Abstract
Malaria is a major public health problem in India and one which contributes significantly to the overall malaria burden in Southeast Asia. The National Vector Borne Disease Control Program of India reported ~1.6 million cases and ~1100 malaria deaths in 2009. Some experts argue that this is a serious underestimation and that the actual number of malaria cases per year is likely between 9 and 50 times greater, with an approximate 13-fold underestimation of malaria-related mortality. The difficulty in making these estimations is further exacerbated by (i) highly variable malaria eco-epidemiological profiles, (ii) the transmission and overlap of multiple Plasmodium species and Anopheles vectors, (iii) increasing antimalarial drug resistance and insecticide resistance, and (iv) the impact of climate change on each of these variables. Simply stated, the burden of malaria in India is complex. Here we describe plans for a Center for the Study of Complex Malaria in India (CSCMi), one of ten International Centers of Excellence in Malaria Research (ICEMRs) located in malarious regions of the world recently funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The CSCMi is a close partnership between Indian and United States scientists, and aims to address major gaps in our understanding of the complexity of malaria in India, including changing patterns of epidemiology, vector biology and control, drug resistance, and parasite genomics. We hope that such a multidisciplinary approach that integrates clinical and field studies with laboratory, molecular, and genomic methods will provide a powerful combination for malaria control and prevention in India.
Keywords: Malaria, Plasmodium, Anopheles, India, Genomics, Epidemiology
1. Malaria in India
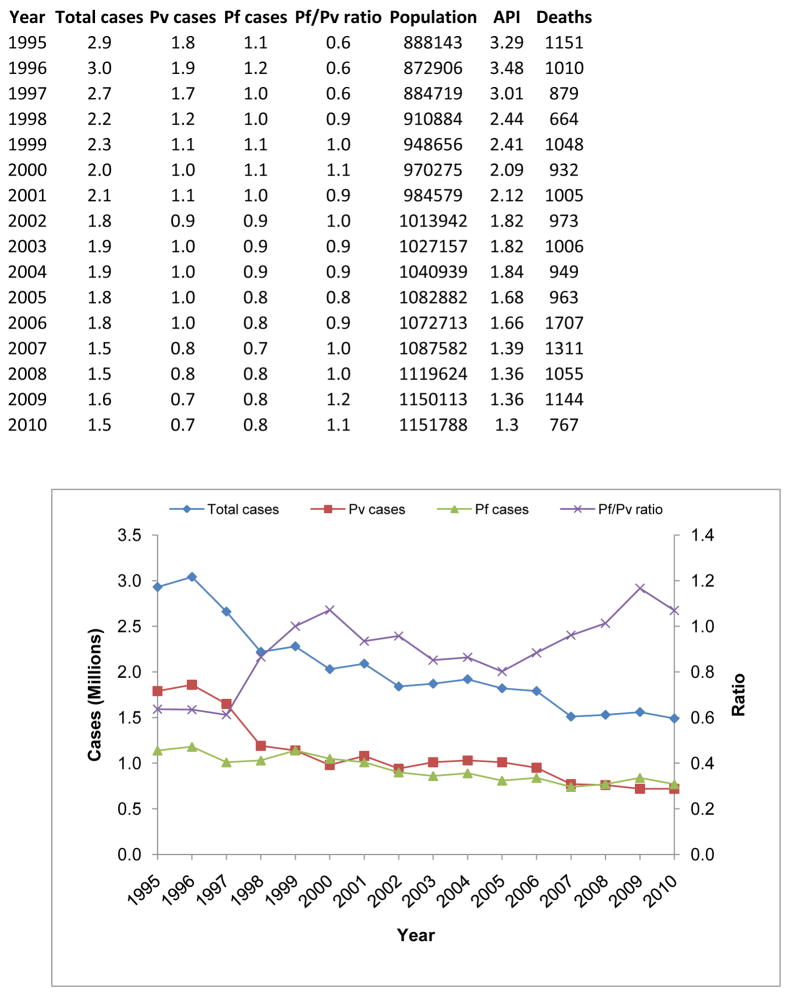
As the second most populous country in the world, with a population exceeding one billion people, India’s public health system faces many challenges including implementation of surveillance programs to accurately estimate and control the national malaria burden. Historically, the highest incidence of malaria in India occurred in the 1950s, with an estimated 75 million cases and 0.8 million deaths per year (World Health Organization, Country Office for India). The launch of the National Malaria Control Program (NMCP) in 1953 resulted in a significant decline in the number of reported cases to <50,000 and no reported mortality, by 1961. Despite its near elimination in the mid-1960’s, malaria resurged to ~6.45 million cases in 1976. Since then, confirmed cases have gradually decreased to 1.6 million cases and ~1,100 deaths in 2009 (Figure 1). Recently, it has been suggested that the malaria incidence is between 9 to 50 times greater than reported (reviewed in Hay et al., 2010), with a ~13-fold underestimation of malaria-related mortality (Dhingra et al., 2010). Such claims reinforce the need for robust and comprehensive epidemiological surveillance studies across the country (Singh et al., 2009) to determine the actual burden.
Figure 1.
Malaria cases and changing species pattern in India during the years 1995–2010. The total number of reported malaria cases has decreased since 1995, primarily through a reduction in the number of reported P. vivax cases. Currently, there is a 1:1 ratio of P. falciparum to P. vivax cases.
2. Indian malaria parasites and their vectors
2.1 India has a diverse topography, ecology and climate
India’s expansive geography and diverse climate supports ideal environments for sustaining malaria parasites and their vectors. The climate varies from tropical monsoon in the south of the country to temperate in the north, but has four major climatic zones: mountain climate, tropical wet climate, tropical dry climate, and subtropical humid climate. Such climatic variation is due to a sharp temperature gradient caused by atmospheric changes in wind circulation and precipitation, lending to seasonally-dependent asymmetric heating patterns of India’s peripheral bodies of water and land. The average annual temperature in India varies with the altitude, as the foothills and mountainous regions near the Himalayas and Western Ghats average 20°C, while coastal areas are tropical and humid, averaging above 30°C annually. The monsoon strikes the Indian southwestern coast in June every year, marking the beginning of the rainy season. Monsoon bursts drastically change the average daily rainfall and shape the overall annual rainfall in most regions of India. Remarkably, it is not uncommon for Meghalaya and the coastlines of Kerala, Karnataka, Goa, and Maharashtra to receive >250cm of rainfall each year, while some areas in Rajasthan receive <25cm each year. Such climatic diversity influences the distribution of vectors and species of malaria parasite; as a result, malaria in India takes a number of different forms, including forest/tribal malaria, urban/slum malaria, industrial malaria, and plains malaria.
2.2 Malaria parasite species in India
The two major human malaria species in India are Plasmodium falciparum and P. vivax; P. malariae has been reported in the eastern India state of Orissa (Sharma et al., 2006), while P. ovale appears to be extremely rare if not absent. Intriguingly, the two major infecting species vary in proportion across India. For example, the southern state of Tamil Nadu suffers from P. vivax, P. falciparum is the dominant parasite in Orissa, and mixed-species infections are prevalent in the west (e.g., Gujarat state). Although P. falciparum and P. vivax are unevenly distributed across India, this is not due to Anopheles vector restriction (even though the vector species also differ in geographical distribution, see below) since both species are transmitted by the same vectors. Historically, P. vivax has been the major infecting species; however, over the past several years P. vivax cases have decreased: the ratio of P. falciparum versus P. vivax malaria was 0.41 in 1985, gradually increasing to 0.60 by 1995, and shifting to 1.01 by 2010 (Singh et al., 2004a,b) (Figure 1). In states where P. falciparum and P. vivax co-circulate, fluctuating proportions of the two species complicate diagnosis and treatment. Treatment is based upon the primary species identified in an infection by standard microscopy diagnosis, subjecting all species in a single host to the same drug treatment, likely contributing to this surge in drug-resistant parasite strains.
Approximately 65% of those at risk for becoming infected with malaria in Southeast Asia are individuals residing in India (WHO 2010). The central and eastern regions of India report the most malaria (Figure 2), particularly the eastern states of Orissa, West Bengal, and Jharkhand, the central states of Chhattisgarh and Madhya Pradesh, and the western states of Gujarat, Karnataka and Rajasthan, with the largest number of deaths reported in Orissa (Joshi et al., 2008). Malaria cases in India are reported throughout the year, since a perfect combination of average temperature (15–30°C), rainfall and precipitation-inducing conditions persist across the different parts of the country over all the seasons. With increasing ecological and man-made environmental change (e.g. urbanization, construction of dams, agricultural intensification, deforestation) malaria in India is exhibiting general trends from rural to urban malaria, from forest to plain malaria, and from industrial to travel malaria (Sharma et al., 2006).
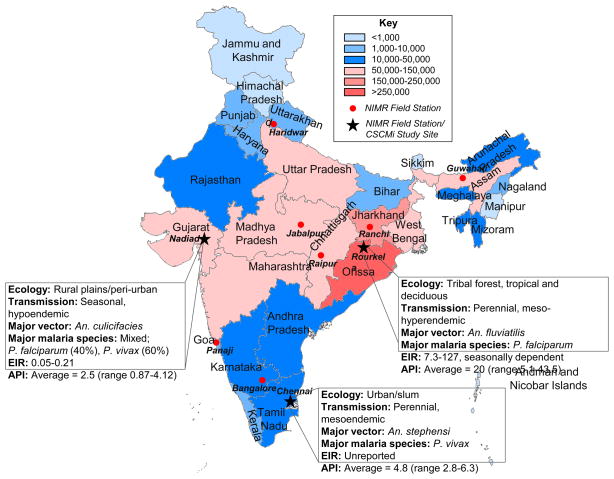
Figure 2.
Malaria endemicity in India. State boundaries are color-coded according to total malaria endemicity (see key). Data taken from the National Vector Borne Disease Control Programme (http://www.nvbdcp.gov.in/) for year 2010. NIMR field stations are indicated as red dots. The three NIMR field stations incorporated as part of the CSCMi are: Nadiad (Gujarat), Rourkela (Orissa) and Chennai (Tamil Nadu), each with different eco-epidemiological profiles as shown. EIR: entomological inoculation rate; API: annual parasite incidence.
2.3 Malaria vectors in India
The diverse malaria epidemiology in India is mirrored by high diversity of malaria vector species, most of which exist as complexes comprising several cryptic species that vary in vectorial capacity (Dash et al., 2007; Singh et al., 2009). In fact, close to 60 morphologically distinct species have been described in India; six of these have been found to vector malaria (Table 1), although An. culicifacies is responsible for 60–65% of the malaria burden (Goswami et al., 2006; Dash et al., 2008; Singh et al., 2009). As argued by Singh et al., (2009), this level of diversity makes India an ideal ‘model’ to study interactions amongst host-vector-parasite and the environment to both fully understand malaria and develop sustainable solutions.
Table 1.
Distribution of principal species of malaria vectors in India.
| Primary vectors | No. sibling species recorded | No. sibling species in India | Members | Ecological distribution | Species to be studied as part of CSCMi |
|---|---|---|---|---|---|
| An. stephensia | - | - | - | Urban | Yes |
| An. culicifacies s.l. | 5 | 5 | A, B, C, D, E | Rural/peri-urban | Yes |
| An. fluviatilis s.l. | 4 | 4 | S, T, U, V | Foothills/forest | Yes |
| An. minimus s.l. | 3 | 1 | An. minimus, An. harrisoni, E | Northeastern states | Yes |
| An. dirus s.l. | 7 | 2 | D, E | Northeastern states | Yes |
| An. sundaicus s.l. | 3 | 1 | New cytotype D | Andaman-Nicobar Islands | No |
No description of An. stephensi sibling species is available. However, two races, i.e. the vector ‘type form’ and non-vector ‘var. mysoriensis’ are often reported. The ‘type form’ is found in urban areas, while ‘var. mysoriensis’ is found in rural areas. Another form, ‘intermediate’ is also suggested. Adapted from Singh et al., 2009.
The geographically widespread vector, An. culicifacies, comprises a complex of five morphologically indistinguishable sibling species: A, B, C, D, and E (Goswami et al., 2006; Adak et al., 1999). Though these sibling species are physically indistinguishable, they differ physiologically in terms of vectorial capacity (Adak et al., 1999). Of the three sibling species tested, species A was found be the most susceptible to infection, as measured by oocyst abundance and sporozoite maturation; species B was the least susceptible to infection, bordering on being a non-vector (Adak et al., 1999). Similar complexity exists for the other principle malaria vectors in India. For example, An. fluviatilis s.l. exists as a complex of four sibling species (S, T, U, and V) (Subbarao et al., 1994), An. minimus s.l. a complex of three sibling species (Singh et al., 2010 ) (An. minimus, An. harrisoni and E) and An. dirus a complex of at least seven sibling species two of which, An. baimaii and An. elegans, occur in India (Dash et al., 2008).
3. Malaria control programs in India
3.1 National Vector Borne Disease Control Programme (NVBDCP)
The National Vector Borne Disease Control Programme (NVBDCP) is the agency responsible for the prevention and control of all vector-borne diseases in India, including malaria. It is one of the technical departments of the Directorate General of Health Services under the Ministry of Health and Family Welfare and is responsible for framing technical guidelines and policies, and monitoring implementation through regular reports on malaria control. The NVBDCP goals are to develop a well-informed and self-sustained health care system in India with equitable access to quality health care and to ensure that the program activities are in accord with the Millennium Development Goal of halting and reversing the incidence of malaria and other vector-borne diseases by the year 2015.
The major strategies being pursued by the NVBDCP to help achieve its objectives are: (i) disease management through early case detection and complete treatment, (ii) integrated vector management (IVM) to reduce the risk of vector-borne transmission; and (iii) supportive interventions which include communicating behavior change, capacity building and monitoring and evaluation of programs. To facilitate disease management, fever treatment depots (FTDs) exist at the village level. The FTDs are diagnostic stations for the collection of blood slides from febrile patients, with an Annual Blood Examination Rate (ABER) target set by NVBDCP at ≥10% for screening the Indian population. An important initiative recently introduced by the National Rural Health Mission (NRHM) is the provision of village-based Accredited Social and Health Activist (ASHAs), personnel that have been trained in malaria diagnosis by rapid diagnostic tests (RDTs), and antimalarial drug administration.
The NVBDCP has introduced the use of RDTs to help facilitate early detection and also the deployment of insecticide-treated bed nets in high-risk regions for prevention. The NVBDCP achieves evaluation of its programs in collaboration with the National Institute of Malaria Research (NIMR), one of the permanent institutes of the Indian Council of Medical Research (ICMR; under the Department of Health Research, Ministry of Health and Family Welfare, Government of India). While the NVBDCP undertakes the fortnightly domiciliary operational surveillance of malaria across India, NIMR provides technical support to the national program for the control of malaria. Thus NIMR, through its ten field stations (see below), evaluates new insecticides and diagnostic kits, conducts clinical trials, and monitors resistance to insecticides among vectors and drug therapy among parasites. The institute has also established quality assurance of malaria RDTs for NVBDCP.
3.2 National Institute of Malaria Research, Indian Council of Medical Research
The National Institute of Malaria Research (NIMR) was established in 1977 and is the only research institute in India completely devoted to malaria research. The primary task of the NIMR is to find solutions to the problems of malaria through basic, applied, and operational field research. The institute also plays a key role in resource development through training workshops and transfer of technologies for malaria control. Field evaluations of new insecticides, bio-larvicides, insecticide-impregnated bed nets, antimalarial medicines and parasite diagnostic kits are also carried out, and many of these research results are incorporated by the NVBDCP to strengthen their control initiatives. The NIMR has a network of 10 field units spread across India that serve as units for testing these new technologies (Figure 2). Each site has a different eco-epidemiological profile and can be used to study the variety of different forms of malaria, including tribal malaria, plain malaria, urban/slum malaria, forest malaria and industrial malaria.
3.3. Challenges for controlling malaria in India
The three most important challenges for malaria control in India are difficulties in control implementation and logistics, insecticide resistance, and antimalarial drug resistance. As the world’s second most populous country, India’s challenge is to design and restructure its health system to fulfill the needs of its large population. Several commentaries and research reports have emerged in the last several years highlighting the ongoing struggle of adequate infectious disease surveillance and control across India, including malaria surveillance (Sharma 2007; Hay et al., 2007; Dhingra et al., 2010; John et al., 2011).
Chemical insecticides against adult mosquitoes are among the most effective malaria control tools yet developed. Indoor residual spraying (IRS) with insecticides continues to be a mainstay of malaria control, having been responsible for often spectacular reductions in disease incidence last century and for the elimination of malaria from many countries (Trigg et al., 1998; Shiff 2002; Mabaso et al., 2004). In India, IRS with Dichlorodiphenyltrichloroethane (DDT) reduced cases from 75 million to just 0.1 million in 1966 (Sharma 1999). More recently, insecticide-treated bed nets (ITNs) have become a leading tool for malaria control (Lengeler 2004; Wakabi 2007). Because of these historic and contemporary successes, the major international efforts currently underway to comprehensively control and even globally eradicate malaria involve enormous up-scaling of IRS and ITN deployment (Roberts and Enserink 2007; Feachem and Sabaot 2008; Anon 2008; Grabowsky 2008). Indoor house-spraying and insecticide-treated bed nets are among the cheapest, most effective and best proven methods of controlling malaria globally, and in India. Unfortunately, mosquitoes can rapidly evolve resistance to all currently approved classes of public health insecticides. As was seen last century, one of the major challenges to these new efforts is the evolution of insecticide resistance in Anopheles populations (Trigg et al., 1998; Shiff 2002; Hemingway et al., 2002; N’Guessan et al., 2007; Nauen 2007; Kelly-Hope et al., 2008). Insecticide-resistant mosquitoes were one of the main hurdles faced by the ultimately unsuccessful Global Malaria Eradication plan in the middle of last century (Davidson and Zahar 1973; Harrison 1978; Trigg et al., 1998; Shiff 2002; Hemingway et al., 2002; Nauen 2007; Kelly-Hope et al., 2008), and present-day experience reconfirms this experience.
DDT, hexachlorocyclohexane (HCH), and malathion are used to control malaria throughout India, especially in rural areas. However, the development of insecticide resistance threatens to halt these once effective methods of control and prevention. In particular, growing insecticide resistance in the predominant malaria vectors such as An. culicifacies and An. stephensi is a major concern (Singh et al., 2009). While strategies and capacity for resistance surveillance exist through the NIMR and the NVBDCP, insecticide resistance is increasing in India. Increasing insecticide resistance surveillance, especially in India’s rural regions, is a necessity to prevent the dissemination of double and triple resistant strains. Yet, even as control programs intensify, so too will selection for resistance. Understanding how this will impact malaria transmission across different eco-epidemiological contexts is imperative for malaria control. Without this knowledge there is only partial insight into the sustainability of current control programs and the utility of prospective resistance management strategies.
Malaria imposes a major Indian global health burden in large part because the Plasmodium parasite readily evolves drug resistance. Resistance of P. falciparum to chloroquine (CQ) was first reported in 1973, and resistance likely originated from neighboring countries (Thailand, Burma, Bangladesh), which reported CQ-treatment failures earlier (Shah et al., 2011). Resistance then spread across India, particularly with migrant workers travelling to the west and south, with treatment failures increasing significantly between 1978 and 2007. CQ resistance is now widespread in India, but with geographic clustering of resistance areas (Shah et al., 2011). A national antimalarial drug policy was introduced in 1982, with sulfadoxine/pyrimethamine (SP) established as the treatment in CQ resistant areas. In 2004, artesunate plus SP replaced SP alone as the second-line drug for use in CQ-treatment failure. In 2007, artesunate plus SP became the first line treatment in high-risk districts with identified CQ resistance, and in 2010 this treatment became the recommended first-line treatment throughout India (Shah et al., 2011).
There have been a few case reports of CQ resistant P. vivax in India (Valecha 2009), but systematic trials from across the country have reported 100% efficacy of standard dose CQ treatment. Thus, CQ resistant P. vivax appears not to be a serious concern in India, despite over 50 years of chloroquine use (Shah et al., 2011). Why resistance is not present despite selection pressure is an interesting question in its own right, not least because it is unclear whether resistance is just a matter of time, or whether there is something different about P. vivax in India which prevents resistance evolution, in very sharp contrast to the experience with CQ against P. falciparum.
Thus the major threat today is the potential for resistance to arise in P. falciparum against artesunate or its partner drug, SP. Reduced efficacy of SP treatment was observed in some recent surveys in the north-east (Shah et al., 2011), but molecular markers for SP resistance are well known so that survelliance is possible. The greater challenge is surveillance for artesunate tolerance or resistance. Less sensitive parasites have been detected in SE Asia (Dondorp et al., 2009), and must be considered as an imminent threat to India. However, surveillance for artesunate resistance is a challenge. Once evolution has generated enough treatment failure to arouse suspicions of resistance, the spread of resistance is probably well advanced in an area. For new drugs like artesunate, little can be known in advance about the underlying resistance mechanisms. Moreover, diverse genetic mechanisms can be responsible for resistance in different regions, against different drugs and indeed in different malaria parasite species.
4. The Center for the Study of Complex Malaria in India
The Center for the Study of Complex Malaria in India (CSCMi) is a partnership between Indian and U.S.-based investigators to develop the knowledge, tools and evidence-based strategies needed to support the intervention and control programs of Indian government organizations, and to build research capacity in India and help train the next generation of malaria and mosquito vector biologists. Our research focus is the analysis of mixed-species and mixed-genotype infections (complex malaria), with respect to their eco-epidemiological profiles, transmission capabilities, and potential impact on drug resistance. To capture the eco-epidemiological diversity in India, three NIMR field stations have been selected as sentinel research sites for this study (Figure 2). The Rourkela field station (Orissa), in the east of the country, has predominantly P. falciparum in forest/riparian ecology, while the urban Chennai field station (Tamil Nadu) in the south of the country has predominantly P. vivax, and the Nadiad field station (Gujarat State) in the western plains has both P. vivax and P. falciparum transmission.
4.1 A five-year malaria epidemiology survey
Disease surveillance for malaria in India is generally facilitated by passive case detection (PCD) and active case detection (ACD) of fever cases fortnightly. However, these methods do not account for individuals who are asymptomatic, or present symptomatically but are missed by active surveillance. In addition, statistics from the treatment of malaria cases in the private sector are hard to come by. Consequently, the malaria prevalence rate in India may be underestimated or inaccurate, making it difficult to implement effective control strategies. Furthermore, due to this incomplete scope of detection, malaria parasite diversity and the distribution of malaria drug resistance on the Indian subcontinent may not be optimally characterized.
A major component of the CSCMi is to survey the circulating Plasmodium parasite populations in three different eco-epidemiological regions in India. Fundamental to attaining our primary objectives of how complex malaria in India influences disease outcome, vector transmission, and drug resistance is the collection and analysis of parasites that cause both symptomatic and asymptomatic malaria. Towards this aim, we will be running two interconnected epidemiological studies, a Community Study that uses active case detection to estimate the incidence and prevalence of asymptomatic malaria within each field site, and a Clinic Study that recruits individuals through passive case detection, focusing on symptomatic malaria and the detection of drug resistant parasites. A powerful relational database and data management system will facilitate data collection and storage, and interpretation of research results.
4.2 Determining the ecological and evolutionary determinants of malaria transmission
A key objective of the CSCMi is to advance ecological and evolutionary understanding of diverse malaria transmission in India and use this knowledge to progress towards sustainable management of adult mosquito vectors. By pooling the substantial expertise in entomology and intervention trials in India with the expertise in vector and parasite ecology and evolution in the United States, this work will contribute substantially to malaria control in India and generate novel insights into basic biological processes applicable to disease processes globally.
First, research will be conducted to quantify the role of environmental conditions in determining malaria transmission intensity (risk) in different eco-epidemiological contexts, including the future effects of climate change. The rationale behind this work is that most of the key components of R0 (basic reproduction number) for vector borne diseases are temperature dependent, including the rate of development of mosquitoes (and hence under many conditions, mosquito densities) and the rate of development of malaria parasites inside mosquitoes. However, the standard relationships used to describe the influence of temperature on key mosquito and parasite life history traits derive from research done in the early part of last century with a mishmash of species. Examining the interaction of key mosquito species with relevant locally derived parasites will provide valuable insights into precisely how temperature determines malaria transmission intensity. This entomological research is key for estimating malaria risk now from climate maps and also for determining the likely impact of altered environmental conditions, particularly due to climate change.
Second, we plan to evaluate the evolutionary responses of key mosquito vectors to the increasing adoption of insecticide-based interventions, quantifying the implications for malaria transmission in different eco-epidemiological contexts. A first step to resistance management is resistance surveillance. The recent introduction of long-lasting treated bed nets into India provides an exciting opportunity to look at bed net-driven resistance evolution, particularly since genetic resistance mechanisms are already present in Indian mosquitoes (Singh et al., 2009). Resistance management requires an understanding of the sensitivity of the resistance phenotypes revealed in standard surveillance to different environmental conditions, an understanding of the fitness costs of resistance, and then assessment of the consequences of resistance for malaria transmission and of alternative strategies. We aim to investigate these issues with a range of lab and field studies on resistant and susceptible mosquito lines.
Together these projects combine novel research and capacity building on entomology, vector biology and ecology, and the impact of vector management and control strategies on disease transmission. In so doing they address a need recently identified by the World Health Organization to evaluate the effectiveness of approaches such as IVM to simultaneously control disease, respond to ecological change and reduce pesticide use and insecticide resistance, in different settings (WHO 2009).
4.3 Genomics and drug resistance
Malaria imposes a major global health burden in large part because the Plasmodium parasite readily evolves drug resistance. In P. falciparum resistance has arisen against all classes of first line antimalarial drugs and several have had to be withdrawn from use in many countries. CQ, for instance, is no longer recommended for treatment of P. falciparum in India, and has been replaced with the more expensive artemisinin-based combination therapy (ACT). It is now accepted as inevitable by the World Health Organization that all drugs will eventually fail in the face of resistance evolution. The only solution to this failure is to shift to new therapies or reformulations of old ones when resistance becomes too widespread.
In the CSCMi we propose to use new technologies, including next generation genomics, to study antimalarial drug resistance in India. The approaches we propose here are designed to develop tools necessary for improved surveillance for resistance against drugs in use in India now, and not least for P. vivax as well as P. falciparum. Vivax malaria is a critical part of the Indian malaria picture, but globally, the genetic analysis of resistance in the species lags far behind that of P. falciparum. These tools will also be applicable to the detection and analysis of resistance to drug classes not yet in therapeutic use (e.g. ACT for P. vivax, next generation compounds or formulations for P. falciparum). This future-proofing is an important aspect of our aims.
First, we plan to use new generation sequencing technologies to detect evidence of the early signs of failure of ACT in P. falciparum and chloroquine in P. vivax in India. The approach we propose to investigate uses deep sequencing to identify the presence of ‘tolerant’ or ‘resistant’ parasite clones present in low levels in a patient – clones which are rare because resistance is newly arisen and associated with fitness costs. [Note this is the worst case scenario for traditional methods because if it is rare it will take weeks to be detected (and so could look like a re-infection) and it will not affect the rate of parasite clearance immediately post-treatment]. Deep sequencing of a polymorphic marker will enable detection of rare clones (Juliano et al., 2010) even if the less-susceptible clone is at a frequency of 5 in 2000 parasites, whereas conventional PCR methods cannot, since they struggle to find alleles at frequencies <1%. The presence of a clone which is less sensitive to treatment than a wild-type clone is identified through a change in its frequency as a patient transitions from an untreated to treated state. The parasites from that patient can then be subject to standard in vitro testing, or analysis of candidate resistance markers.
A second aim of the CSCMi genomics and drug resistance project deals specifically with P. vivax and has the goal of developing a map of common P. vivax SNPs (single nucleotide polymorphisms) segregating in the Indian subcontinent. This will provide preliminary data for a haplotype map that can be used for association studies of drug resistance. Vivax malaria is a key component and critical part of the malaria picture in India, but research into this species lags far behind that of P. falciparum, especially as far as genetic diversity and genomic studies are concerned. This paradox is most likely due to the refractoriness of P. vivax to in vitro culture, and unique aspects of its biology that include low patient parasitemia and a dormant ‘hypnozoite’ form in the liver. Currently only a single reference genome sequence of an isolate of P. vivax from El Salvador is available for the malaria research community (Carlton et al., 2008). The lack of genetic sequence data from additional P. vivax genomes undoubtedly hampers whole genome analyses and elucidation of the unique biology associated with the species. In particular genetic diversity within P. vivax populations contributes directly to drug resistance and antigenic variation, allowing the parasite to evolve immunity to antimalarial compounds and to evade the host immune response. Knowledge of this genetic variation is critical for the development of intervention strategies including drugs and vaccines. In contrast to P. falciparum, which is thought to have undergone one or more bottlenecks in recent history (Joy et al., 2002), P. vivax is thought to have had a more stable demographic past (Mu et al., 2003), which could mean that P. vivax may exhibit a greater degree of genetic polymorphism within populations and greater divergence among populations in disparate geographic locations (Gupta et al., 2011). This has important implications as it suggests that widespread sampling of P. vivax in different geographical regions is necessary in order to understand the diversity and population structure of the species in different areas. Specifically, the availability of sequence data from Indian isolates will provide the context for understanding where sequence conservation or divergence is critical, and provide insights into sequence/function relationships, with the ultimate aim of generating a genetic diversity map of P. vivax in India that can be used for association studies of drug resistance.
As part of the CSCMi, we plan to develop a next generation sequencing core at the NIMR to enable deep sequencing of Plasmodium parasites, for both the multiplicity of infection studies and P. vivax whole genome sequencing described above. Precisely which NGS platform we intend to install at NIMR remains to be decided, although several have obvious advantages for implementing in a field location (Table 2).
Table 2.
Next generation sequencing platforms and their suitability for endemic country field settings. All six platforms are currently either being sold or expected to be sold in India shortly.
| Platform | Read type | Run time | Computing requirements | Cost | Suitability for a field setting |
|---|---|---|---|---|---|
| Roche 454 GS FLX | Long read | 10 hr | Desktop | $$$ | Potential: low computational requirements, but high capital and running costs |
| Roche 454 GS Junior | Long read | 10 hr | Desktop | $$$$ | Potential: low computational requirements, but high running costs |
| Illumina GAII/HiSeq | Short read | 5 days | Cluster | $ | Problematic: short reads, high computational requirements, high capital cost |
| Illumina MiSeq | Short read | 26 hr | Desktop | $$ | Promising: low cost, short run time, small footprint |
| Life Technologies SOLiD 4/5500 | Short read | 8 days | Cluster | $ | Problematic: short reads, unusual bioinformatics analysis, computational requirements high |
| Life Technologies Ion Torrent ‘318’ chip | Long read | 2 hr | Desktop | $$ | Promising: low cost, short run time, simple mechanics, small footprint, long reads |
Short read: sequences <150 bp; Long read: sequences >150bp. $: <$0.5/Mb; $$: ~$1/Mb; $$$: >$7/Mb.
5. Conclusion
Malaria is a major public health problem in India, the world’s largest democracy and its second most populous country. We intend to leverage the existing strong relationship between Indian and U.S scientists to develop a Center for the Study of Complex Malaria in India. The CSCMi is a collaborative scientific research center working towards the goal of enhancing malaria intervention and control programs in India.
Acknowledgments
We thank the previous NIMR Directors Professor A. P. Dash and Dr. V. K. Dua for facilitating the CSCMi, and the Director General of the Indian Council of Medical Research, Dr. V. M. Katoch, for project permissions and encouragement. We are also indebted to Dr. Hema Joshi who was central to the project before her untimely death in March 2010. We also thank CSCMi Project Coordinator Dr. Lalitha Ramanathapuram and Dr. A.C. Dhariwal, Director of the National Vector Borne Disease Control Programme, Government of India, for input and comments. This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) grant U19AI089676. P.L.S. was supported by a Fogarty International Center/NIH U.S. Global Health Postdoctoral Scientist Fellowship 3D43TW007884-03S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aparup Das, Email: aparup@mrcindia.org.
Anupkumar R. Anvikar, Email: anvikar@rediffmail.com.
Lauren J. Cator, Email: laurencator@gmail.com.
Ramesh C. Dhiman, Email: dhimanrc@icmr.org.in.
Alex Eapen, Email: alexeapen@yahoo.com.
Neelima Mishra, Email: neelima@mrcindia.org.
Bhupinder N. Nagpal, Email: b_n_nagpal@hotmail.com.
Nutan Nanda, Email: nutanmrc@yahoo.co.in.
Kamaraju Raghavendra, Email: kamarajur2000@yahoo.com.
Andrew F. Read, Email: a.read@psu.edu.
Surya K. Sharma, Email: suryaksharma@gmail.com.
Om P. Singh, Email: singh@mrcindia.org.
Vineeta Singh, Email: vineetas_2000@yahoo.com.
Photini Sinnis, Email: psinnis@jhsph.edu.
Harish C. Srivastava, Email: hcrsv_52@rediffmail.com.
Steven A. Sullivan, Email: steven.sullivan@nyu.edu.
Patrick L. Sutton, Email: patrick.sutton@nyu.edu.
Matthew B. Thomas, Email: mbt13@psu.edu.
Neena Valecha, Email: neenavalecha@gmail.com.
References
- Adak T, Kaur S, Singh OP. Comparative susceptibility of different members of the Anopheles culicifacies complex to Plasmodium vivax. Trans R Soc Trop Med Hyg. 1999;93(6):573–577. doi: 10.1016/s0035-9203(99)90052-4. [DOI] [PubMed] [Google Scholar]
- Anonymous. Rolling back malaria - the next ten years. Lancet. 2008;372:1193. doi: 10.1016/S0140-6736(08)61494-4. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang’a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455(7214):757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Curr Sci. 2007;92:1571–1578. [Google Scholar]
- Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: challenges and opportunities. J Biosci. 2008;33(4):583–592. doi: 10.1007/s12038-008-0076-x. [DOI] [PubMed] [Google Scholar]
- Davidson G, Zahar AR. The practical implications of resistance of malaria vectors to insecticides. Bull WHO. 1973;49:475–483. [PMC free article] [PubMed] [Google Scholar]
- Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, Bassani DG, Suraweera W, Laxminarayan R, Peto R. Adult and child malaria mortality in India. Lancet. 2010;376:1768–1774. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Eng J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem R, Sabaot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- Goswami G, Raghavendra K, Nanda N, Gakhar SK, Subbarao SK. PCR-RFLP of mitochondrial cytochrome oxidase subunit II and ITS2 of ribosomal DNA: markers for the identification of members of the Anopheles culicifacies complex (Diptera: Culicidae) Acta Trop. 2005;95(2):92–99. doi: 10.1016/j.actatropica.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Goswami G, Singh OP, Nanda N, Raghavendra K, Gakhar SK, Subbarao SK. Identification of all members of the Anopheles culicifacies complex using allele-specific polymerase chain reaction assays. Am J Trop Med Hyg. 2006;75(3):454–460. [PubMed] [Google Scholar]
- Grabowsky M. The billion-dollar malaria moment. Nature. 2008;451:1051–1052. doi: 10.1038/4511051a. [DOI] [PubMed] [Google Scholar]
- Gupta B, Srivastava N, Das A. Inferring the evolutionary history of Indian Plasmodium vivax from population genetic analyses of multilocus DNA fragments. Molecular Ecology. 2011 doi: 10.1111/j.1365-294X.2012.05480.x. in press. [DOI] [PubMed] [Google Scholar]
- Harrison G. Mosquitoes, malaria and man: A History of hostilities since 1880. New York: Dutton; 1978. [Google Scholar]
- Hay SI, Gething PW, Snow RW. India’s invisible malaria burden. Lancet. 2010;376:1716–1717. doi: 10.1016/S0140-6736(10)61084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the Global Clinical Burden of Plasmodium falciparum Malaria in 2007. PLoS Med. 2007;7(6) doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Joy DA, Feng X, Mu J, Furuya T, Chotivanich K, Krettli AU, Ho M, Wang A, White NJ, Suh E, Beerly P, Su XZ. Early origin and recent expansion of Plasmodium falciparum. Science. 2003;300:318–321. doi: 10.1126/science.1081449. [DOI] [PubMed] [Google Scholar]
- John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–269. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- Joshi H, Prajapati SK, Verma A, Kang’a S, Carlton JM. Plasmodium vivax in India. Trends Parasitol. 2008;24(5):228–235. doi: 10.1016/j.pt.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Juliano JJ, Porter K, Mwapasa V, Sem R, Rogers WO, Ariey F, Wongsrichanalai C, Read A, Meshnick SR. Exposing malaria in-host diversity and estimating population diversity by capture-recapture using massively parallel pyrosequencing. Proc Natl Acad Sci. 2010;107(46):20138–20143. doi: 10.1073/pnas.1007068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–398. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- Lengeler C. Cochrane Database of Systematic Reviews. 2004. Insecticide-treated bed nets and curtains for preventing malaria; p. CD000363. 000310.001002/14651858.CD14000363.pub14651852. [DOI] [PubMed] [Google Scholar]
- Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- Mu J, Joy DA, Duan J, Huang Y, Carlton J, Walker J, Barnwell J, Beerli P, Charleston MA, Pybus OG, Su XZ. Host Switch Leads to Emergence of Plasmodium vivax Malaria in Humans. Mol Biol Evol. 2005;22(8):1686–1693. doi: 10.1093/molbev/msi160. [DOI] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance areas, Benin. Emerg Infect Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L, Enserink M. Malaria: Did They Really Say. Eradication? Science. 2007;318:1544–1545. doi: 10.1126/science.318.5856.1544. [DOI] [PubMed] [Google Scholar]
- Sallum MA, Peyton EL, Wilkerson RC. Six new species of the Anopheles leucosphyrus group, reinterpretation of An. elegans and vector implications. Med Vet Entomol. 2005;19(2):158–199. doi: 10.1111/j.0269-283X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- Shah NK, Dhillon GPS, Dash AP, Arora U, Meshnick S, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;1:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Tyagi PK, Padhan K, Upadhyay AK, Haque MA, Nanda N, Joshi H, Biswas S, Adak T, Das BS, Chauhan VS, Chitnis CE, Subbarao SK. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100(10):917–925. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sharma VP. Current scenario of malaria in India. Parasitologia. 1999;41:349–353. [PubMed] [Google Scholar]
- Sharma VP. Battling the malaria iceberg with chloroquine in India. Malaria J. 2007;6:105. doi: 10.1186/1475-2875-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malaria J. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff C. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15:278–293. doi: 10.1128/CMR.15.2.278-293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Kataria O, Singh MP. The changing dynamics of Plasmodium vivax and P. falciparum in central India: trends over a 27-year period (1975–2002) Vector Borne Zoonotic Dis. 2004;4(3):239–248. doi: 10.1089/vbz.2004.4.239. [DOI] [PubMed] [Google Scholar]
- Singh N, Nagpal AC, Saxena A, Singh MP. Changing scenario of malaria in central India, the replacement of Plasmodium vivax by Plasmodium falciparum (1986–2000) Trop Med Int Health. 2004;9(3):364–371. doi: 10.1046/j.1365-3156.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- Singh OP, Nanda N, Dev V, Bali P, Sohail M, Mehrunnisa A, Adak T, Dash AP. Molecular evidence of misidentification of Anopheles minimus as Anopheles fluviatilis in Assam (India) Acta Tropica. 2010;113:241–244. doi: 10.1016/j.actatropica.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25(10):452–457. doi: 10.1016/j.pt.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Subbarao SK, Nanda N, Vasantha K, Dua VK, Malhotra MS, Yadav RS, Sharma VP. Cytogenetic evidence for three sibling species in Anopheles uviatilis (Diptera: Culicidae) Ann Entomol Soc Am. 1994;87:116–121. [Google Scholar]
- Subbarao SK, Vasantha K, Adak T, Sharma VP, Curtis CF. Egg-float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Med Vet Entomol. 1987;1:265–271. doi: 10.1111/j.1365-2915.1987.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Trigg PI, Kondrachine AV. Commentary: Malaria control in the 1990s. Bull WHO. 1998;76:11–16. [PMC free article] [PubMed] [Google Scholar]
- Valecha N. Recent developments in malaria chemotherapy. Proc Natl Acad Sci. 2009;79:23–35. [Google Scholar]
- Wakabi W. Africa counts greater successes against malaria. Lancet. 2007;370:1895–1896. doi: 10.1016/S0140-6736(07)61796-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2007 http://www.whoindia.org/LinkFiles/Malaria_Country_Profile-Malaria.pdf.
- World Health Organization. WHO Integrated Vector Management Working Group Meeting Reports 2009. 2009. [Google Scholar]