Abstract
Objectives
Osteoarthritis (OA) is associated with cell death and extracellular matrix degradation in articular cartilage. Autophagy is an essential cellular homeostasis mechanism that was found to be deficient in aging and OA cartilage. This study determined whether pharmacological inhibition of the mammalian target of rapamycin (mTOR), a key inhibitor of autophagy, has disease-modifying activity in experimental OA.
Methods
Experimental OA was induced by transection of the medial meniscotibial ligament and the medial collateral ligament in 2-month old C57Bl/6 mice (n=36). Rapamycin (1 mg/kg weight/day) (n=18 mice) or DMSO vehicle control (n=18 mice) was administered intraperitoneally for 10 weeks. Histopathological changes in articular cartilage and synovium were examined by using semiquantitative scoring systems. Rapamycin effects on mTOR signaling, autophagy, cartilage homeostasis and inflammation were analyzed by immunohistochemistry and immunofluorescence staining.
Results
Rapamycin affected the mTOR signaling pathway in mouse knee joints as indicated by inhibition of ribosomal protein S6 phosphorylation, a target of mTOR and activation of LC3, a main marker of autophagy. The severity of cartilage degradation was significantly (P < 0.01) reduced in the rapamycin treated group compared to the control group and this was associated with a significant (P < 0.05) decrease in synovitis. Rapamycin treatment also maintained cartilage cellularity, and decreased ADAMTS-5 and IL-1β expression in articular cartilage.
Conclusions
These results suggest that rapamycin, at least in part by autophagy activation, reduces the severity of experimental OA. Pharmacological activation of autophagy may be an effective therapeutic approach for OA.
Keywords: mTOR, rapamycin, autophagy, cartilage, osteoarthritis
INTRODUCTION
Osteoarthritis (OA), the most common aging-related joint pathology, is characterized by degradation of cartilage extracellular matrix (ECM) and reduced cartilage cellularity.[1] While changes in the articular cartilage appear to be critical in OA initiation and progression, other joint tissues are invariably involved.[2] Chondrocytes, the only cell population of adult articular cartilage, are capable of responding to structural changes in the surrounding cartilage matrix but the capacity of the adult articular chondrocytes to regenerate the normal cartilage matrix architecture is limited and declines with aging, due to cell death and abnormal responsiveness to anabolic stimuli.[3] Previous attempts at treating established OA, for example by inhibiting ECM-degrading enzymes, failed to show efficacy or were associated with adverse events in clinical trials.[4] As an alternative approach, protection of cells against failure of homeostasis mechanisms, which results in global changes in gene expression, may have potential for greater efficacy.
Autophagy is a cellular homeostasis mechanism that plays an essential role in energy and nutrient regulation, and in the removal of damaged and dysfunctional macromolecules and organelles.[5,6] At the cellular level, failure of autophagy results in increased production of reactive oxygen species, abnormal gene expression, and can lead to cell death.[7] Consequences of autophagy failure at the tissue and organismal level are neurodegeneration, cardiomyopathies, abnormal skeletal development, and premature death.[8–10]
Mammalian target of rapamycin (mTOR) is an important suppressor of autophagy, functioning upstream of the autophagy (Atg) proteins and is centrally regulated by multiple upstream signaling pathways involving PI3-kinase/Akt and AMP-activated protein kinase.[11–13] Imbalances in the mTOR pathway are also involved in obesity, diabetes, inflammatory diseases and cardiac hypertrophy, and pharmacological intervention of mTOR has been proposed as a potential treatment for these conditions.[14] Rapamycin, a lipophilic macrolide antibiotic, that is used as an immunosuppressive drug in solid organ transplantation, can induce autophagy in a variety of cell types.[15,16] In addition, rapamycin treatment extends lifespan in mice,[17] and protects against aging-related pathology in brain and heart.[18–21]
In articular cartilage, which is characterized by a very low rate of cell turnover autophagy would appear to be essential to maintain cell survival and function. Previously, we demonstrated that autophagy is a constitutively active and apparently protective process for the maintenance of cartilage homeostasis. Reduced expression of autophagy regulators was observed in joint aging and OA in humans and mice, and this was accompanied by an increase in chondrocyte apoptosis.[22]
Collectively, these observations suggest that compromised autophagy may contribute to the development of OA. The objective of this study was to establish proof-of-principle that pharmacological enhancement of autophagy may have disease-modifying activity in experimental OA.
MATERIALS AND METHODS
Experimental osteoarthritis in mice
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute. Experimental OA was induced in 2 months old male C57Bl/6J mice by transection of the medial meniscotibial ligament and the medial collateral ligament (MMTL+MCL) in the right knee.[23] The left knee was not subjected to surgery and used as a control. The animals were euthanized 10 weeks after the knee surgery.
Rapamycin treatment
Two independent experiments were performed. Each experiment included a total of 18 mice (9 mice treated with rapamycin and 9 mice receiving vehicle). Rapamycin was obtained from LC Laboratories (Woburn, MA), dissolved in Dimethyl Sulfoxide (DMSO) at 25 mg/ml and stored at −20 °C. For injection, the stock solution was diluted in phosphate buffered saline (PBS). Mice received daily intraperitoneal injections of rapamycin at 1 mg/kg body weight/dose in a total injection volume of 0.3 ml for 10 weeks and control animals received the DMSO vehicle at 0.4% in a total injection volume of 0.3 ml.
Histological analysis of mouse knee joints
Knee joints (total n=72) from mice with experimental OA and vehicle treatment (n=18), experimental OA and rapamycin treatment (n=18) and sham condition (n=18/each group) were harvested. The joints were fixed in 10% zinc-buffered formalin (Z-Fix; Anatech, Battle Creek, MI) for 24 hours, decalcified in TBD-2 (Shandon, Pittsburgh, PA) for 48 hours, followed by paraffin embedding. Serial sections (4 µm) were cut, stained with Safranin O-fast green, and examined for histopathological changes using a semiquantitative scoring system.[24] In this system the scores are defined as follows: 0=normal cartilage, 0.5=loss of proteoglycan with an intact surface, 1=superficial fibrillation without loss of cartilage, 2=vertical clefts and loss of surface lamina (any % or joint surface area), 3=vertical clefts/erosion to the calcified layer lesion for 1–25% of the quadrant width, 4=lesion reaches the calcified cartilage for 25–50% of the quadrant width, 5=lesion reaches the calcified cartilage for 50–75% of the quadrant width, 6=lesion reaches the calcified cartilage for >75% of the quadrant width.
Histological analysis of inflammation
Synovium from mice with experimental OA and vehicle treatment (N=16) and rapamycin treatment (N=11) was examined using a grading system for synovial inflammation.[25] Three parameters, hyperplasia/enlargement of synovial lining cell layer, activation of resident cells/synovial stroma and inflammatory cell infiltration were graded as absent (grade 0), slight (grade 1), moderate (grade 2) or severe (grade 3).
Cartilage cellularity
Knee joint sections were stained with Hematoxylin and Eosin. In cartilage from knees with experimental OA (vehicle and rapamycin treated mice), 3 pictures were taken under 40X magnification, representing the center of the femoral condyle that is not covered by the menisci as well as the medial and lateral femoral condyles. Total number of cells in each section was counted.[26]
Immunohistochemistry
Sections from paraffin-embedded joints were first deparaffinized in the xylene substitute Pro-Par Clearant (Anatech) and rehydrated in graded ethanol and water. For antigen unmasking, sections in 10 mM sodium citrate buffer (pH 6.0) were heated in a microwave oven and kept at 80–85°C for 1.5 minutes. Slides were cooled for 20 minutes at room temperature after antigen unmasking. After washing with phosphate buffered saline (PBS), sections were blocked with 5% serum for 30 minutes at room temperature. Phospho-rpS6 antibody (Cell Signalling Technology, Boston, MA, 1:100 dilution), ADAMTS-5 (Abcam, Cambridge, MA, 5 µg/ml) and IL-1β (Santa Cruz Biotechnology, Santa Cruz, CA, 1:100) were applied and incubated overnight at 4°C. After washing with PBS, sections were incubated with biotinylated goat anti-rabbit secondary antibody for 30 minutes at room temperature, and then incubated using Vectastain ABC-AP alkaline phosphatase (Vector Laboratories, Burlingame, CA) for 30 minutes. Slides were washed, and sections were incubated with alkaline phosphatase substrate for 20–30 minutes.
Immunofluorescence
Paraffin-embedded samples were deparaffinized in the xylene substitute Pro-Par Clearant (Anatech) and rehydrated in graded ethanol and water. After washing with phosphate buffered saline (PBS), sections were blocked with 5% serum for 30 minutes at room temperature and then incubated with rabbit anti-LC3 polyclonal antibody (1:50) (Abgent, San Diego, CA) overnight at 4°C. After washing with PBS, sections were incubated with Alexa Fluor 488 anti-rabbit IgG used for secondary antibody for 30 minutes. Finally, slides were washed and mounted with Prolong Gold Antifade Reagent (Invitrogen, Carlsbad, CA).
Quantification of ADAMTS-5 positive cells
In cartilage from mice subjected to experimental OA and treated with vehicle or rapamycin, three pictures were taken under 40× magnification, showing the center of the femoral condyle that is not covered by the menisci as well as the medial and lateral femoral condyles. Total number of chondrocytes was counted [26] and then, the total number of ADAMTS-5-positive cells was counted in each section. Results are expressed as the percentage of ADAMTS-5 positive cells.
Statistical analysis
Statistically significant differences between two groups were determined with t- tests. The results are reported as mean ± S.D. P values less than 0.05 were considered significant.
RESULTS
Systemic administration of rapamycin modulates mTOR signaling and autophagy in mouse knee joints
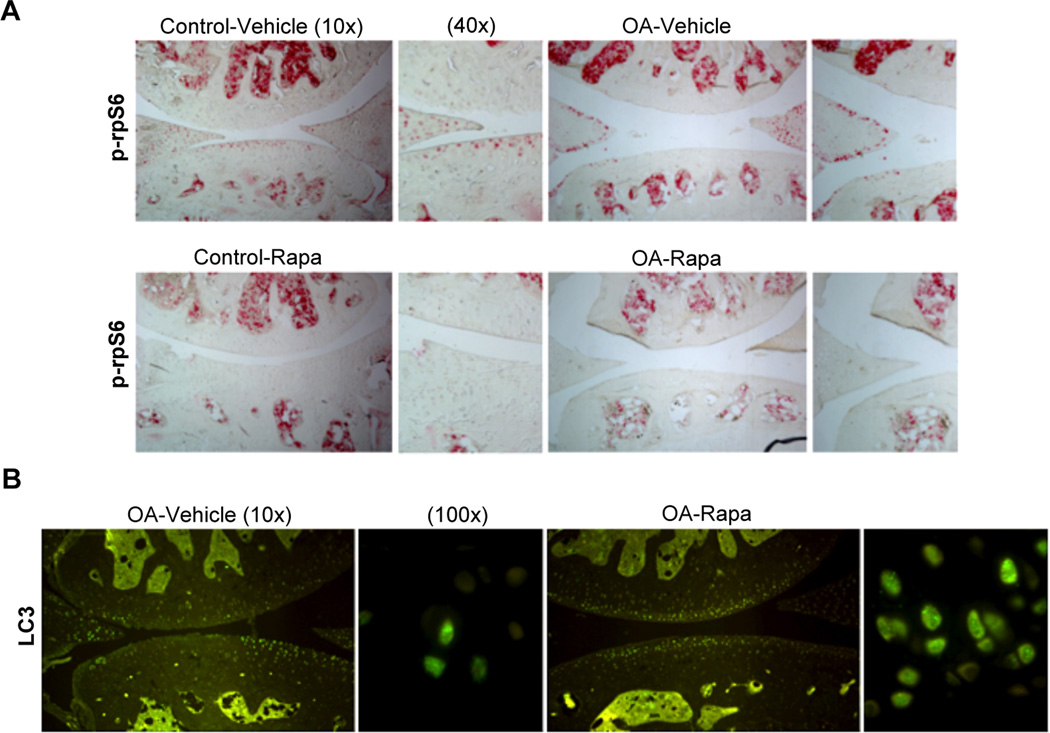
First, we evaluated the effect of intraperitoneal rapamycin on the mTOR signaling pathway in mouse knee joints by determining the phosphorylation levels of ribosomal protein S6 (phospho-rpS6), a downstream target of mTORC1.[27] Rapamycin treatment suppressed rpS6 phosphorylation in articular cartilage and menisci in the knee joints compared to vehicle treated mice (Figure 1A). To determine whether the mTOR inhibition by rapamycin promotes autophagy, knee joint sections were stained with LC3 antibody. We found an increase in LC3 expression after rapamycin treatment. This increase was correlated with an increase in LC3 puncta, indicating strong activation of autophagy in articular cartilage (Figure 1B). These results indicate that the intraperitoneal administration of rapamycin caused the expected effects mTOR signaling and autophagy activation in knee joints.
Figure 1. Systemic administration of rapamycin modulates mTOR signaling and autophagy in mouse knee joints.
A, Knee joints from C57Bl/6J mice were collected 10 weeks after knee destabilization or and treatment with rapamycin (rapa) or vehicle (n=3/each). Contralateral knees were used as a control. Sections were analyzed by immunohistochemistry for phosphorylation of ribosomal protein rpS6. Magnification: ×10 and ×40. B, Knee joints from 2 months old C57Bl/6J mice after OA surgery treated with vehicle (n=4) or rapa (n=4) were analyzed by immunofluorescence for LC3. Magnification: 10× and 100×.
Rapamycin reduces severity of experimental OA
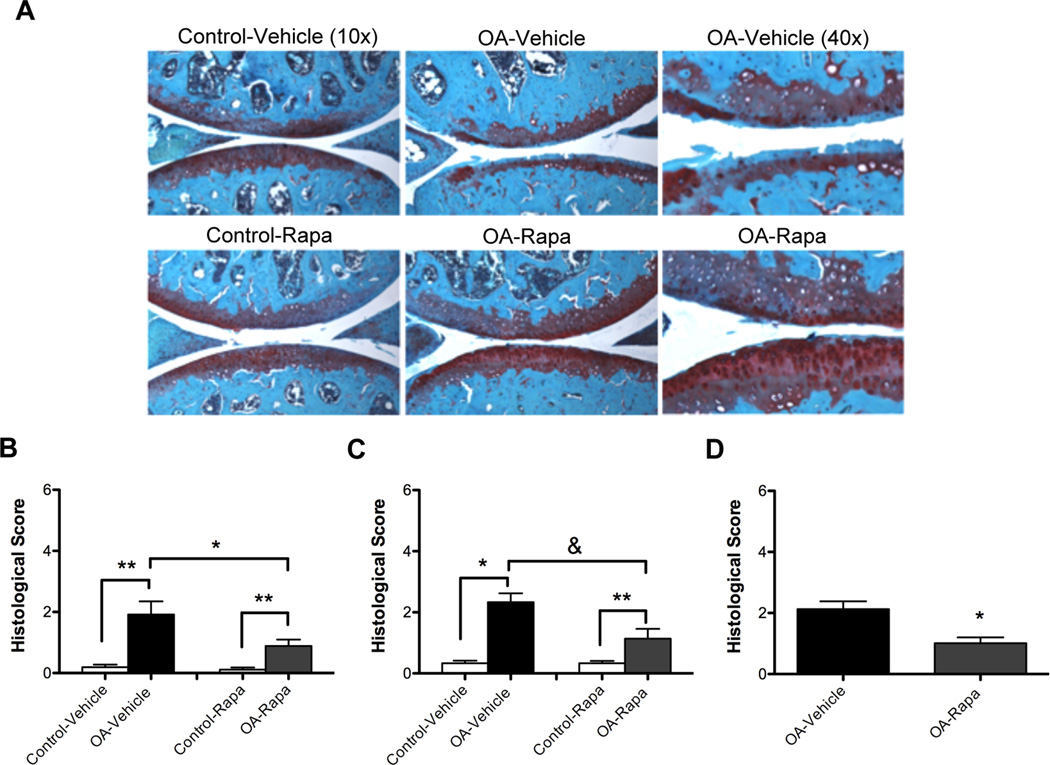
To investigate the role of mTOR in the development of OA, we performed surgery on knee joints of C57Bl/6J mice to destabilize the medial meniscus by transection of the medial meniscotibial ligament and the medial collateral ligament. One day after the surgery, daily intraperitoneal injections of rapamycin (1 mg/kg body weight/day; injection volume 0.3 ml) or vehicle (DMSO 0.4%; injection volume 0.3 ml) were given for 10 weeks. We performed two independent studies, each including 9 mice in the rapamycin group and 9 mice in the vehicle group (n=36 total mice). Mouse knee joints in the vehicle group exhibited significant cartilage degeneration, with proteoglycan depletion, loss of surface lamina and fibrillations. Rapamycin treatment decreased the severity of these OA-like changes (Figure 2A). We did not observe any structural changes in the control knees in vehicle or rapamycin-treated mice. Analysis of OA pathology by a semiquantitative scoring system indicated a significant decrease in the severity of the OA-like changes after rapamycin treatment (P < 0.05) compared to vehicle-treated mice in both studies (Figure 2B, 2C). Comparison of the combined scores from the 2 experiments showed a significant decrease in cartilage pathology (P < 0.01) by approximately 50% after rapamycin treatment compared to vehicle control (Figure 2D).
Figure 2. Rapamycin reduces severity of experimental OA.
Two months old C57Bl/6J mice were subjected to OA surgery in right the knee. The left knee was not subjected to surgery and used as control. Two separate experiments were performed. Each experiment included 18 mice, with 9 receiving rapamycin (rapa) and 9 receiving vehicle. A, Knee joints were analyzed by staining with Safranin O. Magnification:10x, 40x. B–C, Histological scores for the two separate experiments. Values are mean ± SD. * = P < 0.01 versus control-vehicle and control-rapa; ** = P < 0.01 versus OA-vehicle. D, Histological scores after combining both experiments (n= 36) showed a significant decrease in OA scores after rapa treatment. Values are the mean ± SD. * = P < 0.01 versus OA-vehicle.
Rapamycin maintains cartilage cellularity and reduces ADAMTS-5 expression in experimental OA
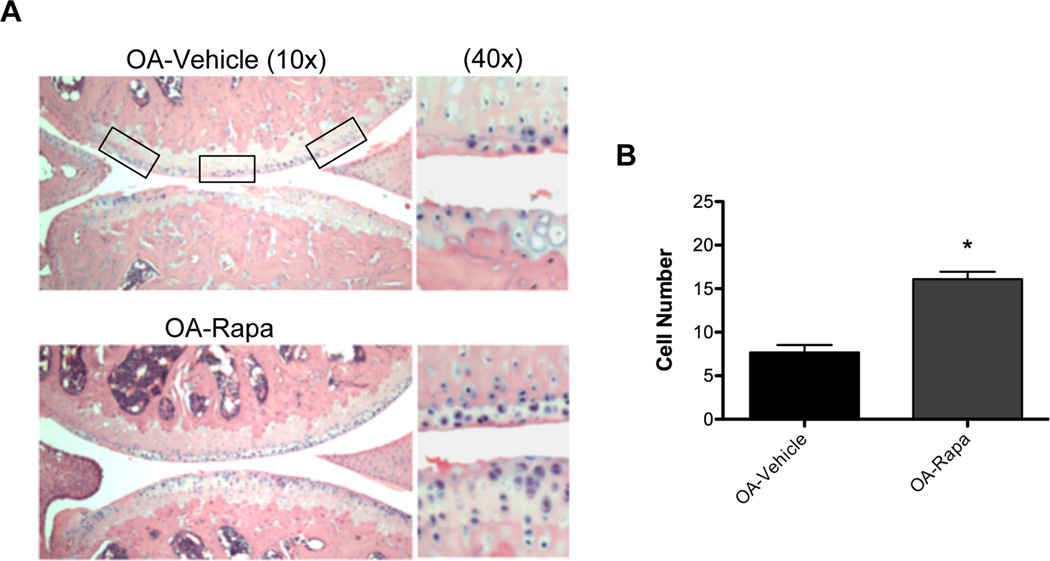
To determine the mechanism of action of rapamycin in experimental OA, we analyzed cartilage cell density in mouse knee joints. We observed preservation of cellularity after rapamycin treatment (Figure 3A). This difference was significant (P < 0.001) compared to vehicle-treated mice (Figure 3B).
Figure 3. Rapamycin prevents reduction in cartilage cellularity during experimental OA.
A, Knee joint sections from C57Bl/6J mice with or without OA surgery under treatment with rapamycin (rapa) or vehicle (n=6 each) were stained with Hematoxylin Eosin (H&E). Magnification: 10× and 40×. B, Quantitative analysis of cell number showed a significant increase of cellularity after rapa treatment compared to vehicle treatment. Values are the mean ± SD. * = P < 0.001 versus OA-vehicle.
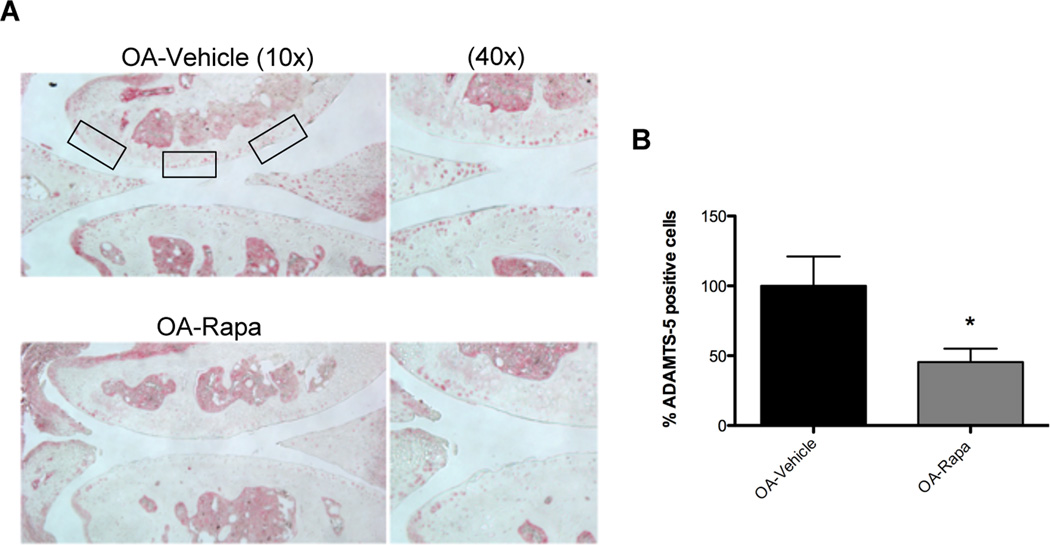
Immunohistochemistry was performed for ADAMTS-5, a main proteinase responsible for aggrecan degradation in articular cartilage.[28] ADAMTS-5 was expressed by a lower number of chondrocytes in rapamycin-treated mice compared with vehicle-treated mice with experimental OA (Figure 4A). This result was significant (P < 0.05) compared to vehicle treated knee joints (Figure 4B). These findings indicate that rapamycin protects against cell loss and extracellular matrix damage by reducing ADAMTS-5 expression.
Figure 4. Rapamycin reduces ADAMTS-5 expression. A.
Knee joints from C57Bl/6J mice were collected 10 weeks after OA surgery and treatment with rapamycin (rapa) or vehicle (n=5 each). Sections were analyzed by immunohistochemistry for ADAMTS-5. Magnification: 10× and 40×. B, Quantitative analysis of ADAMTS-5 positive cells. Total cell number in three fields and ADAMTS-5 positive cells were counted and the percentage of ADAMTS-5 positive cells was calculated. Results show a significant decrease in ADAMTS-5 after rapa treatment compared to vehicle. Values are mean ± SD. * = P < 0.05 versus OA-vehicle.
Effect of rapamycin on inflammation in experimental OA
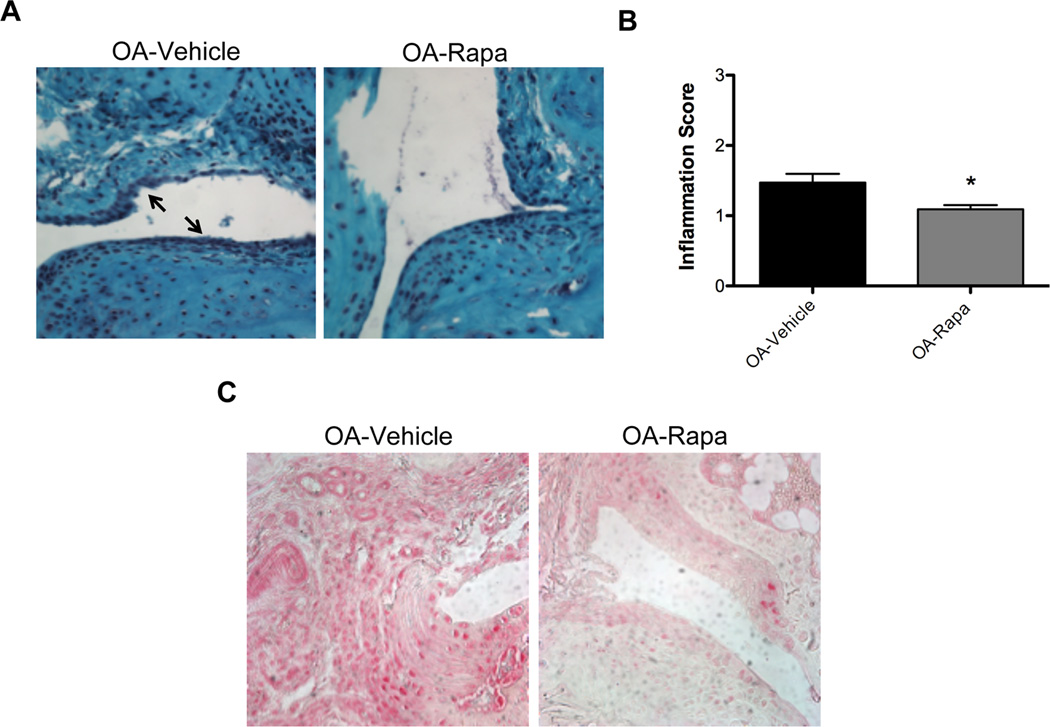
To study the effect of rapamycin on inflammation in the experimental OA model, we examined histopathological changes using a semiquantitative grading system to measure inflammation in synovial tissue. The results indicate a decrease in synovial inflammation in response to rapamycin treatment compared to vehicle-treated mice (Figure 5A). The decrease in the severity of inflammation after rapamycin treatment compared to vehicle-treated mice was significant (P < 0.05) (Figure 5B). Furthermore, IL-1β expression in synovial tissue was reduced after rapamycin treatment (Figure 5C). Thus, in addition to protective effects on articular cartilage, rapamycin also reduces the severity of synovitis and the synovial tissue level of IL-1β in mouse knees with experimental OA.
Figure 5. Effect of Rapamycin on inflammation in experimental OA.
A, Synovium from mice after experimental OA and vehicle (N= 16) or rapamycin (rapa, N= 11) treatment was analyzed by staining with Safranin O. Magnification: 40×. B, The histological score for inflammation was significantly decreased after rapa treatment. Values are the mean ± SD. * = P < 0.05 versus OA-vehicle. C, Synovium sections from vehicle or rapa treated mice (N=5 each) were analyzed by immunohistochemistry for IL-1β. Magnification: 40×.
DISCUSSION
Substantial progress has been achieved in understanding pathogenesis pathways in established OA and a large number of drug candidates were effective in OA animal models. However, thus far no clinical trials showed disease-modifying activity of a large number of drug candidates tested.[4] A potential explanation is that therapeutic agents were highly specific. For example, inhibiting one of a large number of ECM-degrading enzymes may not provide sufficient impact to change the overall course of the disease. Recent advances in understanding mechanisms in other aging-related diseases has led to the concept that failure of cellular homeostasis mechanisms, such as autophagy,[5,6] leads to global changes in gene expression and can ultimately cause cell death and extracellular matrix destruction, two main features of OA-affected cartilage. Based on this notion, we previously analyzed human OA joints and joints from experimental OA models and observed a reduced expression in autophagy regulators that was associated with increased cell death.[22]
These observations motivated the present study to test whether activation of autophagy can reduce severity of experimental OA. For this study we selected rapamycin, a specific and widely used inhibitor of mTOR signaling pathway, which regulates autophagy initiation. Rapamycin, a lipophilic macrolide antibiotic, used as an immunosuppressive drug, induces autophagy in a variety of cell types, extends lifespan and protects against aging-related diseases in mice.[15–21]
Our results show that systemic administration of rapamycin, inhibited mTOR signaling pathway in articular cartilage, as reflected by reduced phosphorylation of ribosomal protein S6, which integrates processes of protein translation with cell growth and cell proliferation.[29] Treatment of cells with rapamycin blocks S6K phosphorylation, and inhibits S6K1 activation.[30] Furthermore, rapamycin activated autophagy as indicated by increased LC3-II expression, the most specific autophagosomal marker in articular cartilage.[31,32] These results confirmed the effect of systemically administered rapamycin on mTOR and autophagy pathways in articular cartilage.
In the experimental OA model, we observed that rapamycin treatment caused a significant reduction in OA severity by approximately 50%. The principal parameter measured in the OA scoring system is the depth of the cartilage lesions and thus reflects cell loss and extracellular matrix degradation. To address the potential mechanism by which rapamycin mediates this beneficial effect, we examined cartilage cellularity and levels of ADAMTS-5, a major aggrecan-degrading enzyme.[33] The number of chondrocytes in knees with experimental OA in rapamycin treated mice was significantly increased compared with the vehicle treated mice. Furthermore, ADAMTS-5 expression was significantly decreased in cartilage from rapamycin-treated mice with OA. The observed reduction in cartilage lesion depth and width could be mediated by a reduction in the expression of ADAMTS-5.
OA in humans and in experimental models is associated with inflammatory changes in synovium.[34] In the present study, rapamycin also reduced the severity of synovitis. This was associated with a reduction in IL-1β expression after rapamycin treatment in synovium. These results are consistent with previous studies where rapamycin significantly decreased synovial inflammation, and protected against bone loss and cartilage destruction in an inflammatory arthritis model.[35,36]
The observed effects of rapamycin treatment on cartilage degradation, ADAMTS5 and IL-1β expression, and synovial inflammation are a consequence of mTOR inhibition. Rapamycin is a highly specific inhibitor of mTOR and there is no evidence that has off-target effects on other enzymes.[37,38]. It is well established that mTOR inhibition induces autophagy but as direct and indirect consequences of mTOR inhibition other signaling pathways are affected, such as PI3K/Akt signaling pathway.[39] In regard to the role of autophagy activation in the effect observed in the present study, it is possible that rapamycin restores the suppressed autophagy, which we previously observed in experimental and human OA,[22] and as a result, inhibits cell death. The induction of cell death is a well-known effect of defective autophagy,[40] and the preservation of cartilage cellularity observed in the present study can be due to autophagy activation.
The reduction of IL-1β and ADAMTS5 expression observed after rapamycin treatment in the OA model could be due to the removal of aggregation prone-proteins that stimulate production of reactive oxygen species (ROS). Both ROS accumulation and mTORC1 activation are associated with accelerated aging and the development of aging-associated pathologies.[41,42] Conversely, reduction of ROS levels by antioxidants or as a result of mTORC1 inhibition causes an extension of lifespan.[43]
One important limitation of rapamycin as a therapeutic molecule in humans is its immunosuppressive effects. The clinical feasibility of using mTOR inhibitors for the treatment of OA is supported by recent advances in the development of new and safer rapamycin analogs. The treatment with rapamycin analogs with a short half-life might reduce systemic exposure to mTOR inhibitors and may show an improved safety profile compared with the lead compound.[44]
The present study is the first to establish efficacy of rapamycin in an animal model of OA. We used young mice subjected to surgical knee stabilization. This is a widely used model for this purpose. For further preclinical development of autophagy activators it will be necessary to also test other animal species and older animals to more realistically model the human condition.
In summary, we report that rapamycin, at least in part by autophagy activation, reduces the severity of experimental OA. These results suggest that pharmacological inhibition of mTOR by rapamycin may be a potentially effective therapeutic approach for OA.
Acknowledgements
The authors acknowledge Diana Brinson, Jean Valbracht and Lilo Creighton for technical assistance.
Funding
This study was supported by National Institutes of Health Grants AG007996, AR058954, RR027577 and the Sam and Rose Stein Endowment Fund. B. Caramés was supported by Postdoctoral Fellowship “Anxeles Alvariño”, Secretaria Xeral I+D+i, Xunta de Galicia, Spain.
Footnotes
Competing interest
None
Ethics approval
This study was conducted with the approval of the Institutional Animal Care and Use Committee at The Scripps Research Institute.
REFERENCES
- 1.Takayama K, Ishida K, Matsushita T, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 3.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–1401. doi: 10.1016/j.joca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through Cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural Cell s causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata M, Lu T, Furuya T, et al. Regulation of intra cellular accumulation of mutant Huntingtin by Beclin1. J Biol Chem. 2006;281:14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 11.Settembre C, Arteaga-Solis E, McKee MD, et al. Proteoglycan desulfation determines the efficiency of chondrocyte autophagy and the extent of FGF signaling during endochondral ossification. Genes Dev. 2008;22:2645–2650. doi: 10.1101/gad.1711308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Guan KL. Expanding TOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 14.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Shigemitsu K, Tsujishita Y, Hara K, et al. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma Cell. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 16.Sabers CJ, Martin MM, Brunn GJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian Cell. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar S, Rubinsztein DC. Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS J. 2008;275:4263–4270. doi: 10.1111/j.1742-4658.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan T, Rawal P, Wu Y, et al. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inuzuka Y, Okuda J, Kawashima T, et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. doi: 10.1161/CIRCULATIONAHA.109.871137. [DOI] [PubMed] [Google Scholar]
- 22.Caramés B, Taniguchi N, Otsuki S, et al. Autophagy is a Protective Mechanism in Normal Cartilage and its Aging-related Loss is Linked with Cell Death and Osteoarthritis. Arthritis and Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamekura S, Hoshi K, Shimoaka T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Krenn V, Morawietz L, Häupl T, et al. Grading of chronic synovitis-a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced Cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekim B, Magnuson B, Acosta-Jaquez HA, et al. mTOR kinase domain phosphorylation promotes mTORC1 signaling, Cell growth, and Cell cycle progression. Mol Cell Biol. 2011;31:2787–2801. doi: 10.1128/MCB.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 29.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 31.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima N, Yamamoto A, Matsui M, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 34.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 35.Cejka D, Hayer S, Niederreiter B, et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 36.Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med. 2010;16:352–358. doi: 10.2119/molmed.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi S, Kishimoto T, Kamata S, et al. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 2007;98:726–733. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawahara T, Asthana S, Kneteman NM. M-TOR Inhibitors: What Role in Liver Transplantation? J Hepatol. 2011 doi: 10.1016/j.jhep.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Cirstea D, Hideshima T, Rodig S, et al. Dual inhibition of akt/mammalian target of rapamycin pathway by nanoparticle albumin-bound-rapamycin and perifosine induces antitumor activity in multiple myeloma. Mol Cancer Ther. 2010;9:963–975. doi: 10.1158/1535-7163.MCT-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 42.Stanfel MN, Shamieh LS, Kaeberlein M, et al. The TOR pathway comes of age Biochim. Biophys. Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonawitz ND, Chatenay-Lapointe M, Pan Y, et al. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner R, Mollison KW, Liu L, et al. Rapamycin analogs with reduced systemic exposure. Bioorg Med Chem Lett. 2005;15:5340–5343. doi: 10.1016/j.bmcl.2005.06.106. [DOI] [PubMed] [Google Scholar]