Abstract
Limiting energy availability via diet or physical activity has health benefits; however, it is not known if these interventions have similar effects on the development of cancer. Two questions were addressed: 1) does limiting energy availability by increasing physical activity have the same effect on mammary carcinogenesis as limiting caloric intake, and 2) are host systemic factors, implicated as risk biomarkers for breast cancer, similarly affected by these interventions? Female Sprague Dawley rats were injected with 50 mg 1-methyl-1-nitrosourea/kg body weight at 21 days of age and randomized to one of five groups (30 rats/gp): 1) sham running wheel control; 2) restricted fed to 85% of the sham-control, 3 and 4) voluntary running in a motorized activity wheel (37 m/min) to a maximum of 3500 m/day or 1750 m/day, and 5) sedentary ad libitum fed control with no access to a running wheel. The three energetics interventions inhibited the carcinogenic response, reducing cancer incidence (P=0.01), cancer multiplicity (P<0.001), and cancer burden (P<0.001), while prolonging cancer latency (P=0.004) although differences among energetics interventions were not significant. Of the plasma biomarkers associated with the development of cancer, the energetics interventions reduced bioavailable insulin-like growth factor-1(IGF-1), insulin, interleukin-6, serum amyloid protein, tumor necrosis factor-α, and leptin and increased IGF binding protein 3 (IGFBP3) and adiponection. Plasma fasting glucose, C-reactive protein, estradiol, and progesterone were unaffected. The plasma biomarkers of greatest value in predicting the carcinogenic response were: adiponectin > IGF-1/IGFBP-3 > IGFBP-3 > leptin > IGF-1.
Keywords: physical activity, dietary energy restriction, mammary carcinogenesis, adipokines, insulin like growth factors, chronic inflammation
Introduction
Over 60% of the population of the United States is currently classified as overweight or obese based on body mass index, and given the link between excess risk for a number of chronic diseases including cancer, there has been intense interest in defining the role of energetics in the development of cancer (1, 2). One cancer site that has been the focus of extensive investigation is the breast and for this site, the relationships are complex. While obesity is associated with increased risk for post-menopausal breast cancer, risk for the disease in pre-menopausal women is either unaffected or slightly decreased by obesity (3–7). However, either weight loss in overweight/obese women or increased levels of physical activity in pre- or post-menopausal women have been reported to be protective against breast cancer (8–10). Thus, a key but often unappreciated consideration in energetics and cancer research is the identification of a daily level of net energy available to an organism that protects against the development of cancer relative to the level of net energy availability that promotes the development of cancer (11). Net energy availability is defined as the amount of energy (kcal) available to an organism based on dietary energy intake after the needs for maintenance, digestion, and physical activity are met, a condition also referred to as energy balance. Net energy available can be stored as glycogen and fat and/or channeled into growth processes, including those associated with the development of cancer.
The term physical activity describes any type of work involving skeletal muscle contraction that results in a quantifiable expenditure of energy; whereas, exercise refers to a specific plan of physical activity designed to improve physical fitness (12–14). In this experiment, rats were allowed to run in a motorized activity wheel in response to food reward which permitted us to carefully titrate energy intake with energy expenditure. Nonetheless, running was voluntary and was also limited so as not to exceed either 1750 or 3500 m/day, thus this was an investigation of the effects of physical activity, not exercise.
Mechanistic inquiries of the relationship between energetics and cancer risk can be divided into the investigation of host systemic factors and cell autonomous processes, and undoubtedly they are related and both are important to the full understanding of how various interventions impact breast cancer risk (15, 16). Nonetheless, given the current state of knowledge of these processes, each area is quite broad and involves a large number of components that require examination. For that reason, the focus of the work reported herein was on host systemic factors. Four families of plasma analytes were studied because physical activity and dietary energy restriction have been reported to exert effects on them and these same analytes have been implicated in the carcinogenic process (17–23). They are: glucose homeostasis, chronic inflammation, secreted cytokines (myokines/adipokines), and sex steroid hormones.
The experiment reported was designed to evaluate the overall effect of very modest differences of energy availability to the host on the post initiation phase of chemically induced mammary carcinogenesis. Differences in energy availability were achieved by varying energy intake or energy expenditure. Two questions were addressed: 1) does limiting energy availability by increasing energy expenditure via physical activity have the same effect on mammary carcinogenesis as limiting food intake, and 2) does limiting energy availability via physical activity versus dietary energy restriction have the same effects on host systemic factors that have been implicated as risk factors for breast cancer?
Materials and Methods
Chemicals and Reagents
Carcinogen: 1-methyl-1-nitrosourea (MNU) was obtained (Ash Stevens, Detroit, MI) and stored at −80°C prior to use. The following kits and reagents were used to conduct the experiments: glucose-hexokinase liquid stable reagent (Thermo Fisher Scientific Inc., Waltham, MA); enzyme-linked immunosorbent assay (ELISA) C-reactive protein (CRP) kit (Helica Biosystems, Inc., Fullerton, CA); multiplex and signalplex kits for insulin, leptin, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) and insulin growth factor-1 (IGF-1) as well as ELISA kit of adiponectin (Millipore, Billerica, MA); insulin growth factor binding protein 3 (IGFBP-3) ELISA kit (Mediagnost, Reutlingen, Germany); commercial ELISA kits for serum amyloid P (SAP), 17-beta-estradiol, and progesterone (GeneWay Biotech, San Diego, CA); purified pelleted diet (Test Diet, Division of Land O’Lakes Purina Feed, LLC, Richmond, IN).
Experimental Design
Female Sprague Dawley rats were obtained (Charles River, Wilmington, MA) at 20 days of age. At 21 days of age, rats were injected with 50 mg MNU/kg body weight, i.p., as previously described (24). Rats were individually housed in solid bottomed polycarbonate cages equipped with a motorized running wheel and food delivery system. At 28 days of age, 1 week after carcinogen injection, rats were assigned by stratified randomization using body weight to one of five groups (30 rats/group): 1) sham running wheel control fed using a pellet dispenser (PFD-Ctl; 2) restricted fed to 85% of the sham-control (DER), 3 and 4) voluntary running in a motorized activity wheel (37 m/min) to a maximum of 3500 m/day (WR-HIGH) or 1750 m/day (WR-LOW), and 5) sedentary ad libitum fed control with no access to a running wheel (Ad Lib Ctl). For all rats other than the AD Lib-Ctl, total daily food allotment was based on body mass (body weight, g0.75) as detailed in references (25–28). The rats ran at a constant speed (37 m/min) and the running wheel was stopped by an auto-brake when distance run reached either 3500m or 1750m/day or when the 12 hour allotted time to run was reached in day.
Rats were fed a purified pelleted diet that is a modification of AIN-76A (Test Diet, Division of Land O’Lakes Purina Feed, LLC, Richmond, IN). Food pellets were distributed based on distance run as a positive reinforcement of running behavior. A computer device attached to the activity wheel monitored distance run and pellets delivered which were recorded daily.
Throughout the experiment, rats were weighed daily and were palpated twice weekly for the detection of mammary tumors. At necropsy, rats were skinned and the skin to which mammary gland chains were attached was examined under translucent light for detectable mammary pathologies. All grossly detectable mammary gland pathologies were excised and processed for histological classification. Mammary pathologies were categorized based criteria published in (29, 30). Only histopathologically confirmed mammary carcinomas are reported since they represented > 98% of the pathologies that were excised at necropsy (Supplementary Figure S1). The experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee and conducted according to the committee guidelines.
Blood collection and plasma biomarker analyses
Blood collection
Following an overnight fast, rats were euthanized over a 3-hour time interval, between 8 and 11 a.m., via inhalation of gaseous carbon dioxide. The sequence in which rats were euthanized was stratified across groups so as to minimize the likelihood that order effects would masquerade as treatment associated effects. After the rat lost consciousness, blood was directly obtained from the retro-orbital sinus and gravity fed through heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA) into EDTA coated tubes (Becton Dickinson, Franklin Lakes, NJ) for plasma. The bleeding procedure took approximately 1 min/rat. Thereafter, the unconscious rat was euthanized by cervical dislocation. Plasma was isolated by centrifugation at 1000 × g for 10 min at room temperature.
Plasma biomarkers
IGF-1, IGFBP-3, insulin, glucose, CRP, SAP, IL-6 and TNFα, adiponectin, leptin, progesterone, 17-β estradiol in plasma were determined using a commercially available ELISA (Mediagnost, Reutingen, Germany; GenWay Biotech, San Diego, CA) or Multiplex kits (Millipore, Billerica, MA) as previously described (31, 32).
Statistical Analyses
Differences among groups were evaluated as follows: incidence of mammary adenocarcinomas by chi-square analysis (33), the number of mammary adenocarcinomas per rat (multiplicity) by one-way analysis of variance (ANOVA) after square root transformation of tumor count data (33), cancer burden per rat by one-way ANOVA after log transformation of tumor mass per rat (33, 34), and cancer latency by life table analysis (33, 34). Differences in final body weight and circulating analytes were evaluated by one-way ANOVA with post hoc comparisons by the method of Bonferroni (34). For the analysis of plasma analytes between two levels of mammary carcinogenic responses, data were evaluated by t-test. All analyses were performed using Systat statistical analysis software, version 13 (Systat Software, Inc., Chicago, IL). The principle component analysis of the plasma analytes was performed using Partek (Partek, Inc., St. Louis, MO).
Results
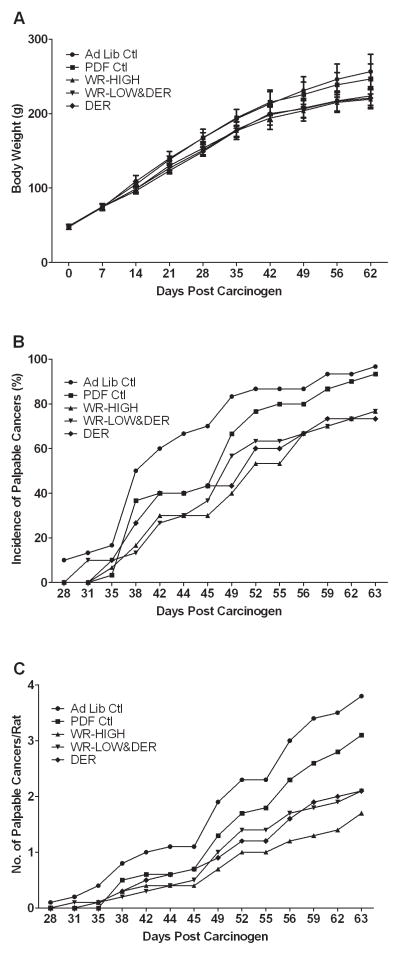
Rats that ran in activity wheels and/or that were provided a restricted number of dietary calories had similar final body weights and body weight gains per day (Table 1 and Figure 1A). In order to have similar overall net energy availability among the energy intervention groups, animals in the WR-HIGH group ate more diet than rats that were only DER (P<0.002) and the caloric intake of the WR-LOW group was intermediate to that of either the WR-HIGH group or the rats that were only DER, but neither difference was statistically significant. As intended, both food intake and weight gain of the energetics intervention groups were lower than that observed in either control group. Final body weights mirrored the same patterns observed among groups in food intake and body weight gain (Table 1). The rats ran a distance equivalent to achieving caloric restriction of approximately 7% or 3.5%, for WR-HIGH and WR-LOW respectively. These data support the net energy availability equivalence of the three energetics interventions that were investigated.
Table 1.
Effect of wheel running and dietary energy restriction on body weight, body weight gain, food eaten and running distance
| Dietary treatment | Ad Lib Ctl | PDF- Ctl | WR-HIGH | WR-LOW & DER | DER | Overall P |
|---|---|---|---|---|---|---|
| Final Body Weight (g) | 259 ± 24a | 249 ± 21a | 221 ± 13b | 226 ± 13b | 223 ± 15b | < 0.001 |
| Body Weight Gain (g/day) | 2.94 ± 0.47a | 2.82 ± 0.32a | 2.36 ± 0.22b | 2.48 ± 0.24b | 2.54 ± 0.25b | < 0.001 |
| Food Eaten (g/day) | 15.5 ± 1.6a | 14.7 ± 0.75b | 13.4 ± 0.6c | 13.0 ± 0.42c,d | 12.6 ± 0.3d | < 0.001 |
| Exercise (m/day) | ----- | ----- | 3139 ± 339a | 1623 ± 168b | ---- | < 0.001 |
Values are means ± SD (N = 30/gp). Data were analyzed by One-way ANOVA with Bonferroni Post Hoc Test. Values within a row with different superscripts (a, b, c or d) are statistically different from each other (P < 0.05). Value with superscript (c,d) is not statistically different from values with superscripts (c or d). Ad Lib, ad libitum; Ctl, control; PDF, paired-fed; WR, wheel running; DER, dietary energy restriction.
Figure 1.
Effect of physical activity and/or dietary energy restriction on body weight gain and the carcinogenic response. (A) Body weight gain. Values at each time point are means ± SD (N = 30). (B) Incidence of palpable mammary cancer, which was calculated using the number of cancer-bearing rats divided by total number of rats for each group. (C) Number of palpable mammary carcinoma number per rat, which was calculated using the total number of cancer divided by total number of rats for each group. Ad Lib, ad libitum; Ctl, control; PDF, paired fed; WR, wheel running; DER, dietary energy restriction.
The carcinogenic response that was observed in each intervention group is shown (Table 2 and Figure 1B and 1C). As noted in the Materials and Methods section, what distinguished the two control groups was that the ad libitum fed control group had access to food 24 hours per day; whereas, the pellet dispenser fed-control group only received food over the 12 hour run cycle when the wheel running rats earned their respective food rewards and the total amount of food allotted was computed to meet their energy requirements based on body mass. While there were numerical differences in the carcinogenic response in the two control groups, none of these differences reached the level of statistical significance. Similarly, all three energetics interventions inhibited the carcinogenic response, reducing cancer incidence, cancer multiplicity, and cancer burden, while prolonging cancer latency (Table 2). However, while numerical differences in the carcinogenic response existed among the three energetics interventions, none of these differences was of sufficient magnitude to reach the level of statistical significance.
Table 2.
Effect of wheel running and dietary energy restriction on the carcinogenic response in mammary gland
| Dietary treatment | Ad Lib Ctl | PDF- Ctl | WR-HIGH | WR-LOW & DER | DER | Overall P |
|---|---|---|---|---|---|---|
| Cancer Incidence (%) | 97.0a | 100.0a | 80.0b | 77.0b | 77.0b | 0.010 |
| Cancer Multiplicity (No. carcinomas/rat) | 4.8 ± 2.8a | 3.6 ± 2.5a,b | 2.0 ± 1.9b | 2.4 ± 2.0b | 2.6 ± 2.4b | < 0.001 |
| Cancer Burden (Ave. cancer mass/rat (g)) | 6.16 ± 6.73a (3.65 – 8.68) | 3.33 ± 4.74a,b (1.56 – 5.10) | 1.82 ± 3.37b (0.57 – 3.08) | 2.24 ± 3.42b (0.96 – 3.52) | 3.15 ± 5.02b (1.27 – 5.02) | 0.001 |
| Cancer Latency (day) | 43a (40 – 46) | 48a,b (44 – 51) | 52b (49 – 55) | 51b (48 – 54) | 50b (47 – 54) | 0.001 |
Values are means ± SD (N = 30/gp) for cancer multiplicity and cancer burden; the numbers in parentheses for cancer burden and cancer latency are 95% confidence intervals. Data were analyzed by Fisher’s exact test (cancer incidence), and contrasts in a Kruskal-Wallis test with Conover-Inman analysis for pairwise comparisons among groups (cancers per rat and cancer burden) and survival analysis for cancer latency. The data for cancers per rat were square root transformed before analysis. Values within a row with different superscripts (a, and b) are statistically different from each other (P < 0.05). Values with superscripts (a,b) are not statistically different from values with superscripts (a or b). Ad Lib, ad libitum; Ctl, control; PDF, paired fed; WR, wheel running; DER, dietary energy restriction.
Four families of plasma biomarkers investigated that have been implicated in the development of cancer are: 1) glucose homeostasis, 2) chronic inflammation, 3) secreted adipokines, and 4) sex steroid hormones. The effect of the energetics interventions on these plasma biomarkers are shown (Table 3). For each biomarker category, there were no statistically significant differences in plasma analyte concentrations between the two control groups, although numerical differences did exist.
Table 3.
Effect of wheel running and dietary energy restriction on plasma analytes
| Dietary treatment | Ad Lib Ctl | PDF-Ctl | WR-HIGH | WR-LOW & DER | DER | Overall P |
|---|---|---|---|---|---|---|
| Glucose homeostasis | ||||||
| IGF-1 (ng/ml) | 361 ± 69a | 366 ± 86.2a | 262 ± 44.0b | 270 ± 48.6b | 260 ± 66.3b | < 0.001 |
| IGFBP-3 (ng/ml) | 110 ± 22.4a | 115 ± 26.7a | 132 ±24.8b | 125 ± 23.6a,b | 121 ± 23a,b | 0.006 |
| IGF-1/IGFBP-3 | 3.30 ± 0.33a | 3.20 ± 0.31a | 2.01 ± 0.27b | 2.18 ± 0.32b | 2.14 ± 0.32b | < 0.001 |
| Insulin (pg/ml) | 1387 ± 187a | 1258 ± 160a | 833 ± 223b | 1050 ± 543b | 1011 ± 193b | < 0.001 |
| Glucose (mg/dl) | 141 ± 28.0 | 139 ± 24.0 | 141 ± 36.0 | 139 ± 23.8 | 136 ± 40.8 | 0.964 |
| Inflammation | ||||||
| IL-6 (pg/ml) | 62 ± 10.9a,b | 60 ± 9.53a,b | 66 ± 9.4a | 64 ± 10.4a | 56 ± 10.3b | 0.002 |
| SAP (μg/ml) | 424 ± 91.8a | 396 ± 52.4a | 339 ± 67.5b | 337 ± 37.0b | 336 ± 51b | < 0.001 |
| CRP (μg/ml) | 487 ± 224 | 488 ± 213 | 476 ± 105 | 476 ± 174 | 405 ± 149 | 0.344 |
| TNFα (pg/ml) | 9.5 ± 1.0a | 9.2 ± 1.1a | 8.1 ± 1.2b | 8.2 ± 0.9b | 7.9 ± 1.0b | < 0.001 |
| Adipokines | ||||||
| Adiponectin (μg/ml) | 14 ± 4.73a | 15 ± 2.17a,b | 18 ± 8.1a,b | 16 ± 6.0a,b | 19 ± 6.4b | 0.019 |
| Leptin (pg/ml) | 2106 ± 291a | 2135 ± 312a | 1088 ± 154b | 1099 ± 177b | 1104 ± 130b | < 0.001 |
| Sex Steroid Hormones | ||||||
| Estradiol (pg/ml) | 18 ± 8.9 | 17 ± 9.0 | 16 ± 7.9 | 16 ± 9.2 | 16 ± 8.3 | 0.917 |
| Progesterone (ng/ml) | 18 ± 11.1 | 18 ± 12.9 | 23 ± 14.5 | 22 ± 14.6 | 20 ± 8.6 | 0.408 |
Values are means ± SD (N = 30/gp). Data were analyzed by one-way ANOVA with Bonferroni Post Hoc Test. Values within a row with different superscripts (a and b) are statistically different from each other (P < 0.05). Values with superscripts (a,b)are not statistically different from values with superscripts (a or b). Ad Lib, ad libitum; Ctl, control; PDF, paired fed; WR, wheel running; DER, dietary energy restriction.
Relative to the control groups, the energetics interventions resulted in favorable changes in several biomarkers associated with glucose homeostasis (insulin, IGF-1, IGFBP-3 and IGF-1/IGFBP3), but the numerical differences among the energetics interventions were small and did not reach the level of statistical significance. Only plasma glucose was unaffected across both the control and intervention groups.
For markers of chronic inflammation, none of the differences in IL-6, TNF-α, CRP, or SAP were significantly different between control groups; whereas, all biomarkers, with the exception of CRP, were significantly different in the intervention groups versus the control groups. CRP was not affected by control or intervention group status. IL-6 was differentially affected by the energetics intervention, being elevated by wheel running and suppressed by caloric restriction.
The third family of markers assessed was the adipokines. Leptin is produced by adipocytes and is regarded as a reliable indicator of body fat content (35). The levels of leptin were similar in the two control groups and markedly reduced by each energetics intervention, but the plasma concentration of leptin was not different among the three energetics interventions. On the other hand, the plasma concentration of adiponectin was similar in both control groups and slightly elevated by the energetics interventions, an effect that was statistically significant only for the dietary energy restricted group.
The fourth family of plasma biomarkers assessed was the sex steroid hormones, i.e. 17-β estradiol and progesterone. No differences in plasma concentrations of these hormones were observed across control or intervention groups.
In an effort to identify the effect of the energetics interventions on plasma biomarkers that may be involved in mediation of cancer inhibitory activity from those that are not causally involved, a series of additional analyses were undertaken. These analyses were considered hypothesis generating so that only raw P-values (not adjusted for multiple comparisons) are reported. All 150 rats in this experiment were identified as being either cancer bearing or cancer-free (Supplementary Table S1). For statistical analysis, this distinction was used to create a coding variable that served as the independent variable for a t-test on each plasma biomarker that was assessed. Of the plasma biomarkers evaluated, significant differences occurred in animals with or without cancer for IGF-1, the ratio of IGF-1 to IGFBP3, adiponectin, leptin, and SAP. These findings are summarized only for the analytes that were significantly different in cancer bearing versus cancer free rats (Table 4). The average concentrations of each plasma analyte in animals with or without cancer, irrespective of their treatment group assignment is also shown (Table 4). A second parameter by which the carcinogenic response is assessed in this breast cancer model is cancer multiplicity. The median number of cancers per rat in the 150 rats studied was 2.0. Therefore, rats with < the median value were assigned a coding variable value of 1; whereas, rats harboring 2 or more cancers were assigned a score of 2. The results of the t-test analyses on each plasma analyte are shown (Supplementary Table S2). Plasma analytes for which values were statistically different in animals < the median or ≥ the median for cancer multiplicity were IGFBP-3, IGF-1/IGFBP3, adiponectin, leptin, and SAP (Table 4). A third parameter by which the carcinogenic response is assessed in this cancer model is cancer burden, i.e. cancer mass per rat. The median cancer burden in all 150 rats was 1.058 g/rat. Using the same approach for creating a coding variable as used for cancer multiplicity, the plasma analyte data were then evaluated by t-test (Supplementary Table S3). Significant differences were observed for IGFBP-3, and adiponectin (Table 4). The fourth parameter by which the carcinogenic response is assessed is cancer latency. The median time to detection of the first palpable mammary cancer, irrespective of treatment group assignment was 49 days. Using the same approach to coding variable construction as described above, the plasma variables for which the effect was significant for cancer latency < versus ≥ the median (Supplementary Table S4) were: IGFBP3, IGF-1/IGFBP3, and adiponectin. A weighted score for each plasma analyte that had predictive value for one or more parameters in the carcinogenic response is shown (Table 4). The score is weighted in that it is the summation of the t-statistic on which probabilities were computed. The rank order (descending) of the scores for these analytes in predicting overall carcinogenic response was: adiponectin > IGF-1/IGFBP-3 > IGFBP-3 > leptin> SAP > IGF-1.
Table 4.
Plasma analytes predictive of the carcinogenic response
| Plasma Analyte | Condition | Incidence | Multiplicity | Burden | Latency | Weighted Score |
|---|---|---|---|---|---|---|
| IGF-1 (ng/ml) | < Median | 256 | ------ | ------ | ------ | 4.48 |
| ≥ Median | 311 | ------ | ------ | ------ | ||
| IGFBP-3 (ng/ml) | < Median | ------ | 127 | 128 | 115 | 8.51 |
| ≥ Median | ------ | 117 | 113 | 125 | ||
| IGF-1/IGFBP-3 | < Median | 2.06 | 2.41 | ------ | 2.70 | 9.31 |
| ≥ Median | 2.65 | 2.64 | ------ | 2.46 | ||
| Adiponectin (μg/ml) | < Median | 21.0 | 18.9 | 19.0 | 13.9 | 18.08 |
| ≥ Median | 15.6 | 15.2 | 13.8 | 18.4 | ||
| Leptin (pg/ml) | < Median | 1127 | 1377 | ------ | ------ | 7.76 |
| ≥ Median | 1568 | 1570 | ------ | ------ | ||
| SAP (μg/ml) | < Median | 342 | 348 | ------ | ------ | 4.62 |
| ≥ Median | 371 | 376 | ------ | ------ |
For cancer incidence the condition was the absence or presence of cancer. For cancer multiplicity the median number of cancer per rat was 2; for cancer burden (cancer mass/rat in gram) the median was 1.058; median cancer latency was 49 days post carcinogen. T- statistic score is the sum of the absolute value of t-statistics for plasma analytes that were statistically predictive of a difference in the carcinogenic response.
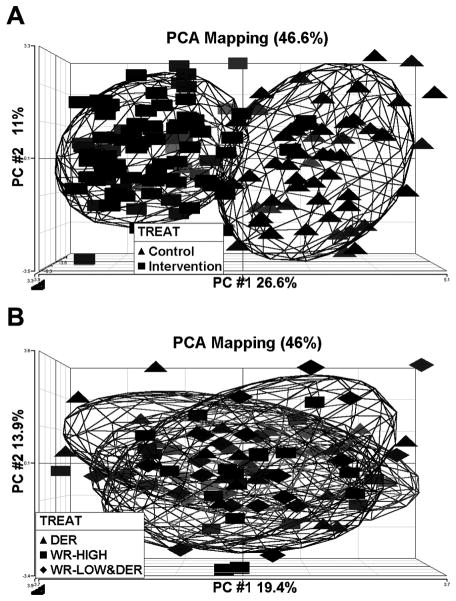
The plasma analyte data were also subjected to unsupervised cluster analysis using principal components analysis. The analysis was done twice, first with the data from all groups and second using only data from the three energetic intervention groups. There was complete separation of the control groups from the energetic intervention groups with the first component accounting for 26.6% of the variability and the second component accounting for 11.0% of the variability (Figure 2A). There was extensive overlap of plasma markers among the 3 energetic intervention groups with limited discriminatory ability with the first component accounting for 19.4% of the variability and the second component accounting for 13.9% of the variability (Figure 2B). These findings indicate that the three energetics interventions had similar effects on the plasma analytes that were assessed.
Figure 2.
Principle Component Analysis (PCA) of all plasma analytes. (A) Pooled control (pooled data of ad libitum fed control and paired fed control versus pooled intervention (pooled data of wheel running-high, wheel running-low and dietary energy restriction treatments). (B) Comparison among three intervention groups, dietary energy restriction (DER), wheel running high (WR-HIGH), and wheel running low and dietary energy restriction (WR-LOW&DER).
Discussion
The experiment reported in this paper begins to break new ground by assessing the question of whether physical activity exerts effects on carcinogenesis by mechanisms that differ from limiting caloric intake. For this experiment, risk for cancer was set high at an early age by administration of a dose of carcinogen that induces mammary cancer in essentially all treated rats within 10 weeks of injection, and the effects of three energetics interventions were compared. All three energetics interventions were modest, 15%, and a threshold slightly above the 10% level of energy restriction at which it is usually possible to detect effects on the carcinogenic process using an N=30 rats/ group in this model system. There were also modest differences in energy availability between the 2 control groups since the PDF-control group was allotted a daily amount of food prescribed based on the recommended energy requirements for the rat (25–28); whereas, the ad libitum fed control group experienced a modest over consumption of calories that occurs in rodents given ad libitum access to food. To minimize variation among groups of wheel running animals, physical activity was at a constant intensity using a motorized activity wheel, and distance run was limited to either 1750 or 3500 m/day. These levels of physical activity, for a rodent, were modest in that they required either 7 or 3.5% of the daily metabolic energy requirement for a rat. Comparatively, for a 5′5″ woman weighing 55 kg, this amount of physical activity would be equivalent to about 120 kcal, a level of physical activity that would be rated as light. Under these modest and highly relevant conditions, limiting energy availability by caloric restriction alone or in combination with wheel running, reduced all aspects of the carcinogenic response. While there was numerical evidence that protection was greater with wheel running, neither factorial ANOVA or regression of cancer endpoints on distance run rendered sufficient evidence that these differences were not due to chance alone (data not shown). Similarly, there were numerical differences between the ad libitum fed control group and the pellet dispenser control group, but none of these differences reached the level of statistical significance.
A number of plasma analytes were consistently reduced (IGF-1, IGF1/IGFBP3, insulin, IL-6, SAP, leptin, and TNFα), or increased (IGFBP-3 and adiponectin) by all energetics interventions relative to control animals (Table 3). In order to determine the role of host systemic factors, the plasma analytes that were measured were evaluated for their ability to predict various aspects of the carcinogenic response. Those analyses, which are shown (Supplementary tables S1–S4) and that are summarized (Table 4), were highly instructive. Among the 150 rats studied, plasma adiponectin was by far the most predictive of the presence or absence of cancer or of cancer multiplicity, cancer burden, or cancer latency above or below the median response irrespective of treatment group assignment. The literature about adiponectin and breast cancer is mixed and may well relate to whether targets of adiponectin are misregulated in the cancers on which it is predicted to exert an effect. Another aspect of adipokine biology that merits mention is that until recently it was thought that adiponection was only synthesized and secreted by adipocytes. However, it is now clear that skeletal muscle can also synthesize and secrete adiponectin (36). Thus if adiponectin plays a causal role in cancer protection, it can be envisioned that physical activity, depending on its effects on adipose tissue and muscle, could exert effects over and above those attributed to energy restriction alone. However, to observe such effects consistently might require a planned regime of physical activity intensity and duration to improve physical fitness, i.e. exercise, but that was not the intent of this investigation. Biologically available IGF-1, estimated as the ratio of IGF-1 to IGFBP3, was the second most predictive plasma biomarker. The IGF-1 bioavailability observation is consistent with a significant literature that indicates that IGF-1 is either a marker of effect or a causal agent (37, 38).
While the goal of establishing causal relationships is elusive and infrequently achieved, great insight can be obtained through the elimination of potential causal mechanisms. Such was the case in this experiment. Our analyses failed to provide compelling evidence for the involvement of two families of plasma analytes in accounting for the protective effects of the energetic interventions on the carcinogenic response, namely markers of chronic inflammation (CRP, IL-6, TNFα, and SAP) and of sex steroid metabolism (estrogen and progesterone). However, in making this observation, it must be recognized that estrogen and progesterone play a critical role in breast carcinogenesis and that CRP, as a marker of chronic inflammation, has been reported to predict long term survival following treatment for breast cancer (23). Thus, the data indicate that these plasma markers are unlikely to be playing a prominent role in determining the carcinogenic response in the biological text of the experiment described (Tables 3 and 4). As we have recently reported, it is critical not to fixate on a sole mechanism in trying to account for health benefits of energy restriction or physical activity. Nonetheless, these data, while consistent with that premise, do give weighted direction to promising areas for cellular and molecular inquiry.
Concluding remarks
The findings of this study provide strong evidence that limiting energy availability by about 15% is protective against mammary cancer and that the magnitude of protection is similar whether or not increased physical activity is a component of the strategy used to limit energy availability. The host systemic data point to the plasma adipokines, specifically adiponectin, and factors regulating glucose homeostasis as serving as markers of the protected state, but this does not establish causality. There was also evidence that markers of chronic inflammation were modulated by the energetics interventions investigated, but those differences appeared to be without predictive value for protection against mammary cancer. Similarly, circulating levels of estrogen and progesterone were not predictive, perhaps because of the cyclic and asynchronous changes that occur in these hormones in a rodent population as studied here. While some work has reported that physical activity and dietary energy restriction are likely to operate via different mechanism in protecting against cancer, the data indicate that for the host systemic factors related to glucose homeostasis, chronic inflammation, adipokine secretion and sex hormone metabolism, that both interventions had similar effects (Figure 2).
Many people want to know how much they can eat and how physically active they must be to maintain a body weight for height that is consistent with a lower risk for cancer or conversely, what level of excess net energy availability can be tolerated without increasing cancer risk. As reported here, relatively small differences in body weight gain were associated with marked protection against cancer, with or without increased physical activity. The fact that small differences such as these occur naturally in populations of individuals who have the same height but that differ in levels of physical activity and body weight, underscores the importance of current recommendations to maintain body weight in the normal range for height and to prevent adult weight gain and to do this by eating in moderation and being physically active.
Supplementary Material
Acknowledgments
Grant Support
This work was supported in part by United States Public Health Services Grant CA100693 from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.International Agency for Research on Cancer. Physical Activity, Weight Control, and Cancer. Lyon: IARC Press; 2002. [Google Scholar]
- 2.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 3.Trentham-Dietz A, Newcomb PA, Egan KM, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11(6):533–42. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 4.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13(4):325–32. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Margolis KL, Adami HO, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women’s Health Initiative (United States) Am J Epidemiol. 2007;166(7):752–9. doi: 10.1093/aje/kwm137. [DOI] [PubMed] [Google Scholar]
- 6.Friedenreich CM. Physical activity and breast cancer risk: the effect of menopausal status. Exerc Sport Sci Rev. 2004;32(4):180–4. doi: 10.1097/00003677-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Eng SM, Gammon MD, Terry MB, et al. Body size changes in relation to postmenopausal breast cancer among women on Long Island, New York. Am J Epidemiol. 2005;162(3):229–37. doi: 10.1093/aje/kwi195. [DOI] [PubMed] [Google Scholar]
- 8.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42(8):636–47. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A. Cancer Prevention and Management through Exercise and Weight Control. 1. Boco Raton: CRC Press; 2006. [Google Scholar]
- 10.Physical Activity Guidelines Advisory Committee. Physical Activity guidelines Advisory Committee Report, 2008. Washington DC: US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 11.Thompson HJ, Jiang W, Zhu Z. Energetics and Cancer: Exploring a Road Less Traveled. In: McTiernan A, editor. Physical Activity, Dietary Calorie Restriction, and Cancer. 1. Springer Science + Business Media, LLC; 2011. pp. 55–67. [Google Scholar]
- 12.Thompson HJ. Pre-clinical investigations of physical activity and cancer: a brief review and analysis. Carcinogenesis. 2006;27(10):1946–9. doi: 10.1093/carcin/bgl117. [DOI] [PubMed] [Google Scholar]
- 13.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46(2–3):135–41. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 15.Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61(9):895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin PJ. Host-related factors in breast cancer: an underappreciated piece of the puzzle? J Clin Oncol. 2008;26(20):3299–300. doi: 10.1200/JCO.2007.15.4526. [DOI] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101(9):630–43. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Roos AJ, Ulrich CM, Ray RM, et al. Intentional weight loss and risk of lymphohematopoietic cancers. Cancer Causes Control. 2010;21(2):223–36. doi: 10.1007/s10552-009-9453-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Jiang W, McGinley J, Wolfe P, Thompson HJ. Effects of dietary energy repletion and IGF-1 infusion on the inhibition of mammary carcinogenesis by dietary energy restriction. Mol Carcinog. 2005;42(3):170–6. doi: 10.1002/mc.20071. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Jiang W, Sells JL, et al. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1920–9. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson HJ, Zhu Z, Jiang W. Weight control and breast cancer prevention: are the effects of reduced energy intake equivalent to those of increased energy expenditure? J Nutr. 2004;134(12 Suppl):3407S–11S. doi: 10.1093/jn/134.12.3407S. [DOI] [PubMed] [Google Scholar]
- 22.Thompson HJ, Wolfe P, McTiernan A, Jiang W, Zhu Z. Wheel running-induced changes in plasma biomarkers and carcinogenic response in the 1-methyl-1-nitrosourea-induced rat model for breast cancer. Cancer Prev Res (Phila) 2010;3(11):1484–92. doi: 10.1158/1940-6207.CAPR-10-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 24.Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16(10):2407–11. doi: 10.1093/carcin/16.10.2407. [DOI] [PubMed] [Google Scholar]
- 25.CHERNIKOFF TN, KLEIBER M, SMITH AH. Metabolic rate of female rats as a function of age and body size. Am J Physiol. 1956;186(1):9–12. doi: 10.1152/ajplegacy.1956.186.1.9. [DOI] [PubMed] [Google Scholar]
- 26.KLEIBER M. Energy metabolism. Annu Rev Physiol. 1956;18:35–52. doi: 10.1146/annurev.ph.18.030156.000343. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. Nutrient Requirements of the Laboratory Animals. 4 Revised. Washington, D.C.: National Academy Press; 1995. [Google Scholar]
- 28.Kleiber M. The Fire of Life: an introduction to animal energetics. Revised. Malabar: Robert E. Krieger Publishing Company; 1975. [Google Scholar]
- 29.Singh M, McGinley JN, Thompson HJ. A comparison of the histopathology of premalignant and malignant mammary gland lesions induced in sexually immature rats with those occurring in the human. Lab Invest. 2000;80(2):221–31. doi: 10.1038/labinvest.3780025. [DOI] [PubMed] [Google Scholar]
- 30.Thompson HJ, Singh M, McGinley J. Classification of premalignant and malignant lesions developing in the rat mammary gland after injection of sexually immature rats with 1-methyl-1-nitrosourea. J Mammary Gland Biol Neoplasia. 2000;5(2):201–10. doi: 10.1023/a:1026495322596. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z, Jiang W, Thompson MD, McGinley JN, Thompson HJ. Metformin as an energy restriction mimetic agent for breast cancer prevention. J Carcinog. 2011;10:17. doi: 10.4103/1477-3163.83043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Z, Jiang W, McGinley JN, et al. Mammary Gland Density Predicts the Cancer Inhibitory Activity of the N-3 to N-6 Ratio of Dietary Fat. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-11-0175. [DOI] [PubMed] [Google Scholar]
- 33.Sokal RR, Rohlf FJ. Biometry the principles and practice of statistics in biological research. 3. New York: W.H. Freeman; 1995. [Google Scholar]
- 34.Snedecor GW, Cochran WG. Statistical Methods. 8. Ames, IA: Iowa State University Press; 1989. [Google Scholar]
- 35.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Chewchuk S, Lavigne C, et al. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297(3):E657–E664. doi: 10.1152/ajpendo.00186.2009. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999;51 (Suppl 3):34–41. doi: 10.1159/000053160. [DOI] [PubMed] [Google Scholar]
- 38.Pollak M. IGF-I physiology and breast cancer. Recent Results Cancer Res. 1998;152:63–70. doi: 10.1007/978-3-642-45769-2_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.