Abstract
Endothelium lining luminal surface of blood vessels is the key target and barrier for vascular drug delivery. Nanocarriers coated with antibodies or affinity peptides that bind specifically to endothelial surface determinants provide targeted delivery of therapeutic cargoes to these cells. Endothelial targeting consists of several phases including circulation in the bloodstream, anchoring on the endothelial surface and, in some cases, intracellular uptake and trafficking of the internalized materials. Dynamic parameters of the vasculature including the blood hydrodynamics as well as surface density, accessibility, membrane mobility and clustering of target determinants modulate these phases of the targeting, especially anchoring to endothelium. Further, such controlled parameters of design of drug nanocarriers as affinity, surface density and epitope specificity of targeting antibodies, carrier size and shape also modulate endothelial targeting and resultant sub-cellular addressing. This article reviews experimental and computational approaches for analysis of factors modulating targeting nanocarriers to the endothelial cells.
Keywords: antibody surface density, carrier concentration, cell adhesion molecules, endothelium, hydrodynamics, nanocarriers, optimization, targeted drug delivery
Introduction
Targeted delivery of diagnostic and therapeutic compounds to sites of pathology is an important medical goal. Targeting can be achieved with a variety of specifically formulated carriers including liposomes, droplets, microbubbles, dendrimers, polimersomes, and polymer particles, which are being developed for diverse drug delivery and diagnostic imaging applications [1–5]. In exploitation of biochemical molecular recognition events and receptor-ligand interactions occurring at the nano-scale that can be utilized to enhance localized carrier binding, coupling of carriers to molecules such as antibodies (Abs) or affinity peptides provides targeting to specific molecules expressed on the surface of the cells requiring a particular intervention. This approach also permits increasing the binding specificity of delivered therapeutic agents while minimizing potential toxicity [2,6–9].
In this context, the vasculature is both highly accessible and convenient route and target for drug delivery [10–15]. The vasculature is lined by endothelial cells, which form a thin (1–2 μm thick) monolayer throughout the blood vessel lumen. This monolayer forms a unique tissue, endothelium that regulates vascular tone, blood fluidity, and transport of cellular and non-cellular blood components, including white blood cells [16–19]. Endothelial dysfunction and injury play an important role in pathogenesis of a plethora of disease conditions including the prevalent cardiovascular maladies (atherosclerosis, thrombosis, hypertension, ischemic disorders such as acute myocardial infarction and stroke), as well as pulmonary (acute lung injury, pneumonia and pulmonary hypertension) and other diseases including metabolic, diabetic and cancer pathologies. Pathological factors of the endothelial microenvironment typical of these conditions (e.g., abnormalities of blood flow, influx of reactive oxygen species and cytokines and activated white blood cells) pathologically alter and damage endothelial cells [16,17,20]. Endothelial cells therefore represent important targets for clinical intervention. However, most known therapeutic agents themselves do not present intrinsic affinity to endothelial cells. Therefore, despite high accessibility to circulating blood, only a minor fraction of injected drugs get to and act in the endothelium. Suboptimal (mildly put) drug delivery represents an especially acute challenge in case of interventions using biotherapeutics (enzymes, genetic materials), which are labile and require specific addressing to given sub-cellular compartments: plasmalemma, vesicles, cytosol or the nucleus. In order to achieve targeted delivery to endothelial compartments, drugs or their carriers are conjugated with molecules having affinity to specific endothelial determinants, providing optimal anchoring and subsequent sub-cellular delivery. Of note, pathological endothelial alterations in many cases are manifested by surface exposure of molecular structures that represent potential targeting entities, such as endothelial cell adhesion molecules [20,21].
In theory, the ability of a targeted carrier to reach a specific tissue via vascular delivery depends on both biological parameters affecting hemodynamics, blood flow and the endothelial cell surface as well as parameters inherent to the carrier design. Parameters such as the identity of the endothelial molecular target to which the cargo will be addressed, its surface density, function, regulation and involvement in pathology, the affinity of the targeting vector (e.g., antibody) providing binding of the drug carrier, its density on the carrier surface, carrier size, and flow dynamics at the interface between the cell and the carrier are key factors regulating targeting. Categorically, previous studies in vitro and in vivo have addressed the impact of certain design parameters and physiological conditions on the targeting of carriers of different composition (e.g., polystyrene vs poly(lactic-co-glycolic acid) or PLGA) [22], size and shape (0.1–10 μm, spherical vs elliptical disks) [23], and under different shear stress levels (e.g., 1 and 5 dyne/cm2) [24]. While not exhaustive, these experiments have indicated the similar performance of model polystyrene carriers compared to biocompatible PLGA carriers [22], the higher specificity and efficiency of micrometer-range elongated carriers over submicron spherical carriers [23], and that the most efficient EC targeting occurs under shear stress of 1 dyne/cm2, representative of blood flow in arterioles and venules [24].
These studies exemplify how quantifiable biological parameters need be evaluated, such as the level of shear stress that carriers must withstand to stay firmly bound to endothelial cells while exposed to blood flow, and the accessibility and degree of surface expression of the target molecule. These may largely depend on the general and local pathophysiological state for a particular illness or disease [24–29]. Individualized targeted carrier features can include their chemical composition, size, shape, affinity for the targeting molecule, and their instillation at a particular carrier bulk concentration [3,4,22,23,30]. Selection of such parameters is essential to effective use of drug delivery vehicles to meet the treatment or imaging requirements for each intended clinical application.
The major contributions to our understanding targeted carrier-based drug delivery come from testing multiple targeting parameters, each one presenting distinct features relevant to carrier binding from the bloodstream. There has also been work performed using a single targeting parameter (e.g., carrier size or surface antibody density) whose particular features conform to the various factors which control carrier targeting in a broad spectrum. The domain for such analysis includes in vitro and in vivo experimentation and in silico computer simulation. Following an experimental logic based on quantification of nanocarrier interactions with target cells allows identification of those dynamic factors which control nanocarrier binding to vascular endothelium. For instance, a molecular target can be expressed on the cell surface both under a normal physiological status (naïve or quiescent endothelial cells) and during pathology (activated endothelium), allowing it to serve as a prospect for targeted drug delivery in either prophylactic or therapeutic interventions. Its structural parameters (e.g., length, flexibility and localization in the endothelial luminal surface) may be such that the presence or absence of the cell glycocalyx influences its accessibility to nanocarriers. It may be subject to a differential regulation (e.g., conformation, expression level, interaction with the cytoskeleton regulating the cell shape and morphology) depending on the shear flow to which the target endothelial cells are subjected, representative of different vascular beds (e.g., capillaries, venules, and arterioles) or pathological status (disregulation of vascular tone, abnormal hematocrit, presence of atheromatous plaques, leaky tumor vasculature, etc.). It may be capable of mobility in the plasmalemma, e.g., clusterization and localization in specific plasma membrane domains such as vesicular invaginations driving intracellular delivery by endocytosis of nanocarriers within endothelial cells.
There exist significant gaps in the literature pertaining to available cell-surface expressed molecular targets, their affinity, spatial representation on the endothelial cell surface as well as specific nanocarrier effects such as those of exotic shape variants such as filaments or disks, and the general lack of physiological studies that included relevant hemodynamic data (e.g., measures of blood flow and hematocrit from which shear stress can be determined). As a result, the discussion herein is restricted to spherical particles, well-characterized molecular recognition targets and relevant quantitative examination of the role of carrier affinity in targeting the endothelium, the role of hemodynamic factors in anchoring carriers to endothelial cells and the role of glycocalyx in targeting. Given the current state of the art in quantitative studies directed at assessing nanocarrier targeting to vascular endothelium, there is an obvious and critical need for additional comprehensive studies involving in vitro and in vivo biomedical aspects of delivery and binding as well as computation-based simulation providing predictive results for high-throughput assessment to further enable delivery optimization for future clinical application.
Carriers for targeting drugs to endothelium
Targeted delivery of drugs to endothelium holds promise to improve clinical management of many diseases [25,30–34]. A wide range of approaches have been examined in the design and implementation of colloid based drug carrier systems. The current focus of research in this area has generated a broad spectrum of carriers [3,4,35–41]. However, our ability to understand and predict biological performance in terms of both circulation time and target specificity remains a challenge [42]. Theoretically, the clinical goals can be achieved by coating drug carriers with affinity ligands providing anchoring to the endothelial surface molecules [24,28,30,43–46]. On the other hand, endothelial cells represent the first tissue barrier encountered by circulating drugs and drug carriers en route to extravascular therapeutic targets such as tumor cells, neurons, or cardiomyocytes. In order to facilitate extravasation, one can try to anchor drug carriers specifically to endothelial molecules involved in processes that transfer blood components into tissues [5,10,32,44].
Both these goals can be achieved using targeted nanocarriers, i.e., artificial containers for drugs, coated with antibodies, affinity peptides and other ligands binding to specific endothelial epitopes. A carrier provides high drug loading capacity, protects drugs from inactivation by the body, protects the body against side effects of drugs en route to therapeutic targets, optimizes drug pharmacokinetics and provides a modular platform for targeting, capitalizing on multivalent binding numerous copies of affinity ligands to cellular counterparts. Small-scale drug carriers (nanoparticles and microparticles with diameters of 100 nm to a few ten μm) [47,48] include liposomes [49] and other lipid-based carriers such as micelles [40,41,50], lipid emulsions and lipid-drug complexes [51], polymer-drug conjugates [52], dendrimers [53], polymer microspheres [27,54,55], non-spherical carriers including discoid, filamentous and more exotic geometries Cai, 2007 150/id;Discher, 2002 187/id;Geng, 2007 162/id;Simone, 2007 190/id}, and various ligand-targeted products such as immunoconjugates [56]. Applications of drug delivery systems in experiments and clinical medicine include: (A) circulating drug reservoir in the blood compartment; (B) oxygen delivery systems; (C) blood-pool imaging; (D) passive targeting (i.e., targeting pathologies with leaky vasculature such as solid tumors, inflammatory and infectious sites, spleen, lymph nodes); and (E) active targeting (i.e. ligand coupling, antibody-mediated targeting, folate-mediated targeting, targeting tumor vasculature and microenvironment [57,58]).
Strategic encapsulation of therapeutic or diagnostic agents (i.e., cargoes) to be delivered within biocompatible carriers confers protection against rapid cargo degradation in the bloodstream and permit its controlled release [15,52,59–61]. The additional coupling of such carriers to targeting ligands enables the biological recognition of cell-surface expressed elements necessary for specific, effective targeted drug delivery [10–14,29,62,63]. A variety of carriers having different structures allow encapsulation and controlled release of diverse drugs and imaging probes, surface properties, charge, elasticity and life time in circulation. In order to be useful for intravascular delivery without danger of entrapment in the small vessels (the diameter of capillaries is ~5 micron), carriers must be submicron in size in at least in one dimension. Coating particles with highly hydrophilic polymers such as polyethylene glycol (PEG) improves carrier solubility, circulation profile and longevity in the bloodstream, as well as reduces immune reactions to the carriers and their cargoes. Furthermore, several types of non-spherical carriers including discs [23] and flexible filomicelles [50] demonstrate superior circulation profiles due to alignment with flow guiding them to avoid excessive collisions with blood and vascular cells.
Considerable attention has been devoted to defining the binding chemistry for attaching ligands such as antibodies, peptides, and other molecules onto the surface of nanocarriers. Generally, the surface density of a specific ligand on carriers is a controllable manufacturing parameter. Torchilin et al. [46] described a procedure for coupling ligands to polyethylene glycol chains incorporated in liposomes and providing an additional sterical freedom for antibody to interact with target epitopes. The simplest mode of attachment of affinity ligands (e.g., antibodies for specific receptors) involve straightforward incubation to uniformly coat the carrier [64]. From a hydrodynamics perspective, the two modes of attaching the ligands are expected to result in vastly different adhesion mechanics because of the stark difference in the size of the lubrication layer when the carrier is close to the cell surface. Systematic experimental studies of the role of a flexible spacer remain to be reported. It is also possible that the complex dynamical response of the endothelial surface layer attenuates this contrast [65], but further experimental confirmation must be performed. This will integrate further with the study of other controllable manufacturing parameters including the specific material used to produce the carrier shell (phospholipids, synthetic polymer lipids) and the ideal carrier size, which may warrant different targeting approaches for optimal responses.
Quantification of individual factors such as binding affinity, effects of shear stress, bulk concentration and other indices controlling their binding are as yet indeterminate. Therefore, while such design parameters including geometry, flexibility and stealth characteristics do in part dictate how effectively carriers reach and anchor on the target endothelial molecules, these factors as yet escape formal definition as dynamic factors controlling nanocarrier targeting. The use of unreasonably high quantities of the carrier can lead to problems of carrier toxicity, metabolism and elimination, or biodegradability. Drug potency can be an issue for very large therapeutic molecules such as proteins, particularly if small carrier diameters are desirable for reasons of biodistribution. Drug solubility can be an important consideration in liposomal systems: hydrophilic drugs can be readily entrapped within the liposome aqueous interior, but neutral hydrophobic drugs or those with intermediate solubilities tend to be rapidly released in the presence of plasma proteins or cell membranes [42]. Hence there is a need to optimize targeting efficiency, specificity, and circulation times of nanocarriers.
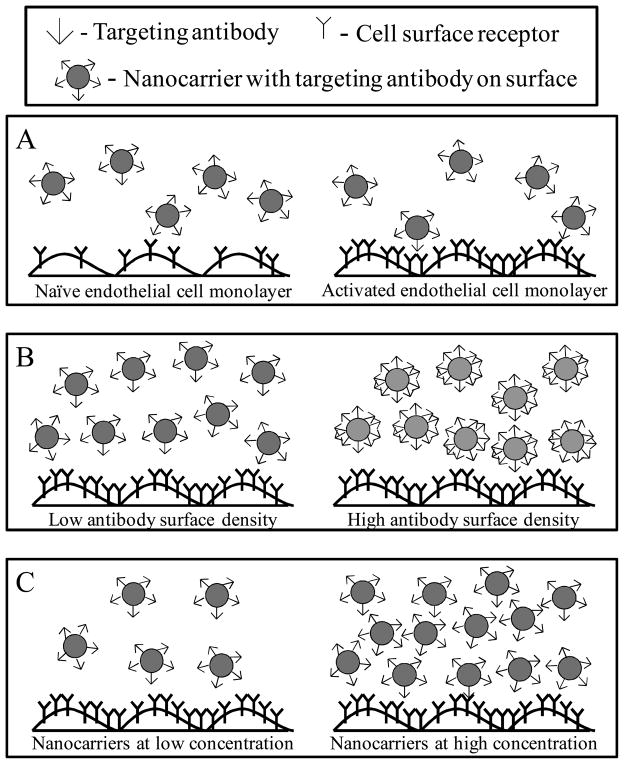
We focus particularly on endothelial targets under physiological status (quiescent cells) and during pathology (activated endothelium), which serve as a model for targeted drug delivery in prophylactic vs. therapeutic interventions (Figure 1). Structural parameters (e.g., length and localization in the endothelial luminal surface) and presence or absence of the cell glycocalyx affect accessibility of nanocarriers to the cell surface. Additional layers of differential regulation (e.g., conformation, expression level, interaction with the cytoskeleton regulating the cell shape and morphology) are important in optimizing delivery systems depending on the shear flow to which the target endothelial cells are subjected, representative of different vascular beds (e.g., capillaries, venules, and arterioles) or pathological status (vasodilation or vasoconstriction, hematocrit, presence of atheroma plaques, leaky tumor vasculature, etc). Subsequent to binding and arrest, the mobility of targets in the plasmalemma, localization in vesicular invaginations driving intracellular delivery by endocytosis, also impact targeting efficacy.
Figure 1.
Individually tunable parameters for nanocarrier targeting to vascular endothelium including A) endothelial cell expression level of surface receptors, B) on surface density of antibody on carriers and C) bulk concentration of nanocarriers
Optimizing Binding Affinity
The tunable design parameters (namely, size, shape, type, method of functionalization etc.) involved in the synthesis of functionalized nanocarriers necessitates a multi-parameter optimization for achieving efficacious targeting in drug delivery applications [48]. Rational design of functionalized nanocarriers faces many challenges owing to the complexities of molecular and geometric parameters surrounding receptor-ligand interactions and nanocarriers [23,37,38,54,66,67], lack of accurate characterization of hydrodynamic, physico-chemical barriers for nanocarriers uptake/arrest [68–72], nanoparticle-cell membrane force interactions and their influence on particle trafficking [73], and uncertainty in targeting environment in vivo [42,68,74]. Among the factors impacting the design of nanocarriers and therapeutic agents are: (1) binding affinity [22,75]; (2) multivalency or the average number of receptor-ligand bonds per bound nanocarriers [75–79]; (3) in vivo targeting, measured as percentage of injected dose accumulated after intravenous injection [22,55,80], and hemodynamics [38].
Parameters such as the identity of the endothelial molecular target [24,32,81–83], its surface density (Figure 1A) [36], function [14,84,85], regulation and involvement in pathology [86,87], the affinity of the targeting vector (e.g., antibody) driving the drug carrier [22,51,84], its density on the carrier surface (Figure 1B) [24,36], carrier size [23,54,55,61], carrier concentration in the bloodstream [36] (Figure 1C) and flow dynamics at the interface between the cell and the carrier [24,88,89] are key points regulating targeting.
A central objective is to establish a unified framework which will predict a priori the relative efficacy of targeting to endothelial cells, facilitating experimental design of drug delivery vehicles with optimal features for each particular application. Such a framework can be developed from the input of testing experimentally multiple target determinants, each one presenting distinct features relevant to nanocarrier binding from the bloodstream or, what is more amenable, using a single target determinant whose particular features conform to the various parameters which control nanocarrier targeting in a broad spectrum.
Endothelial Target Molecules
Endothelial cells express a number of molecules on their surface that can be used for anchoring drug carriers [90,91]. These molecules are localized in specific areas or endothelial plasmalemma. For example, in the normal adult vasculature, glycoproteins including thrombomodulin and angiotensin-converting enzyme localize on the apical luminal surface [56,92], platelet-endothelial adhesion molecule-1 (PECAM-1, CD31) and VE-cadherin localize in the intercellular junctions [93], and aminopeptidase P and PV1 glycoprotein localize in endothelial caveoli [94], specific dynamic invaginations of the plasma membrane with diameter close to 70 nm. This differential localization dictates different accessibility to circulating carriers. Further, endothelial cells in different vascular areas are enriched in specific determinants. For example, pulmonary capillary endothelium contains several times more angiotensin converting enzyme and thrombomodulin on the luminal surface than do endothelial cells present in large arteries [2,7,26]. Endothelial cells in the cerebral vasculature and are relatively enriched in the glycoprotein transferrin receptor [95], which is also present at high levels in hepatic cells [96].
Pathological factors including abnormal blood flow (e.g., insufficient flow under ischemia), oxidative stress and inflammatory agents (e.g., cytokines) alter endothelial phenotype [81,97,98]. In particular, surface density of some “normal” endothelial molecules such as thrombomodulin and angiotensin converting enzyme are greatly reduced in pathologically altered endothelial cells [81]. In contrast, numerous “pathological” markers are exposed at higher levels in disease states. For example, aminopeptidase N and proteins involved in pathological angiogenesis (VEGF-receptor, integrins) are exposed in endothelial cells in tumor and post-ischemic vasculature [99]. Inflammation causes relatively rapid exposure of P-selectin [100] and delayed exposure of E-selectin [101], VCAM-1 [99] and up-regulation of ICAM-1 in endothelium [99,102] (Figure 1A); all these molecules are involved in transmigration of circulating leukocytes in the inflammation foci.
From the drug delivery standpoint, endothelial cell adhesion molecules, either constitutive or inducible, represent a good target for anchoring carriers of anti-inflammatory and anti-thrombotic agents. Molecules expressed in tumors and sites of angiogenesis are excellent targets for diagnosis and treatment of these conditions [83], whereas molecules expressed by normal endothelium (e.g., PECAM-1) seems attractive for delivery of prophylactic interventions.
A variety of endothelial epitopes including PECAM-1 and others are being explored as potential molecular targets for drug delivery in a variety of disease states. In this article we will make cursory mention of many of them. However, for purposes of focus in the context of understanding the dynamic factors controlling vascular targeting, we place particular emphasis on the Intercellular Adhesion Molecule 1 (ICAM-1). ICAM-1 is a well known endothelial determinant which can be used as a convenient and representative prototype target for modeling a plethora of nanocarrier-assisted drug delivery parameters [82,85,86,90,103]. Polymer carriers targeted to ICAM-1 by antibodies (anti-ICAM carriers) bind specifically to endothelial cells in culture and in vivo [11,21,26,29,84,91,97,104–107]. Numerous studies have shown the efficacy of targeting carriers and conjugates to endothelial ICAM-1 for a variety of applications [6,23,25,26,32,91,92,105,106,108].
ICAM-1 is an Ig-like transmembrane glycoprotein that serves as a leukocyte anchor and is functionally involved in inflammation and thrombosis [11,109–111]. In opposition to other endothelial determinants located in small plasmalemma invaginations or caveoli (such as PV-1 [112]) and other molecules enriched in the cell-cell border (such as PECAM-1 [93]), ICAM-1 is expressed more diffusely in the apical luminal surface of endothelial cells [109,110]. This feature makes ICAM-1 an easily accessible target for carriers of a wide range of sizes (e.g., 100 nm to 1 μm diameter) delivered intravascularly in vivo or applied in cell culture studies [10,11,21,102]. In addition, ICAM-1 is expressed both in quiescent and activated endothelial cells, and its expression is enhanced during pathology [109,110]. This feature further adds to its utility in the study of targeted nanocarrier delivery in either prophylactic or therapeutic settings [109,110]. For instance, anti-ICAM antibodies and anti-ICAM protein conjugates and carriers bind specifically to endothelial cells in culture and in vivo models, and this targeting increases with pro-inflammatory insults such as treatment with TNFα [11,21,26,29,102,113–118]. Vascular immunotargeting of ICAM-1 has been shown to provide delivery of imaging and therapeutic agents to endothelial cells [11,26,29,102,113,117,118]. Anti-ICAM-targeted ultrasound contrast agents bind to activated endothelial cells, facilitating imaging of acute cardiac transplant rejection [105]. ICAM-1-targeted isotopes detect acute inflammation sites [118]. Anti-ICAM immunoliposomes bind to endothelial cells in vitro and in animal studies [114]). Anti-ICAM delivers therapeutic and reporter enzymes to endothelial cells in vitro and in vivo [11,26,102]. In addition, blocking ICAM-1 by targeted carriers attenuates inflammation, a beneficial secondary effect [119,120].
After intravenous injection in vivo, anti-ICAM antibodies and carriers accumulate in highly vascularized organs. Intrapulmonary binding is commonly high in this setting since the lung i) presents the first major capillary bed encountered post intravenous injection, ii) contains ~30% of endothelium in the body, and iii) receives nearly the entirety of the cardiac venous blood output [10,11,21,102]. Local infusions of antibodies or carriers targeted to cell adhesion molecules have been shown to favor cardiac, cerebral and other vessel uptake [121]. Endothelial cells efficiently internalize multivalent anti-ICAM carriers [11,21,26,119]. Multivalency of such carriers causes ICAM-1 clustering and induces a unique endocytic pathway, CAM-mediated endocytosis [11,21,26]. This constitutes the bases for intracellular delivery of therapeutics via ICAM-1 targeting. For instance, delivery of the enzyme catalase by anti-ICAM carriers confers endothelial antioxidant protection [11,21,26,120], and ICAM-1-targeted delivery of recombinant acid sphingomyelinase attenuates genetic sphingomyelin storage in cell models for the lysosomal disorder, Niemann-Pick Disease [11,91]. Hence, ICAM-1 is particularly well established as a highly suitable target useful for careful study to determine an understanding of the specific dynamic factors controlling targeting nanocarriers to vascular endothelium.
Muro et al. reported that 100 nm diameter anti-ICAM carriers possessing ~250 antibodies/carrier (~7000 antibodies/μm2), bound with high affinity and capacity to cultured endothelial cells (70 pM, Bmax ~300 particles/cell) [22]. As generally expected, the maximum binding sites available to carrier-coupled antibodies was much lower than that of free antibodies, i.e. 7.5×104 vs. 1.6×106 antibody/cell, found for ICAM-1 [22]. This is likely due to the fact that free antibodies can potentially interact with every target molecule in the plasma membrane, whereas only those antibodies properly oriented onto the surface of the carrier facing the cell membrane can engage that target. Binding to endothelial cells in the case of 100 nm carrier targeted to ICAM-1 is more than 100 times higher than that of micron anti-ICAM carriers [29,87,113,122]. It is apparent that carrier size is an important determinant of the efficiency of targeting. Other important variables such as antibody affinity and density on the carrier surface, carrier concentration, and shear rate (previously uncharacterized systematically) and endothelial cell surface structure also contribute to these differences, which must be studied in the context of hydrodynamic effects.
Other types of high affinity nanoparticles with multivalent interactions have also been studied recently [51,75]. Haun and Hammer [43] investigated the kinetic rate constants of attachment and detachment of 210 nm nanocarriers as a function of receptor density, ligand density on surface, and flow shear rate, and identified a time dependence of the detachment rate due to multivalent binding. Ho et al. [123] studied the effect of antibody surface coverage on equilibrium binding constants by measuring fractional coverage of bound nanocarriers (80 nm in diameter) as a function of carrier concentrations; by fitting their experimental data, they observed linear dependence of Kα on antibody surface coverage, leading them to conclude that the system was dominated by monovalent interactions. Immunotargeting of liposomes to activated vascular endothelial cells has been advocated as a strategy for site-selective delivery in the cardiovascular system by Spragg et al. [34]. A potential molecular target for such delivery is E-selectin, an endothelial-specific cell surface molecule expressed at sites of activation in vivo and inducible in cultured human umbilical vein endothelial cells (HUVEC) by treatment with cytokines such as recombinant human interleukin 1b (IL-1b). Liposomes of various types (classical, sterically stabilized, cationic, pH-sensitive), each conjugated with murine monoclonal antibody that recognizes the extracellular domain of E-selectin, bound selectively and specifically to IL-1b-activated HUVEC at levels up to 275-fold higher than to unactivated HUVEC. E-selectin-targeted immunoliposomes appeared in acidic, perinuclear vesicles 2–4 hr after binding to the cell surface, consistent with internalization via the endosome/lysosome pathway [34]. Despite such previous studies, a comprehensive understanding of the determinants of nanocarrier binding to endothelial cells in vitro and in vivo is lacking, but progress has been made in computation-based simulation.
Thermodynamic models quantifying the binding affinities of carriers to cells have been reported [124]. Nanocarrier binding to cancer cells was recently studied where, human epidermal growth factor receptor 2 (HER2) overexpressing cancer cells were used as the target for anti-HER2 coated nanocarriers. The antibody conjugation density was varied and the cellular binding was quantified using flow cytometric assay. Using this method, the authors were able to quantify binding strength, allowing them to better understand whether Nanocarriers were bound by monovalent or multivalent interactions. The binding data for each formulation were fitted to Langmuir isotherms, and based on the theory presented, it was concluded that the system studied behaved in a manner consistent with monovalent binding [123]. Liu et al. [89] developed a computational model to calculate the absolute binding affinities between functionalized nanocarriers and the endothelial cell surface. In this work is presented an equilibrium model for quantifying the effect of glycocalyx in mediating the interaction of functionalized nanocarriers with endothelial cells. In this model, nanocarrier adhesion is governed by the interplay between three physical parameters, namely, glycocalyx resistance, flexural rigidity of receptors, and receptor–ligand bond stiffness. The authors demonstrated that they can quantitatively reproduce the experimental binding affinities and provide quantitative descriptions for the multivalency in nanocarrier binding, as well as for the degree of clustering of antigens. The study identified two interesting parameters: glycocalyx resistance and antigen flexural rigidity; both of which reduce binding of nanocarriers and alter the sensitivity of the nanocarrier binding constant to changes in temperature. The effect of antibody surface coverage of the nanocarrier on binding simulations reveals a threshold value below which the nanocarrier binding affinities reduce drastically and drop lower than that of single anti-ICAM-1 molecule to ICAM-1. The computed trend describing the effect of antibody surface coverage on binding agreed remarkably well with experimental results of in vivo targeting of the anti-ICAM-1 coated nanocarriers to pulmonary endothelium in mice. Model results were further validated through close agreement between computed nanocarrier rupture force distribution and measured values in Atomic Force Microscopy experiments [89].
Hemodynamic Factors and Glycocalyx Effects on Carrier Anchoring
In general, our understanding of factors regulating carrier targeting to ICAM-1 as well as other endothelial targets is incomplete. Anti-ICAM affinity and surface density on carriers have been determined to a limited extent [14,24,29,36,87,107]. In many studies, only micron-scale, and not submicron, or nano-scale, carriers have been utilized [11,29,113,122], thus limiting our understanding of carrier size effects on vascular targeting. There are also few studies which have conducted for quantitative analysis of carrier targeting to any endothelial antigen, including factors that control binding of targeted carriers to endothelial cells in static or flow-perfused endothelial cell culture [24].
Targeted carriers diffuse in fluids and, hence, collide with the endothelial cell surface. The efficiency of this process is not sufficiently characterized, but due to size difference with free antibodies, antibody-targeted carriers may diffuse slower, and this may affect targeting. This effect may be offset by the greater momentum of carriers in flowing blood, which may promote more frequent wall collisions in the vasculature. Similarly, the hydrodynamic conditions apply forces that act to detach carriers anchored on the endothelial surface, and these are higher than the forces imposed against free antibodies. This total force applied depends on the shear stress, which varies from relatively low 1 dyne/cm2 in capillaries to 100 dyne/cm2 in large arteries. The multivalent character of antibody-targeted carriers facilitates high affinity anchoring to endothelial cells. (see, for instance, [22]), even in the face of large hydrodynamic forces.
When a targeted carrier approaches the endothelial surface, the fluid flow geometry consists of a nanometer-scale gap between the carrier surface and the cell membrane surface and the micron-sized channel in which the bulk flow occurs. This narrow gap is occupied by the endothelial cell surface layer, or glycocalyx, which in recent years has emerged as a structure of fundamental importance to a broad range of phenomena that determine cardiovascular health and disease [31,33]. Compelling evidence continues to emerge suggesting that the glycocalyx surface layer on vascular endothelial cells plays a determining role in numerous physiological processes including inflammation, microvascular permeability, and endothelial mechanotransduction. Previous research has shown that enzymes degrade the glycocalyx [68], whereas inflammation causes shedding of the layer [125]. New understanding of the functional significance of the glycocalyx has been made possible through recently developed experimental techniques that are capable of directly probing the glycocalyx in vivo. Damiano et al. showed that the hydrodynamically relevant endothelial cell glycocalyx surface layer observed in microvessels, is a fundamental determinant of the hydrodynamic and mechanical environment at the endothelial cell surface, in vivo [31,126–129].
The gel-like endothelial glycocalyx layer, usually extends hundreds of nanometers on the cell exterior in vivo, is an important structure affecting many cardiovascular diseases [31,126]. Recently a large number of studies has been devoted to the study of the mechanical and biochemical properties of glycocalyx. It has been observed in vivo experiments that the presence of the glycocalyx played a key role in mediating the binding of nanocarriers to the endothelial cell surface [68]. Potter and Damiano [128,129] concluded that the glycocalyx layer observed in vivo is nearly absent in in vitro conditions. However, it is still unclear whether the glycocalyx layer is completely absent or partially exist in in vitro conditions, and how will it affects the binding of nanocarriers to the endothelial cell surface. Weinbaum et al. modeled the glycocalyx as a transport barrier, as a porous hydrodynamic interface in the motion of red and white cells in microvessels, and as a mechanotransducer of fluid shearing stresses to the actin cortical cytoskeleton of the endothelial cell [69].
Lipowsky et al. [68] reported on the role of glycocalyx in endothelial cell adhesion in which the binding of fluorescently labeled microspheres (0.1 μm diameter) coated with antibody to ICAM-1 was studied in postcapillary venules. The authors noted that the carrier adhesion to endothelial cells increased dramatically after the removal of glycocalyx infusion of the venule with heparinase. The authors concluded that the glycocalyx serves as a barrier to adhesion and that its shedding during natural activation of endothelial cells may be an essential part of the inflammatory response [68]. Vink et al. [130] noted that the capillary endothelial surface layer can selectively reduce plasma solute distribution volume influencing the penetration of anionic molecules suggesting that multiple factors may influence the penetration of the barrier, including molecular size, charge, and structure. Finally, damage of the endothelial glycocalyx has been shown to decrease vascular barrier function and lead to protein extravasation and tissue edema, loss of nutritional blood flow, and an increase in platelet and leucocyte adhesion [31,126].
The presence of the glycocalyx [65,131] and the receptor-ligand bonds tend to alter the nature of the flow at this length scale. The interaction between the receptor-ligand bonds and the endothelial cell surfaces can be treated in simulation as an interaction between surfaces coated with polymer brushes separated by a nanometer-scale channel [132–134] and subjected to simple shear flow. Specifically, the density of macromolecular elements, including those specific receptors participating in carrier binding, which form the glycocalyx is comparable to the density of ligands functionalized on the microcarrier. The flow in the glycocalyx can therefore be considered explicitly at a continuum level as a porous medium [69,135], and the deformation of the receptor-ligand pairs then does not alter significantly the flow in the nanometer-scale gap [134].
In the near-contact region between a nanocarrier and the endothelial cell surface, the dynamics of adhesion are strongly influenced by the physical chemistry of receptor-ligand interactions [136]. Single molecule force spectroscopy experiments have provided precise characterization of the specific receptor-ligand interactions for leukocyte adhesion to the endothelium. Hanley and co-workers [137] have shown that the characteristics of P-selectin binding to P-selectin glycoprotein ligand-1 can be accurately described by the Bell model [138,139]. Our own atomic force spectroscopy experiments to measure ICAM-1 and ICAM-1 antibody mAb R65 binding confirm the applicability of a mesoscale model for receptor-ligand interactions [89]. Since the dynamics of adhesion are strongly influenced by the physical chemistry of receptor-ligand interactions [136], in our treatment, in the near-contact region between the nanocarrier and the endothelial cells, receptor-ligand pairs have been tested for bond formation in a stochastic fashion, according to their deviation length-dependent binding kinetics. In addition to bond formation, we allow a pre-formed bond to break. The length of each spring specifies the instantaneous force and torque exerted by that bond on a volume element of the carrier, and also its probability for breakage per unit time. This force and torque lead to translational (i.e., axial) and rotational motion of the nanocarrier, which are counteracted by those receptor-ligand pairs that remain intact. Our result is to describe the kinetics of ligand-receptor (single biomolecular) bond formation and failure, from which we can simulate the effect of antibody surface coverage on nanocarrier binding (Figure 2). Our model suggests that the dominant effect of changing antibody surface coverage is through a change in multivalent interactions. Shear flow and the presence of the glycocalyx do not alter the computed threshold of antibody surface coverage. The simulations describing the effect of antibody surface coverage on nanocarrier binding agrees very closely with experimental results of in vivo targeting of anti-ICAM-1 coated nanocarriers to pulmonary endothelium in mice as well as to human umbilical vein endothelial cells in culture [89].
Figure 2.
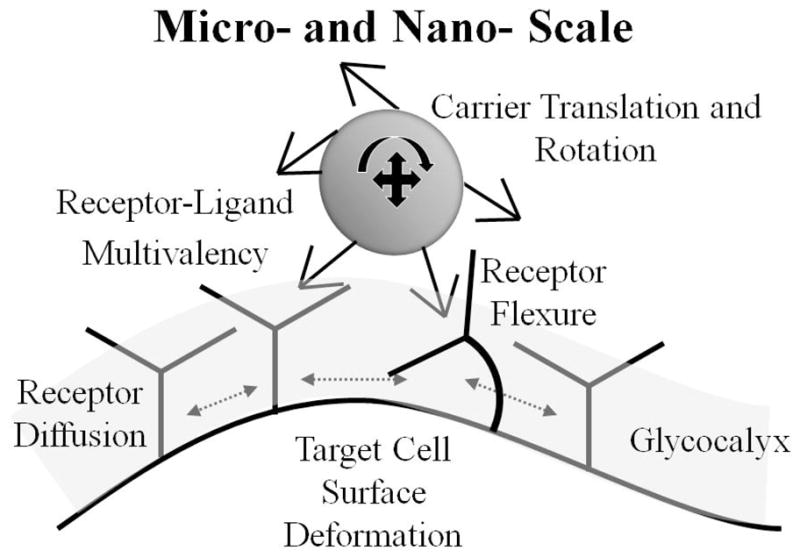
Schematic of micro- and nano-scale factors in targeted nanocarrier binding to endothelial cells.
The nanocarrier trajectory as it approaches the cell surface is important to binding. As the carrier approaches the glycocalyx, the “near-wall” effects dominate the nanocarrier motion. The relevant forces include non-specific (osmotic stress, glycocalyx resistance repulsion, steric repulsion between the ligands functionalized on the carrier and the cell membrane etc) and receptor-ligand forces, which act on specific surface elements of the carrier. These effects can be compounded by lateral diffusion of receptors on the endothelial cell membrane coupled with cell membrane deformation. As evidence of these events, two recent modeling studies have explored the formation of a spatial annulus pattern of receptor-ligand complexes in binding of 100 nm particles (carriers, viruses) to cells [89,140]. However, direct validation through super-resolution visualization experiments has not yet been realized.
The local mechanical environment dictates certain aspects of carrier binding. As the functionalized nanocarrier approaches the endothelial cell wall, it experiences a velocity gradient, and non-uniform force distribution because of the localized action of receptor-ligand bond forces tugging the carrier. Near the wall, the ligands on the nanocarrier contact the receptors on the activated endothelium through a highly specific-binding process. This interaction may mediate carrier-attachment to, or carrier-rolling along, the surface of the endothelium. Without firm arrest occurring, the carrier continues to move under the influence of the hydrodynamic force and torque exerted locally by the flow, which influences the binding interactions between the carrier and the cell surface [24,141–143]. Flow can thus cause the breakage of the bonds and tear nanocarriers free from the cell surface [24]. The adhesion of leukocyte mediated by selectins under flow has been extensively studied in both experiments and numerical simulations [28,136]. A threshold shear stress was identified for the rolling of leukocytes and recently was explained by catch-slip arguments [144].
As a model to define factors controlling the interplay between carrier motion in the bloodstream and its interactions with molecular targets in the endothelial wall, Calderon et al. used 1 μm carriers coated with ICAM-1 monoclonal antibody at different surface densities 370–4100 Ab/μm2 [24]. Carriers were perfused at different shear rates over resting or activated endothelial cells, expressing minimum vs. maximum ICAM-1 levels, to determine carrier rolling, binding and detachment. Even at 0.1 Pa and 4100 Ab/μm2, carriers attached only to activated cells (21 fold increase over resting cells), ideal for specific drug targeting to sites of pathology. Binding was increased by raising the Ab surface density on the carrier, and as a consequence of decreased rolling velocity. Carrier binding was stable even under a high shear stress: carriers with 1100 and 4100 Ab/μm2 withstand shear stress over 3 Pa without detaching from the cells [24].
In a different study, Calderon et al. focused on parameters such as the carrier dose and density of targeting molecules on the carrier surface [36]. Using radioisotope tracing they assessed the influence of these parameters on the biodistribution of model polymer carriers targeted to ICAM-1 (125I-anti-ICAM carriers) in mice. Increasing the surface antibody density enhanced specific accumulation in the pulmonary vasculature (a preferential endothelial target) with minimally reduced non-specific hepatic and splenic uptake. Increasing the carrier dose enhanced lung accumulation and decreased liver and spleen uptake. Regardless of the parameter varied, lung accumulation correlated positively with the total injected dose of carrier-bound Ab. Binding to activated endothelial cells reached saturation levels with all surface antibody densities and carrier concentrations. With quiescent cells, carriers reached a 3-fold lower saturation level, even at high carrier concentration and Ab density, and carriers with low Ab density did not reach saturation, reflecting affinity below threshold [36].
Eniola et al. [27] focused on elucidating the effect of particle size along with hemodynamics, blood rheology, and vessel size on the adhesion efficiency of targeted polymeric spheres to inflamed endothelium in vitro via parallel plate flow chamber assays. They found that the binding efficiency of biotinylated, multivalent sialyl Lewisx (sLex) carbohydrate coated spheres to the endothelium from blood flow generally increased with increasing particle size, wall shear rate and channel height for particle sizes from 100 nm up to 10 μm. However, nano-sized particles showed minimal adhesion to the endothelium from blood flow in small to medium-sized venules and arteries when compared to micron-sized spheres. Overall, the data suggested that spheres 2–5 μm in size are optimal for targeting the wall in medium to large vessels relevant in several cardiovascular diseases [38].
These studies and others [89] are representative of how physical forces imposed by blood flow and the concomitant receptor diffusion events redistribute the spatial receptor densities as well as the local curvature of the cell membrane in response to flow, receptor-ligand forces, and stochastic (random collisions) effects. Overall, these effects determine the individual carrier trajectory near the endothelial membrane, including rolling and motion arrest or detachment. For these studies, as in the case of many relevant endothelial determinants, hydrodynamic effects of local flow conditions modulate ICAM-1 expression and, hence, targeting can be even further complicated. Abnormal flow and pro-inflammatory factors increase the exposure of inducible ICAM-1 in pathological areas (e.g., inflammation foci) [17,84,97,104]. From the standpoint of dynamics of binding to a target anchor, flow forces may facilitate (enhanced mixing and number of productive collisions with endothelial cells) or suppress (enhanced detaching force) targeting of anti-ICAM carriers to endothelial cells. Flow parameters may affect endothelial cell targeting due to their effect on the endothelial cell phenotype [6,17,81,84,97,104–107,145–147]. Hydrodynamic forces can induce endocytic signaling and increase the number of endocytic vesicles in endothelium, which might facilitate carrier uptake and, hence, increased drug delivery [6,81,147]. On the other hand, endothelial cells in vivo and grown under flow in culture elongate and align in the flow direction [81,147,148]. This is helped by actin recruitment into flow-induced stress fibers, which may inhibit its role in endocytosis and final drug delivery after binding events have occurred [81,147,148]. Other groups, for example [23,37,54,66,142], have also reported factors related to subsequent internalization, especially, geometric parameters (i.e. size and shape).
Among the factors impacting targeting, geometric parameters (i.e. size and shape) have been shown to influence the processes of binding and the subsequent internalization [23,37,54,66,142]. With respect to non-spherical shaped carriers, the latter aspect, namely mode of internalization, is an important factor in targeting and delivery considerations. Phagocytosis is a principal component of the body’s innate immunity in which macrophages internalize targets in an actin-dependent manner. Using polystyrene particles of various sizes and shapes, Mitragotri et al studied phagocytosis by alveolar macrophages and reported a surprising finding that particle shape, not size, plays a dominant role in phagocytosis [37,54,66]. All shapes were capable of initiating phagocytosis in at least one orientation. However, the local particle shape, measured by tangent angles, at the point of initial contact dictates whether macrophages initiate phagocytosis or simply spread on particles. The local shape determines the complexity of the actin structure that must be created to initiate phagocytosis and allow the membrane to move over the particle. Failure to create the required actin structure results in simple spreading and not internalization. Particle size primarily impacts the completion of phagocytosis in cases where particle volume exceeds the cell volume [37,54,66].
Muro et al. studied the mechanisms that regulate ICAM-1 and PECAM-1 internalization by examining the uptake of anti-PECAM-1 and anti-ICAM-1 conjugates by endothelial cells [149]. The authors concluded that the conjugates must be multimeric, because monomeric anti-ICAM-1 and anti-PECAM-1 are not internalized. Moreover, newly internalized anti-ICAM-1 and anti-PECAM-1 conjugates did not colocalize with either clathrin or caveolin, and immunoconjugate internalization was not reduced by inhibitors of clathrin-mediated or caveolar endocytosis, suggesting that an alternative endocytic pathway was responsible. Amiloride and protein kinase C (PKC) inhibitors, agents known to inhibit macropinocytosis, reduced the internalization of clustered ICAM-1 and PECAM-1. However, expression of dominant-negative dynamin-2 constructs inhibited uptake of clustered ICAM-1. Binding of anti-ICAM-1 conjugates stimulated the formation of actin stress fibers by human umbilical vein endothelial cells (HUVEC). Latrunculin, radicicol and Y27632 also inhibited internalization of clustered ICAM-1, suggesting that actin rearrangements requiring Src kinase and Rho kinase (ROCK) were required for internalization. Interestingly, these kinases are part of the signal transduction pathways that are activated when circulating leukocytes engage endothelial cell adhesion molecules, suggesting the possibility that CAM-mediated endocytosis is regulated using comparable signaling pathways [149].
Macrophages are widely distributed and strategically placed in many tissues of the body to recognize and clear altered and senescent cells, invading particulates, as well as macromolecular ligands via a multitude of specialized plasma membrane receptors. This propensity of macrophages for endocytosis/phagocytosis of foreign particles has provided an opportunity for the efficient delivery of therapeutic agents to these cells with the aid of colloidal drug delivery systems (usually in the form of liposomes, polymeric nanospheres, micelles, and oil-in-water emulsions), following parenteral administration. However, the same properties make it difficult to design long-circulating NCs evading phagocytosis by macrophages [42].
Rational approaches in the design of long-circulating particles have been derived from following examples found in nature. For example, healthy erythrocytes evade the macrophages of the immune system and fulfill their function of transporting oxygen with a life span of 110 to 120 days. A multitude of physicochemical and physiological factors are believed to control the life span of red blood cells. These include surface characteristics (e.g., surface charge, membrane phospholipid composition, surface antigens) as well as bulk properties (e.g., shape and their extent of deformability) Red blood cells may avoid macrophage surveillance with the protection of a barrier of oligosaccharide groups. Furthermore, their deformable nature allows them to bypass the human splenic filtration process. In a different context, microbes seldom passively accept their fate at the hands of phagocytes and have evolved ways of evading intracellular killing to survive within the cell; one example is by escaping the phagosome, which is followed by direct entry into cytoplasm [42].
Recently, using highly stable polymer micelle assemblies known as filomicelles Discher et al. reported circulation of carriers up to one week after intravenous injection, which is about ten times longer than their spherical counterparts and is more persistent than any known synthetic nanoparticle [39,50]. Studies on the effect of the filamentous structures on binding affinity, signaling, and trafficking can provide a unified framework for designing long-circulating carrier in the future. In summary, hydrodynamic forces are expected to affect targeting of carriers in a multiplicity of ways, including interactions with the nano-scale molecular environment of the endothelial cell surface.
Conclusion
Targeting nanocarrier binding to vascular endothelium represents a complex interplay of physical forces, receptor-ligand molecular interactions and binding affinity, and characteristic features of carrier composition such as shape, bulk concentration, surface antibody density, carrier shape and size. Understanding the parametric dependencies of carrier anchoring with the target in relation to these independent factors provides unique opportunities for experimentation and simulation to advance the design and clinical implementation of optimal drug targeting. For instance, intuitively one might expect that increasing the antibody surface density and bulk concentration of carriers would implicitly result in improved binding specificity, faster binding kinetics, and an increase in the number of carriers capable of reaching the target cells. As a rule of thumb, enhanced vascular targeting can be accomplished by i) decreasing carrier size from micron-sized elements to sub-micron sized carriers, noting that decreased carrier size is accompanied by a decrease in drug carrying capacity for targeted delivery and that there is likely a minimum size limit beyond which targeting binding cannot be achieved; ii) by increasing antibody surface density, recognizing that maximal binding efficiency can occur well before maximal surface density is achieved; iii) by increasing bulk concentration of carriers, but this mass effect can also defeat purposes such as limiting toxicity, which motivated vascular targeting; and iv) selectivity of the targeting molecule, as accompanying processes such as upregulation, internalization and cellular trafficking and recycling of various molecules from the cell interior back to the cell surface are not uniform for various binding targets.
The existing evidence is that the true correlation between a particular targeting parameter and the resultant vascular endothelium binding achieved greatly depends on multiple parameters intrinsic to the biological system studied. These can include the local hydrodynamic conditions, the level of expression of the target molecule and other features that may vary between control and pathological conditions. The most readily available data concerning anti-ICAM coated carriers, derived from in vitro, in vivo and in silico experimentation and modeling remain relatively sparse. Thus, a few of the basic dynamic factors controlling targeted carrier adhesion to vascular endothelium are well defined, but only for the highly idealized systems of spherical particles bearing a highly characterized molecular recognition element such as an anti-ICAM antibody. Under such constraints, these governing factors include effects of hydrodynamic forces (e.g., shear stress), carrier size and, to a lesser degree, bulk concentration. The results of the many studies available may allow for utilization of carriers that minimize the amount of targeting Ab to be used or for coupling with delivery of specific cargoes by exploiting targeting capabilities. Still, overall there remains a strong need for more thorough parametric investigation of molecule-specific and geometry-specific platforms in the context of designing therapeutic interventions and diagnostic modalities based on nanocarrier targeting to healthy or diseased vascular endothelium.
Acknowledgments
This work has been supported by NIH grant R01 EB006818 (DME) and R01 HL087036 (VRM).
References
- 1.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 2.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Del. 2005;2:909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 3.Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dziubla TD, Muzykantov VR. Synthetic carriers for vascular delivery of protein therapeutics. Biotechnol Genet Eng Rev. 2006;22:267–298. doi: 10.1080/02648725.2006.10648074. [DOI] [PubMed] [Google Scholar]
- 5.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233(1–2):51–59. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 6.Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13(1):135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 7.Muzykantov VR. Immunotargeting of drugs to the pulmonary vascular endothelium as a therapeutic strategy. Pathophysiology. 1998;5(1):15–33. [Google Scholar]
- 8.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic (R) block copolymers as novel polymer therapeutics for drug and gene delivery. J Controlled Release. 2002;82(2–3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 9.Klibanov AL. Ligand-carrying gas-filled microbubbles: Ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16(1):9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 10.Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, Miletich DJ, Muzykantov VR. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Heart Circ Physiol. 2001;280(6):L1335–L1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 11.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 12.Molema G. Tumor vasculature directed drug targeting: applying new technologies and knowledge to the development of clinically relevant therapies. Pharm Res. 2002;19(9):1251–1258. doi: 10.1023/a:1020312220968. [DOI] [PubMed] [Google Scholar]
- 13.Fan YX, Wong L, Deb TB, Johnson GR. Ligand regulates epidermal growth factor receptor kinase specificity: activation increases preference for GAB1 and SHC versus autophosphorylation sites. J Biol Chem. 2004;279(37):38143–38150. doi: 10.1074/jbc.M405760200. [DOI] [PubMed] [Google Scholar]
- 14.Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophys J. 2005;89(5):3577–3588. doi: 10.1529/biophysj.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11(Suppl 2):S81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 16.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 17.Gimbrone MA., Jr Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155(1):1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simionescu M, Gafencu A, Antohe F. Transcytosis of plasma macromolecules in endothelial cells: a cell biological survey. Microsc Res Tech. 2002;57(5):269–288. doi: 10.1002/jemt.10086. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte PM. Endothelium-derived free radicals: for worse and for better. J Clin Invest. 2001;107(1):23–25. doi: 10.1172/JCI11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 21.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 22.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 23.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon AJ, Muzykantov V, Muro S, Eckmann DM. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46(4):323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S. Delivery of acid sphingomyelinase in normal and Niemann-Pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325(2):400–408. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- 26.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 27.Eniola AO, Hammer DA. Artificial polymeric cells for targeted drug delivery. J Controlled Release. 2003;87(1–3):15–22. doi: 10.1016/s0168-3659(02)00346-2. [DOI] [PubMed] [Google Scholar]
- 28.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys J. 2003;85(4):2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci US A. 2003;100(26):15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008;29(3):377–384. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87(2):300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 32.Chittasupho C, Xie SX, Baoum A, Yakovleva T, Siahaan TJ, Berkland CJ. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur J Pharm Sci. 2009;37(2):141–150. doi: 10.1016/j.ejps.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, Egbrink MGAO. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee KD, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci USA. 1997;94(16):8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batrakova EV, Kabanov AV. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Controlled Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon AJ, Bhowmick T, Leferovich J, Burman B, Pichette B, Muzykantov VR, Eckmann DM, Muro S. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Controlled Release. 2011;150:37–44. doi: 10.1016/j.jconrel.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Controlled Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simone EA, Dziubla TD, Colon-Gonzalez F, Discher DE, Muzykantov VR. Effect of polymer amphiphilicity on loading of a therapeutic enzyme onto protective fulamentous and spherical polymer nanocarrlers. Biomacromolecules. 2007;8(12):3914–3921. doi: 10.1021/bm700888h. [DOI] [PubMed] [Google Scholar]
- 41.Torchilin VP. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71(3):431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 43.Haun JB, Hammer DA. Quantifying nanoparticle adhesion mediated by specific molecular interactions. Langmuir. 2008;24(16):8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 44.Hossen N, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J Controlled Release. 2010;147(2):261–268. doi: 10.1016/j.jconrel.2010.07.100. [DOI] [PubMed] [Google Scholar]
- 45.Shmeeda H, Tzernach D, Mak L, Gabizon A. Her2-targeted pegylated liposomal doxorubicin: Retention of target-specific binding and cytotoxicity after in vivo passage. J Controlled Release. 2009;136(2):155–160. doi: 10.1016/j.jconrel.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511(2):397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 47.D’Aquino R, Harper T, Vas CR. Nanobiotechnology - Fulfilling the promise of nanomedicine. Chem Eng Progr. 2006;102(2):35–37. [Google Scholar]
- 48.Khademhosseini A, Langer R. Nanobiotechnology - Drug delivery and tissue engineering. Chem Eng Progr. 2006;102(2):38–42. [Google Scholar]
- 49.Bakowsky H, Richter T, Kneuer C, Hoekstra D, Rothe U, Bendas G, Ehrhardt C, Bakowsky U. Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim Biophys Acta. 2008;1778(1):242–249. doi: 10.1016/j.bbamem.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Cai S, Vijayan K, Cheng D, Lima EM, Discher DE. Micelles of different morphologies--advantages of worm-like filomicelles of PEO-PCL in paclitaxel delivery. Pharm Res. 2007;24(11):2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 51.Gobbi M, Re F, Canovi M, Beeg M, Gregori M, Sesana S, Sonnino S, Brogioli D, Musicanti C, Gasco P, Salmona M, Masserini ME. Lipid-based nanoparticles with high binding affinity for amyloid-beta1-42 peptide. Biomaterials. 2010;31(25):6519–6529. doi: 10.1016/j.biomaterials.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 52.Duncan R. Polymer-drug conjugates: targeting cancer. In: Muzykantov VT, editor. Biomedical aspects of drug targeting. Kluwer Academic Publishers; Boston: 2003. pp. 193–209. [Google Scholar]
- 53.Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, Minko T. Receptor targeted polymers, dendrimers, liposomes: Which nanocarrier is the most efficient for tumor-specific treatment and imaging? J Controlled Release. 2008;130(2):107–114. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. J Controlled Release. 2010;141(3):320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 58.Shelley SA. Nanobiotechnology cancer’s newest deadly foe. Chem Eng Progr. 2006;102(2):43–48. [Google Scholar]
- 59.Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5(5):449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- 60.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 61.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat Biotechnol. 2003;21(10):1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 62.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49(3):265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 64.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 65.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136(3):239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 66.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci US A. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery (review) Mol Membr Biol. 2010;27(4–6):190–205. doi: 10.3109/09687688.2010.499548. [DOI] [PubMed] [Google Scholar]
- 68.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283(4):H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 69.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci US A. 2003;100(13):7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci US A. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci. 2007;134–135:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 72.Lynch I, Salvati A, Dawson KA. Protein-nanoparticle interactions: What does the cell see? Nat Nanotechnol. 2009;4(9):546–547. doi: 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- 73.Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29(31):4244–4252. doi: 10.1016/j.biomaterials.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 75.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr, Banaszak Holl MM. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chemistry & Biology. 2007;14(1):107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Kaittanis C, Santra S, Perez JM. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv Drug Deliv Rev. 2010;62(4–5):408–423. doi: 10.1016/j.addr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaittanis C, Santra S, Perez JM. Role of nanoparticle valency in the nondestructive magnetic-relaxation-mediated detection and magnetic isolation of cells in complex media. J Am Chem Soc. 2009;131(35):12780–12791. doi: 10.1021/ja9041077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basha S, Rai P, Poon V, Saraph A, Gujraty K, Go MY, Sadacharan S, Frost M, Mogridge J, Kane RS. Polyvalent inhibitors of anthrax toxin that target host receptors. Proc Natl Acad Sci US A. 2006;103(36):13509–13513. doi: 10.1073/pnas.0509870103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rai P, Padala C, Poon V, Saraph A, Basha S, Kate S, Tao K, Mogridge J, Kane RS. Statistical pattern matching facilitates the design of polyvalent inhibitors of anthrax and cholera toxins. Nat Biotechnol. 2006;24(5):582–586. doi: 10.1038/nbt1204. [DOI] [PubMed] [Google Scholar]
- 80.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 81.Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Physiol. 1998;275(4 Pt 1):L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 82.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gosk S, Moos T, Gottstein C, Bendas G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim Biophys Acta. 2008;1778(4):854–863. doi: 10.1016/j.bbamem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 84.Luo GX, Kohlstaedt LA, Charles CH, Gorfain E, Morantte I, Williams JH, Fang F. Humanization of an anti-ICAM-1 antibody with over 50-fold affinity and functional improvement. J Immunol Methods. 2003;275(1–2):31–40. doi: 10.1016/s0022-1759(02)00542-2. [DOI] [PubMed] [Google Scholar]
- 85.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 86.Jevnikar AM, Wuthrich RP, Takei F, Xu HW, Brennan DC, Glimcher LH, Rubin-Kelley VE. Differing regulation and function of ICAM-1 and class II antigens on renal tubular cells. Kidney Int. 1990;38(3):417–425. doi: 10.1038/ki.1990.221. [DOI] [PubMed] [Google Scholar]
- 87.Kiani MF, Yuan H, Chen X, Smith L, Gaber MW, Goetz DJ. Targeting microparticles to select tissue via radiation-induced upregulation of endothelial cell adhesion molecules. Pharm Res. 2002;19(9):1317–1322. doi: 10.1023/a:1020350708672. [DOI] [PubMed] [Google Scholar]
- 88.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J Controlled Release. 2010;146(2):196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Weller GER, Zern B, Ayyaswamy PS, Eckmann DM, Muzykantov VR, Radhakrishnan R. A computational model for nanocarrier binding to endothelium validated using in vivo, in vitro, and Atomic Force Microscopy experiments. Proc Natl Acad Sci US A. 2010;107(38):16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 91.Bloemen PG, Henricks PA, van Bloois L, van den Tweel MC, Bloem AC, Nijkamp FP, Crommelin DJ, Storm G. Adhesion molecules: a new target for immunoliposome-mediated drug delivery. FEBS Lett. 1995;357(2):140–144. doi: 10.1016/0014-5793(94)01350-a. [DOI] [PubMed] [Google Scholar]
- 92.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 93.Newman PJ, Albelda SM. Cellular and molecular aspects of PECAM-1. Nouv Rev Fr Hematol. 1992;34(S):9–13. [PubMed] [Google Scholar]
- 94.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: A pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci US A. 2002;99(4):1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 96.Soda R, Tavassoli M. Liver endothelium and not hepatocytes or Kupffer cells have transferrin receptors. Blood. 1984;63(2):270–276. [PubMed] [Google Scholar]
- 97.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 98.Muro S, Cui XM, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. American Journal of Physiology - Cell Physiology. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 99.Albelda SM. Endothelial and epithelial-cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 100.Kumasaka T, Quinlan WM, Doyle NA, Condon TP, Sligh J, Takei F, Beaudet AL, Bennett CF, Doerschuk CM. Role of the intercellular adhesion molecule-1 (ICAM-1) in endotoxin-induced pneumonia evaluated using ICAM-1 antisense oligonucleotides, anti-ICAM-1 monoclonal antibodies, and ICAM-1 mutant mice. J Clin Invest. 1996;97(10):2362–2369. doi: 10.1172/JCI118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kishimoto TK, Rothlein R. Integrins, ICAMs, and Selectins: Role and Regulation of Adhesion Molecules in Neutrophil Recruitment to Inflammatory Sites. In: August JT, editor. Advances in Pharmacology. Vol. 25. Academic Press; 1994. pp. 117–169. [DOI] [PubMed] [Google Scholar]
- 102.Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am J Pathol. 1995;147(5):1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 103.Christofidou-Solomidou M, Kennel S, Scherpereel A, Wiewrodt R, Solomides CC, Pietra GG, Murciano JC, Shah SA, Ischiropoulos H, Albelda SM, Muzykantov VR. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury: targeting to thrombomodulin, but not to PECAM-1, causes pulmonary thrombosis and neutrophil transmigration. Am J Pathol. 2002;160(3):1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takei Y, Nishimura Y, Kawano S, Nagai H, Ohmae A, Fusamoto H, Kamada T. Expression of ICAM-1 is involved in the mechanism of liver injury during liver transplantation: therapeutic usefulness of the F(ab’)2 fragment of an anti-ICAM-1 monoclonal antibody. Transplant Proc. 1996;28(2):1103–1105. [PubMed] [Google Scholar]
- 105.Villanueva FS, Jankowski RJ, Klibanov S, Pina ML, Alber SM, Watkins SC, Brandenburger GH, Wagner WR. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98(1):1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 106.Weiner RE, Sasso DE, Gionfriddo MA, Thrall RS, Syrbu S, Smilowitz HM, Vento J. Early detection of oleic acid-induced lung injury in rats using (111) In-labeled anti-rat intercellular adhesion molecule-1. J Nucl Med. 2001;42(7):1109–1115. [PubMed] [Google Scholar]
- 107.Weller GE, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann Biomed Eng. 2002;30(8):1012–1019. doi: 10.1114/1.1513565. [DOI] [PubMed] [Google Scholar]
- 108.Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8(6):819–824. doi: 10.1161/01.atv.8.6.819. [DOI] [PubMed] [Google Scholar]
- 109.Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. Network of coupled promoting motions in enzyme catalysis. Proc Natl Acad Sci US A. 2002;99(5):2794–2799. doi: 10.1073/pnas.052005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muro S. Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1. In: Aird WC, editor. Endothelial Biomedicine. Chapter 117. Cambridge University Press; New York: 2007. pp. 1058–1070. [Google Scholar]
- 111.Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13(5):569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 112.Agarwal PK, Geist A, Gorin A. Protein dynamics and enzymatic catalysis: investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A. Biochemistry (Mosc) 2004;43(33):10605–10618. doi: 10.1021/bi0495228. [DOI] [PubMed] [Google Scholar]
- 113.Davies PF. NO flow helps clear murky waters. Circ Res. 1996;78(5):945–946. doi: 10.1161/01.res.78.5.945. [DOI] [PubMed] [Google Scholar]
- 114.Gan HH, Perlow RA, Roy S, Ko J, Wu M, Huang J, Yan S, Nicoletta A, Vafai J, Sun D, Wang L, Noah JE, Pasquali S, Schlick T. Analysis of protein sequence/structure similarity relationships. Biophys J. 2002;83(5):2781–2791. doi: 10.1016/s0006-3495(02)75287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21(24):6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei Z, Costa K, Al Mehdi AB, Dodia C, Muzykantov V, Fisher AB. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res. 1999;85(8):682–689. doi: 10.1161/01.res.85.8.682. [DOI] [PubMed] [Google Scholar]
- 117.Sun RJ, Muller S, Stoltz JF, Wang X. Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur Biophys J. 2002;30(8):605–611. doi: 10.1007/s00249-001-0195-x. [DOI] [PubMed] [Google Scholar]
- 118.Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–2177. doi: 10.1016/s0142-9612(01)00349-0. [DOI] [PubMed] [Google Scholar]
- 119.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci US A. 1997;94(12):6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hopkins AM, Baird AW, Nusrat A. ICAM-1: targeted docking for exogenous as well as endogenous ligands. Adv Drug Deliv Rev. 2004;56(6):763–778. doi: 10.1016/j.addr.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 121.Kim DW, Langille BL, Wong MK, Gotlieb AI. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ Res. 1989;64(1):21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- 122.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ho K, Lapitsky Y, Shi M, Shoichet M. Tunable immunonanoparticle binding to cancer cells: thermodynamic analysis of targeted drug delivery vehicles. Soft Matter. 2009;5:1074–1080. [Google Scholar]
- 124.Agrawal NJ, Radhakrishnan R. Role of glycocalyx in nanocarrier-cell adhesion: Thermodynamic model and Monte Carlo simulations. Journal of Physical Chemistry B. 2007;111(43):15848–15856. doi: 10.1021/jp074514x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. American Journal of Physiology-Heart and Circulatory Physiology. 2004;286(5):H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 126.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34(2):133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 127.Damiano ER. The effect of the endothelial-cell glycocalyx on the motion of red blood cells through capillaries. Microvasc Res. 1998;55(1):77–91. doi: 10.1006/mvre.1997.2052. [DOI] [PubMed] [Google Scholar]
- 128.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102(7):770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 129.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104(11):1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278(1):H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 131.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 132.Grest GS. Normal and shear forces between polymer brushes. In: Granick S, editor. Polymers in Confined Environments. Vol. 138. Springer-Verlag; Berlin: 1999. pp. 149–183. [Google Scholar]
- 133.Klein J. Reduction of Frictional Forces between Solid-Surfaces Bearing Polymer Brushes. Nature. 1994;370:634–636. [Google Scholar]
- 134.Milner ST. Hydrodynamic Penetration into Parabolic Brushes. Macromolecules. 1991;24(12):3704–3705. [Google Scholar]
- 135.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 2000;279(4):F698–F712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- 136.Bhatia SK, King MR, Hammer DA. The state diagram for cell adhesion mediated by two receptors. Biophys J. 2003;84(4):2671–2690. doi: 10.1016/S0006-3495(03)75073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanley W, McCarty O, Jadhav S, Tseng Y, Wirtz D, Konstantopoulos K. Single molecule characterization of P-selectin/ligand binding. J Biol Chem. 2003;278(12):10556–10561. doi: 10.1074/jbc.M213233200. [DOI] [PubMed] [Google Scholar]
- 138.Bell GI, Dembo M, Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 140.Dobrowsky TM, Daniels BR, Siliciano RF, Sun SX, Wirtz D. Organization of cellular receptors into a nanoscale junction during HIV-1 adhesion. PLOS Comp Biol. 2010;6(7):e1000855. doi: 10.1371/journal.pcbi.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Decuzzi P, Gentile F, Granaldi A, Curcio A, Causa F, Indolfi C, Netti P, Ferrari M. Flow chamber analysis of size effects in the adhesion of spherical particles. Int J Nanomedicine. 2007;2(4):689–696. [PMC free article] [PubMed] [Google Scholar]
- 142.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94(10):3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 144.Beste MT, Hammer DA. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc Natl Acad Sci US A. 2008;105(52):20716–20721. doi: 10.1073/pnas.0808213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muro S, Muzykantov VR, Murciano JC. Characterization of endothelial internalization and targeting of antibody-enzyme conjugates in cell cultures and in laboratory animals. Methods Mol Biol. 2004;283:21–36. doi: 10.1385/1-59259-813-7:021. [DOI] [PubMed] [Google Scholar]
- 146.Cardena E. Evaluation of post-traumatic stress disorders. Mil Med. 2001;166(12 Suppl):53–54. [PubMed] [Google Scholar]
- 147.Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993;92(4):2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Al Mehdi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Ross C, Blecha F, Dinauer M, Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83(7):730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 149.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]