar in samples with elevated or reduced Arg expression, suggesting that cadherin-mediated cell adhesion was not affected by the changed levels of Arg.
A) Arg(KR) contained a point mutation of a conserved lysine at the amino acid position 306 to arginine. B) Arg(KR) acted as a weak dominant negative molecule. At low doses (0.2–0.5ng RNA), Arg(KR) did not block phosphorylation of CrkII by Arg (not shown); while at high doses (2ng), Arg(KR) reduced CrkII phosphorylation by Arg (0.25ng) and decreased endogenous CrkII phosphorylation. Arg-MO was used at the 40ng dose. The amount of Arg or Arg(KR) proteins was shown by Western blot using an anti-Arg antibody from Santa Cruz Biotechnology. C) While Arg induced gastrulation defects at 0.25ng (shown here), Arg(KR) did not alter embryonic development at the same dose (not shown). At the 2ng RNA dose, Arg(KR) induced minor axial defects in Xenopus embryos (bottom panel). 40ng Arg-MO was used.
Highlights.
Arg regulates anterior mesendoderm migration and trunk mesoderm convergent extension
Arg modulates membrane protrusive activity
Arg controls dynamic actin remodeling
Arg modulates phosphorylation of actin regulators CrkII and paxillin during gastrulation
Acknowledgments
We thank Dr. Wallingford for providing Utropin-GFP construct and Dr. Jianbo Wang for allowing us to use his confocal microscope. This work is support by NIH grant R01-GM083029 to CC.
Footnotes
Supplemental movies can be viewed at the following website: http://138.26.40.41/changlab
Supplemental movies 1–3. Dynamic protrusive activities in dissociated control mesendodermal cells.
Supplemental movies 4–6. Membrane bleb dynamics in dissociated mesendodermal cells from embryos overexpressing Arg.
Supplemental movies 7–9. Dynamic protrusive activities of dissociated mesendodermal cells from Arg morphant embryos.
Supplemental movie 10. Time lapse movie of cell movements at the edge of the anterior mesendodermal explants from Arg morphants.
Supplemental movies 11–12. Dynamic actin remodeling in whole cells of dissociated control mesendodermal cells.
Supplemental movies 13–15. Dynamic actin remodeling in lamellipodia of dissociated control mesendodermal cells.
Supplemental movies 16–19. Dynamic actin remodeling in dissociated mesendodermal cells from embryos overexpressing Arg.
Supplemental movies 20–21. Dynamic actin remodeling in whole cells of dissociated mesendodermal cells from Arg morphant embryos.
Supplemental movies 22–24. Dynamic actin remodeling in lamellipodia of dissociated mesendodermal cells from Arg morphant embryos.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- Backert S, Feller SM, Wessler S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem Sci. 2007;33:80–90. doi: 10.1016/j.tibs.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Smith JC. Interference with Brachyury function inhibits convergent extension, causes apoptosis, and reveals separate requirements in the FGF and activin signalling pathways. Dev Biol. 1999;213:85–100. doi: 10.1006/dbio.1999.9330. [DOI] [PubMed] [Google Scholar]
- Cousin H, Alfandari D. A PTP-PEST-like protein affects a5b1-integrin-dependent matrix assembly, cell adhesion, and migration in Xenopus gastrula. Dev Biol. 2004;265:416–432. doi: 10.1016/j.ydbio.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Davidson L, Hoffstrom B, Keller R, DeSimone D. Mesendoderm extension and mantle closure during Xenopus laevis gastrulation: combined roles for integrin a5b1, fibronectin and tissue geometry. Dev Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- Davidson L, Marsden M, Keller R, DeSimone D. Integrin a5b1 and fibronectin regulate polarized protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle M, Valles AM, Lentz D, Thiery JP, Boyer B. Mesoderm-independent regulation of gastrulation movements by the Src tyrosine kinase in Xenopus embryo. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Trinkaus JP. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976;99:375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- Feller SM. Crk family adaptors – signaling complex formation and biological roles. Oncogene. 2001;20:6348–6371. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Malaguarnera R, Mandarino A, Messina RL, Sciacca L, Belfiore A, Vigneri R. Role of c-Abl in directing metabolic versus mitogenic effects in insulin receptor signaling. J Biol Chem. 2007;282:26077–26088. doi: 10.1074/jbc.M705008200. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SE, Krishnaswami M, Miller A, Keleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Bio. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. A study of the mechanics of gastrulation. Part I, J Exp Zool. 1943;94:261–318. [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Iioka H, Iemura S, Natsume T, Kinoshita N. Wnt signaling regulates paxillin ubiquitination essential for mesodermal cell motility. Nat Cell Biol. 2007;9:813–821. doi: 10.1038/ncb1607. [DOI] [PubMed] [Google Scholar]
- Kain KH, Klemke RL. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J Biol Chem. 2001;276:16185–16192. doi: 10.1074/jbc.M100095200. [DOI] [PubMed] [Google Scholar]
- Kain KH, Gooch S, Klemke RL. Cytoplasmic c-Abl provides a molecular “Rheostat” controlling carcinoma cell survival and invasion. Oncogene. 2003;22:6071–6080. doi: 10.1038/sj.onc.1206930. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Keller R, Shook D. Gastrulation in Amphibians. In: Stern CD, editor. Gastrulation: From Cells to Embryos. 2004. pp. 171–204. [Google Scholar]
- Keller R, Shook D, Skoglund P. The forces that shape em bryos: physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:1–24. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKα function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKCδ is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Schwartz MA. Integrins regulate the association and phosphorylation of paxillin by c-Abl. J Biol Chem. 1998;273:14225–14230. doi: 10.1074/jbc.273.23.14225. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nie S, Chang C. Regulation of Xenopus gastrulation by ErbB signaling. Dev Biol. 2007a;303:93–107. doi: 10.1016/j.ydbio.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S, Chang C. PI3K and Erk MAPK mediate ErbB signaling in Xenopus gastrulation. Mech Dev. 2007b;124:657–667. doi: 10.1016/j.mod.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gβγ signaling downstream of Wnt-11/Xfz-7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004;24:2573–2583. doi: 10.1128/MCB.24.6.2573-2583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal. 2007;19:1621–1632. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Nagel M, Tahinci E, Winklbauer R, Symes K. Migrating anterior mesodermal cells and intercalating trunk mesodermal cells have distinct responses to Rho and Rac during Xenopus gastrulation. Dev Dyn. 2006;235:1090–1099. doi: 10.1002/dvdy.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol. 2004;166:157–159. doi: 10.1083/jcb.200406151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005.0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Rolo A, Chen X, Gumbiner B, Keller R. Convergence and extension at gastrula requires a myosin IIB dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Mitsi M, Nugent MA, Symes K. PDGF-A interactions with fibronectin reveal a critical role for heparin sulfate in directed cell migration during Xenopus gastrulation. Proc Natl Acad Sci USA. 2009;106:21683–21688. doi: 10.1073/pnas.0902510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Price BMJ, Green JBA, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Song BH, Choi SC, Han JK. Local activation of protein kinase A inhibits morphogenetic movements during Xenopus gastrulation. Dev Dyn. 2003;227:91–103. doi: 10.1002/dvdy.10296. [DOI] [PubMed] [Google Scholar]
- Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Tao Q, Nandadasa S, McCrea PD, Heasman J, Wylie C. G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development. 2007;134:2651–2661. doi: 10.1242/dev.002824. [DOI] [PubMed] [Google Scholar]
- Tickle C, Trinkaus JP. Observations of nudging cells in culture. Nature. 1976;261:413. doi: 10.1038/261413a0. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Miller A, Mooseker M, Koleske A. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proc Natl Acad Sci USA. 2001;98:14965–14870. doi: 10.1073/pnas.251249298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M. Directional mesoderm cell migration in the Xenopus gastrula. Dev Biol. 1991;148:573–589. doi: 10.1016/0012-1606(91)90275-8. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M, Selchow A, Wacker S. Mesoderm migration in the Xenopus gastrula. Int J Dev Biol. 1996;40:305–311. [PubMed] [Google Scholar]
- Woodring PJ, Hunter T, Wang JYJ. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–2626. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the noncanonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Bentley B, Shao R. Distinct angiogenic mediators are required for basic fibroblast growth factor- and vascular endothelial growth factor-induced angiogenesis: the role of cytoplasmic tyrosine kinase c-Abl in tumor angiogenesis. Mol Biol Cell. 2008;19:2278–2288. doi: 10.1091/mbc.E07-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandy NL, Pendergast AM. Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle. 2008;7:444–448. doi: 10.4161/cc.7.4.5452. [DOI] [PubMed] [Google Scholar]
- Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci USA. 2007;104:17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Deckers S, Mayer BJ, Saltiel AR. Direct analysis of the binding of the abl Src homology 2 domain to the activated epidermal growth factor receptor. J Biol Chem. 1993;268:1775–1779. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Sequence alignment of X. laevis, X. tropicalis, human, and mouse Arg proteins. B) The identity table for the above-mentioned vertebrate Arg proteins.
A) Unlike Arg-MO, Arg-MO2 did not effectively block Arg protein production in vivo. B) Arg-MO2 did not interfere with anterior mesendoderm migration on fibronectin. C) Arg-MO2 did not inhibit convergent extension in DMZ explants. D) Embryos injected with Arg-MO2 did not show gastrulation defects. 40ng Arg-MO2 was used in all the experiments.
Animal caps from noggin-injected embryos expressed the pan-neural marker Sox2 and the anterior neural marker Otx2. Wnt8 both caudalizes and dorsalizes the neural tissues, so that the neural crest marker Twist, the hindbrain marker Krox20, and the spinal cord marker HoxB9 were all expressed. Overexpression or knockdown of Arg did not change the expression of any of the markers. The doses of RNAs used were: 10pg Noggin, 20pg Wnt8, and 0.5ng Arg; and 40ng Arg-MO was used.
Dorsal mesodermal cells taken from early gastrula embryos were dissociated in calcium (Ca2+) - and magnesium (Mg2+) - free buffer. The cells were then transferred into the buffer containing Ca2+ and Mg2+ in tissue culture dishes coated with agarose, swirled to the center of the dishes, and either left alone without further disturbance (left column) or placed on the shaker with continuous slow swirling (right column) for 2 to 4 hours. Reaggregation of the cells resulted in formation of cell clusters. Formation of cell clusters was similar in samples with elevated or reduced Arg expression, suggesting that cadherin-mediated cell adhesion was not affected by the changed levels of Arg.
A) Arg(KR) contained a point mutation of a conserved lysine at the amino acid position 306 to arginine. B) Arg(KR) acted as a weak dominant negative molecule. At low doses (0.2–0.5ng RNA), Arg(KR) did not block phosphorylation of CrkII by Arg (not shown); while at high doses (2ng), Arg(KR) reduced CrkII phosphorylation by Arg (0.25ng) and decreased endogenous CrkII phosphorylation. Arg-MO was used at the 40ng dose. The amount of Arg or Arg(KR) proteins was shown by Western blot using an anti-Arg antibody from Santa Cruz Biotechnology. C) While Arg induced gastrulation defects at 0.25ng (shown here), Arg(KR) did not alter embryonic development at the same dose (not shown). At the 2ng RNA dose, Arg(KR) induced minor axial defects in Xenopus embryos (bottom panel). 40ng Arg-MO was used.