Abstract
A complete understanding of complex dynamic cellular processes such as cell migration or cell adhesion requires the integration of atomic level structural information into the larger cellular context. While direct atomic-level information at the cellular level remains inaccessible, electron microscopy, electron tomography and their associated computational image processing approaches have now matured to a point where sub-cellular structures can be imaged in three dimensions at the nanometer scale. Atomic-resolution information obtained by other means can be combined with this data to obtain three-dimensional models of large macromolecular assemblies in their cellular context. This article summarizes some recent advances in this field.
Introduction
Recognition and cooperative interaction among molecules in large assemblies are fundamental for dynamic processes in living cells. Understanding of how these assemblies work often requires structural information at the atomic level. Nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography are well-established approaches for obtaining atomic structures of individual molecules and domains. However, atomic structures of large macromolecular assemblies remain more difficult to obtain with these methods. These complexes can be too large to be amenable to NMR and often exhibit a large degree of flexibility that hampers crystallization attempts.
Electron microscopy has been a powerful tool for investigating biological structures for several decades now, but only recently steps towards achieving its full potential have begun to come to fruition. High-resolution electron microscopy studies of purified macromolecules are starting to rival X-ray crystallography in the resolution achievable and it is now possible to image frozen hydrated cells and tissue sections at close-to-native conditions at resolutions that allow identification and analysis of molecular components [1,2]. Technical advances in electron microscopy equipment, in methods of specimen preparation, and in computational image reconstruction methods have all been essential in enabling the remarkable progress we have witnessed in the last few years. It is now possible to achieve resolutions of 0.5 nm or better not only from two-dimensional crystals [3] or helically symmetrical objects [4], but also from icosahedral virus particles [5] and even from smaller, less symmetric particles [6**,7]. Electron tomography, the most widely applicable method for obtaining three-dimensional information by electron microscopy, can now be combined efficiently with localization and dynamics data from light microscopy [8,9*] and potentially allows investigation of entire mammalian cells at molecular resolution [10], paving the way for structure-based systems biology [11].
Since the potential of the method has been realized, more and more methods are emerging that target efficient incorporation of atomic-level information into reconstructions derived by electron microscopy. Here, we will describe recent advances and open challenges in this field with an emphasis on assembly reconstructions at intermediate resolution (1–3 nm) and tomographic reconstructions of eukaryotic cells as they relate to the actin cytoskeleton, a major determinant of dynamic cellular processes such as directed cell migration and focal adhesion dynamics.
Reconstructions of isolated cytoskeletal assemblies
Many biological assemblies occur naturally in helical form, particularly cytoskeleton filaments. These filamentous structures are not usually amenable to crystallization due to their natural tendency to polymerize. Image processing of electron microscopy data can take advantage of the helical symmetry and can, in principle, achieve near-atomic resolution [4,12,13]. However, owing to the intrinsic flexibility of filamentous actin [14], the structure determination of actin-filament assemblies generally does not extended into the subnanometer range. The notable exception to this rule is the recent three-dimensional structure of rabbit skeletal actin filaments, which was determined at a resolution of better than 0.7 nm [15**]. Electron microscopy reconstructions of actin filaments in complex with binding partners most commonly fall into the 1.2–2.5 nm resolution range. Examples of actin filaments with bound domains of cytoskeletal proteins that were recently solved include actin in complex with tropomyosin [16], myosin binding protein C [17,18], α-actinin [19], eps8 [20], drebrin [21], coronin-1A [22], talin [23], and fimbrin [24]. Three-dimensional structures of actin filaments crosslinked by villin [25] and vinculin [26] as well as of arp2/3-mediated actin branches [27] have been determined by electron tomography at resolutions of about 2.5–3.5 nm.
Fitting of atomic models into intermediate resolution density maps
In the 1–3 nm resolution range that is most generally achievable for actin-based cytoskeletal assemblies, it is possible to map individual subunits and thus to understand the general architecture of the assemblies. This intermediate resolution also gives a solid basis for fitting high-resolution structures of smaller entities into the reconstructions. The resulting models are often referred to as 'pseudo-atomic' models to hint at the fact that the accuracy of the atom positioning is of limited resolution. This is an unfortunate and confusing term because the models are built out of actual atoms and the term ‘pseudo atom’ is often used to denote atom-like representations of entire residues or other groups of atoms in coarse-grained molecular modeling [28], direct phasing approaches [29,30] or nuclear magnetic resonance calculations [31].
Until recently, correlation-based rigid-body fitting [32–34] has been the most commonly used tool to achieve the goal of fitting high-resolution structures into reconstructions from electron microscopy. Because of the increased availability of subnanometer resolution reconstructions where secondary structural elements are often visible as rods (α-helices) and sheets (β-sheets), significant efforts have been directed towards developing ‘flexible’ fitting methods that allow the high-resolution structures to be distorted in some way, subject to different types of constraints, in order to improve the fit with the density [35–49]. However, these flexible fitting methods are not necessarily useful for resolutions above the subnanometer range where secondary structural elements are not discernible. Recent test calculations indicate that the conceptually simpler modular rigid-body fitting of domain structures often surpasses the achievable accuracy of the models obtained by flexible fitting [50**].
The need for validation tools
At intermediate resolution, depending on the shape of the structure, the number of parameters that can be determined can be severely limited. Care must be taken that the number of the degrees of freedom used during the fitting procedure does not exceed the number of independent observations. Otherwise, overfitting will inevitably ensue. Even the fitting of a rigid body using only the six rotational and translational degrees of freedom can lead to ambiguities in the resulting models [51]. Recently developed methods for incorporating data from other data sources such as Förster resonance energy transfer [52*], proteomics [53*], or sparse distance restraints [54*] into the fitting process are helping to resolve some of these ambiguities.
However, it is clear that rigorous and objective statistics-based evaluation criteria are needed to corroborate conclusions drawn from these models. The inherent uncertainties could be expressed by considering the various possible conformational changes and orientations that fit the observed data equally well. A promising step into this direction is the use of statistical tools to obtain confidence intervals for the orientation parameters in modular fitting of rigid body domains, which allows determining ensembles of structures that fit the data equally well [50**]. These ensembles can then be used to estimate the uncertainties in the positioning of the atoms or in interaction surfaces.
Unfortunately, statistical procedures are sometimes applied in a very casual manner to density fitting procedures so that the conclusions deduced are not always reliable. A recent example is the comparison of different scoring functions for the quality of fit [55]. The authors calculate and compare confidence intervals by assuming that all scoring functions follow Gaussian, normal distributions. However, it is well known that, for example, the correlation coefficient, one of the scoring functions analyzed, does not follow a Gaussian distribution at all and needs to be subjected to a variance-stabilizing variable transformation [56] before reliable confidence intervals can be calculated. Since no normality tests were performed for the other scoring functions and a visual inspection of the distributions does not convey a particularly Gaussian shape for any of those, the confidence intervals calculated under the normality assumption can not be considered to be supported by the data.
Cellular electron tomography
Electron tomography is the most widely applicable method for obtaining three-dimensional information of large assemblies. In fact, it is the only method suitable for investigating unique structures such as organelles, cells, and tissues at a relatively high resolution of 4–8 nm. The reasons for the limited resolution as compared to other electron microscopy techniques are manifold. Primary bottlenecks include extremely low signal-to-noise ratios and low contrast in the tomograms. Technical developments are under way to improve upon both of these issues. Phase plates that are mounted inside the microscope can modify the microscope characteristics so that the contrast in tomograms is significantly improved [57][58]. Direct electron detection devices promise to improve the signal-to-noise ratio of the cameras as well as optimize the efficiency of signal detection [59].
Structure determination by electron microscopy and image reconstruction requires the sample to be thin enough to allow transmission of the image forming electrons. This limits the sample thickness to about 500 nm, which is sufficient for small bacteria [60] and viruses [61] but can be problematic for eukaryotic cells. Cryo-sectioning is one method that can produce thin sections [62–65] but tends to produce cutting artifacts. Focused ion beam milling can also produce thin enough sections [9*,66,67] but the technique is still in its infancy. Alternatively, electron tomography can be restricted to thin regions of the cell. Electron cryotomography has first been used to investigate the actin cytoskeleton at the cell edges of Dictyostelium cells [68*,69]. More recently, cell edges of eukaryotic cells have also been investigated [70–72]. The computational tools for interpreting these dense, crowded tomograms are still in their infancy and, for the most part, interpretation is carried out manually. The inherent subjectivity of the process has led to a major controversy in the field [73,74], corroborating the need for objective computational tools in this area.
Extracting structural information from electron tomograms
The primary tool for extracting molecular level information from electron tomograms is currently based on template matching [75–78]. It has, so far, mostly been applied to the detection of isolated macromolecular assemblies such as ribosomes [79,80], but has also been used for detecting filaments [81] and membranes [82,83]. The major challenge in this framework is to distinguish true positive from false positive detections. The detection performance depends on tomogram-specific parameters such as sample thickness, data acquisition settings, and the degree of molecular crowding. It also depends on target-specific parameters, such as abundance in the cell, molecular weights, and cellular abundance of assemblies with similar structural signature competing for detections.
In a recent study, proteomics experiments for detecting the identity and concentrations of cellular proteins of the pathogen Leptospira interrogans were performed and combined with electrontomography-based template matching to detect spatial localizations [78,84]. This experiment allows estimating the detection performance of the template matching approach in light of the proteomics data. The study showed that ribosomes can be discovered at an estimated true positive rate of better than 90% but discovery rates higher than 50% are difficult to achieve for targets of smaller molecular weights, indicating that there is room for improvements. The detection of low abundance target assemblies did not work out at all. A recently introduced alternative approach for detecting macromolecules in cellular tomograms is based on an initial template-free classification using rotation-invariant features of the tomogram, which is then refined using a Gaussian Hidden Markov Random Field [85]. The advantage of this approach is that it does not depend on templates. However, the current performance on simulated data is relatively poor and indicates that further development is necessary before this approach will be a viable alternative to template-based methods.
Correlating high-resolution information with electron tomograms
The quality and resolution of the raw densities of macromolecular assemblies extracted as subvolumes from electron tomograms are generally not good enough for direct structural interpretation or meaningful fitting of atomic models. In order to boost the signal to make this feasible, the subvolumes must be aligned, classified, and averaged. Several approaches have been developed recently to address these issues [86–92]. A recent study uses kernel density estimator self-organizing maps for classification of the extracted subvolumes [93**], which shows very encouraging results not only for the classification step itself but also for cross-validation of template-matching algorithms applied to electron tomograms. Once classification, alignment and averaging is achieved, the quality of the density maps is greatly improved and fitting of high-resolution atomic models can be pursued in an analogous fashion to that used for other electron microscopy reconstructions. Because the resolution tends to be lower than that of single-particle reconstructions (current limit about 2.5 nm), it is of major importance to minimize the degrees of freedom exploited in the fitting process and to employ validation procedures to detect ambiguities.
Concluding Remarks
Electron microscopy and electron tomography, in conjunction with computational tools for integrating atomic-resolution information, are already making it possible to provide a bridge between cell biological function and molecular mechanism. With further improvements in experimental methods and hardware, in conjunction with emerging technologies such as correlative light and electron microscopy [8,9] and iPALM [94,95], these approaches will not only allow high-resolution mapping and interpretation of macromolecular assemblies and cytoskeleton elements in eukaryotic cells but will also allow direct correlation with dynamics information from life-cell imaging. Current bottlenecks include the relatively low signal-to-noise ratio and high noise level of electron tomograms as well as the lack of validation tools for incorporating atomic level information. However, in both areas promising progress has been made in the last few years. In summary, electron microscopy is likely to provide major contributions for defining the detailed spatiotemporal framework that is necessary for pushing the understanding of cell structure and dynamics to the next level. Further technical progress combined with systematic integration of atomic-resolution and dynamics information should allow electron microscopy to be a major player in the future of structural cell biology.
Highlights.
Electron microscopy provides nm-range information on large biological assemblies
Cellular context can be provided by electron tomography
Atomic-resolution information can be combined with both types of data
These approaches provide an essential link between structure and cell function
Acknowledgments
I would like to thank Dr. Dorit Hanein for critically reading the manuscript and for providing valuable input. I thank Dr. Roman Koning for kindly providing the tomogram used in Figure 1. Writing of this article was made possible by support from the National Institutes of Health (grant numbers GM066311 and GM098412) and the NIGMS Cell Migration Consortium..
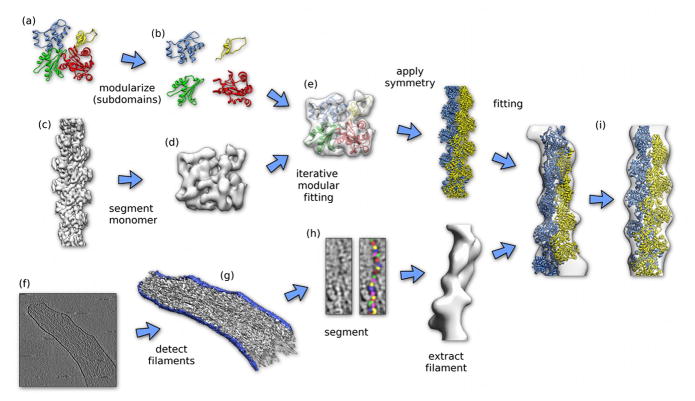
Figure 1. Schematic workflow for correlating atomic-level information with large, dynamic cellular structures. As an example, we place actin atoms into a cellular, actin-rich protrusion of a mouse embryonic fibroblast [71].
A. Structure of actin obtained by X-ray crystallography [96] (pdb accession code: 1atn).
B. Modularization of the structure into the four sub-domains.
C. Density of actin filament at 0.7-nm resolution [15**] (emdb accession code: emd_5168).
D. Single actin monomer density segmented from the filament density using the model-free, three-dimensional watershed procedure [97].
E. Iterative modular fitting [50**] of the actin subdomain structures into the monomer density segmented from the electron microscopy reconstruction of filamentous actin.
F. Slice through a tomogram of an actin-rich protrusion of a mouse embryonic fibroblast [71].
G. Template-based automatic segmentation of tomogram shown in F.
H. Watershed-based, template-free segmentation of filament extracted from tomogram shown in F using a mask derived from G. Note that single actin monomers can be segmented from the density.
I. Result of fitting the actin filament atomic model derived in E into the filament density extracted from the cellular tomogram in F-H. The left hand side shows the fit into the unprocessed extracted density. The right hand side shows the fit after the actin symmetry was applied to the extracted density. Comparison of the symmetrized density with the atomic model indicates a resolution of about 0.4 nm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Harush K, Maimon T, Patla I, Villa E, Medalia O. Visualizing cellular processes at the molecular level by cryo-electron tomography. J Cell Sci. 2010;123:7–12. doi: 10.1242/jcs.060111. [DOI] [PubMed] [Google Scholar]
- 2.Leis A, Rockel B, Andrees L, Baumeister W. Visualizing cells at the nanoscale. Trends Biochem Sci. 2009;34:60–70. doi: 10.1016/j.tibs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Fujiyoshi Y, Unwin N. Electron crystallography of proteins in membranes. Curr Opin Struct Biol. 2008;18:587–92. doi: 10.1016/j.sbi.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–50. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 5.Grigorieff N, Harrison SC. Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopy. Curr Opin Struct Biol. 2011;21:265–273. doi: 10.1016/j.sbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Cong Y, Baker ML, Jakana J, Woolford D, Miller EJ, Reissmann S, Kumar RN, Redding-Johanson AM, Batth TS, Mukhopadhyay A, Ludtke SJ, Frydman J, Chiu W. 4.0-Å resolution cryo-EM structure of the mammalian chaperonin TRiC/CCT reveals its unique subunit arrangement. Proceedings of the National Academy of Sciences. 2010;107:4967–4972. doi: 10.1073/pnas.0913774107. This paper describes a 0.4-nm reconstruction of the chaperonin TRiC/CCT. The remarkable thing here is that a reconstruction that was calculated without enforcing the symmetry, yielded a resolution of 0.47 nm. This is an important proof of concept that near-atomic resolution can be achieved by electron microscopy and image processing, even in the absence of symmetry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, Chiu W. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–8. doi: 10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Hanein D, Volkmann N. Correlative light-electron microscopy. Adv Protein Chem Struct Biol. 2011;82:91–9. doi: 10.1016/B978-0-12-386507-6.00004-X. [DOI] [PubMed] [Google Scholar]
- 9*.Rigort A, Bäuerlein FJ, Leis A, Gruska M, Hoffmann C, Laugks T, Böhm U, Eibauer M, Gnaegi H, Baumeister W, Plitzko JM. Micromachining tools and correlative approaches for cellular cryo-electron tomography. J Struct Biol. 2010;172:169–79. doi: 10.1016/j.jsb.2010.02.011. This paper describes a complete pipeline for correlative electron and light cryo-microscopy, including milling of the sample within a cryo Focused Ion Beam instrument. [DOI] [PubMed] [Google Scholar]
- 10.Noske AB, Costin AJ, Morgan GP, Marsh BJ. Expedited approaches to whole cell electron tomography and organelle mark-up in situ in high-pressure frozen pancreatic islets. J Struct Biol. 2008;161:298–313. doi: 10.1016/j.jsb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkmann N, Hanein D. Electron microscopy in the context of systems biology. In: Gu J, Bourne PE, editors. Structural Bioinformatics. Wiley-Blackwell; 2009. pp. 143–170. [Google Scholar]
- 12.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–55. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 13.Sachse C, Chen JZ, Coureux PD, Stroupe ME, Fändrich M, Grigorieff N. High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J Mol Biol. 2007;371:812–35. doi: 10.1016/j.jmb.2007.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galkin VE, Orlova A, Schröder GF, Egelman EH. Structural polymorphism in F-actin. Nat Struct Mol Biol. 2010;17:1318–1323. doi: 10.1038/nsmb.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467:724–8. doi: 10.1038/nature09372. This paper describes the structure of the actin filament at 0.68 nm resolution. This is the highest resolution structure of filamentous actin obtained so far, providing a much better structural basis for interpreting actin-based assemblies. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Tobacman LS, Mun JY, Craig R, Fischer S, Lehman W. Tropomyosin Position on F-Actin Revealed by EM Reconstruction and Computational Chemistry. Biophys J. 2011;100:1005–1013. doi: 10.1016/j.bpj.2010.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlova A, Galkin VE, Jeffries CM, Egelman EH, Trewhella J. The N-terminal domains of myosin binding protein C can bind polymorphically to F-actin. J Mol Biol. 2011;412:379–86. doi: 10.1016/j.jmb.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mun JY, Gulick J, Robbins J, Woodhead J, Lehman W, Craig R. Electron Microscopy and 3D Reconstruction of F-Actin Decorated with Cardiac Myosin-Binding Protein C (cMyBP-C) J Mol Biol. 2011;410:214–25. doi: 10.1016/j.jmb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galkin VE, Orlova A, Salmazo A, Djinovic-Carugo K, Egelman EH. Opening of tandem calponin homology domains regulates their affinity for F-actin. Nat Struct Mol Biol. 2010;17:614–616. doi: 10.1038/nsmb.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, Le Clainche C, Offenhauser N, Block J, Rottner K, Di Fiore PP, Carlier MF, Volkmann N, Hanein D, Scita G. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol. 2010;8:e1000387. doi: 10.1371/journal.pbio.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grintsevich EE, Galkin VE, Orlova A, Ytterberg AJ, Mikati MM, Kudryashov DS, Loo JA, Egelman EH, Reisler E. Mapping of drebrin binding site on F-actin. J Mol Biol. 2010;398:542–554. doi: 10.1016/j.jmb.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkin VE, Orlova A, Brieher W, Kueh HY, Mitchison TJ, Egelman EH. Coronin-1A stabilizes F-actin by bridging adjacent actin protomers and stapling opposite strands of the actin filament. J Mol Biol. 2008;376:607–13. doi: 10.1016/j.jmb.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras AR, Bate N, Goult BT, Hazelwood L, Canestrelli I, Grossmann JG, Liu H, Putz NS, Roberts GC, Volkmann N, Hanein D, Barsukov IL, Critchley DR. The structure of the C-terminal actin-binding domain of talin. Embo J. 2007;27:458–69. doi: 10.1038/sj.emboj.7601965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galkin VE, Orlova A, Cherepanova O, Lebart MC, Egelman EH. High-resolution cryo-EM structure of the F-actin-fimbrin/plastin ABD2 complex. Proc Natl Acad Sci U S A. 2008;105:1494–8. doi: 10.1073/pnas.0708667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampton CM, Liu J, Taylor DW, DeRosier DJ, Taylor KA. The 3D structure of villin as an unusual F-Actin crosslinker. Structure. 2008;16:1882–91. doi: 10.1016/j.str.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen ME, Kim E, Liu H, Fujimoto LM, Bobkov A, Volkmann N, Hanein D. Three-dimensional structure of vinculin bound to actin filaments. Mol Cell. 2006;21:271–81. doi: 10.1016/j.molcel.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, Hanein D. The structural basis of actin filament branching by Arp2/3 complex. J Cell Biol. 2008;180:887–895. doi: 10.1083/jcb.200709092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Z, Gardner DP, Gutell RR, Ren P. Coarse-grained model for simulation of RNA three-dimensional structures. J Phys Chem B. 2010;114:13497–506. doi: 10.1021/jp104926t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorset DL. Direct phase determination in protein electron crystallography: the pseudo-atom approximation. Proc Natl Acad Sci U S A. 1997;94:1791–4. doi: 10.1073/pnas.94.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunin VY, Lunina NL, Petrova TE, Vernoslova EA, Urzhumtsev AG, Podjarny AD. On the ab initio solution of the phase problem for macromolecules at very low resolution: the few atoms model method. Acta Crystallogr D Biol Crystallogr. 1995;51:896–903. doi: 10.1107/S0907444995005075. [DOI] [PubMed] [Google Scholar]
- 31.Wüthrich K, Billeter M, Braun W. Pseudo-structures for the 20 common amino acids for use in studies of protein conformations by measurements of intramolecular proton-proton distance constraints with nuclear magnetic resonance. J Mol Biol. 1983;169:949–61. doi: 10.1016/s0022-2836(83)80144-2. [DOI] [PubMed] [Google Scholar]
- 32.Volkmann N, Hanein D. Quantitative fitting of atomic models into observed densities derived by electron microscopy. J Struct Biol. 1999;125:176–84. doi: 10.1006/jsbi.1998.4074. [DOI] [PubMed] [Google Scholar]
- 33.Roseman AM. Docking structures of domains into maps from cryo-electron microscopy using local correlation. Acta Crystallogr D Biol Crystallogr. 2000;56:1332–40. doi: 10.1107/s0907444900010908. [DOI] [PubMed] [Google Scholar]
- 34.Chacon P, Wriggers W. Multi-resolution contour-based fitting of macromolecular structures. J Mol Biol. 2002;317:375–84. doi: 10.1006/jmbi.2002.5438. [DOI] [PubMed] [Google Scholar]
- 35.Tama F, Miyashita O, Brooks CL3. Flexible multi-scale fitting of atomic structures into low-resolution electron density maps with elastic network normal mode analysis. J Mol Biol. 2004;337:985–99. doi: 10.1016/j.jmb.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 36.Hinsen K, Reuter N, Navaza J, Stokes DL, Lacapère J-J. Normal mode-based fitting of atomic structure into electron density maps: application to sarcoplasmic reticulum Ca-ATPase. Biophys J. 2005;88:818–27. doi: 10.1529/biophysj.104.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao H, Frank J. Molding atomic structures into intermediate-resolution cryo-EM density maps of ribosomal complexes using real-space refinement. Structure. 2005;13:401–6. doi: 10.1016/j.str.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Suhre K, Navaza J, Sanejouand YH. NORMA: a tool for flexible fitting of high-resolution protein structures into low-resolution electron-microscopy-derived density maps. Acta Crystallogr D Biol Crystallogr. 2006;62:1098–100. doi: 10.1107/S090744490602244X. [DOI] [PubMed] [Google Scholar]
- 39.Velazquez-Muriel JA, Valle M, Santamaria-Pang A, Kakadiaris IA, Carazo JM. Flexible fitting in 3D-EM guided by the structural variability of protein superfamilies. Structure. 2006;14:1115–26. doi: 10.1016/j.str.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Schröder GF, Brünger AT, Levitt M. Combining efficient conformational sampling with a deformable elastic network model facilitates structure refinement at low resolution. Structure. 2007;15:1630–41. doi: 10.1016/j.str.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacs JA, Yeager M, Abagyan R. Damped-dynamics flexible fitting. Biophys J. 2008;95:3192–207. doi: 10.1529/biophysj.108.132357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topf M, Lasker K, Webb B, Wolfson H, Chiu W, Sali A. Protein structure fitting and refinement guided by cryo-EM density. Structure. 2008;16:295–307. doi: 10.1016/j.str.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolley CC, Wells SA, Fromme P, Thorpe MF. Fitting low-resolution cryo-EM maps of proteins using constrained geometric simulations. Biophys J. 2008;94:1613–21. doi: 10.1529/biophysj.107.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan RKZ, Devkota B, Harvey SC. YUP. SCX: coaxing atomic models into medium resolution electron density maps. J Struct Biol. 2008;163:163–74. doi: 10.1016/j.jsb.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusu M, Birmanns S, Wriggers W. Biomolecular pleiomorphism probed by spatial interpolation of coarse models. Bioinformatics. 2008;24:2460–2466. doi: 10.1093/bioinformatics/btn461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–83. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiMaio F, Tyka MD, Baker ML, Chiu W, Baker D. Refinement of protein structures into low-resolution density maps using rosetta. J Mol Biol. 2009;392:181–90. doi: 10.1016/j.jmb.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J, Cheng L, Fang Q, Zhou ZH, Honig B. Building and refining protein models within cryo-electron microscopy density maps based on homology modeling and multiscale structure refinement. J Mol Biol. 2010;397:835–851. doi: 10.1016/j.jmb.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng W. Accurate Flexible Fitting of High-Resolution Protein Structures into Cryo-Electron Microscopy Maps Using Coarse-Grained Pseudo-Energy Minimization. Biophys J. 2011;100:478–488. doi: 10.1016/j.bpj.2010.12.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Volkmann N. Confidence intervals for fitting of atomic models into low-resolution densities. Acta Crystallogr D Biol Crystallogr. 2009;65:679–689. doi: 10.1107/S0907444909012876. This paper describes a statistical procedure that allows obtaining confidence intervals for the orientation parameters of structures fitted into reconstructions derived by electron microscopy. The paper also shows that an iterative modular fitting approach outperforms flexible fitting approaches in most cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkmann N, Hanein D. Docking of atomic models into reconstructions from electron microscopy. Methods Enzymol. 2003;374:204–225. doi: 10.1016/S0076-6879(03)74010-5. [DOI] [PubMed] [Google Scholar]
- 52*.Xu XP, Rouiller I, Slaughter BD, Egile C, Kim E, Unruh JR, Fan X, Pollard TD, Li R, Hanein D, Volkmann N. Three-dimensional reconstructions of Arp2/3 complex with bound nucleation promoting factors. Embo J. 2011 doi: 10.1038/emboj.2011.343. [In Press]. This paper describes the structure of Arp2/3 complex with bound nucleation promoting factors. The iterative modular fitting of the complex components into the density was augmented by a novel probability mapping procedure for FRET distance data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Rout MP, Sali A. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–94. doi: 10.1038/nature06404. This paper describes a procedure for integrating data from electron microscopy, proteomics and biophysical methods into a structure consistent with all data sources. [DOI] [PubMed] [Google Scholar]
- 54*.Campos M, Francetic O, Nilges M. Modeling pilus structures from sparse data. J Struct Biol. 2011;173:436–444. doi: 10.1016/j.jsb.2010.11.015. This paper describes the integration of generalized NMR restraints and parameters from an electron microscopy reconstruction in a common framework to obtain models of helically symmetric bacterial pili. [DOI] [PubMed] [Google Scholar]
- 55.Vasishtan D, Topf M. Scoring functions for cryoEM density fitting. J Struct Biol. 2011;174:333–43. doi: 10.1016/j.jsb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Fisher RA. On the 'probable error' of a coefficient of correlation deduced from a small sample. Metron. 1921;1:1–32. [Google Scholar]
- 57.Murata K, Liu X, Danev R, Jakana J, Schmid MF, King J, Nagayama K, Chiu W. Zernike phase contrast cryo-electron microscopy and tomography for structure determination at nanometer and subnanometer resolutions. Structure. 2010;18:903–12. doi: 10.1016/j.str.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danev R, Kanamaru S, Marko M, Nagayama K. Zernike phase contrast cryo-electron tomography. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.03.013. In Press. [DOI] [PubMed] [Google Scholar]
- 59.Milazzo AC, Moldovan G, Lanman J, Jin L, Bouwer JC, Klienfelder S, Peltier ST, Ellisman MH, Kirkland AI, Xuong NH. Characterization of a direct detection device imaging camera for transmission electron microscopy. Ultramicroscopy. 2010;110:744–7. doi: 10.1016/j.ultramic.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen GJ, Briegel A. How electron cryotomography is opening a new window onto prokaryotic ultrastructure. Curr Opin Struct Biol. 2007;17:260–7. doi: 10.1016/j.sbi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Fu CY, Johnson JE. Viral Life Cycles Captured in Three-Dimensions with Electron Microscopy Tomography. Curr Opin Virol. 2011;1:125–133. doi: 10.1016/j.coviro.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bos E, SantAnna C, Gnaegi H, Pinto RF, Ravelli RB, Koster AJ, de Souza W, Peters PJ. A new approach to improve the quality of ultrathin cryo-sections; its use for immunogold EM and correlative electron cryo-tomography. J Struct Biol. 2011;175:62–72. doi: 10.1016/j.jsb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 63.Al-Amoudi A, Castano-Diez D, Devos DP, Russell RB, Johnson GT, Frangakis AS. The three-dimensional molecular structure of the desmosomal plaque. Proc Natl Acad Sci U S A. 2011;108:6480–6485. doi: 10.1073/pnas.1019469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouchet-Marquis C, Hoenger A. Cryo-electron tomography on vitrified sections: a critical analysis of benefits and limitations for structural cell biology. Micron. 2011;42:152–162. doi: 10.1016/j.micron.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Gruska M, Medalia O, Baumeister W, Leis A. Electron tomography of vitreous sections from cultured mammalian cells. J Struct Biol. 2008;161:384–92. doi: 10.1016/j.jsb.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Hayles MF, de Winter DA, Schneijdenberg CT, Meeldijk JD, Luecken U, Persoon H, de Water J, de Jong F, Humbel BM, Verkleij AJ. The making of frozen-hydrated, vitreous lamellas from cells for cryo-electron microscopy. J Struct Biol. 2010;172:180–90. doi: 10.1016/j.jsb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Marko M, Hsieh C, Schalek R, Frank J, Mannella C. Focused-ion-beam thinning of frozen- hydrated biological specimens for cryo- electron microscopy. Nat Methods. 2007;4:215–217. doi: 10.1038/nmeth1014. [DOI] [PubMed] [Google Scholar]
- 68.Medalia O, Weber I, Frangakis AS, Nicastro D, Gerisch G, Baumeister W. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science. 2002;298:1209–13. doi: 10.1126/science.1076184. [DOI] [PubMed] [Google Scholar]
- 69.Medalia O, Beck M, Ecke M, Weber I, Neujahr R, Baumeister W, Gerisch G. Organization of actin networks in intact filopodia. Curr Biol. 2007;17:79–84. doi: 10.1016/j.cub.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Patla I, Volberg T, Elad N, Hirschfeld-Warneken V, Grashoff C, Fassler R, Spatz JP, Geiger B, Medalia O. Dissecting the molecular architecture of integrin adhesion sites by cryo-electron tomography. Nat Cell Biol. 2010;12:909–15. doi: 10.1038/ncb2095. [DOI] [PubMed] [Google Scholar]
- 71.Koning RI, Zovko S, Bárcena M, Oostergetel GT, Koerten HK, Galjart N, Koster AJ, Mieke Mommaas A. Cryo electron tomography of vitrified fibroblasts: microtubule plus ends in situ. J Struct Biol. 2008;161:459–68. doi: 10.1016/j.jsb.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Urban E, Jacob S, Nemethova M, Resch GP, Small JV. Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol. 2010;12:429–35. doi: 10.1038/ncb2044. [DOI] [PubMed] [Google Scholar]
- 73.Yang C, Svitkina T. Visualizing branched actin filaments in lamellipodia by electron tomography. Nat Cell Biol. 2011;13:1012–3. doi: 10.1038/ncb2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Small JV, Winkler C, Vinzenz M, Schmeiser C. Reply: Visualizing branched actin filaments in lamellipodia by electron tomography. Nat Cell Biol. 2011;13:1013–4. doi: 10.1038/ncb2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Böhm J, Frangakis AS, Hegerl R, Nickell S, Typke D, Baumeister W. From the cover: toward detecting and identifying macromolecules in a cellular context: template matching applied to electron tomograms. Proc Natl Acad Sci U S A. 2000;97:14245–50. doi: 10.1073/pnas.230282097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frangakis AS, Bohm J, Forster F, Nickell S, Nicastro D, Typke D, Hegerl R, Baumeister W. Identification of macromolecular complexes in cryoelectron tomograms of phantom cells. Proc Natl Acad Sci U S A. 2002;99:14153–8. doi: 10.1073/pnas.172520299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Förster F, Medalia O, Zauberman N, Baumeister W, Fass D. Retrovirus envelope protein complex structure in situ studied by cryo-electron tomography. Proc Natl Acad Sci U S A. 2005;102:4729–34. doi: 10.1073/pnas.0409178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck M, Malmström JA, Lange V, Schmidt A, Deutsch EW, Aebersold R. Visual proteomics of the human pathogen Leptospira interrogans. Nat Methods. 2009;6:817–23. doi: 10.1038/nmeth.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandt F, Etchells SA, Ortiz JO, Elcock AH, Hartl FU, Baumeister W. The native 3D organization of bacterial polysomes. Cell. 2009;136:261–71. doi: 10.1016/j.cell.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Ortiz JO, Brandt F, Matias VR, Sennels L, Rappsilber J, Scheres SH, Eibauer M, Hartl FU, Baumeister W. Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J Cell Biol. 2010;190:613–21. doi: 10.1083/jcb.201005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rigort A, Günther D, Hegerl R, Baum D, Weber B, Prohaska S, Medalia O, Baumeister W, Hege H-C. Automated segmentation of electron tomograms for a quantitative description of actin filament networks [Internet] Journal of Structural Biology. 2011 doi: 10.1016/j.jsb.2011.08.012. In Press, Accepted Manuscript:- [DOI] [PubMed] [Google Scholar]
- 82.Lebbink MN, van Donselaar E, Humbel BM, Hertzberger LO, Post JA, Verkleij AJ. Induced membrane domains as visualized by electron tomography and template matching. J Struct Biol. 2009;166:156–61. doi: 10.1016/j.jsb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Lebbink MN, Jimenez N, Vocking K, Hekking LH, Verkleij AJ, Post JA. Spiral coating of the endothelial caveolar membranes as revealed by electron tomography and template matching. Traffic. 2010;11:138–50. doi: 10.1111/j.1600-0854.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- 84.Malmström J, Beck M, Schmidt A, Lange V, Deutsch EW, Aebersold R. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature. 2009;460:762–5. doi: 10.1038/nature08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu M, Beck M, Alber F. Template-free detection of macromolecular complexes in cryo electron tomograms. Bioinformatics. 2011;27:69–76. doi: 10.1093/bioinformatics/btr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartesaghi A, Sprechmann P, Liu J, Randall G, Sapiro G, Subramaniam S. Classification and 3D averaging with missing wedge correction in biological electron tomography. J Struct Biol. 2008;162:436–50. doi: 10.1016/j.jsb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Förster F, Hegerl R. Structure determination in situ by averaging of tomograms. Methods Cell Biol. 2007;79:741–67. doi: 10.1016/S0091-679X(06)79029-X. [DOI] [PubMed] [Google Scholar]
- 88.Förster F, Pruggnaller S, Seybert A, Frangakis AS. Classification of cryo-electron sub-tomograms using constrained correlation. J Struct Biol. 2008;161:276–86. doi: 10.1016/j.jsb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 89.Schmid MF, Booth CR. Methods for aligning and for averaging 3D volumes with missing data. J Struct Biol. 2008;161:243–8. doi: 10.1016/j.jsb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winkler H, Zhu P, Liu J, Ye F, Roux KH, Taylor KA. Tomographic subvolume alignment and subvolume classification applied to myosin V and SIV envelope spikes. J Struct Biol. 2009;165:64–77. doi: 10.1016/j.jsb.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scheres SH, Melero R, Valle M, Carazo JM. Averaging of electron subtomograms and random conical tilt reconstructions through likelihood optimization. Structure. 2009;17:1563–72. doi: 10.1016/j.str.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stölken M, Beck F, Haller T, Hegerl R, Gutsche I, Carazo J-M, Baumeister W, Scheres SHW, Nickell S. Maximum likelihood based classification of electron tomographic data. J Struct Biol. 2011;173:77–85. doi: 10.1016/j.jsb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 93**.Yu Z, Frangakis AS. Classification of electron sub-tomograms with neural networks and its application to template-matching. J Struct Biol. 2011;174:494–504. doi: 10.1016/j.jsb.2011.02.009. This paper describes how kernel density estimator self-organizing maps can be used to classify three-dimensional subtomograms and to narrow down the number of false positives after template matching. [DOI] [PubMed] [Google Scholar]
- 94.Shtengel G, Galbraith JA, Galbraith CG, Lippincott-schwartz J, Gillette JM, Manley S, Sougrat R, Waterman CM, Kanchanawong P, Davidson MW, Fetter RD, Hess HF. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0813131106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- 97.Volkmann N. A novel three-dimensional variant of the watershed transform for segmentation of electron density maps. J Struct Biol. 2002;138:123. doi: 10.1016/s1047-8477(02)00009-6. [DOI] [PubMed] [Google Scholar]