Abstract
The fungal allergen, Alternaria, is specifically associated with severe asthma, including life-threatening exacerbations. To better understand the acute innate airway response to Alternaria, naïve WT mice were challenged once intranasally with Alternaria. Naïve WT mice developed significant BAL eosinophila following Alternaria challenge when analyzed 24 hours later. In contrast to Alternaria, neither Aspergillus nor Candida induced BAL eosinophilia. Gene microarray analysis of airway epithelial cell brushings demonstrated that Alternaria-challenged naïve WT mice had an over 20 fold increase level of expression of “Found in Inflammatory Zone 1” (FIZZ1/Retnla), a resistin-like molecule. Lung immunostaining confirmed strong airway epithelial FIZZ1 expression present as early as 3 hours after a single Alternaria challenge that persisted for at least 5 days and was significantly reduced in STAT6-deficient, but not PAR-2-deficient mice. Bone marrow chimera studies revealed that STAT6 expressed in lung cells was required for epithelial FIZZ1 expression, while in contrast, STAT6 present in bone marrow derived cells contributed to airway eosinophilia. Studies investigating which cells in the non-challenged lung bind FIZZ1 demonstrated that CD45+CD11c+ (macrophages and dendritic cells) as well as collagen-1 producing CD45 negative cells (fibroblasts) can bind to FIZZ1. Importantly, direct administration of recombinant FIZZ1 to naïve WT mice led to airway eosinophilia, peribronchial fibrosis, and increased thickness of the airway epithelium. Thus, Alternaria induces STAT-6 dependent acute airway eosinophila and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. This may provide some insight into the uniquely pathogenic aspects of Alternaria-associated asthma.
Introduction
Though asthma has many clinical, physiologic, and immunologic phenotypes, the majority of asthmatics have environmental allergic triggers including dust mite, cockroach, and mold. Alternaria is an example of a common fungal allergen that is associated with the development of asthma (1). Sensitization to Alternaria alternata is a risk factor for persistence of asthma and fatal/near-fatal asthma (2–8). The spores of Alternaria are known to be a source of outdoor allergens for sensitized individuals, and have also recently been detected at high levels indoors (9). Dispersion of the spores occurs during warm, dry weather periods especially in late summer/early fall and has been associated with epidemic, severe asthma symptoms (2–8). Such clinical associations with Alternaria and asthma are intriguing, but the mechanisms contributing to the pathologic airway responses are still incompletely understood.
Allergic disease including asthma has largely been characterized by dysregulation of adaptive immunity in response to allergens, including Th2 cell differentiation and IgE sensitization. More recently, it has become clear that innate immune responses to allergens in the airway help to shape subsequent adaptive responses (10, 11). For example recent reports have suggested that allergens with high protease activity, such as cockroach and fungal allergens, induce innate inflammatory events and allergen sensitization through a protease activated receptor two (PAR-2) -mediated pathway in the bronchial epithelium (12–14). Investigations into such innate epithelial responses to inhaled allergens may provide important clues to the pathogenesis of asthma. In this study we have investigated whether Alternaria is able to induce an acute Th2-like airway inflammatory response in naïve WT mice via activation of innate epithelial genes. We demonstrate that Alternaria induces a significant acute airway eosinophil response in naïve WT mice that is mediated by innate immune mechanisms distinct from those triggered by protease allergens through PAR-2 on the epithelium. This innate pro-eosinophil inflammatory and pro-remodeling effect of Alternaria in naïve WT mice is not shared with other common fungal allergens such as Aspergillus and Candida suggesting that different allergens trigger distinct innate airway epithelial pathways that contribute to asthma.
Materials and Methods
Mice and airway challenges
Six to eight week- old female naïve C57BL/6 WT mice were administered 100ug of either Alternaria alternata (Lot# 130656), Candida albicans (Lot# 111797), or Aspergillus fumigatus (Lot# 118033) extracts (Greer, Lenoir NC) intranasally in 80ul and killed 24 hours later at which timepoint BAL and lung specimens were processed. For selected experiments naïve WT mice were analyzed 3 hours and 5 days after challenge. Control groups of naïve WT mice were given intranasal challenges of 80uL PBS. In selected experiments PAR-2 deficient or Stat6 deficient mice (Jackson laboratories) on B6 background were administered 100ug of Alternaria alternata extracts intranasally with WT controls as described above. Collagen-1 GFP reporter mice were a gift from Dr. David Brenner and have been previously described (15).
In some experiments, 5ug of recombinant Fizz-1 (Peprotech) or vehicle (PBS) was given intranasally to naïve WT mice every day for five days and mice were killed on day 8. The endotoxin level detected in rFIZZ1 was 0.0051 ng/ml by limulus assay (Lonza). All experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
BAL Cellular Analysis, Lung Processing, and FACS
BAL and lung processing was performed as previously described (16). BAL fluid was obtained by intratracheal insertion of a catheter and five lavages with 0.8 mL of 2% filtered bovine serum albumin (BSA) (Sigma). The right lung was tied off, removed, and snap-frozen in liquid nitrogen for RNA isolation or ELISA. The left lung was instilled with 0.4 mL 4% paraformaldehyde (PFA) and placed in PFA for paraffin embedding and staining. To obtain lung single cell suspensions, lungs were minced and shaken vigorously in RPMI with 2 mg/ml collagenase and 1mg/ml DNAse I for 40 minutes. Lung cells were isolated using a 70-um cell strainer.
BAL cells were incubated with a monoclonal antibody to CD16/CD32 (24G.2) for 10 min to block Fc receptors and then stained with PE-conjugated Siglec-F, FITC-conjugated CD11c, and APC-conjugated Gr-1 (eBiosciences) for 30 minutes. BAL cells were washed with FACS buffer and eosinophils were identified as the SiglecF+ CD11c-population (17). FACS was performed using an Accuri C6 flow Cytometer and analyzed with FlowJo software (Tree Star, Oregon).
ELISA for cytokines and total IgE
ELISA of lung homogenate IL-5 and IL-13 (R&D) was performed as previously described (16). ELISA for IL-33 (R&D) was performed on BAL supernatant. Serum total IgE performed with IgE ELISA kit (BD biosciences) according to the manufacturer’s instructions and all ELISA plates read with a BioRad Model 680 microplate reader.
Isolation of airway epithelial cells
To study which epithelial genes were induced by Alternaria we adapted a technique first developed by Kotaro Sugimoto and Dean Sheppard at UCSF (18) for obtaining airway epithelial cells in mice by bronchial brushing. Prior to performing epithelial brushing, BAL was performed to remove BAL cells. The bronchial brushing was performed using a sterile plastic feeding tube (Solomon Scientific) modified by removal of the rubber bulb, sanding to create roughness, and autoclaving. The tube was inserted into the right main and left main bronchus with gentle brushing and immediately placed in RNAlater (Qiagen).
Microarray and real time RT-PCR analysis
Airway epithelial cells obtained by bronchial brushing were lysed by multiple passages through an 18G needle. RNA was then extracted according to manufacturer’s protocol (Qiagen). Isolated epithelial RNA with sufficient purity (A260/280 = 1.97–2.12) and yield (8.99–12.66 ng/ul) was used for microarray analysis (GeneChip Mouse Gene 1.0 ST, Affymetrix). Gene chip results were confirmed by quantitative real-time PCR and analyzed with Vampire software. Microarray data has been deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34764) and given accession# GSE34764. For whole lung RNA extraction, the lung was homogenized in TRIzol reagent (Invitrogen) and RNA was extracted according to manufacturer’s protocol (Qiagen). RNA yield and purity was measured with the Nanodrop 1000 (Thermo Scientific). Single-strand cDNA was prepared by reverse transcription of 1 µg of total RNA with the SuperScript III kit (Invitrogen). Fizz-1 was quantitated by amplification of cDNA in SYBR Green Supermix (Applied Biosystems) using the following primer pairs: forward, 5'-CCC TTC TCA TCT GCA TCT C-3', reverse, 5'- CAG TAG CAG TCA TCC CAG CA-3'. Triplicates of samples were run with the mean value used for quantification.
WT and STAT6−/− bone marrow chimeras
WT CD45.1 and STAT6−/− (CD45.2+) mice purchased from Jackson labs received Sulfatrim antibiotic (5mL/200mL, Med Vet International) for one week prior and two weeks after receiving donor bone marrow. Recipient mice were irradiated twice with 450R separated by two hours. Donor bone marrow was isolated from both tibias and fibulas and 15 million cells were injected to recipient via tail veins. Mice were rested for six weeks before Alternaria challenge. Efficiency of chimerism was assessed by FACS for congenic markers CD45.1 and CD45.2 on BAL cells.
FIZZ1 lung binding assay
The FIZZ1 lung binding assay was adapted from a previously described method to detect FIZZ1 binding to mouse splenocytes (19). The strategy involved incubating naïve WT lung cells with recombinant FIZZ1 (rFIZZ1) followed by the addition of primary and secondary antibodies. Negative control lung samples were not incubated with rFIZZ1, but were identically processed otherwise.
To determine whether FIZZ1 binding co-localized with lung fibroblasts, single cell suspensions from lungs of naïve WT mice and collagen-1 GFP reporter mice were incubated with 0.5 µg rFIZZ1, or FACS buffer alone, for 60 minutes at 4°C. Cells were washed and incubated with Fc blocking antibody for 30 minutes, stained with APCCD45 and polyclonal rabbit anti-FIZZ1 (Peprotech) for 30 minutes followed by addition of PE-conjugated anti-rabbit Fab fragment (eBiosciences). Cells from WT mice were also stained with FITC-CD11c.
Immunofluorescence
For collagen-1 and Fizz-1 staining, lung samples were de-paraffinized by sequential placement in xylene and ethanol (16). Staining for collagen-1 was performed with a polyclonal antibody (Millipore) at 1:500 concentration and staining for Fizz-1 was performed with rabbit polyclonal antibody (PeproTech) at 1:1,000 concentration. Tyramide Signal Amplification Kit #41 (Invitrogen) was used for fluorescent signal amplification with subsequent DAPI staining (Vector Laboratories). Lung airways were visualized with a DM2500 microscope (Leica Microsystems).
Remodeling Analysis and Epithelial Thickness
Parrifinized lung sections were stained with Masson’s trichrome and the area of peribronchial fibrosis on trichrome-stained sections was quantified by analysis with Image-Pro Plus software (16). All slides were blinded and results are expressed as the area of positive staining per micrometer length of bronchiole basement membrane. Hematoxylin and eosin stained lung sections were used to evaluate the thickness of the epithelium in micrometers was measured from the bottom of the basement membrane to the mucosal surface of the bronchial epithelium. Six individual areas per airway were measured and a minimum of four airways were analyzed per slide. All measurements were done with the Image-Pro Plus software.
Airway hyperresponsiveness
In some experiments, invasive pulmonary function testing was performed using the Flexivent system (Scireq, Canada) and airway resistance analyzed by Scireq Flexivent 5.1 software as previously described (16). Briefly, mice were anesthetized, canulated via the trachea, administered increasing doses of methacholine, and airway resistance measured.
Statistical Analysis
Statistical analysis was performed using Prism Software (Graphpad). The Mann-Whitney test or student’s t-test were used as indicated.
Results
Alternaria induces acute BAL airway eosinophilia and lung Th2 cytokine production in naïve WT mice
We examined the acute airway inflammatory response to fungal allergens Alternaria, Aspergillus, and Candida in naïve WT mice. One day after a single intranasal administration of these allergen extracts, only mice receiving Alternaria developed significant airway eosinophilia (Fig. 1A). Over 30% of the BAL cells were eosinophils (Siglec-F+ CD11c- cells) after acute Alternaria exposure compared with less than 1% found after instillation of the same dose of Aspergillus, Candida, or control PBS (Fig. 1A). The total number of BAL eosinophils were significantly elevated only in the Alternaria-treated mice (Fig. 1A). Alternaria exposure induced acute airway eosinophilia in a dose-response manner. Naïve WT mice challenged with increasing doses of Alternaria (10ug, 50ug, and 100ug) had increases in both percent and total airway eosinophilia with escalating doses (Fig. 1B). Thus, Alternaria specifically induces airway eosinophilia within 24hrs of a single exposure in naïve WT mice in a dose-dependent manner.
Figure 1. Single acute airway exposure of Alternaria in naïve WT mice induces early eosinophilia and Th2 cytokine production.
Naïve mice received a single intranasal challenge with 100ug of Alternaria, Candida, or Aspergillus extract or PBS and BAL performed 24 hours later (A) for eosinophil percent by FACS (left) and absolute eosinophil counts (right). Eosinophils were defined as Siglec-F+ CD11c- cells. Naïve mice were given intranasal administrations of 10ug, 50ug, or 100ug Alternaria extract and BAL performed one day later (B). BAL eosinophil percent (left) and total number (right) were analyzed at the given dose. FACS plots representative of 4–7 mice per group and dose. Eosinophil numbers for individual mice shown. ELISA for BAL IL-33, Lung IL-5 and IL-13 (C) at 3hr, 6hr, and 1d after Alternaria challenge (4 mice per group). PBS is from 3hr time point. *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001 compared with PBS, t-test.
Cytokines including IL-33, IL-5, and IL-13 contribute to Th2-type responses. We measured levels of these cytokines in the BAL and lung at 3, 6, and 24 hours after Alternaria challenge (Fig. 1C). BAL levels of IL-33 and lung levels of IL-5 and IL-13 were increased after Alternaria challenge compared with PBS challenge. Levels of Il-33 peaked at 3 hours and trended down by 24 hours. Lung levels of IL-5 and IL-13 were increased maximally at 6hr. Total serum IgE levels were not significantly different between Alternaria and PBS challenged mice (mean 27.9 ng/ml Alternaria vs. 17.5 ng/ml PBS, 3–4 mice per group). Overall, this suggests that Alternaria induces rapid Th2 cytokine production in addition to innate eosinophilia.
Bronchial brushing to identify Alternaria-induced epithelial mediators
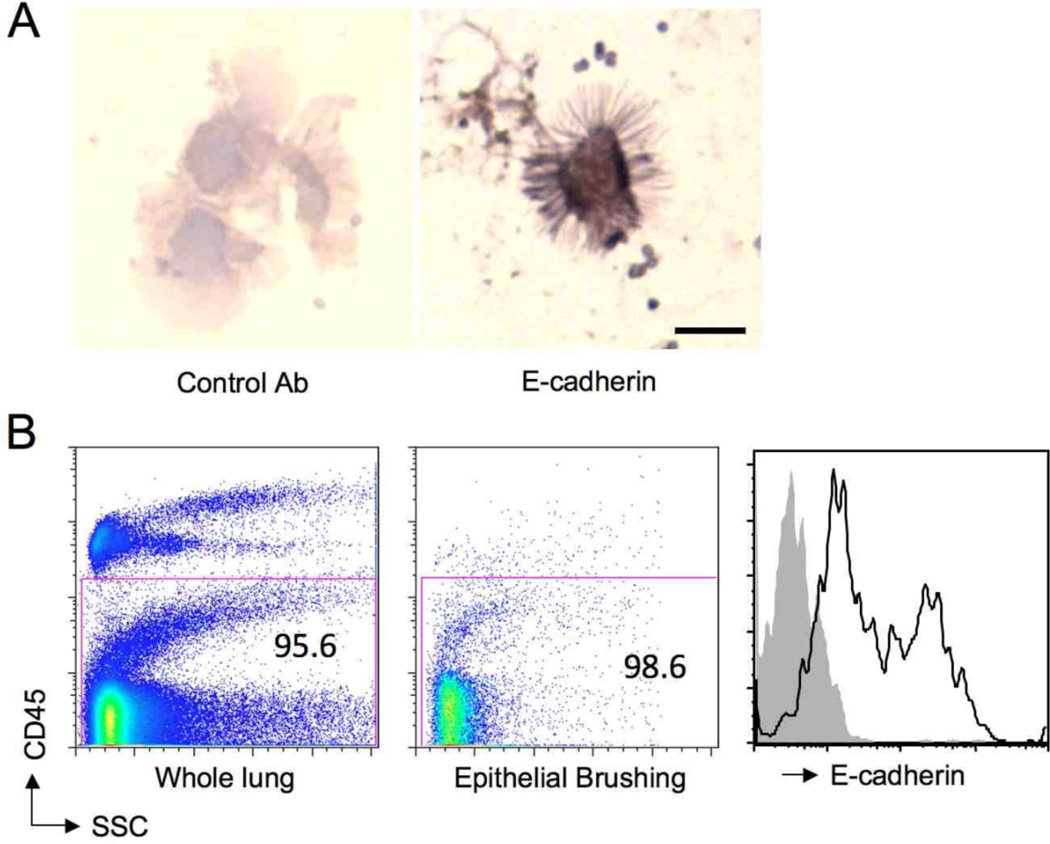
In order to identify genes that may be upregulated in the epithelium during the acute response to a single challenge with Alternaria, we employed a technique utilizing a bronchial brush to obtain cells from the airway (18). Cytospun cells from bronchial brushings had the ultrastructural appearance of ciliated airway epithelial cells and immunostained positive for the epithelial marker E-cadherin (Fig. 2A). Compared with whole lung cell suspensions that contained both CD45 positive and CD45 negative cells, the cells from the airway brushing were nearly all CD45 negative by FACS (Fig. 2B). Additionally, nearly all of the live airway brushing CD45 negative cell population expressed E-cadherin compared with control antibody staining (Fig. 2B). These bronchial brushing airway epithelial cells were used to identify genes induced by acute exposure of naïve WT mice to Alternaria.
Figure 2. Airway epithelial cells obtained by bronchial brushing.
Cells collected by brushing of large airways in naïve WT mice were analyzed by morphology and immunostained for E-cadherin (A). Scale bar 10 um. Airway brushing cells pooled from 6 mice were analyzed by FACS (B, middle) and compared with lung cells derived from naïve WT mouse lung (left) and stained for E-cadherin (right). Grey shaded region on histogram represents isotype control.
Found in inflammatory Zone 1 (FIZZ1) is highly induced in the airway epithelium in naïve WT mice after acute Alternaria exposure
Utilizing the technique from Fig. 2, we isolated epithelial RNA from Alternaria and PBS challenged mice for microarray analysis. As shown in Fig. 3A, FIZZ1 (Retnla) was induced approximately 20-fold after Alternaria exposure in the expression array and subsequently confirmed by qPCR to be induced over 200 fold compared with PBS (Fig. 3A & B). Other genes with highly elevated transcript levels included some critically involved in mucus production including MUC5AC (8-fold) and Clca3 (9-fold). As mucus production occurs exclusively in the epithelium, the fact that these genes were highly induced in Alternaria-challenged mice supports our airway brushing characterizing an epithelial transcriptome. Chitinase genes Ym1 (Chi3l3) and Chi3l1 are members of a family of proteins increasingly associated with severe asthma and were induced five fold (20, 21). Interestingly, many other genes significantly upregulated in the epithelium of Alternaria exposed mice compared with PBS included muscle-related genes and structural proteins that may represent epithelial-mesenchymal transition proteins such as MYO18b, a marker for myocyte differentiation (22).
Figure 3. Epithelial brushing microarray and lung FIZZ1 expression after acute Alternaria airway challenge.
Mice received Alternaria or PBS one time and underwent bronchial brushing 24 hours later and subsequent RNA isolation for microarray analysis (A). Confirmatory qPCR performed on bronchial brush samples for FIZZ1 (B). Lung sections from mice challenged with either Alternaria, Candida, Aspergillus extracts or PBS and immunofluorscent staining performed for FIZZ1 at 24 hours after challenge (C) and 3 hours (D, left) or 5 days (D, right) after one challenge with Alternaria. Scale bars 100 um. *p < 0.01, 3 mice per group (A), 3–4 mice per group in (B).
To visualize FIZZ1 expression in the Alternaria challenged airway, we performed immunofluorescent staining of lung sections. As expected, FIZZ1 was highly expressed in the epithelium of Alternaria-challenged mice compared with PBS (Fig. 3C). Some subepithelial cells also expressed FIZZ1 though in significantly lower frequency compared with epithelial expression. FIZZ1 expression was detected in the epithelium of lung sections as early as 3hr after a single Alternaria challenge and remained elevated for five days (Fig. 3D). In contrast, we detected minimal expression of epithelial FIZZ1 in lung sections from WT mice challenged one time with fungal allergens other than Alternaria such as Aspergillus and Candida. Thus, airway epithelial FIZZ1 is specifically and highly induced early after exposure of mice to Alternaria and persists for days after one challenge.
Alternaria-induced epithelial FIZZ1 expression and BAL eosinophilia is STAT-6 dependent
The transcription factor STAT6 is critical to IL-4/IL-13 signaling and Th2 cell development (23), but little is known about an acute role in airway disease in naïve WT mice. To investigate this, WT and STAT6-deficient (STAT6−/−) mice were challenged with a single dose of Alternaria. Dramatically, FIZZ1 expression was nearly absent in the epithelium of STAT6−/− mice (Fig. 4A). Lung FIZZ1 mRNA levels were also significantly reduced in acute Alternaria challenged STAT6−/− mice (Fig. 4B). Additionally, BAL eosinophilia was strongly reduced in STAT6−/− mice compared with WT mice (Fig 4C). This suggests that both FIZZ1 expression and the acute airway inflammatory events induced by Alternaria are dependent on STAT6.
Figure 4. Alternaria-induced lung FIZZ1 expression and early eosinophilia is dependent on STAT6, but not PAR-2.
WT, STAT6−/−, or PAR-2−/− mice were challenged with Alternaria and FIZZ1 immunofluorescent staining performed on lung sections (A); scale bar 100 um. FIZZ1 mRNA levels measured in whole lung samples from WT, STAT6−/−, or PAR-2−/− mice (B). Percent BAL eosinophils by FACS (left) and total numbers (right) for WT, STAT6−/−, or PAR-2−/− mice (C). 4–6 mice in each group, Scale bars 100um, *p < 0.005 and **p < 0.01 compared with WT mice, t test.
Fungal allergens are known to possess significant protease activity and early inflammatory responses in the airway can be impaired by addition of protease inhibitors (12, 24). Additionally, Alternaria has been shown in-vitro to induce the pro-allergic cytokine TSLP in epithelial cells through protease activated receptor 2 (PAR-2) suggesting that other epithelial derived factors such as FIZZ1 may be regulated by PAR-2 (14). To test this, we challenged WT and PAR-2-deficient mice with a single dose of Alternaria. In contrast to STAT6−/− mice, PAR-2 deficient mice had only a slight reduction in the level of FIZZ1 expression in the epithelium of stained lung sections and no difference at the lung transcript level (Fig. 4B). Additionally, the BAL eosinophilia was unchanged in PAR-2 deficient mice compared with WT mice. This suggests that PAR-2 is not critical for FIZZ1 epithelial expression or acute airway eosinophilia after Alternaria challenge.
Bone marrow-derived STAT6 contributes to Alternaria-induced airway eosinophilia
To address whether STAT6 expressed in lung structural cells or bone marrow derived cells contributed to airway eosinophilia and FIZZ1 expression after Alternaria challenge, we performed bone marrow chimera studies with WT and STAT6 deficient mice. Six weeks after WT mice and STAT6 deficient mice were irradiated and received either WT or STAT6 bone marrow transplantation, mice were challenged once with Alternaria and analyzed 24 hours later. We utilized congenic WT mice (CD45.1) to determine the efficiency of chimerism in the lung (Figure 5A). FACS analysis of BAL cells revealed 94–98% efficiency of chimerism in the lung in all groups. We next determined the level of BAL eosinophils in these mice (Figure 5B). WT CD45.2 mice that received WT CD45.1 bone marrow developed significant airway eosinophilia when measured 24 hours after one Alternaria challenge. STAT6 deficient recipients that received WT bone marrow had similar levels of airway eosinophilia compared with the WT only group. In contrast, WT mice that received STAT6 deficient bone marrow had significantly reduced eosinophilia after Alternaria challenge. This suggests that STAT6 expressed in hematopoetic cells contributes to the innate eosinophilia after single Alternaria challenge.
Figure 5. Bone marrow STAT6 contributes to Alternaria-induced eosinophilia, but FIZZ1 expression is dependent on STAT6 in lung structural cells.
WT (CD45.1 and CD45.2) and STAT6−/− mice were irradiated and injected i.v. with WT or STAT6−/− bone marrow and challenged once with Alternaria 6 weeks later. FACS analysis of BAL cells for % of donor bone marrow cells in airway (A). Total BAL eosinophils in WT and STAT6−/− bone marrow chimeric mice 24 hours after single Alternaria challenge (B). FIZZ1 immunofluorescent staining performed on lung sections (C); scale bar 100 um. Representative of 8–10 mice per group (A&C), *p<.05, Mann-whitney, 8–10 mice per group (B).
Alternaria-induced epithelial FIZZ1 is dependent on STAT6 expression in lung cells
We performed immunofluorescent staining for FIZZ1 in lung sections from WT and STAT6 deficient bone marrow chimeras that received a single challenge with Alternaria. Stained lung sections from WT CD45.2 mice that received WT CD45.1 bone marrow revealed strong FIZZ1 staining in the airway epithelium (Figure 5C). Further, WT recipients that received STAT6 deficient bone marrow had similar levels of FIZZ1 staining in the airway epithelium compared with WT recipients that received WT bone marrow. However, STAT6 deficient mice that received WT bone marrow had significantly reduced FIZZ1 staining in the airway epithelium after Alternaria challenge. This suggests that STAT6 in lung cells are required for Alternaria-induced FIZZ1 expression.
FIZZ1 binds to inflammatory and structural cells in the lung
The receptor for FIZZ1 is currently unknown, so we used a FIZZ1 lung-binding assay (19) to identify cell types in the lung that bind to FIZZ1 (Fig. 6A). Single cell suspensions from digested lung were gated on either leukocytes (CD45+) or structural cells (CD45−). Both lung CD45+ cells (Fig. 6B) and lung CD45− cells (Fig. 6C) bound to FIZZ-1. In the lung CD45+ cell population, CD45+CD11c+ cells (comprised of both macrophages and dendritic cells) displayed significant FIZZ1 binding (Fig. 6B). In contrast, analysis of CD45+CD11c- cells revealed a smaller population that bound FIZZ1 (Fig. 6B). The CD45− population also displayed FIZZ1 binding (6C). As the lung CD45− population is composed of structural cells including fibroblasts, we then performed the same FIZZ1-binding assay using single cell suspensions from digested lungs from collagen-1 GFP reporter mice in which lung fibroblasts strongly express GFP (15) and determined that the CD45− col1+ population bound to FIZZ1 (Fig. 6D). These studies suggest that several cell types (macrophages, dendritic cells, fibroblasts) within the naïve lung may bind and respond to FIZZ1. However, not all cell types in the lung bind FIZZ1 as only a small population of lung CD45+CD11c- cells bound FIZZ1.
Figure 6. rFIZZ1 binds to leukocytes and structural cells isolated from naïve WT mouse lung.
Single cell suspensions from two naïve WT mouse lungs were incubated with rFIZZ1 followed by staining as shown (A). Lung cells were stained with CD45 and CD11c. CD45 positive cells (left) were gated and CD11c+ and CD11c− populations (right) were analyzed for FIZZ1 binding (B). CD45− cells were gated analyzed for FIZZ1 binding (C). CD45− cells from Col-1 GFP mice were gated on collagen-1 positive cells and analyzed for FIZZ1 binding (D). Lung cells analyzed from pooled samples from two mice and are results of two independent experiments.
Intranasal rFIZZ1 administration leads to airway eosinophilia, increased epithelial thickness, and fibrosis
To evaluate possible roles of FIZZ1 in the airway, we administered recombinant FIZZ1 (rFIZZ1) to naïve WT mice for 5d and performed BAL and histologic analysis 3 days later. Mice that received rFIZZ1 had elevated levels of eosinophils in the airways compared with mice that received only PBS (Fig. 7A). These mice also had evidence of increased epithelial thickness (Fig. 7B), a feature associated with severe asthma (25). Elevated levels of peribronchial fibrosis detected by trichrome staining and increased collagen-1 immunofluorescent staining were present in lung sections from mice that received rFIZZ1 compared with those that received PBS (Fig. 7C). To determine whether these changes were associated with increased airway reactivity, we performed invasive pulmonary testing in mice receiving rFIZZ1 or PBS but did not detect a difference in airway resistance after increasing doses of methacholine (Fig. 7D). These data suggest that FIZZ1 may have many roles in the airway including promoting eosinophilia, epithelial changes, and peribronchial fibrosis.
Figure 7. Exogenous FIZZ1 induces airway eosinophila, epithelial thickening and fibrosis.
Naïve WT mice received intranasal rFIZZ1 or vehicle (PBS) for five consecutive days and BAL and lung were analyzed three days later. Total BAL eosinophils enumerated (A). Epithelial thickness measured in H&E lung sections from mice receiving rFIZZ (right) or PBS (middle) (B). Scale bars, top row 100 um; bottom row 50 um. Lung sections stained with trichrome and scored (C, left and top row) or collagen-1 (bottom row); scale bars 100 um. (D) Balb(c) mice were challenged with intranasal rFIZZ1 or PBS for five days and invasive airway resistance measured after increasing doses of methacholine. 4 mice per group (A & D), 19–25 airways per group (B), 23–25 airways per group (C). *p < 0.05, t test. **p < 0.0001, ***p < 0.001, Mann-whitney.
Discussion
Alternaria has been associated with the development, persistence and severity of asthma (4). There is currently very little known regarding reasons behind the unique pathogenicity of Alternaria. In this study we demonstrate that naïve WT mice developed significant BAL eosinophila following Alternaria challenge. In contrast to Alternaria, neither Aspergillus nor Candida induced BAL eosinophilia. Gene microarray analysis of airway epithelial cell brushings demonstrated that Alternaria-challenged naïve WT mice had a 20 fold increase level of expression of “Found in Inflammatory Zone 1” (FIZZ1/Retnla), a resistin-like molecule whose increased expression in airway epithelium was confirmed by qPCR. Additional genes that were highly induced by Alternaria in airway epithelium included those involved in mucus expression (MUC5AC, Clca3) and chitinase genes (Yim1 and Chi3l1). Lung immunostaining confirmed strong airway epithelial FIZZ1 expression present as early as 3 hours after a single Alternaria challenge that persisted for at least 5 days and was significantly reduced in STAT6-deficient, but not PAR-2-deficient mice. Bone marrow chimera studies revealed that STAT6 expressed in lung cells was required for epithelial FIZZ1 expression. In contrast, STAT6 present in bone marrow derived cells contributed to airway eosinophilia. Direct administration of recombinant FIZZ1 to naïve mice lead to airway eosinophilia, peribronchial fibrosis, and increased thickness of the airway epithelium. Studies investigating which cells in the lung bind FIZZ1 demonstrated that both fibroblasts (CD45−collagen-1+) and leukocytes in the lung (CD45+), in particular CD45+CD11c+ macrophages and dendritic cells, bound to FIZZ1. Thus, Alternaria induces STAT-6 dependent acute airway eosinophila and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. This may provide some insight into the uniquely pathogenic aspects of Alternaria-associated asthma.
Our studies have also identified that the innate acute eosinophilic response to Alternaria is mediated by STAT6 and not PAR-2. This is in contrast to a report of mice challenged with intranasal purified protease from a different fungal allergen Aspergillus that did not require STAT6 for development of early eosinophilic airway inflammation at 18 hours but did require intact allergen protease activity (24). In our studies, we utilized whole allergen extracts from Alternaria and Aspergillus instead of purified protease from Aspergillus and this may have contributed to the different results. Others have suggested a PAR-2 mediated pathway may drive inflammatory events induced by protease allergens, including Alternaria (12–14, 26). We did not detect a reduction in eosinophilic inflammation in PAR-2 deficient mice in response to Alternaria exposure, instead our data suggests a STAT6-mediated pathway. In vitro studies have shown that PAR-2 is important in Alternaria-induced bronchial epithelial cell activation and production of TSLP (12, 14), but as demonstrated in our study, in vivo Alternaria-challenged PAR-2 deficient mice may use alternate STAT-6 dependent pathways. Studies have also examined the role of PAR-2 in the adaptive immune response in mice challenged with a non-fungal cockroach allergen. These studies demonstrated that cockroach challenge did not induce an acute innate airway eosinophilia in WT mice (26). However, cockroach challenge in PAR-2 deficient mice did result in reductions in airway inflammation in response to cockroach after 3 challenges over 17 days (13). Thus, differences in the results of our studies (which do not demonstrate dependence on PAR-2) and others may be related to our use of in vivo models (as opposed to in vitro studies), the time-points studied (acute innate 24 hr vs late adaptive immune response), and the allergen utilized (Alternaria vs cockroach).
We detected early increases in IL-33, IL-5, and IL-13 in the lung after one Alternaria challenge. This is consistent with a recent report showing that Alternaria induces the release of the pro-Th2 cytokine IL-33 within a few hours in-vivo and was dependent upon extracellular ATP inducing calcium influx in epithelial cells (27). The same study showed that mice receiving a single challenge with Alternaria extract had elevated lung levels of Th2 cytokines IL-5 and IL-13 that were nearly absent in Myd88 and ST2 (IL-33 receptor) deficient mice when measured twelve hours later. This work highlights the complexity of the innate response to Alternaria and the multiple pathways involved including TLR/IL-1 family signaling. We chose a 24 hour time-point following Alternaria challenge for gene micro-array analysis as we were interested in determining which genes expressed at this time point might be responsible for persistent airway inflammation and remodeling. Indeed we identified both FIZZ-1 and members of the chitinase gene family being expressed at this time point, both candidate genes for remodeling. Given the very early rise (within a few hours) of Th2 cytokines detected in the lung in our studies as well as others (27), the 24 hour time point we used for microarray analysis was likely not optimal for detection of cytokine and chemokine genes induced in the initial few hours following Alternaria challenge (e.g. TSLP, IL-25, IL-33).
To identify genes that are upregulated in the airway epithelium in vivo after a single Alternaria challenge, we obtained airway epithelial cells using bronchial brushings, an adapted method initially developed by Kotaro and Dean Sheppard (18). The airway epithelial cell morphology, positive E-cadherin staining, absence of CD45, and transcript signature that includes mucus genes strongly suggests that the predominant cell type obtained by brushing was airway epithelium. The most highly expressed transcript, FIZZ1 has also been detected in airway epithelium using a systemic adaptive immune sensitization protocol with OVA in alum and subsequent OVA challenge detecting FIZZ-1 expression 2–3 weeks later (28, 29), but has not previously been reported as being induced following an innate stimulus with Alternaria. FIZZ1 is a resistin-like molecule that shares homology with human resistin and is known to be induced during Th2 mediated inflammation (30). FIZZ1 is expressed primarily in the inflamed airway epithelium as well as by alternatively activated macrophages after allergen challenge or helminth infection (19, 28). The expression of FIZZ1 in the lung is regulated by STAT-6 following one exposure to Alternaria in naïve mice at 24 hours as demonstrated in this study, as well as at later timepoints following exposure to allergen (2 weeks) or bleomycin (7 days) (29, 31). The receptors for FIZZ1 remain elusive though reports suggest that signaling occurs through Bruton’s-tyrosine kinase (BTK) and Notch1 (32, 33). Using a previously published FIZZ1 capture assay (19), we identified that both lung CD11+ cells (include macrophages and dendritic cells) and lung fibroblasts bound to FIZZ1. This is consistent with a previous report that found rFIZZ1 could bind to splenic macrophages and dendritic cells (19), and also extends the observation to lung fibroblasts which have not previously been reported to bind to FIZZ1. Although the FIZZ1 binding assay has limitations in terms of sensitivity, the levels of FIZZ1 binding we have detected in the lung are similar to that reported in splenic macrophages and dendritic cells. Prior reports have shown that FIZZ1 can induce myofibroblast differentiation including collagen-1 and alpha-smooth muscle actin production in vitro, suggesting that structural cells can respond to FIZZ1 directly (34, 35).
We found that rFIZZ1 given to the airways of naïve mice leads to eosinophil accumulation. This is consistent with previous reports suggesting that FIZZ1 regulates eosinophil chemotaxis in the GI tract (36, 37). It is possible that FIZZ1 participates in the early recruitment of eosinophils after Alternaria challenge, but given the relatively low magnitude of eosinophilic response induced by rFIZZ1 compared to that induced by Alternaria, it is likely that other mediators such as IL-5 and eotaxin play larger roles. Previous reports have suggested that FIZZ1 dampens helminth-induced Th2 type inflammation (19, 38), but may promote features of remodeling (34, 35, 39). Airway remodeling is an important feature of asthma and previous reports have suggested that FIZZ1 can induce lung collagen deposition and myofibroblast differentiation (34, 35, 39). Additionally, repetitive intranasal administration of recombinant FIZZ1 was noted to induce fibrotic changes in a lung granuloma model (40). Thus, FIZZ1 may have several roles in the lung depending on the cell types involved and stage of the inflammatory response. Our data as well as others have suggested an early pro-inflammatory and pro-remodeling role of FIZZ1 (35, 37, 39, 40), but others have suggested an anti-inflammatory role during more chronic Th2 responses (19, 38). Further work will be required to fully elucidate the multiple functions of FIZZ1 in the lung during chronic inflammatory responses.
In summary, we have characterized a unique acute eosinophilic airway response to Alternaria that is STAT6 dependent and associated with significant upregulation of FIZZ1 in airway epithelium. Further, exogenous FIZZ1 induced airway eosinophilia, epithelial changes, and airway fibrosis. This underscores the potential importance of FIZZ1 in asthma and airway remodeling, and could possibly translate to a role of related human resistin molecules in human asthma.
Acknowledgements
We acknowledge the UCSD microarray core for processing the epithelial microarray.
Footnotes
Grant support: NIH grant 1K08AI080938-01A1 to T.A.D. and AI 38425, AI 70535, AI 72115 to D.H.B., and NIAID U19 AI077439 to D.S.
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 3.Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, Xuan W, Downie SR, Tobias A, Peat JK. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–459. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 4.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 6.Lyons TW, Wakefield DB, Cloutier MM. Mold and Alternaria skin test reactivity and asthma in children in Connecticut. Ann Allergy Asthma Immunol. 2011;106:301–307. doi: 10.1016/j.anai.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plaza V, Serrano J, Picado C, Cosano J, Ancochea J, de Diego A, Martin JJ, Sanchis J. Clinical characteristics of the fatal and near-fatal asthma in Alternaria alternata sensitized patients. Med Clin (Barc) 2003;121:721–724. doi: 10.1016/s0025-7753(03)74076-7. [DOI] [PubMed] [Google Scholar]
- 8.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999;103:709–711. doi: 10.1016/s0091-6749(99)70247-2. [DOI] [PubMed] [Google Scholar]
- 9.Salo PM, Arbes SJ, Jr., Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–L614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page K, Ledford JR, Zhou P, Dienger K, Wills-Karp M. Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respir Res. 2010;11:62. doi: 10.1186/1465-9921-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Doherty M, Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW, Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrin modulates airway hyperresponsiveness by regulating intra-epithelial mast cells. J Clin Invest. 2012 doi: 10.1172/JCI58815. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, Lester LA, Gern JE, Lemanske RF, Jr., Nicolae DL, Elias JA, Chupp GL. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salamon M, Millino C, Raffaello A, Mongillo M, Sandri C, Bean C, Negrisolo E, Pallavicini A, Valle G, Zaccolo M, Schiaffino S, Lanfranchi G. Human MYO18B, a novel unconventional myosin heavy chain expressed in striated muscles moves into the myonuclei upon differentiation. J Mol Biol. 2003;326:137–149. doi: 10.1016/s0022-2836(02)01335-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Khurana Hershey GK. Signal transducer and activator of transcription signals in allergic disease. J Allergy Clin Immunol. 2007;119:529–541. doi: 10.1016/j.jaci.2007.01.004. quiz 542-523. [DOI] [PubMed] [Google Scholar]
- 24.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med. 2007;176:138–145. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day SB, Zhou P, Ledford JR, Page K. German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2. J Innate Immun. 2010;2:495–504. doi: 10.1159/000317195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr., Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 30.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol. 2004;173:3425–3431. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- 32.Su Q, Zhou Y, Johns RA. Bruton's tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J. 2007;21:1376–1382. doi: 10.1096/fj.06-6527com. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong L, Wang SJ, Camoretti-Mercado B, Li HJ, Chen M, Bi WX. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. J Asthma. 2008;45:648–653. doi: 10.1080/02770900802126941. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207. doi: 10.1016/j.jaci.2008.10.017. e1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr., Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010;185:5539–5548. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 40.Ito T, Schaller M, Raymond T, Joshi AD, Coelho AL, Frantz FG, t. Carson WF, Hogaboam CM, Lukacs NW, Standiford TJ, Phan SH, Chensue SW, Kunkel SL. Toll-like receptor 9 activation is a key mechanism for the maintenance of chronic lung inflammation. Am J Respir Crit Care Med. 2009;180:1227–1238. doi: 10.1164/rccm.200906-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]