Abstract
Background
Smoking of synthetic cannabinoid-enhanced “herbal incense” is an emerging substance abuse problem. The indole-derived cannabinoids identified in these products were originally developed as research tools and are structurally distinct from cannabinoids in the cannabis plant. Although abused by humans, most published research on this class of compounds has been performed in vitro. The purpose of this study was to evaluate a novel series of 1-pentyl-3-phenylacetylindoles in mice.
Methods
The potencies of these analogs to produce the cannabinoid agonist effects of antinociception, hypothermia and suppression of locomotion were evaluated in ICR mice. The major structural manipulations in the present series included the type of substituent (i.e., unsubstituted, methyl, methoxy, chloro, bromo, and fluoro) and the position of the substituent on the phenyl ring (i.e., 2-, 3- or 4-position).
Results
Potencies of this series of phenylacetylindoles for each cannabinoid effect were highly correlated with CB1 receptor affinities reported previously. Active compounds produced a profile of effects that resembled that exhibited by Δ9-tetrahydrocannabinol (THC). The most critical factor affecting in vivo potency was the position of the substituent. Whereas compounds with 2- and 3-phenylacetyl substituents were efficacious with good potencies, 4-substituents resulted in compounds that had poor potency or were inactive.
Conclusions
These results suggest that phenylacetylindoles with good CB1 binding affinity share pharmacological properties with THC in mice; however, they also emphasize the complexity of molecular interactions of synthetic cannabinoids with CB1 receptors and suggest that scheduling efforts based solely upon structural features should proceed with caution.
Keywords: Aminoalkylindoles, Cannabinoid indoles, Herbal incense, Structure-activity relationship, Synthetic cannabinoids
1.0 Introduction
The complexity of the central nervous system cannabinoid (CB1) receptor’s ligand recognition and signaling has become increasingly recognized over the last few decades with the discovery of such structurally diverse cannabinoid agonists as classical cannabinoids [e.g., Δ9-tetrahydrocannabinol (THC)], bicyclic synthetic cannabinoids (e.g., CP55,940), aminoalkylindoles (e.g., WIN55,212-2), and endogenous cannabinoids (e.g., anandamide) and CB1 receptor antagonists (e.g., rimonabant). Despite their diverse chemical structures, all of these cannabinoids bind to the CB1 receptor and, in the case of agonists, activate it (for a review, see Thakur et al., 2005). Until recently, cannabinoid abuse and dependence in humans had been restricted to plant-derived cannabinoids such as THC, the primary psychoactive constituent of marijuana (Gaoni and Mechoulam, 1964); however, within the last decade, synthetic cannabinoids have been sprayed onto plant material, which is subsequently packaged and sold as “herbal incense” (Vardakou et al., 2010). Although labeled “not for human consumption,” these products are smoked, resulting in a marijuana-like high as well as other physiological effects, some of which may differ from those of marijuana (e.g., elevated blood pressure, vomiting; Schifano et al., 2009; Young et al., 2011; Zimmermann et al., 2009). In addition, the chemical structure of the indole-derived cannabinoids contained in these products and that of THC are distinct to the extent that these products are not detected by standard drug tests for marijuana (Vardakou et al., 2010). Since the Drug Enforcement Agency (DEA) identifies drugs by their chemical structure in the scheduling process, they remained legal until earlier this year. In March 2011, the DEA issued an emergency edict banning five representative synthetic cannabinoids: JWH-018, JWH-200, JWH-073, CP-47,497, and cannabicyclohexanol (DEA, 2010). At the same time, states began to pass their own laws against these and other designer drugs. In an attempt to bypass these laws, however, illicit manufacturers have made slight changes in chemical structure of the cannabinoids contained in these products which allow retention of the desired intoxicating properties. This ongoing change in the active cannabinoids contained in these products has presented continuing detection problems for forensic and law enforcement personnel (Lindigkeit et al., 2009).
JWH-018 [naphthalen-1-yl-(1-pentylindol-3-yl)methanone; “street” names include Spice and K2], the first cannabinoid to be identified in this type of illicit herbal product, was originally synthesized by John Huffman’s group for research purposes in the early 1990’s as part of a large series of indole- and pyrrole-derived cannabinoids (JWH prefix; Huffman et al., 1994) based upon the structural template of WIN55,212-2, the prototypic aminoalkylindole cannabinoid (D’Ambra et al., 1992; Eissenstat et al., 1995). Other cannabinoids within the JWH indole-derived series, those from Alex Makryannis’ (AM prefix) group, bicyclic cannabinoids (CP prefix), and unique structural variations thereof have been identified in confiscated herbal products (Hudson and Ramsey, 2011). Since scientists synthesized these compounds for use in exploration of the structural requirements for binding and activation of CB1 and CB2 receptors, most of the published research with them has been done in vitro (Atwood et al., 2010; Huffman and Padgett, 2005; Manera et al., 2008), while studies containing results from behavioral tests are relatively limited (Brents et al., 2011; Järbe et al., 2011; Vann et al., 2009; Wiley et al., 1998; Wiley et al., 1995). The emerging abuse problem, however, has placed an emphasis on the need for further evaluation of the in vivo pharmacology of indole-derived cannabinoids in order to better understand how these compounds interact with cannabinoid and noncannabinoid receptors, as well as to complete a more comprehensive characterization that may be useful for understanding their pharmacology and toxicology, for drug scheduling processes and, eventually, for development of treatment for individuals who abuse them (Vandrey et al., 2011).
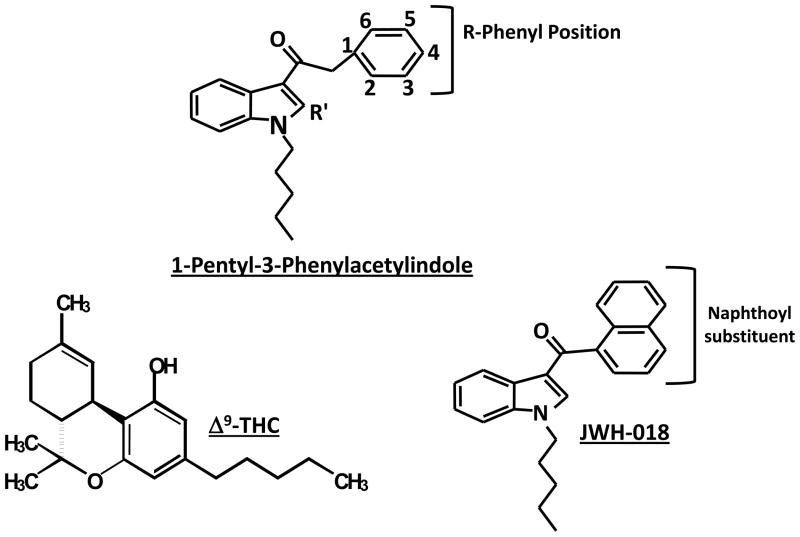
The purpose of the present paper is to report data on the potencies of selected indole-derived cannabinoids in a battery of tests in mice that are sensitive to the effects of psychoactive cannabinoids, including locomotor activity, antinociception (tail flick assay), and rectal temperature. In these tests, prototypical cannabinoid agonists produce the characteristic profile of antinociception, hypothermia, and suppression of locomotion (Martin et al., 1991). These 1-pentyl-3-phenylacetylindole cannabinoids retain the indole and pentyl side chain of JWH-018 (i.e., Spice/K2), but contain a phenylacetyl in place of JWH-018’s naphthoyl and vary in the position of different substituents attached to this phenylacetyl group (Figure 1).
Figure 1.
Chemical structures of THC and JWH-018. The structural template for the 1-pentyl-3-phenylacetylindoles is also shown, with positions of R and R′ groups indicated (see Tables 1 and 2).
2.0 Methods
2.1 Subjects
Male ICR mice (25–32g), obtained from Harlan (Dublin, VA) and housed in groups of five, were used for assessment of locomotor suppression, antinociception, and hypothermia. Different groups of mice (n=5–6 per dose) were used for testing each dose of each compound in this battery of procedures. These mice had free access to food and water when in their home cages. All animals were kept in a temperature-controlled (20–22°C) environment with a 12-hour light-dark cycle (lights on at 7 a.m.). The in vivo studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
2.2 Apparatus
Measurement of spontaneous activity in mice occurred in standard locomotor activity chambers interfaced with a Digiscan Animal Activity Monitor (Omnitech Electronics, Inc., Columbus, OH). A standard tail-flick apparatus and a digital thermometer (Fisher Scientific, Pittsburgh, PA) were used to measure antinociception and rectal temperature, respectively.
2.3 Drugs
Cannabinoid indoles (synthesized at Clemson University) were mixed in a vehicle of ethanol, emulphor (Rhone-Poulenc, Inc., Princeton, NJ) and saline in a 1:1:18 ratio. All injections were administered i.v. in the tail vein at a volume of 0.1 ml/10 kg.
2.4 Procedures
Each mouse was tested in three in vivo assays: locomotor activity, tail flick, and rectal temperature (Martin et al., 1991). Prior to injection, rectal temperature and baseline latency in the tail flick test were measured in the mice. The latter procedure involved exposing the mouse’s tail to an ambient heat source (i.e., bright light) and recording latency (in s) for tail removal. Typical control latencies were 2–4 s. A 10 s maximal latency was used in order to avoid damage to the mouse’s tail. After measurement of temperature and baseline tail flick latency, mice were injected i.v. with vehicle or drug. Five min later they were placed into individual activity chambers for 10 min. Spontaneous activity was measured as the total number of beam interruptions during the entire session. Tail-flick latency was measured at 20 min post-injection. A 10-s maximum test latency was used to avoid damage to the tail. Rectal temperature was measured at 30 min after injection. Whenever quantity of compound allowed, a full dose-effect curve determination in the test battery was conducted; however, insufficient quantities of some of the compounds resulted in probe tests with a single dose.
2.5 Data Analysis
Rectal temperature values were expressed as the difference between control temperature (before injection) and temperature following drug administration (Δ°C). Spontaneous activity was measured as total number of photocell beam interruptions during the 10-min session and was expressed percent inhibition of the control (vehicle) group’s activity. Antinociception was calculated as percent of maximum possible effect{% maximal possible effect = [(test − control latency)/(10 − control)] × 100}. Based on data obtained from numerous previous studies with cannabinoids, maximal cannabinoid effects in each procedure were estimated as follows: 90% inhibition of spontaneous activity, 100% MPE in the tail flick procedure, and −6 °C change in rectal temperature. ED50s were defined as the dose at which half maximal effect occurred. For drugs that produced one or more cannabinoid effects, ED50s were calculated separately using least-squares linear regression on the linear part of the dose-effect curve for each in vivo measure, plotted against log10 transformation of the dose. For the purposes of potency comparison, potencies were expressed as μmol/kg.
3.0 Results
The CB1 and CB2 receptor binding data shown in each of the two tables have been presented and discussed extensively in a previous publication (Huffman et al., 2005a). Their inclusion here is for ease of reference in comparison with the new in vivo data, which represent the main thrust of the paper.
Table 1 presents binding and pharmacological data for unsubstituted and methyl- and methoxy-phenylacetylindole analogs, as well as comparison data for THC and JWH-018. Substitution of a phenylacetyl (JWH-167) for the naphthoyl in JWH-018, the prototypic abused synthetic indole-derived cannabinoid, resulted in a 10-fold decrease in CB1 receptor affinity that was accompanied by a 7-fold reduction in average in vivo potency. Addition of a methyl at the 2 position of the indole core (JWH-205) further decreased potencies in the locomotor and antinociception assays (~10-fold), but did not affect potency for producing hypothermia. Methylation at the 2-position of the phenylacetyl group (JWH-251) increased average potency 2-fold compared to JWH-167 whereas methylation at 4-position (JWH-208) decreased it 3.6-fold. With additional 2-methylation of the indole core (JWH-209), potency was decreased even further (see Fig. 1 for numbering system). In the methoxy series (Table 1), addition of a methoxy group at the 2-phenylacetyl (JWH-306) and 3-phenylacetyl (JWH-302) positions increased average potencies compared to the comparable unsubstituted analogs, JWH-205 and JWH-167, respectively. Addition of a methoxy group at the 4-position resulted in greatly decreased potency (e.g., locomotor and antinociception measures for JWH-202) or elimination of cannabimimetic effects (e.g., antinociception for JWH-201). Neither JWH-201 nor JWH-202 produced the full profile of cannabinoid effects.
Table 1.
Cannabinoid receptor binding and in vivo effects of 1-pentyl-3-phenylacetylindoles, unsubstituted and with methyl and methoxy substituents at various positions on the phenyl ring.
| Compound | * R | * R′ | Affinities (nM)a | In Vivo ED50s (μmol/kg)** | Avg. Potency | |||
|---|---|---|---|---|---|---|---|---|
| CB1 | CB2 | SA | %MPE | RT | ||||
| Δ9-THC | ----- | ----- | 41b (2) | 36c (10) | 0.9 d | 2.6 d | 2.6 d | 2.0 |
| JWH-018 | Naphthoyl | H | 9d (5) | 3 (3) | 0.3d | ~ 0.09d | 1.8d | 0.7 |
| JWH-167 | Phenyl | H | 90 (17) | 159 (14) | 1.6 (0.9–2.6) | 1.3 (0.7–2.3) | 13 (7–23) | 5.3 |
| JWH-205 | Phenyl | CH3 | 134 (23) | 180 (9) | 19 (9–34) | 13 (9–19) | 13 (9–16) | 15 |
| JWH-251 | 2-Methylphenyl | H | 29 (3) | 146 (36) | 0.9 (0.6–1.6) | 0.9 (0.6–1.3) | 6 (3–9) | 2.6 |
| JWH-252 | 2-Methylphenyl | CH3 | 23 (3) | 19 (0.6) | NT | NT | NT | --- |
| JWH-208 | 4-Methylphenyl | H | 179 (10) | 570 (127) | 2.8 (0.9–9) | 16 (9–25) | 38 (22–63) | 18.9 |
| JWH-209 | 4-Methylphenyl | CH3 | 746 (49) | 1353 (270) | 39 (21–75) | 57 (33–99) | 81 (42–153) | 59 |
| JWH-250 | 2-Methoxyphenyl | H | 11 (2) | 33 (3) | NT | NT | NT | --- |
| JWH-306 | 2-Methoxyphenyl | CH3 | 25 (1) | 82 (11) | 87% (2.9 μg/kg) | 1.1 (0.9–1.4) | 1.1 (0.9–1.7) | 1.1 |
| JWH-302 | 3-Methoxyphenyl | H | 17 (2) | 89 (15) | 0.6 (0.3–1.2) | 0.9 (0.6–1.2) | 3 (2.1–4.2) | 1.5 |
| JWH-253 | 3-Methoxyphenyl | CH3 | 62 (10) | 33 (3) | NT | NT | NT | --- |
| JWH-201 | 4-Methoxyphenyl | H | 1064 (21) | 444 (14) | 84% (90 μg/kg) | 35% (90 μg/kg) | −2.3 (90 μg/kg) | --- |
| JWH-202 | 4-Methoxyphenyl | CH3 | 1678 (62) | 645 (6) | 26 (11–51) | 51 (29–97) | −2.5 (86 μg/kg) | 38.5 |
See Figure 1.
SA = spontaneous activity, %MPE = % maximum possible antinociceptive effect, RT = rectal temperature, NT = not tested. Values shown are ED50s (95% confidence intervals). Single dose tests are indicated by magnitude of effect and dose (in parentheses). Average potency was calculated as the average of all ED50 values for the compound.
(Huffman et al., 2005a) CB1 and CB2 affinities of all 1-pentyl-3-phenylacetylindoles presented first in this publication. Values shown are Ki (± SEM).
Table 2 presents binding and in vivo data for phenylacetylindoles with chloro, bromo or fluoro substitution at various positions on the phenyl group. As was seen with methylphenylacetyl and methoxyphenylacetyl substitutions, position of the chloro substituent affected potency. For example, substitution of 2- and 3-chlorophenylacetyl groups (JWH-203 and JWH-237, respectively) increased average potency compared to the unsubstituted phenyl analog (JWH-167). Substitution at the 2-position (JWH-203 and JWH-204) resulted in the best potencies, albeit potency for hypothermia production was inordinately lower than for the other two measures, particularly for JWH-203. Movement of the chloro substitution from the 2-position to the 3-phenylacetyl position (JWH-237) decreased potencies for locomotor suppression and antinociception by 15- and 10-fold, respectively, compared to JWH-203; however, potency for producing hypothermia was decreased 2-fold. Determination of potencies for JWH-303, and the 4-substituted analogs, JWH-206 and JWH-207, was not possible due to insufficient quantities of each compound for testing a range of doses. For each of these compounds, a single probe dose was assessed. An i.v. dose of 30 mg/kg JWH-303 (3-chlorophenyl with 2-methylation of the indole) produced the full profile of cannabinoid effects in the test battery whereas an i.v. dose of 10 mg/kg of JWH-207 and JWH-206, containing 4-chlorophenyl substituents with and without 2-methylation of the indole core, respectively, did not elicit cannabinoid activity.
Table 2.
Cannabinoid receptor binding and in vivo effects of 1-pentyl-3-phenylacetylindoles with chloro, bromo and fluoro substituents at various positions on the phenyl ring.
| Compound | * R | * R′ | Affinities (nM)a | In Vivo ED50s (μmol/kg)** | Avg. Potency | |||
|---|---|---|---|---|---|---|---|---|
| CB1 | CB2 | SA | %MPE | RT | ||||
| JWH-203 | 2-Chlorophenyl | H | 8 (0.9) | 7 (1) | 0.1 (0.09–0.2) | 0.3 (0.2–0.6) | 6 (5–6) | 2.1 |
| JWH-204 | 2-Chlorophenyl | CH3 | 13 (0.7) | 25 (1) | 0.8 (0.3–1.7) | 0.6 (0.6–0.8) | 2 (1.4–2.5) | 1.1 |
| JWH-237 | 3-Chlorophenyl | H | 38 (10) | 106 (2) | 1.5 (0.9–3) | 3 (3–6) | 3 (2.6–6) | 2.5 |
| JWH-303 | 3-Chlorophenyl | CH3 | 117 (10) | 138 (12) | 90% (85 μg/kg) | 100% (85 μg/kg) | −4 (85 μg/kg) | --- |
| JWH-206 | 4-Chlorophenyl | H | 389 (25) | 498 (37) | 76% (29 μg/kg) | inactive (29 μg/kg) | inactive (29 μg/kg) | --- |
| JWH-207 | 4-Chlorophenyl | CH3 | 1598 (134) | 3723 (110) | Inactive (28 μg/kg) | inactive (28 μg/kg) | inactive (28 μg/kg) | --- |
| JWH-249 | 2-Bromophenyl | H | 8 (2) | 20 (2) | 1 (0.5–2) | 0.3 (0.3–0.5) | 1 (0.8–1.3) | 0.8 |
| JWH-305 | 2-Bromophenyl | CH3 | 15 (2) | 29 (5) | 1.5 (0.2–7.5) | 1.8 (1.3–2.5) | 5 (5–8) | 2.8 |
| JWH-248 | 4-Bromophenyl | H | 1028 (39) | 657 (19) | Inactive (26 μg/kg) | inactive (26 μg/kg) | inactive (26 μg/kg) | --- |
| JWH-304 | 4-Bromophenyl | CH3 | 3363 (332) | 2679 (688) | NT | NT | NT | --- |
| JWH-311 | 2-Fluorophenyl | H | 23 (3) | 39 (3) | 2.5 (out of range) | 1.2 (0.9–1.9) | ~ 9 μg/kg | 4.2 |
| JWH-314 | 2-Fluorophenyl | CH3 | 39 (2) | 76 (4) | NT | NT | NT | --- |
| JWH-312 | 3-Fluorophenyl | H | 72 (7) | 91 (20) | 6 (5–7) | 1.9 (1.2–2.5) | 5.6 (5.3–5.9) | 4.5 |
| JWH-315 | 3-Fluorophenyl | CH3 | 430 (24) | 182 (23) | NT | NT | NT | --- |
| JWH-313 | 4-Fluorophenyl | H | 422 (19) | 365 (92) | Inactive (9 μg/kg) | inactive (9 μg/kg) | inactive (9 μg/kg) | --- |
| JWH-316 | 4-Fluorophenyl | CH3 | 2862 (670) | 781 (105) | NT | NT | NT | --- |
The effects of substitutions of bromine or fluorine at various positions on the phenyl ring paralleled results obtained with chlorine substitution. Of all of the phenylacetylindoles shown in this paper, JWH-249 (bromine substitution at the 2-phenylacetyl position) produced CB1 affinity and an average potency in mice that most closely matched values obtained with JWH-018. Addition of a 2-methyl to the indole core (JWH-305) decreased average potency by 3.5-fold, without changing the overall profile of effects. A bromine at the 4-position of the phenylacetyl group dramatically attenuated affinity (420-fold) [JWH-304]. This compound was not tested in vivo. Removal of the 2-methyl substituent of the indole from JWH-304 resulted in a compound (JWH-248) that was inactive at an i.v. dose of 10 mg/kg. Fluorine substitution at the 2-phenylacetyl position (JWH-311) produced decreases in potencies compared to other substitutions at the same position (i.e., bromine in JWH-249, chlorine in JWH-203, and methyl in JWH-251), although the resulting compound was slightly more potent than the unsubstituted analog (JWH-167). Changing the fluorine substituent from 2- to 3-position of the phenylacetyl group (JWH-312) produced little change in average potency despite the 3-fold decrease in CB1 receptor affinity whereas movement of the fluorine substituent to the 4-position (JWH-313) resulted in an inactive compound at an i.v. dose of 3 mg/kg. Insufficient supply did not allow testing of higher doses of JWH-313.
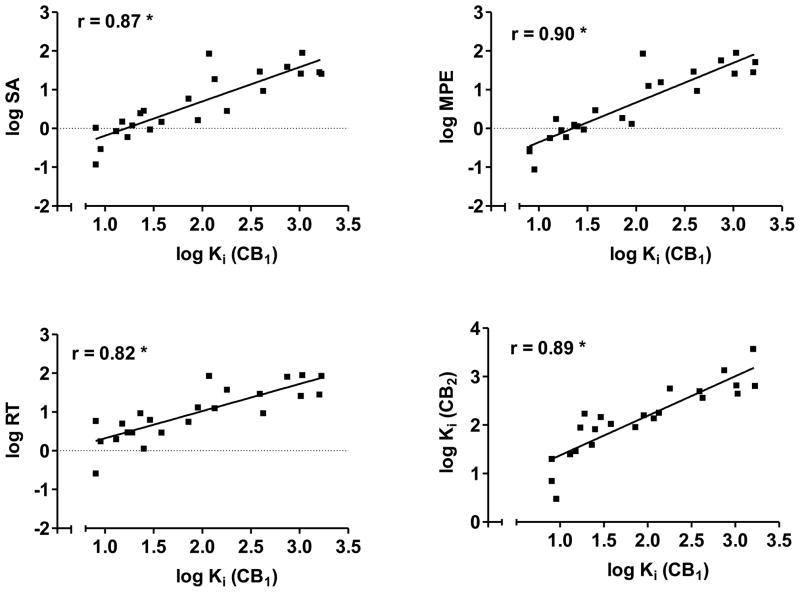
As shown in Figure 2, CB1 receptor affinity is significantly correlated with potencies for decreasing locomotor activity (Fig. 2, top left panel) and for producing antinociception (Fig. 2, top right panel) and hypothermia (Fig. 2, bottom left panel). In addition, CB1 and CB2 receptor affinities are highly correlated with each other (Fig. 2, bottom right panel). These results suggest that structural features of these 1-pentyl-3-phenylacetylindoles that influenced CB1 receptor affinity also affected their potency for producing cannabimimetic activity in mice.
Figure 2.
Scatterplots and regression lines for CB1 affininities (log Ki) plotted against log ED50 for each of the three in vivo tests (SA = spontaneous activity, top left panel; MPE = % maximum possible antinociceptive effect, top right panel; RT = change in rectal temperature, bottom left panel). The bottom right panel shows scatterplot and regression line for the relationship between CB1 and CB2 binding affinities (log Kis). Pearson product-moment correlations are shown for the two measures graphed in each panel. * indicates significant correlation (p<0.05).
4.0 Discussion
Structure-activity relationship analysis of CB1 and CB2 receptor binding affinities for this series of 1-pentyl-3-phenylacetylindoles has been presented previously (Huffman et al., 2005a). Briefly, major findings of this analysis include the following findings: (1) attenuated CB1 receptor affinity for 1-pentyl-3-phenylacetylindoles compared to their 1-pentyl-3-naphthoylindole congeners (Huffman et al., 2005b); (2) tendency for greater CB1 and CB2 receptor affinities for 2-substituted phenylacetylindoles than for 3-substituted analogs; (3) decreased CB1 and CB2 receptor affinities with 2-methyl substitution on the indole core as compared with unsubstituted analogs in the same series; and (4) poor to negligible CB1 and CB2 receptor affinities of 4-substituted phenylacetylindoles. In addition, some of the phenylacetylindoles showed CB1 receptor selectivity (e.g., JWH-251 and JWH-302), which is in contrast with the typical CB2 receptor selectivity that has been reported for several series of indole-derived cannabinoids (Huffman, 2005; Huffman et al., 2005b).
In the mouse test battery, 3-phenylacetylindoles produced the same profile of effects (i.e., locomotor suppression, antinociception and hypothermia) as other classes of plant-derived and synthetic cannabinoids, including THC, bicyclic cannabinoids, and aminoalkylindoles (Compton et al., 1992a; Compton et al., 1992b; Martin et al., 1991). Potency for producing hypothermia tended to be less than or equal to potency for decreasing locomotion for 1-pentyl-3-phenylacetylindoles, as well as for THC and JWH-018. Changing the substituent on the phenyl ring, or its position from the 2- to 3-position did not alter this tendency, but moving it to the 4-position essentially eliminated activity. On the other hand, the indole-derived cannabinoids exhibited greater relative antinociceptive potencies (compared to their hypothermic potencies), a property that was not shared by THC in the present study. Together, these results suggest that the pharmacological properties of THC and indole-derived synthetic cannabinoids are similar, but not identical. Anecdotal and case reports from human users of synthetic cannabinoids also have noted similarities and differences in the subjective and physiological effects of marijuana and “herbal incense” products (Schifano et al., 2009; Schneir et al., 2011; Vardakou et al., 2010).
Despite differences in the relative potencies for the various effects of cannabinoids, structure-activity relationship analysis for the pharmacological effects of the 3-phenylacetylindoles in mice paralleled trends observed for CB1 receptor binding, as has also been reported previously for classical cannabinoids (Compton et al., 1993) and naphthoylindoles (Wiley et al., 1998), suggesting that the cannabinoid effects of 1-pentyl-3-(phenylacetyl)indoles are similarly mediated by the CB1 receptor. The major structural manipulations in the present series included the phenylacetyl substituent (i.e., unsubstituted, methyl, methoxy, chloro, bromo, and fluoro), the position of the substituent on the phenyl ring (i.e., 2-, 3- or 4-position), and presence of a methyl group at the 2-position of the indole core. Of these factors, the position of the substituent on the phenylacetyl group was the most critical factor affecting in vivo potency of these compounds. Whereas compounds with 2- and 3-phenylacetyl substituents were efficacious with good potencies in the mouse test battery, 4-substituents resulted in compounds that had poor potency or were inactive. Similar positional biases were observed when CB1 receptor affinities of a series of 1-alkyl-2-aryl-4-(1-naphthoyl)pyrroles with methyl, methoxy, fluro, and chloro substitutions were evaluated. For each substitution, affinities were best with substitution at the 2-position of the pyrrole substituent, with intermediate affinities for the 3-position and with the least affinity for compounds with 4-substitutions (Huffman et al., 2006), suggesting that positional substitutions following conversion of the indole template to a pyrrole produce similar changes in affinities as deletion of one of the naphthoyl rings (i.e., conversion to a phenylacetyl) with the same positional substitutions on the opposite side of the molecule (as in the present series). Hence, the position of ring substituents on either side of the molecule appears to be important for CB1 receptor affinity and in vivo potency for indole-derived cannabinoids, as it has been shown to be for synthetic cannabinoids based upon a THC structural template (Martin et al., 1999). For example, compounds with halogen (e.g., iodo, bromo, fluoro) or nitrogen substitutions at the C2- and 4-aryl positions (on either side of the C3 pentyl side chain of THC) had less CB1 receptor affinity and were less potent in vivo than compounds with similar substitutions at the terminal end of the side chain (Martin et al., 1993). 2-Methylation of the indole core tended to decrease potency, albeit this effect did not occur in all cases (e.g., JWH-203 vs. JWH-204).
In contrast with the substantial effect of position of the phenylacetyl substituent on affinity and in vivo activity, the nature of the substituent was of lesser importance in determination of activity in the mice, although it did affect potencies. Average potencies ranged from 0.8 – 2.8 μmol/kg for bromo, methoxy, chloro, and methyl substitutions at the 2- and 3-positions of the phenylacetyl group. Fluoro substitution at these positions slightly decreased average potency (4.2 – 4.5 μmol/kg) whereas unsubstituted compounds were even less potent (5.3 – 15 μmol/kg). Although the molecular mechanism for decreased potency with fluoro substitution compared to other halogens cannot be definitively determined from the data presented here, the enhanced electronegativity of fluorine may play a role, as fluoro substitution for the hydroxy group of THC has also been shown to decrease CB1 receptor affinity and in vivo potency in a series of fluorinated classical cannabinoid analogs (Crocker et al., 2007). Interestingly, JWH-018, an unsubstituted 1-pentyl-naphthoylindole, was more potent than any of the compounds in the present series, emphasizing the role of aromatic stacking (i.e., two phenyl rings in naphthoyl versus one in the phenylacetyl series) in the CB1 receptor affinity and in vivo potency of indole-derived cannabinoids (Huffman et al., 2003; Huffman et al., 2005b). It is also noteworthy that the most potent indole-derived cannabinoid, JWH-018, was the first to be detected in synthetic cannabinoids, suggesting that the manufacturers of these products are knowledgeable chemists and have read the scientific literature.
Given similarities in the in vivo pharmacology of THC and the 3-phenylacetylindoles, a significant translational issue is the extent to which this series of synthetic cannabinoids might be expected to produce marijuana-like intoxication if ingested by humans. Although some of the compounds presented here produce cannabinoid effects in the test battery in mice, efficacy in producing the tetrad profile (i.e., locomotor suppression, antinociception, hypothermia and catalepsy) is not entirely selective for psychoactive cannabinoids. For example, a number of drugs (e.g., morphine, diazepam) produce one or more of the tetrad effects and certain antipsychotics (e.g., haloperidol, clozapine) exhibit all four effects (Wiley and Martin, 2003). On the other hand, THC discrimination has been shown to possess greater pharmacological specificity for psychoactive cannabinoids (Barrett et al., 1995) and results from this procedure are predictive of cannabis-like intoxication in humans (Balster and Prescott, 1992). Although insufficient supply prevented testing most of the compounds in this more selective assay, three compounds from this series have been tested in mice trained to discriminate THC from vehicle, with results suggesting that CB1 binding affinity predicts substitution for THC in this indole series (Vann et al., 2009). Hence, JWH 204 [1-pentyl-2-methyl-3-(2-chlorophenylacetyl)indole; CB1 Ki = 13 nM] substituted with good potency for THC. JWH-205 [1-pentyl-2-methyl-3-(phenylacetyl)indole] also produced THC-like effects, but potency was more moderate, as is consistent with the poorer CB1 affinity of this compound (Ki = 134 nM). In contrast, JWH-202 [1-pentyl-2-methyl-3-(4-methoxyphenylacetyl)indole; Ki = 1678 nM] failed to substitute for THC. These results are consistent with those of previous reports in which WIN55,212-2 and other indole- and pyrrole-derived cannabinoids that are active in the mouse profile tests produce THC-like effects in drug discrimination in rodents (Compton et al., 1992a; Wiley et al., 1998) and monkeys (Wiley et al., 1995). CB1 receptor mediation of the discriminative stimulus effects of aminoalkylindoles is indicated by rimonabant reversal (Järbe et al., 2011).
In summary, the in vivo data presented here show that 1-pentyl-3-phenylacetylindoles with good CB1 binding affinity share pharmacological properties with THC in mice. Combined with the results of probe tests with three of the compounds with a range of CB1 binding affinities, the present data also suggest that the compounds in this series that elicit a THC-like profile in a test battery in mice would be likely to produce cannabimimetic discriminative stimulus effects in rodents and would be predicted to be marijuana-like in humans, should they be synthesized and distributed illicitly. These data provide an empirical basis for decisions concerning scheduling of these and similar indole-derived synthetic cannabinoids; however, together with the results of previous structure-activity relationship studies with cannabinoids, they also emphasize the complexity of molecular interactions of synthetic cannabinoids with CB1 receptors and suggest that scheduling efforts based solely upon structural features should proceed with caution.
Acknowledgments
5.1 Role of funding source
Funding for this study was provided by National Institute on Drug Abuse Grants DA-031988, DA-03672, and DA-03590; NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The expert technical assistance of Ramona Winckler is acknowledged. The authors thank Dr. Brian Thomas for his careful reading and insightful comments on the initial draft. This manuscript is dedicated to the memory of Dr. Billy Martin.
Footnotes
5.2 Contributors
John Huffman designed and synthesized the compounds used in the study. Billy Martin and Jenny Wiley designed the in vivo experiments and wrote the protocol. Julie Marusich organized and compiled the data into an initial draft for presentation at the 2011 meeting of the College on Problems of Drug Dependence. Jenny Wiley wrote the first draft of the manuscript. All authors contributed to and, with the exception of Billy Martin, have approved the final manuscript.
5.3 Conflicts of interst
All of the authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta-9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta-9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992a;263:1118–1126. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992b;260:201–209. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Crocker PJ, Mahadevan A, Wiley JL, Martin BR, Razdan RK. The role of fluorine substitution in the structure-activity relationships (SAR) of classical cannabinoids. Bioorg Med Chem Lett. 2007;17:1504–1507. doi: 10.1016/j.bmcl.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, et al. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J Med Chem. 1992;35:124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration. Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I. Fed Reg. 2010;75:71635–71638. [PubMed] [Google Scholar]
- Eissenstat MA, Bell MR, D’Ambra TE, Alexander EJ, Daum S, Ackerman J, Gruett M, Kumar V, Estep KG, Olefirowicz EM, Wetzel J, Alexander MD, Weaver J, Haycock D, Luttinger D, Casiano F, Chippari S, Kuster J, Stevenson J, Ward SJ. Aminoalkylindoles: structure-activity relationships of novel cannabinoid mimetics. J Med Chem. 1995;38:3094–3105. doi: 10.1021/jm00016a013. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3:466–478. doi: 10.1002/dta.268. [DOI] [PubMed] [Google Scholar]
- Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. [Google Scholar]
- Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR. 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor. Bioorg Med Chem. 2003;11:539–549. doi: 10.1016/s0968-0896(02)00451-0. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR. 1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: New high affinity ligands for the cannabinoid CB(1) and CB(2) receptors. Bioorg Med Chem Lett. 2006;16:5432–5435. doi: 10.1016/j.bmcl.2006.07.051. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005a;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005b;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta-9-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A. Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem. 2008;8:370–387. doi: 10.2174/138955708783955935. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Semus SF, Lin S, Marciniak G, Grzybowska J, Charalambous A, Makriyannis A. Pharmacological evaluation of iodo and nitro analogs of delta-8-THC and delta-9-THC. Pharmacol Biochem Behav. 1993;46:295–301. doi: 10.1016/0091-3057(93)90356-x. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, Saha B, Razdan RK. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J Pharmacol Exp Ther. 1999;290:1065–1079. [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Schifano F, Corazza O, Deluca P, Davey Z, Di Furia L, Farre M, Flesland L, Mannonen M, Pagani S, Peltoniemi T, Pezzolesi C, Scherbaum N, Siemann H, Skutle A, Torrens M, Van Der Kreeft P. Psychoactive drug or mystical incense? Overview of the online available information on Spice products. Int J Culture Mental Health. 2009;2:137–144. [Google Scholar]
- Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2010;40:296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Thakur GA, Nikas SP, Makriyannis A. CB1 cannabinoid receptor ligands. Mini Rev Med Chem. 2005;5:631–640. doi: 10.2174/1389557054368772. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.07.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta-9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta-9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2011 Jul 28; doi: 10.1016/j.ajem.2011.05.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold. Dtsch Arztebl Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]