Summary
Positron emission tomography (PET) can be used to image tumor proliferation when combined with appropriate labeled tracers, such as the thymidne analog [18F]-3’-deoxy-3’-fluorothymidine (FLT). While thymidine kinase 1 is the principal mechanism of cell trapping, other variables, including the cellular level of native thymidine may need to be considered.
Commentary
In this issue of Clinical Cancer Research, Zhang and colleagues (1) studied the use of [18F ]-3’-deoxy-3’-fluorothymidine (FLT) and positron emission tomography (PET) in xenografts to measure tumor growth. Variations in the level of native thymidine affected the avidity of the tumor for the tracer in this study.
PET is now routinely used in clinical oncology and generally employs fluorodeoxyglucose (FDG) to detect tumors and assess their metabolic activity. While FDG measures one aspect of the energetic pathway, other tracers have been developed to assess different aspects of metabolism in tumors and normal tissues. FLT, a thymidine analog, was developed to measure cell proliferation, since it is trapped in the DNA synthetic pathway (2). Previous PET work actually utilized thymidine itself, labeled with 11C, but the 20 minute half-life of the radionuclide and the rapid cleavage of the glycosidic linkage limited the routine use of this tracer. FLT, like FDG, is now synthesized commercially and is distributed to centers doing imaging research. It must be kept in mind, PET simply measures the distribution of activity within a human or animal and many parameters can affect tracer uptake and the interpretation of the images produced. The work presented by Zhang et al. highlights one paramount issue, competition for native thymidine, but other matters must be pondered to fully exploit the role of FLT. These include the metabolism and clearance of FLT by glucuronidation, transporters of FLT into the cell, activity of thymidine kinase 1 (TK1) and dephosphorylation of metabolically trapped FLT. The uptake of FLT by inflammatory cells, species differences, and the issue of the blood-brain barrier may also need consideration.
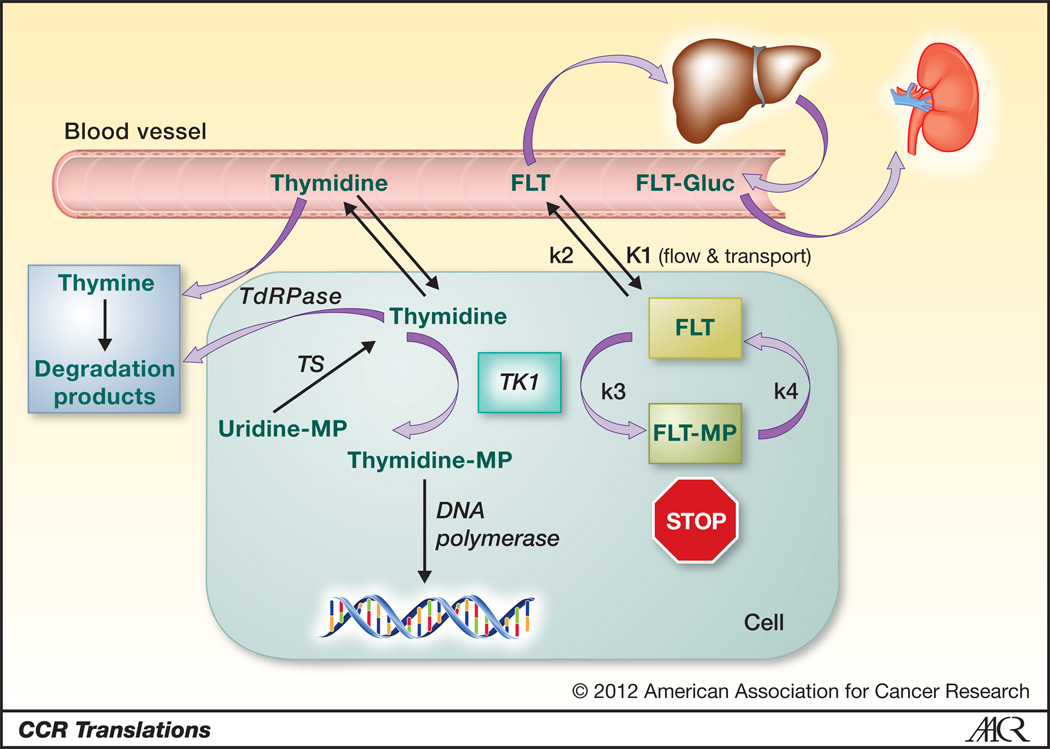
In the simplified model of FLT retention, it is taken up from the circulation by tumors and transported into the cell where it is trapped by phosphorylation by cytosolic TK1 (Figure 1) (3). TK1 has been demonstrated to show increased activity when cells are going through S phase, thus FLT retention reflects proliferative activity. Dynamic imaging and measures of FLT flux into the tumors requires the assessment of the level of the tracer in the circulation, which is obtained from blood samples or imaging of a vessel. This is combined with a time-activity curve obtained over the tumor to calculate the metabolic rate (using measurements of K1, k2 and k3). The phosphorylation of FLT by TK1 is represented in k3, but dephosphorylation (k4) is also possible. Fortunately, this is generally a slow process, but with prolonged imaging times this can affect the retention of the tracer. Simplifying the use of FLT, including assessment of investigational therapeutic agents, most centers solely obtain a static image done around 60 minutes after the injection of the tracer. The static image provides the standardized uptake value (SUV), which compares tracer activity in the tumor to the injected dose divided by the weight. Clearly, the easy measurement of SUV hides all the other metabolic variables mentioned. In most, but not all tumors, the pure measurement of SUV has been found to correlate with other measurements of tumor proliferation, such as Ki-67, obtained from biopsies (4). Furthermore, changes in SUV have been found to be useful in some studies assessing treatment response both in mice and humans, as shown in the study by Zhang et al. and others (5). The limitations of simple measurements, such as SUV, clearly need to be considered, since unexpected variations in FLT uptake may lead to erroneous conclusions.
Fig. 1.
FLT and thymidine uptake and metabolism are diagrammed including the phosphorylation by thymidine kinase 1 (TK1), synthesis of thymidine by thymidylate synthase (TS), and degradation by thymidine phosphorylase (TdRPase). Thymidine, but not FLT, is incorporated by DNA polymerase. FLT is metabolized to its glucuronide in the liver and excreted by the kidney. The rate constants used in modeling of FLT uptake (K1, k2, k3, and k4) are indicated.
While FLT resists degradation, its metabolism varies a great deal in different mammalian species. In rodents and dogs it is excreted unchanged in the urine. In humans, it is glucuronidated in the liver and approximately 25% (range about 15–43%) of FLT appears as the glucuronide in the blood by one hour (6). Whether this is an issue in repeated studies in individual humans under varying clinical makeup, requires further study. Regardless, marked liver retention is seen from this metabolism, limiting the use of FLT when hepatic tumors are present. Another important species difference is the natural level of thymidine in the blood is over 100-fold higher in mice and rats than found in dogs and humans (7). In some preclinical studies the high level of thymidine in rodents has decreased tracer uptake. The reliance of the paper by Zhang et al. on murine xenografts may alter the ability to fully translate their results to patients.
From the blood stream FLT and thymidine are transported across the cell membrane by both equilibrative (ENT1 and ENT2) and concentrative transporters (CNT1 and CNT3), which have different affinities for thymidine and FLT (8). The added complexity of the conformation of the transporters and how these differences may affect FLT retention still require further study. Once in the cell the exogenous thymidine and FLT obtained from the blood, mix with the endogenously synthesized pool of thymidine. In the implanted cell lines studied by Zhang and colleagues, this pool was shown to vary significantly and compete for the trapping of FLT. How common a problem this is in tumors clinically remains to be demonstrated. Most of the thymidine used by cells in DNA synthesis comes from endogenous synthesis. Once in the cell the FLT can be metabolically trapped by phosphorylation by TK1. As noted above, TK1 and cell proliferation generally increase concomitantly, but the control of TK1 is complex and includes post-translational mechanisms. It should be noted; drugs interfering with the endogenous synthesis of thymidine can produce rapid increases in TK1 activity and lead to marked retention of labeled thymidine and FLT (9). This “flare” phenomena can be used in pharmacodynamic measurements of the affect of agents that block thymidylate synthase, such as 5FU and nolatrexed.
In vivo one needs to consider normal distribution of FLT in various organs. Little FLT is seen in the brain because of the low proliferative rate and the inability of thymidine and FLT to cross the intact blood-brain barrier. When the blood-brain barrier is broken down in tumors high contrast is seen compared to normal brain parenchyma (10). FLT retention may be seen in the brain when breakdown occurs because of tissue necrosis or when inflammation is present. Inflammatory cells anywhere in the body, which are often proliferating, can retain FLT and incorrectly suggest that a tumor has spread. On the other hand, some investigators have demonstrated FLT can be used to image the effects of immunotherapy (11).
This brief summary highlights many of the variables that can alter the tumor retention of FLT. Fortunately, many of these become less important when one compares a baseline and post-treatment scan in a single patient. Zhang and colleagues still found the FLT retention readily measured the affect of treatment when used in tumors that demonstrated increased FLT uptake at baseline. While FLT appears promising in assessing the response to treatment, investigators need to understand its mechanism of retention. This information is important in properly interpreting the results obtained with any tracer in oncology, for imaging tumor growth is not as simple as the PET scans look.
Acknowledgments
This work was partially supported by the National Cancer Institute grants CA39566 and CA22453.
References
- 1.Zhang CC, Yan Y, Li W, Kuszpit K, Painter C, Zhang Q, et al. [18F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-1433. [DOI] [PubMed] [Google Scholar]
- 2.Shields A, Grierson J, Dohmen B, Machulla H-J, Stayanoff J, Lawhorn-Crews J, et al. Imaging Proliferation In Vivo with [F-18]FLT and Positron Emission Tomography. Nature Medicine. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 3.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43(9):1210–1217. [PubMed] [Google Scholar]
- 4.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44(9):1426–1431. [PubMed] [Google Scholar]
- 5.Sohn H-J, Yang Y-J, Ryu J-S, Oh S, Im K, Moon D, et al. [18F]Fluorothymidine Positron Emission Tomography before and 7 Days after Gefitinib Treatment Predicts Response in Patients with Advanced Adenocarcinoma of the Lung. Clin Cancer Res. 2008;14:7423–7429. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- 6.Shields AF, Briston DA, Chandupatla S, Douglas KA, Lawhorn-Crews J, Collins JM, et al. A simplified analysis of [18F]3'-deoxy-3'-fluorothymidine metabolism and retention. Eur J Nucl Med Mol Imaging. 2005;32(11):1269–1275. doi: 10.1007/s00259-005-1813-0. [DOI] [PubMed] [Google Scholar]
- 7.Li K, Clarke S, Rivory L. Quantitation of plasma thymidine by high-performance liquid chromatography--atmospheric pressure chemical ionization mass spectrometry and its application to pharmacodynamic studies in cancer patients. Analytica Chimica Acta. 2003;486:51–61. doi: 10.1016/j.jchromb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Plotnik DA, McLaughlin LJ, Chan J, Redmayne-Titley JN, Schwartz JL. The role of nucleoside/nucleotide transport and metabolism in the uptake and retention of 3'-fluoro-3'-deoxythymidine in human B-lymphoblast cells. Nucl Med Biol. 2011;38(7):979–986. doi: 10.1016/j.nucmedbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells P, Aboagye E, Gunn RN, Osman S, Boddy AV, Taylor GA, et al. 2-[11C]thymidine positron emission tomography as an indicator of thymidylate synthase inhibition in patients treated with AG337. J Natl Cancer Inst. 2003;95(9):675–682. doi: 10.1093/jnci/95.9.675. [DOI] [PubMed] [Google Scholar]
- 10.Muzi M, Spence AM, O'Sullivan F, Mankoff DA, Wells JM, Grierson JR, et al. Kinetic analysis of 3'-deoxy-3'-18F-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47(10):1612–1621. [PubMed] [Google Scholar]
- 11.Aarntzen EH, Srinivas M, De Wilt JH, Jacobs JF, Lesterhuis WJ, Windhorst AD, et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3'-fluoro-3'-deoxy-thymidine ([18F]FLT) PET imaging. Proc Natl Acad Sci U S A. 2011;108(45):18396–18399. doi: 10.1073/pnas.1113045108. [DOI] [PMC free article] [PubMed] [Google Scholar]