Abstract
Human studies indicate augmented myocardial lipid metabolism in females, and that sex and obesity interact to predict myocardial fatty acid oxidation and storage. Altered lipid dynamics precede cardiomyopathies, and many studies now address high fat diets. Conversely, caloric restriction (CR), is the most studied model for longevity and stress resistance, including protection against myocardial ischemia. However, no information exists on the effects of long-term caloric restriction (CR) on triacylglyceride (TAG) content and dynamics in the heart. This study explored the effects of CR, sex and age on TAG dynamics in mouse hearts. Male and female SVJ129 mice were fed either normal (ND) or CR diet for 3 or 10 months. In 5-month-old mice, CR similarly decreased cardiac TAG in males (ND: 25.5 +/− 4.5 nanomoles/mg protein; CR: 12.6 +/− 2.7, P<0.05) and females (ND: 30.1 +/− 4.4; CR: 13.7 +/− 1.2) (no significant differences in TAG content were seen between sexes). CR reduced the contribution of exogenous palmitate to oxidative metabolism in males and females, by 15% and 11% respectively, versus ND, without affecting cardiac workload. CR also induced a larger reduction in TAG turnover in male (68%) than female hearts (38%). Interestingly, in 5 month old male mice, CR reproduced the lower TAG turnover rates of middle-aged males (ND 13-month-old male = 423 +/−76 nanomoles/mg protein/min). Thus, long term CR reduces TAG pool dynamics. Despite reduced content, hearts of female mice subjected to CR retained a more dynamic TAG pool than males, while males respond with greater metabolic remodeling of cardiac lipid dynamics.
Keywords: long term caloric restriction, triacylglyceride, long-chain fatty acids
1. Introduction
Age related decline in cardiac function is a major risk factor for cardiovascular disease in the elderly. The prevalence of cardiomyopathy, characterized by left ventricular hypertrophy, increases with age, in approximately ⅓ of men and ½ of women by age 70 [1]. Fatty Acid Oxidation (FAO) declines with age in rodent and humans. In rodent studies, FAO significantly declined in senescent myocardium [2-3]. In aging but not senescent myocardium, Kates et al (2003) showed a decline in fatty acid uptake and FAO in humans, although not significant [4-5]. In addition to the primary effects of normal aging, secondary effects are caused increases in myocardial TAG store or the progression of clinical disorders such as diabetes and hypertension [6-7]. In the senescent myocardium, aging was related to whole body and myocardial insulin resistance, independent of obesity [8]. Studies show that the secondary effects of myocardial lipid accumulation, from an imbalance of fatty acid import, utilization, and storage promotes genetic and age related, acquired, cardiomyopathies [9].
Researchers have identified several proteins and transcriptions factors involved in FAO, long chain fatty acid (LCFA) utilization/storage, and LCFA transport that are also thought to have involvement in age related changes in the myocardium. Peroxisome proliferator-activated receptor-α (PPARα) is a transcription factor that regulates genes involved in fatty acid oxidation (FAO) and lipid storage [10-11]. CD36, a fatty acid transport protein, expression increases with age and was shown to be a mediator in metabolic, function, and structural alteration in the aged heart [12]. Aging increases the susceptibility to diseases such as diabetes, cardiomyopathy, cancer, neurodegenerative diseases, and inflammation [12]. Metabolic syndrome, the mirror opposite of CR, is results from dietary excess leading to poor health and a shortened life span [13]. Aging reduces the ability of heart tissue to respond to adverse conditions. Mild overexpression of SIRT1, an NAD-dependent deacetylase, protected the heart from oxidative stress suggesting that SIRT1 activity could retard aging by conferring stress resistance to the heart in vivo [14]. A recent study showed that overexpressing diacylglycerol acyl transferase 1 (DGAT1) resulted in myocyte steatosis and progressive deterioration of myocardial structure and function over time [15]. At 12 weeks of age, this transgenic mouse showed reduced diastolic dysfunction and by 52 weeks of age cardiac wall thickening and chamber dilation was observed. Clearly, research has shown that altered lipid metabolism and subsequent accumulation is related to age related cardiomyopathy in mice [13, 15].
Caloric restriction (CR) is well known to be a nutritional intervention and has beneficial effects on longevity in rodent and non-human primate models [16-18]. CR has also been shown to decelerate declines in functional capacities, such as cardiomyopathy, nephropathy, diabetes, hypertension, among others, increasing longevity [16, 18-19]. CR has been used as a nutritional intervention for cardiomyopathy. Pathology of heart tissue from rats on a restricted diet received a lower histologic grade for cardiomyopathy [20]. Reducing a mismatch between LCFA and storage would be beneficial in treating age related cardiomyopathy. Mitochondrial oxidative stress and continuous production of free radicals are attributed to the progression of aging [16], and rodent studies of CR reduced oxidative damage to cellular components due to oxidative damage of lipids, proteins and nucleic acids [21]. In rat heart and liver tissue, the level of oxidative damage to mitochondria was lower after long term CR [16-17]. However the mechanisms by which CR enhances longevity continue to remain unclear and widely debated.
Proposed mechanisms of long term CR involve changes in transcription and cellular signaling pathways through nuclear receptors. Two nuclear receptors, which modulate fatty acid metabolism, have emerged as targets for CR, peroxisome proliferator-activated receptor-γ coactivator-1 α and β (PGC1α and PGC1β), and PPAR-α [22]. During short-term fasting, exercise, or times of stress, glycogen stores release glucose and long LCFA are released from adipocytes. Peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-α) has been shown to be a central regulator of adaptive response to caloric deprivation by regulating genes involved in gluconeogensis and FAO in response to fasting [23]. Indeed, long-term CR abrogates the age-dependent decline in skeletal muscle aerobic function by preventing loss of mitochondrial oxidative capacity, which may be related to increases in PGC-1α expression and activity [24]. Importantly, during long-term CR ATP consuming processes are switched off, including FA uptake and oxidation [24]. PPARα, a nuclear receptor that is activated by LCFA and known to regulate FAO, is down regulated in muscle after a 48 hour fast.
Little information exists on the effects of long-term CR on myocardial TAG stores. Brief periods of fasting, or acute caloric deprivation, result in phospholipid depletion, membrane remodeling and triacylglyceride accumulation in the myocardium [25-26]. Previous studies have implicated the loss of activity changes in PPARα, PGC1α, and mTOR activity as a possible mechanism to for age related changes in lipid metabolism [22, 27-28]. While previous studies show changes in enzyme expression, there is little or no information available for the effects of long-term CR, or limited total calorie intake, on in vivo triacylglyceride (TAG) content and importantly, real time TAG dynamics in the heart. Recent studies in transgenic mouse models have shown that PGC-1α and PPARα are key potentiators of TAG dynamics and content in the heart [10, 29]. Human studies indicate augmented myocardial lipid metabolism in females, and that sex and obesity may influence myocardial fatty acid oxidation and storage [30]. Animal models of increased FA uptake result in 2-11 fold elevation in myocardial TAG stores and accumulation of TAG is thought to be deleterious [11, 31]. Interestingly, both obesity and sex have been shown to modulate myocardial blood flow, substrate metabolism, nitric oxide/superoxide balance, and can act as predictors of cardiac disease [30, 32-33]. However, previous studies have been limited in distinguishing sex based differences from underlying disease states versus sex differences that can alter myocyte response to a disease state [34].
This paper explores the primary effects of age and sex on TAG dynamics in myocardium after long-term CR in the 129/SvJ mouse, a strain shown to develop age related cardiomyopathy [35]. In response to long-term CR, there was a reduction of endogenous lipid stores and a significant decrease in TAG turnover, cycling of exogenous LCFA through endogenous TAG stores. Interestingly, TAG dynamics in the female myocardium did not decrease to the same extent as in the male myocardium, despite similarly reduced TAG content. Thus, the female myocardium preserved more involvement of the endogenous TAG stores in lipid metabolism and as a source of LCFA for β-oxidation [10].
2. Materials and Methods
2.1 Mice and Diets
129/SvJ mice were chosen to study the effects of CR on primary aging. This strain of mouse is known to develop age related cardiomyopathy [35]. Male and female 8 week-old 129/SvJ mice were weighed and then randomly assigned to normal diet (ND) or CR for the duration of the experiment (AIN-93M Diets No. F05312 and F05314 40% dietary reduction, Bioserv). ND consisted of 90 kcal/week of a chemically defined control diet, which provides ∼10% fewer calories than are normally assumed to be required by a mouse. For acclimation purposes during the first 2 weeks of all the experiments, mice were fed with ND or CR (25% kcal/week reduction). After that the CR diet was changed to 40% kcal/week reduction for the remainder of the experiments. The main composition for the ND and CR pellets is described in Appendix Table I. The ND is higher on the percentage of carbohydrates, whereas, the CR is higher on percentage of protein, fat and fiber. Mice were fed 3 times per week: CR (40%) received 4, 4 or 6 pellets each time compared to ND mice which received 7,7 or 11 pellets each time. In all experiments, mice were housed individually. All animal experiments were approved by the Institutional Animal Care and Use Committee. Younger mice were sacrificed at 5 months of age and hearts were excised for perfusion (ND: male N = 5, female N = 4; CR: male N = 6, female N = 4). Middle age mice were sacrificed at 13 months of age and hearts were excised for perfusion (ND: male N = 10, female N = 6; CR: male N = 6, female N = 7).
Fasting blood plasma levels of ketone bodies, glucose, triglycerides, and cholesterol were determined as previously described [36].
2.2 Isolated heart protocols
Following the feeding protocol, animals were heparinized (50 U/10 g, i.p.) and anesthetized with ketamine (80 mg/kg, i.p.) plus xylazine (12 mg/kg, i.p.). Hearts were excised and perfused in retrograde fashion with modified Krebs-Henseleit buffer (118.5 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4 and 1.2 mM KH2PO4) maintained at 37 °C, equilibrated with 95% O2/5% CO2 and containing 0.4 mM unlabeled palmitate/ complexed to albumin (3:1 molar ratio) and 10 mM glucose. A water-filled latex balloon was fitted into the left ventricle and set to a diastolic pressure of 5 mmHg. Left ventricular developed pressure (LVDP) and heart rate (HR) were continuously recorded with a pressure transducer and digital recording system (Powerlab, AD Instruments, Colorado Springs, CO). At the end of each experiment, hearts were frozen in liquid N2 cooled tongs and stored at −80 °C.
For TAG dynamics, isolated hearts from both ND and CR mice were perfused in a 14.1 T NMR magnet at baseline workload. After collection of 13C-NMR background signals of naturally abundant 13C (1.1%), hearts were switched to buffer containing 0.4 mM [2,4,6,8,10,12,14,16 - 13C8] palmitate (Isotec, Inc., Miamisburg, OH) plus 10 mM unlabeled glucose.
2.3 NMR spectroscopy and tissue chemistry
NMR measurements of TAG turnover were performed on intact beating hearts that were performed as previously described [37]. Sequential, proton-decoupled 13C NMR spectra were acquired (2 min each) with natural 13C abundance correction using previously reported NMR methods [37].
Carbon-13 enrichment of TAG in the intact heart was monitored from the NMR signal at 30.5 ppm from the TAG methylene groups and TAG turnover was calculated from total TAG content and enrichment over time [29, 37-38]. Kinetic analysis of dynamic 13C-spectra from intact, beating hearts was performed as previously reported from our laboratory [10, 29, 37-38].
Lipid extracts were obtained from heart samples and TAG quantified by colorimetric assay, as previously described (Wako Pure Chemical Industries) [10]. TAG was isolated, saponified, and the fractional 13C enrichment of the fatty acids assessed by liquid chromatography/mass-spectrometry (LC/MS) analysis. LCFA content in TAG, of carbon lengths 12-18, was determined by LCMS and values are reported as a percentage of total LCFA. Total TAG turnover (nanomoles TAG/min/mg protein) was quantified from 13C enrichment rates and the endpoint 13C enrichment of TAG over the time course of the experiment, as previously described [29, 37-38].
In vitro high-resolution 13C NMR spectra of perchloric acid tissue extracts reconstituted in 0.5 mL of D2O were collected with a 5 mm 13C probe (Bruker Instruments, Billerica, MA). Analysis was performed to determine fractional enrichment of [2-13C] acetyl CoA [39, 40]. Due to tissue availability, fractional enrichment of [2-13C] acetyl CoA data represent a subset of hearts used for TAG analysis (5 month old mice: ND: male N = 4, female N = 3; CR: male N = 5, female N = 3; 13 month old mice: ND: male N = 4, female N = 4; CR: male N = 3, female N = 4).
2.4 Statistical Analysis
Statistical comparisons of group mean values was performed using a 23 full factorial design on natural logs that was implemented with weighted least squares method, with sex, diet and age of mice as the factors. Student t multiple comparison method was used in each case. Statistical significance was determined at the 5% probability level, p < 0.05.
3. Results
3.1 Isolated Mouse Heart Hemodynamics and Blood Chemistry
Rate pressure product, RPP, was not significantly different between groups at baseline work (5 month old: Male: ND, 30,000 ± 5,000 beats • mm Hg per minute; CR, 33,000 ± 6,000; Female: ND, 28,000 ± 5,000; CR, 32,000 ± 2,000; 13 month old: Male: ND, 27,000 ± 4,000 beats • mm Hg per minute; CR, 30,000 ± 6,000; Female: ND, 30,000 ± 4000; CR, 33,000 ± 4,000). Oxygen consumption was similar for all groups (data not shown). Thus despite the significant differences in lipid dynamics, the metabolic demand was also similar between groups.
Fasting blood chemistry was similar for 13-month-old mice on a CR diet and ND (Figure 1). Consistent with previous phenotypic reports for the 129/SvJ strain of mouse, these mice do not show impaired insulin sensitivity Supplemental Table. With no differences seen in the blood chemistry of the CR and ND mice in this study, it is unlikely the mice in the current study have impaired glucose or insulin sensitivity [41-44].
Figure 1.
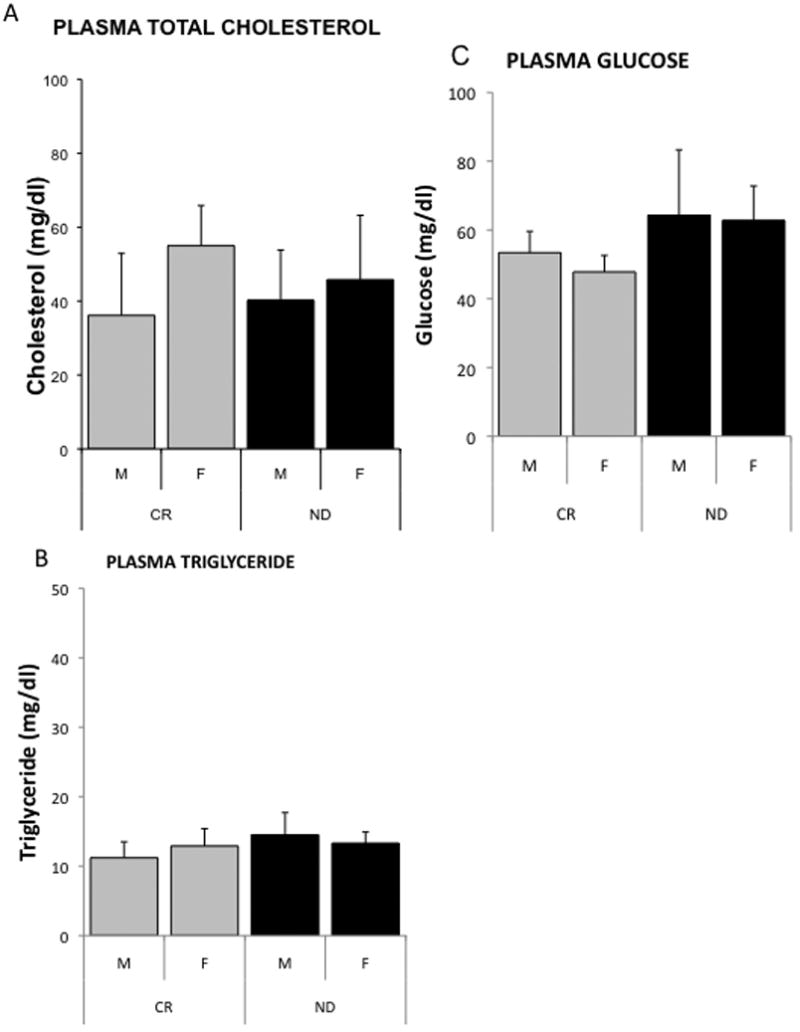
Plasma concentrations of cholesterol (A), triglyceride (B) and glucose (C) in fasted 13-month-old male (m) and female (f) mice fed a calorically restricted (CR) or normal diet (ND).
3.2 Enrichment of acetyl CoA from 13C palmitate
In female and male myocardium, mice on a 3-month CR diet the 13C fractional enrichment of acetyl CoA from 13C palmitate significantly decreased. After 3 months of caloric restriction, there was an adaptive shift in enrichment of acetyl CoA away from palmitate as a source for energy production presumably towards glucose oxidation (Figure 2). However, after 10 months on a calorically restricted diet observed differences in enrichment of acetyl CoA from 13C palmitate were not present as compared to mice on a normal diet. Aging normalized adaptive shifts in myocardial glucose and palmitate oxidation in response to caloric restriction. While diet and age produced shifts in enrichment of acetyl CoA from 13C palmitate, there were no apparent differences seen between sexes.
Figure 2.
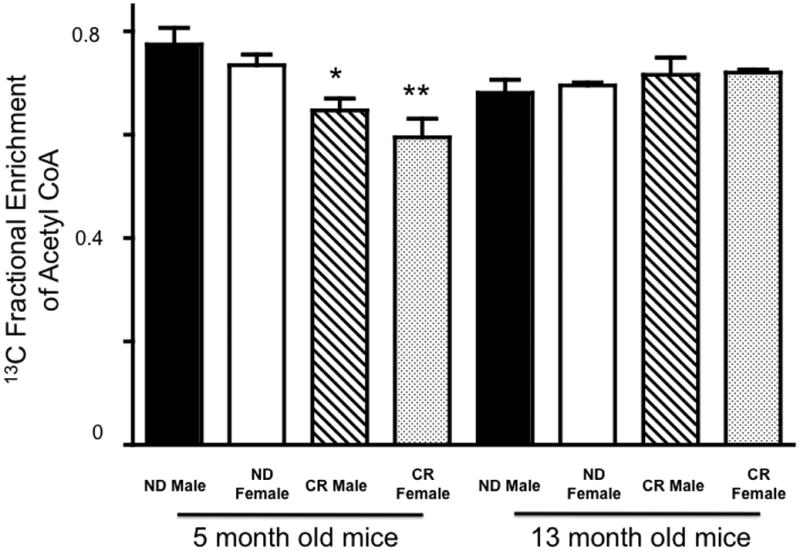
13C fractional enrichment of acetyl CoA from mice fed a normal diet or calorically restricted. Mean +/− SEM. Mean +/− SEM. *, p < 0.05 compared to 5 month old ND male; **, p < 0.05 compared to 5 month old ND female.
3.3 13C Enrichment of TAG Stores and TAG Content
Dynamic NMR spectroscopy, end point TAG concentration, and endpoint percent enrichment of TAG allowed for monitoring the amount of TAG enriched with 13C labeled over time (Figure 3). For both CR and ND myocardium, incorporation of 13C labeled LCFA continued to increase over time. The incorporation of 13C labeled LCFA in TAG stores proved significantly less in the younger CR mice than mice on a ND. In both male and female myocardium, the rate at which the TAG pool was enriched with 13C palmitate was slower for mice on a CR diet, thus the percentage of 13C enrichment of the TAG pool was less for CR myocardium (Table). While no differences in the incorporation of 13C labeled LCFA occurred between sexes at 5 months of age (Figure 3A, Table), age related sex differences were elucidated in myocardium from mice at 13 months of age (Table).
Figure 3.
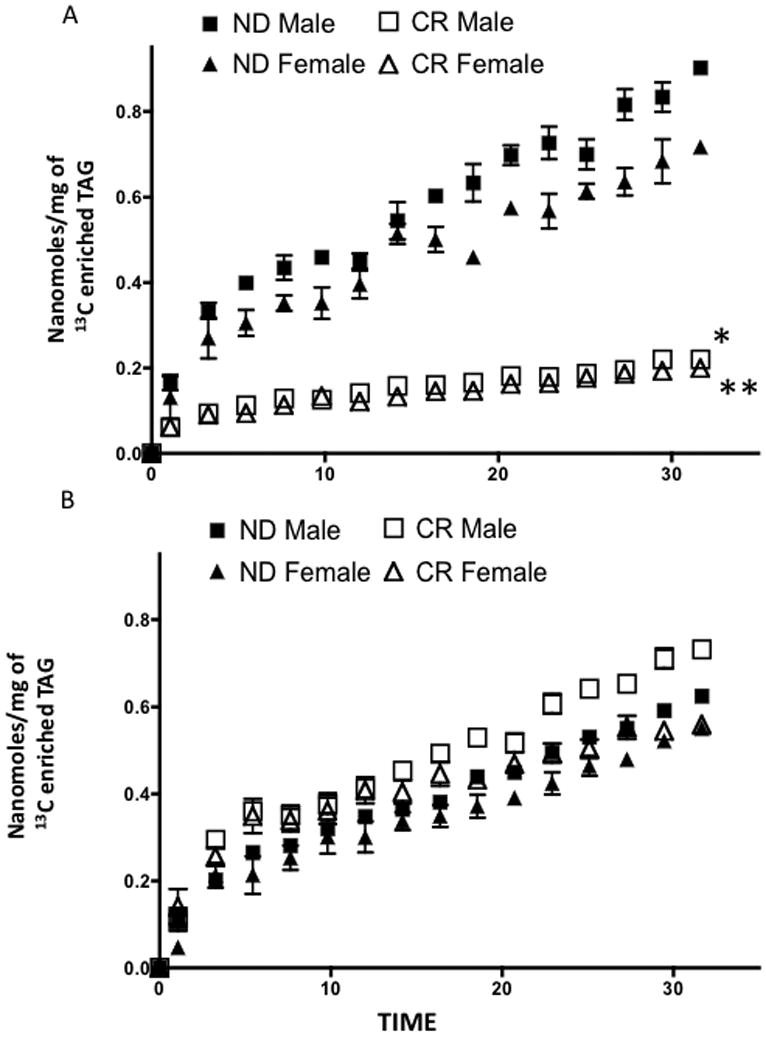
13C enrichment of TAG stores by 13C palmitate over the time course of experiment. A. 5-month-old mice fed a normal diet or calorically restricted diet 3 months. Mean +/− SEM. B. 13-month-old mice fed a normal diet or calorically restricted diet 10 months. Mean +/− SEM. *, p < 0.05 compared to ND male; **, p < 0.05 compared to ND female; †, p < 0.05 compared to 5 month ND male.
Table.
The rate of incorporation of [2,4,6,8,10,12,14,16-13C8] palmitate into the TAG pool, nanomoles/mg 13C enriched TAG/minute.
| Normal Diet Male | Normal Diet Female | Caloric Restriction Male | Caloric Restriction Female | |
|---|---|---|---|---|
| 5 month old mice nanomoles/mg 13C enriched TAG/min | 0.0193 ± 0.0008 | 0.0155 ± 0.0002 | 0.0041 ± 0.0002* | 0.0037 ± 0.0002** |
| 13 month old mice nanomoles/mg 13C enriched TAG/min | 0.0141 ± 0.0003* | 0.0118 ± 0.0007** # | 0.0154 ± 0.0005† | 0.0096 ± 0.0007‡ |
p < 0.05 compared to 5 month old ND male
p < 0.05 compared to 5 month old ND female
p < 0.05 compared to 5 month old CR male
p < 0.05 compared to 13 month old CR male
p < 0.05 compared to 13 month old ND male.
In middle-aged mice, the rates of incorporation of 13C palmitate in TAG stores were the similar for each group (Table). Observed differences in incorporation of 13C palmitate in 5-month-old mice were not seen in 13-month-old mice (Figure 3B).
On a ND, 5-month-old male and female myocardium showed no significant differences in TAG content (Figure 4A). But, as expected, long-term caloric restriction significantly depleted TAG stores in mice after 3 month CR. Only male myocardium on a ND showed a significant age related decrease in TAG content middle-aged (Figure 4B). There are no other significant differences in TAG content in middle-aged myocardium.
Figure 4.
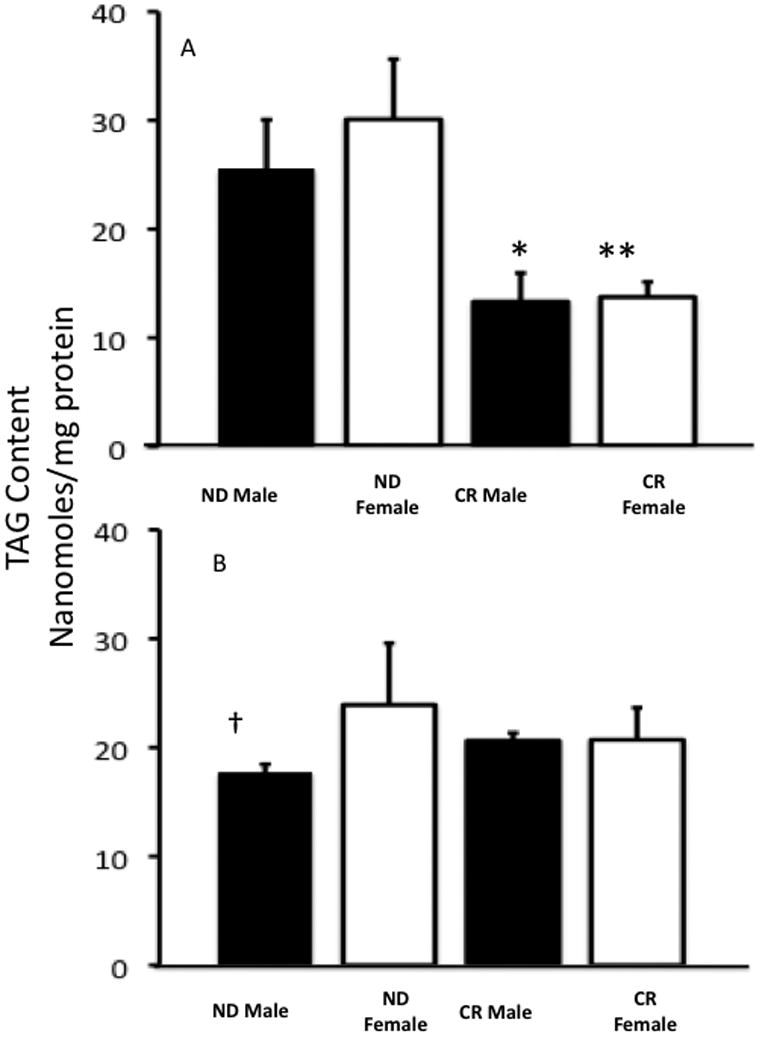
Tag content, nanomoles/mg protein. A. 5-month-old mice fed a normal diet or calorically restricted diet 3 months. Mean +/− SEM. B. 13-month-old mice fed a normal diet or calorically restricted diet 10 months. Mean +/− SEM. *, p < 0.05 compared to ND male; **, p < 0.05 compared to ND female; †, p < 0.05 compared to 5 month ND male and 5 month old ND female.
3.4 TAG dynamics
Caloric restriction for 3 months significantly reduced TAG turnover in both male and female myocardium (68% and 38% reduction, respectively), suggesting caloric restriction resulted in phenotype with a decreased reliance on endogenous LCFA (Figure 5). The decrease in TAG turnover reflected a decrease in shuttling of LCFAs through the TAG pool and a decrease in reliance of the TAG pool as a source for LCFA to fuel β-oxidation. TAG turnover significantly decreased in both 5-month-old male and female myocardium (Figure 5). While the mean differences between 5-month-old male and female myocardium did not reflect a significant reduction in TAG turnover, the TAG turnover from 5-month-old female myocardium on a CR diet was greater than 5-month-old male myocardium on a CR diet (Figure 5). By comparison, female mice relied on endogenous LCFA to a larger extent than myocardium from male mice, indicated by a smaller degree of the reduction in turnover.
Figure 5.
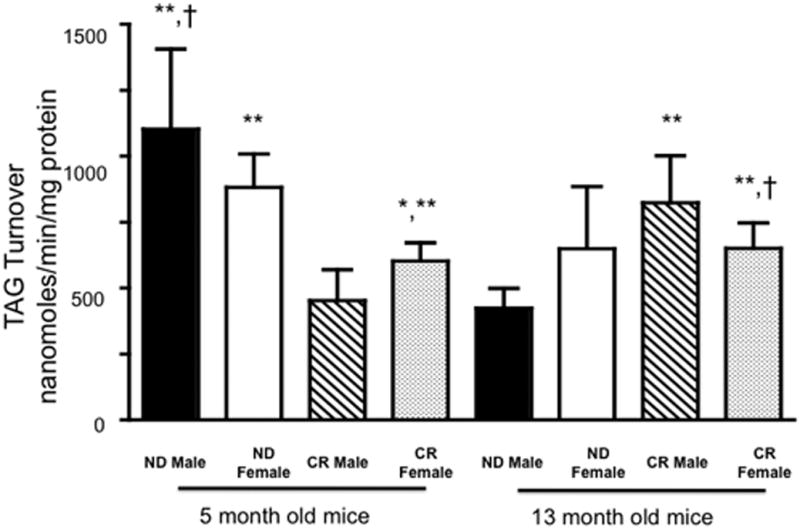
TAG turnover, nanomoles/min/mg protein from mice fed a normal diet or calorically restricted. Mean +/− SEM. *, p < 0.05 compared to 5 month old ND female; **, p < 0.05 compared to 5 month CR male; †, p < 0.05 compared to 13 month old ND male.
A higher rate of turnover in hearts of female mice showed a greater involvement of 13C labeled palmitate. Despite gross changes in TAG turnover following CR and significant differences between sexes, the male and female myocardium continued to have similar enrichment of acetyl CoA from 13C palmitate following CR (Figures 2 and 5).
The phenotype of the calorically restricted 5-month-old male mice was reproduced in the middle-aged male mice on a normal diet. The TAG turnover in the male CR mice was similar to middle-aged male mice on a normal diet (Figure 5). As male mice age, TAG turnover significantly decreased along with the ability to access stored LCFA. While 3 month CR significantly decreased TAG turnover in female myocardium, a similar difference is not seen in middle-aged female myocardium. In females, aging normalized TAG turnover dynamics. Importantly, as the mice aged, the myocardium of females retained the lipid dynamics of the ND while in male myocardial TAG turnover was reduced (Figure 5).
4. Discussion
Changes in substrate oxidation and lipid handling have been suggested to contribute to various cardiomyopathies [45]. Cardiac hypertrophy results in a metabolic shift reverting to a fetal pattern, where there is less reliance on exogenous fats and a greater dependence on carbohydrates [38]. Pressure overload early cardiac failure in rats resulted in reduced TAG storage and mobilization with limited availability of endogenous fats for oxidation [38]. Elucidating how sex and age influences lipid metabolism is an important distinction because recent findings showing that in normal hearts fatty acyl units from the TAG pool are preferentially oxidized [10].
In the current study, we show caloric restriction significantly depletes endogenous TAG stores in 5-month-old mice. This contrasts remarkably to short term or acute caloric restriction and starvation where others report an increase in TAG stores [25]. Consistent with a slower rate of 13C enrichment of TAG, TAG turnover is significantly reduced in response to caloric restriction in both male and female myocardium. Interestingly, turnover in female myocardium does not decrease to the same extent as male myocardium. This would suggest that myocardium from female mice preserve involvement of endogenous TAG in lipid metabolism compared to myocardium from male mice. In the normal heart, the TAG pool is highly dynamic and integral to β-oxidation. Loss of the ability to the access the TAG pool could prove to be deleterious for male myocardium. Female myocardium shows a continued ability to access the TAG pool regardless of diet and age, which could prove to be cardioprotective.
LCFA enter the myocardium where by they can then be stored as TAG or undergo β-oxidation in the mitochondria to provide energy (Figure 6). The endogenous TAG stores are integral to supplying LCFAs for β-oxidation [10]. In the current model of caloric restriction, in younger mice we see a significant deficiency in the ability to access LCFA in the endogenous TAG pool as a source for β-oxidation. This would be consistent with a model where there is a shift towards glucose as a source for energy i.e. during β-adrenergic stimulation or hypertrophy [29]. The initial physiological responses to CR are adaptive metabolic shifts from LCFA as sources for acetyl CoA for energy presumably to glucose (Figure 2). Cycling of LCFA through the TAG pool significantly drops (Figure 5). As mice age, the diet induced differences seen in acetyl CoA enrichment from 13C palmitate are no longer apparent. However, differences in TAG turnover are still evident. Aging in male mice results in a phenotype similar to CR mice at 5 months old. Thus aging is associated with significant decreases in the ability to access the TAG pool as a source of LCFA for energy. Aging normalizes the initial adaptive responses to a calorically restricted diet. For female mice, age results in a TAG turnover similar to that of female mice on a CR diet.
Figure 6.
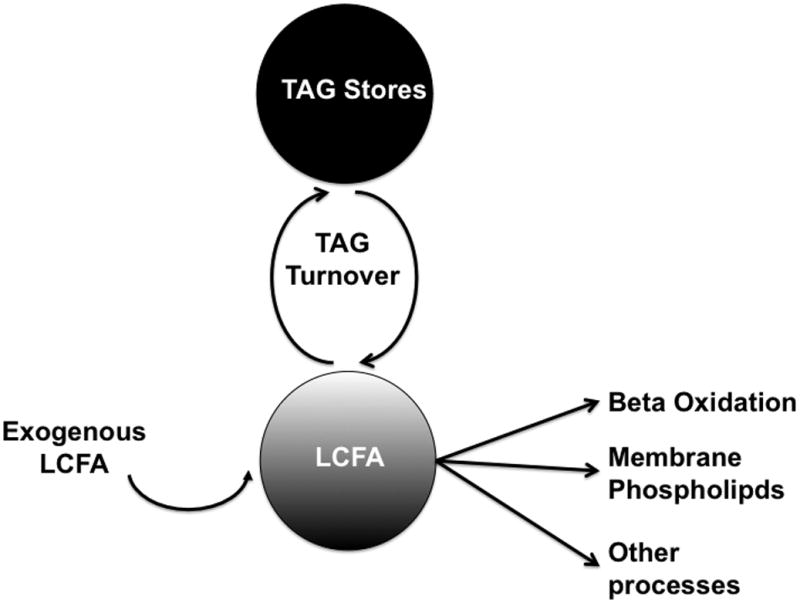
Fate of long chain fatty acids.
CR in a younger mouse promotes a shift away from oxidation of labeled 13C LCFA towards unlabeled substrates, presumably towards glucose (Figure 2). In young mice this shift is favorable reducing the risk factors for cardiovascular disease and extending mortality [18, 22]. However, when CR is implemented in rodents of advanced age the effects are harmful decreasing survival rate [18]. Our current results may suggest a mechanism for such a decrease in survival rates. TAG turnover was decreased in middle-aged mice on a normal diet, comparable to that of young mice on a CR diet. Likely in young mice, there are shifts in energy production as well as other physiological shifts that accommodate a decrease in TAG turnover. As the younger mouse ages, the physiology of the mouse shifts allowing it to exist with a decreased TAG turnover and continue to maintain FAO. However, if CR is implemented in a middle-aged, or aged, mouse where TAG turnover is already low, TAG turnover would drop to a greater degree and decrease the utilization of TAG derived LCFA. It is likely this shift would be deleterious, decreasing survival.
Changes in TAG content and turnover could be a result of the duration, 3 or 10 months, of caloric restriction or age. It is indeed it is possible that the longer duration of a calorically restricted diet can result in metabolic shifts that affect TAG content and the rate of turnover. Thus the longer length of a calorically restricted diet allows the myocardium more time to metabolically adapt to the diet. Indeed other researchers have shown how acute CR, or fasting, actually increases TAG content by mobilizing lipid stores, where longer CR did not show elevated TAG content (Figure 4A&B) [25]. Aging has been shown to result in reductions in PPARα [46]. As a result, expression of PPARα target genes involved in fatty acid us was reduced, which lead to ceramide accumulation and age associated cardiac hypertrophy [46]. Reduction in the expression of PGC-1β was associated with a decrease in ERRα DNA-binding activity which may lead to metabolic disturbances that link skeletal muscle mitochondrial efficiency and aging [47]. With the expression of transcription factors that regulate lipid metabolism being sensitive to aging, we would expect those significant changes in TAG turnover in middle-aged and aging myocardium. For male and female mice on a normal diet, the current study showed significant changes in TAG turnover as a result of age (Figure 5). A similar age dependent decrease in TAG content was shown for male myocardium on a normal diet, but not female myocardium (Figure 4). Age related differences are also evident in the incorporation of 13C palmitate into the TAG pool (Table). The significant decreases in TAG turnover and TAG content is presumably due to age related changes in the expression of PPARα and PGC1α and β, transcription factors involved in the control of glucose and lipid metabolism.
Hyyti et al. (2010) show that there are clearly defined age induced shifts in myocardial substrate oxidation, particularly relative to contributions to the citric acid cycle by LCFA and ketones [5]. Their data show that there are age related impairments to myocardial fatty acid and ketone oxidation. Gene expression data from their work suggest that there is an age related deficiency in PPARα expression. In vitro gene array analysis doe not allow for conclusions to be made regarding ligand specific enzyme activity. The current work provides ex vivo data of TAG dynamics of caloric restriction from an intact beating heart where previous work has been shown to have changes in PPARα and PGC-1α expression. Our current study is consistent with previous data showing that PPARα expression augments TAG turnover [10] and the current study suggesting that in middle-aged male myocardium, TAG turnover becomes compromised.
Consistent with studies of other parameters involved in lipid metabolism, the current study suggests that female myocardium handles LCFA differently than males. The previous findings from human positron emission tomography (PET) studies and calculation of FA utilization are largely based on FA uptake. Peterson et al. (2008) show that the female sex predicted greater FA uptake in the heart, lower glucose uptake and utilization [30]. Studies in rat liver suggest that CD36 is hormonally and nutritionally regulated [51-53]. Koonen et al (2007) showed that an age-dependent increase in expression CD36 is a causative factor in age related cardiomyopathy [12]. The female sex exhibited greater CD36 mRNA expression [48]. Both studies suggest female sex predicts an increased rate of FA uptake. While previous findings are important, the current study allows for direct analysis of 13C labeled LCFA storage and turnover with in the TAG pool. Importantly, our results are consistent with these previous suggesting that not only does the female heart take up FA at a different rate than male myocardium; storage of LCFA and cycling through endogenous TAG stores also occur at different rates.
Sex related differences in TAG turnover beg the question whether TAG turnover is under sex related hormonal control and whether aging normalizes these differences due to decrease of estrogen in the post-menopausal myocardium. Interestingly, in this study the pre-menopausal, 5 month old, female TAG turnover does not decrease to the same extent as in the male myocardium, despite similarly reduced TAG content. Thus, the pre-menopausal female myocardium preserved more involvement of the endogenous TAG stores in lipid metabolism and as a source of LCFA for β-oxidation. In contrast, post-menopausal myocardium shows no significant differences with age matched male myocardium, suggesting normalization between sexes after menopause. A possible mechanism for this could be loss of PGC1α activity through the loss of estrogen related receptors [50]. Post trauma-hemorrhage decline of PGC-1α post trauma is rescued with estradiol or when an estrogen receptor-β agonist was delivered [34]. Another study implicating a role for estrogen in regulating heart fatty acid dynamics showed that estrogen had a significant effect in the expression of lipoprotein lipase [51]. Djouadi et al. (1998) showed that PPARα deficient mice displayed hepatic and myocardial lipid accumulation in both sexes, where male PPARα null mice showed a more severe phenotype [52]. Chronic administration of estrogen in PPARα null mice improved defects in lipid metabolism [52]. Herrero et al. (2005) showed that hormone replacement therapy in postmenopausal women resulted in higher levels of myocardial fatty acid uptake and FAO compared to age matched men [53]. When these studies are considered together with the current findings, it is enticing to speculate that the loss of estrogen in females with age directly affects myocardial lipid turnover dynamics, thereby making the TAG pool in the female myocardium less dynamic, or more like male myocardium.
5. Conclusions
General health is a function of age [52]. In mice, neural and immune declines are evident by middle age (12 months) [54-55]. The current manuscript investigates the changes in myocardial lipid dynamics as a function of age. Furthermore, this work evaluates the effect of nutritional intervention on lipid dynamics in the aging myocardium in both sexes. Long-term caloric restriction is an effective nutritional intervention to promote longevity and reduce oxidative stress [16, 18, 21]. Animal studies of long-term CR report increased whole body FAO and LCFA synthesis rates [56] and CR increased lipogenic gene expression (FAS, ACC1, SREBP-1, and PPARγ) in the liver and adipose tissue [56]. Yet, little if any information exists to date on the how long-term CR affects LCFA storage in the heart, as TAG. Interestingly, this study shows that the female myocardium traffics LCFA differently than male myocardium. With CR, TAG content and turnover decreases in both male and female myocardium. However female mice continue to rely on endogenous TAG stores as a source for FAO to a greater degree than male myocardium. The continued reliance of endogenous TAG stores by female myocardium could be at the center of observed differences between male and female myocardium having cardioprotective effects and being mediated by estrogen receptor signaling.
Supplementary Material
Highlights.
The current manuscript compares the effects of gender and age on TAG dynamics in the myocardium after long-term caloric restriction.
Long-term CR results in a reduction of endogenous lipid stores and a significant decrease in TAG turnover.
In female myocardium, TAG turnover does not decrease to the same extent as in male myocardium.
The female myocardium preserves TAG involvement in lipid metabolism.
Acknowledgments
This work was funded by the following grants: R37HL49244, P01AG27211, and ULRR029879.
Glossary
- ACC1
acetyl CoA carboxylase
- CR
caloric restriction
- DGAT1
diacylglycerol acyl transferase 1
- ERRα
estrogen related receptor α
- FAS
fatty acyl CoA synthase
- FA
fatty acid
- FAO
fatty acid oxidation
- LCFA
long-chain fatty acid
- ND
normal diet
- NMR
nuclear magnetic resonance
- SREBP-1
sterol response element binding protein 1
- TAG
triacylglyceride
- PGC-1α
Peroxisome proliferator-activated receptor-γ coactivator-1 α
- PGC1β
Peroxisome proliferator-activated receptor-γ coactivator-1 β
- PPARα
Peroxisome proliferator-activated receptor-α
Footnotes
Disclosures: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghali JK, III, Liao Y, Cooper RS. Left ventricular hypertrophy in the elderly. Am J Geriatr Cardiol. 1997;6:38–49. [PubMed] [Google Scholar]
- 2.Abu-Erreish GM, Neely JR, Whitmer JT, Whitman V, Sanadi DR. Fatty acid oxidation by isolated perfused working hearts of aged rats. Am J Physiol. 1977;232:E258–62. doi: 10.1152/ajpendo.1977.232.3.E258. [DOI] [PubMed] [Google Scholar]
- 3.McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovas Res. 1993;27:2222–8. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- 4.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, et al. Impact of aging on substrate metabolism by the human heart. Am Coll Cardio. 2003;41:293–9. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 5.Hyyti OM, Ledee D, Ning XH, Ge M, Portman MA. Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am J Pysiol Heart Circ Physiol. 2010;299:H868–75. doi: 10.1152/ajpheart.00931.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–12. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 7.Rijzewijk LJ, van der Meer RW, Smit JWA, Diamant M, Bax JJ, Hammer S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardilol. 2008;52:1793–9. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 8.Bhashyam S, Parikh P, Bolukoglu H, Shannon AH, Porter JH, Shen YT, et al. Aging is associate dwith myocardial insulin resistance and mitochondrial dysfunction. Am J Phyiol Heart Circ. 2007;293:H3062–71. doi: 10.1152/ajpheart.00163.2007. [DOI] [PubMed] [Google Scholar]
- 9.van der Meer RW, Rijzewijk LJ, Diamant M, Hammer S, Schär M, Bax JJ, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–22. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 10.Banke NH, Wende AR, Leone TC, O'Donnell JM, Abel ED, Kelly DP, et al. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARα. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor α (PPARα) signaling in gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol. 2002;34:1249–57. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- 12.Koonen DPY, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, et al. CD36 Expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–47. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- 13.Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 14.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 15.Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, et al. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hyperten. 2011;57:216–22. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–17. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- 17.Lambert AJ, Portero-Otin M, Pamplona R, Merry BJ. Effect of ageing and caloric restriction on specific markers of protein oxidative damage and membrane peroxidizability in rat liver mitochondria. Mech Ageing Dev. 2004;125:529–538. doi: 10.1016/j.mad.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Forster MJ, Morris P, Sohal R. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–2. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masoro EJ. Dietary restriction and aging. JAGS. 1993;41:994–99. doi: 10.1111/j.1532-5415.1993.tb06767.x. [DOI] [PubMed] [Google Scholar]
- 20.Keenan KP, Smith PF, Hertzog P, Soper K, Ballam GC, Clark RL. The effects of overfeeding and dietary restriction on Sprague-dawley rat survival and early pathology biomarkers of aging. Toxicol Path. 1994;3:300–15. doi: 10.1177/019262339402200308. [DOI] [PubMed] [Google Scholar]
- 21.Shoal RS, Woodrush R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor γ coactivator 1 in caloric restriction and other models of longevity. J Gerontology: Biol Sci. 2005;60A:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 23.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1α deficiency causes multi-system energy metabolic derangements; muscle dysfunction, abnormal weigh control and hepatic steatosis. Plos Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J. 2005;19:1320–22. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer RW, Hammer S, Smit JWA, Frolich M, Bax JJ, Diamant M, et al. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes. 2007;56:2849–53. doi: 10.2337/db07-0768. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Cheng H, Mancuso DJ, Gross RW. Caloric restriction results in phospholipid depletion, membrane remodeling, and triacylglycerol accumulation in murine myocardium. Biochem. 2004;43:15584–94. doi: 10.1021/bi048307o. [DOI] [PubMed] [Google Scholar]
- 27.Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–48. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Linford NJ, Beyer RP, Gollahon K, Krajcik RA, Malloy VL, Demas V, et al. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–88. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, et al. The transcriptional coactivator PGC-1α is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–96. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, et al. Impact of gender on myocardial metabolic response to obesity. JACC: Cardiovasc Imag. 2008;1:424–33. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou SH, Lee YC, Huang CF, Wang YR, Yu HP, Lau YT. Gender-specific effects of caloric restriction on the balance of vascular nitric oxide and superoxide radical. Cardiovas Res. 2010;87:751–9. doi: 10.1093/cvr/cvq095. [DOI] [PubMed] [Google Scholar]
- 33.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–81. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovas Res. 2007;75:478–86. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Yan L, Gao S, Ho D, Ge H, Wang C, Tian Y, et al. Abstract 17173: Caloric restriction protects against aging cardiomyopathy, but not longevity. Circulation. 2011 Nov;124:A17173. [Google Scholar]
- 36.Sato N, Vatner SF, Shen YT, Kudej RK, Ghaleh-Marzban B, Uechi M, et al. Effects of cardiac denervation on development of heart failure and catecholamine desensitization. Circulation. 1997;95:2130–40. doi: 10.1161/01.cir.95.8.2130. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Amer J Physiol Endocrinol Metab. 2006;290:E448–E455. doi: 10.1152/ajpendo.00139.2005. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell JM, Fields AD, Sorokina N, Lewandowski ED. Absence of endogenous lipid oxidation in heart failure exposes limitations for triacylglycerol storage and turnover. J Mol Cell Cardiol. 2008;44:315–22. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, White LT, Doumen C, Daminco LA, LaNoue KF, Alpert NM, et al. Kinetic analysis of dynamic 13C NMR spectra: metabolic flux, regulation, and compartmentation in hearts. Biophys J. 1995;69:2090–102. doi: 10.1016/S0006-3495(95)80080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malloy CR, Sherry AD, Jeffrey FMH. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1988;265:6964–6971. [PubMed] [Google Scholar]
- 41.Lin X, Yue P, Chen Z, Schonfeld G. Hepatic triglyceride contents are genetically determined in mice results of a strain survey. J Physiol Gastrointest Liver Physiol. 2005;288:G1179–89. doi: 10.1152/ajpgi.00411.2004. [DOI] [PubMed] [Google Scholar]
- 42.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SH, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–99. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonfeld G. MPD: Schonfeld1 Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, Maine USA: Oct, 2011. Strain survey of liver and plasma lipids and blood glucose levels in 11 inbred strains of mice. http//phenome.jax.org. [Google Scholar]
- 44.Champy MF, Selloum M, Zeitler V, Jung B, Rousseau S, Pouilly L, et al. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008 May;19:318–3. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 45.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced CPT1 activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-Calvo R, Serrano L, Barroso E, Coll T, Palomer X, Camins A, et al. Peroxisome proliferator-activated receptor α down-regulation is associated with enhanced ceramide levels in age-associated cardiac hypertrophy. J Gerontol A Biol Sci Med Sci. 2007;62:1326–36. doi: 10.1093/gerona/62.12.1326. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Calvo R, Jové M, Coll T, Camins A, Sánchez RM, Alegret M, et al. PGC-1β down-regulation is associated with reduced ERRα activity and MCAD expression in skeletal muscle of senescence-accelerated mice. J Gerontol A Biol Sci Med Sci. 2006;61:773–80. doi: 10.1093/gerona/61.8.773. [DOI] [PubMed] [Google Scholar]
- 48.Cheung L, Andersen M, Gustavsson C, Odeberg J, Fernandez-Perez L, Norstedt G, et al. Hormonal and nutrition regulation of alternative CD36 transcripts in rat liver—a role for growth hormone in alternative exon usage. BMC Molec Biol. 2007;8:60. doi: 10.1186/1471-2199-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahlberg N, Rico-Bautista E, Fisher RM, Wu X, Cheung L, Flores-Morales A, et al. Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinol. 2004;145:1972–79. doi: 10.1210/en.2003-0874. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, et al. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Deschamps A, Korach KS, Murphy E. Estrogen-enhanced gene expression of lipoprotein lipase in heart is antagonized by progesterone. Endocrinology. 2008;149:711–16. doi: 10.1210/en.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Djouadi F, Weinheimer CJ, Saffitz J, Pitchford C, Bastin J, Gonzalez FJ. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J Clin Invest. 1998;102:1083–91. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, et al. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005;12:574–81. doi: 10.1016/j.nuclcard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Kohman RA, Crowel B, Kusnecov AW. Differential sensitivity to endotoxin exposure in young and middle-age mice. Brain Behav Immun. 2010;24:486–92. doi: 10.1016/j.bbi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. Detailed analysis of the behavior ad memory performance of middle-aged male and female CD-1 in a 3D maze. Behav Brain Res. 2009;187:312–26. doi: 10.1016/j.bbr.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 56.Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Amer J Physiol Endocrinol Metab. 2010;298:E108–16. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


