Abstract
Memory CD8+ T cells are an important component of the adaptive immune response against many infections, and understanding how antigen-specific memory CD8+ T cells are generated and maintained is crucial for the development of vaccines. We recently reported the existence of memory-phenotype, antigen-specific CD8+ T cells in unimmunized mice (“virtual memory” or VM cells). However, it was not clear when and where these cells are generated during normal development, nor the factors required for their production and maintenance. This issue is especially pertinent given recent data showing that memory-like CD8 T cells can be generated in the thymus, in a “bystander” response to IL-4.
Here we show that the size of the VM population is reduced in IL-4R-deficient animals. However, the VM population appears first in the periphery and not the thymus of normal animals, suggesting this role of IL-4 is manifest following thymic egress. We also show that the VM pool is durable, showing basal proliferation and long term maintenance in normal animals, and also being retained during responses to unrelated infection.
Introduction
Conventional memory CD8 T cells are generated in response to foreign antigens, following the production and contraction of the effector response (1–4). However, memory-like CD8 T cells can also be generated by homeostatic mechanisms, without TCR engagement with foreign peptide/MHC ligands: In response to T cell deficiency, naïve T cells proliferate and differentiate into memory-phenotype cells, in the process of lymphopenia-induced proliferation (5–11). Such “homeostatic memory” CD8 T cells express numerous phenotypic and functional traits of conventional memory CD8 T cells, including their ability to effectively control infection (1, 12, 13). While initially studied in the context of induced lymphopenia (1, 14–16), additional studies suggested that physiological lymphopenia occurs during the neonatal period in mice, and induces production of homeostatic memory T cells (14, 16–20). Finally, recent studies from our group and others have identified a third mechanism for generating memory-like CD8 T cells, which involves the response of thymocytes to the cytokine IL-4. This pathway was identified in mouse models in which the thymic NK-T (or NK-T like) pool was enlarged, resulting in elevated frequencies of IL-4-producing cells. Reactivity to IL-4 leads to upregulation of the transcription factor Eomes and acquisition of memory-phenotype by “bystander” CD8+ mature thymocytes (21–25). Although the mechanism for generation of “bystander memory” CD8 T cells was initially identified in genetically manipulated mice, a similar population was observed in some conventional mouse strains (e.g. BALB/c), suggesting such cells may also contribute to the CD8 memory pool.
Using peptide/MHC tetramers in combination with magnetic enrichment protocol (26–28) we were able to show that unimmunized mice contain a population of antigen-specific memory-phenotype CD8+ T cells (28). Similar populations have subsequently been reported by others (29–31). These cells, which we termed “Virtual memory” (VM) T cells, express multiple phenotypic and functional characteristics of memory CD8 T cells, and were found both in unimmunized conventional mice and also in germ-free animals. These and other data suggested that the VM population was not generated via conventional priming. However, it was unclear whether these cells arise from lymphopenia-induced proliferation or as bystander memory cells produced in the thymus. In addition, previous studies did not address whether the VM pool was maintained during normal T cell homeostasis and during conventional immune responses.
In this report we demonstrate that both bulk memory CD8 T cells and the VM population are reduced in IL-4R deficient C57BL/6 mice. However, we also show that production of VM cells initiates in the periphery and not the thymus arguing that VM cells are not generated exclusively as IL-4 induced thymic memory CD8 T cells. We also show that the VM population appears during a period of neonatal lymphopenia, and is sustained throughout adulthood. Furthermore, the VM pool is maintained during a CD8 T cell response to unrelated antigens, suggesting these memory-like cells are not a consequence of immunological naiveté.
Material and methods
Mice
C57BL/6 (B6), T cell receptor alpha-deficient (TCRα−/−), and Interleukin-4 receptor alpha (CD124) deficient (IL-4Rα−/−) B6 mice were purchased from the Jackson laboratory. Female B6 mice were bred with male TCRα−/− B6 to generate TCRα+/− –B6 mice. All mice were maintained in specific pathogen-free conditions at the University of Minnesota (Twin Cities). All animal protocols were approved by the Institutional Animal Use and Care Committees at the University of Minnesota.
Magnetic bead enrichment and flow cytometry
Spleen, major lymph nodes and thymus were harvested from 1 to 25-week-old mice as indicated. Tissues were injected with 1X collagenase D (Roche, Germany) and minced to generate single cell suspension. Tetramer binding cells (from thymus or pooled spleen and lymph nodes) were isolated by magnetic bead enrichment as previously described in detail (28). Tetramers generated with Kb contained epitopes from vaccinia B8R (TSYKFESV), OVA (SIINFEKL), HSVgB (SSIEFARL) or the following MCMV-derived peptides: M38 (SSPPMFRV), M57 (SCLEFWQRV), m-139 (TVYGFCLL) or IE3 (RALEYKNL). Db based tetramers contained epitopes from LCMV GP33 (KAVYNFATC) or MCMV M45 (HGIRNASFI). Monomers and tetramers were generated as previously described (32). The MCMV epitope based tetramers were a generous donation of Maire Quigley and David Price (NIH) and Chris Snyder (Thomas Jefferson University). Phenotypic analysis was done by staining with antibodies to CD19 (clone 6D5 Biolegend), CD11b (clone M I/70 eBiosciences), CD11c (clone), F4/80 (Invitrogen), CD3ε (clone 145-2C11, eBiosciences), CD4 (clone RM 4–5, BD Biosciences), CD8 (Invitrogen), CD44 (clone IM7, eBiosciences), CD122 (BD Biosciences). Data was acquired using LSR II (BD Biosciences) and analysis was performed using FlowJo software (Tree Star).
Viral infection
Six to 10-week-old B6 mice were infected via intraperitoneal injection with 2×10^5 PFU of LCMV Armstrong (kindly provided by Dr. David Masopust, University of Minnesota). Spleens were harvested 30 to 45 days later for magnetic bead enrichment using GP33/Db and B8R/Kb tetramers.
BrdU incorporation assays
Mice were injected i.p. with 1mg BrdU (in PBS), then maintained on BrdU laced drinking water (0.8 mg/ml, with 2% sucrose to offset bitterness) for 14–16 days. For bulk analysis, thymocytes and pooled spleen and lymph node cells were prepared and stained for surface markers, followed by fixation, permeabilization and intracellular staining for BrdU following manufacturers instructions (Becton Dickinson). In parallel, the remaining spleen and lymph node sample was subject to tetramer pulldown (using a cocktail of PE-labeled B8R/Kb, M57/Kb and HSV-gB/Kb tetramers) prior to staining for surface markers and BrdU.
Statistics
A two-tailed, unpaired student’s t-test was performed on the indicated data samples. P values are displayed within figures. P values of <0.05 are considered significant.
Results
Virtual memory CD8 T cells are detected among CD8 T cells of diverse specificities, and are not a property of dual reactive T cells
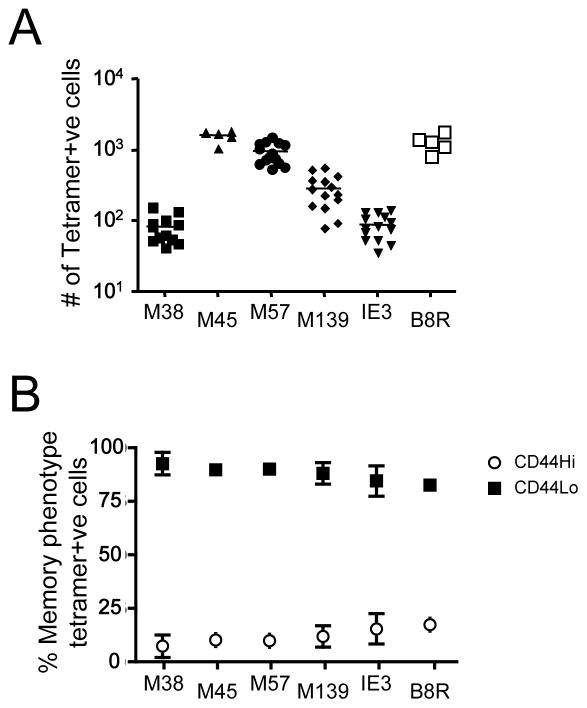
Our previous studies using a peptide/MHC tetramer enrichment procedure revealed the presence of foreign antigen-specific memory-phenotype CD8 T cells (VM cells) in unimmunized conventional and germ-free B6 strain mice (28). In that report, VM cells were detected among the precursor pool specific for three well characterized peptide/MHC complexes (OVA/Kb, B8R/Kb and HSVgB/Kb). However, each of these antigens induce dominant immune responses in their respective systems, and it was possible that the VM population is solely a feature of cells responding to immunodominant epitopes. Also, a previous study using a similar experimental approach concluded that foreign-antigen specific CD8 T cells in unimmunized mice were uniformly of a naïve phenotype (27). To explore this further we used peptide/MHC tetramer enrichment (26) to assess the VM frequencies for five epitopes recognized in the B6 response to MCMV. These represent the three most immunodominant specificities (M45/Db being dominant, followed by M139/Kb and M57/Kb) and two epitope (M38 and IE3, both Kb restricted) which are barely detectable in the acute MCMV response (although the response is higher during chronic infection) (33, 34). This analysis includes the M45/Db specificity studied in the report by Obar et al. (27). The precursor frequencies, in unimmunized animals, for these antigens varied depending on the specificity studied, and were in rough correlation with their reported immunodominance during acute MCMV infection (Fig. 1A). Significantly, VM cells (CD44hi) could be detected for each specificity, and there was no notable correlation between the frequency of VM cells and the immunodominance characteristics of the specificity examined (Fig. 1B). Such data extend our previous observations and indicate VM cells are consistently found within CD8 T cells of varying specificities, precursor numbers and immunodominance characteristics.
Figure 1. VM cells are detected among diverse foreign-antigen specific precursor pools.
Peptide MHC-I tetramer-based enrichment was performed on spleen and lymph node cells from unprimed B6 mice using PE and APC-coupled tetramers representing five epitopes from MCMV (M38/Kb, M45/Db, M57/Kb, M139/Kb, and IE-3/Kb) and one from vaccinia virus (B8R/Kb). (A) shows the number of tetramer positive CD8+ T cells in the bound fraction. (B) represents the percentage of each tetramer positive CD8+ T pool that are either memory (CD44hi/CD122+) or naïve (CD44lo/dCD122−). The results in A and B and are compiled from at least 3 individual experiments.
The fact that VM cells are reliably seen for multiple specificities in unprimed animals makes it unlikely that VM cells were induced by prior encounter with the foreign antigen recognized by these cells. However, a significant fraction of T cells express dual TCRs, as consequence of incomplete TCRα-chain allelic exclusion – a phenomenon reported to occur for up to 30% of T cells (35, 36). It was possible that VM cells represent dual reactive T cells, primed against one foreign antigen but bearing a second TCR for an antigen not yet encountered. To test this hypothesis, we explored the frequency of VM cells in T cells from TCRα+/− animals, which are limited to a single functional TCRα-chain. Frequencies of memory within the bulk (Fig. 2A) and B8R/Kb tetramer-binding CD8 T cells (Fig. 2A–C) were similar for normal and TCRα+/− animals, arguing against dual reactivity as a likely basis for the appearance of VM cells.
Figure 2. VM cells are not dual TCR reactive T cells.
Cells from lymph nodes and spleen were obtained from TCRα +/− B6 and wild type B6 mice, and stained for CD8, CD44, and CD122. Magnetic enrichment was performed using B8R/Kb tetramers. (A) Contour plots of both bulk CD8 T cells and B8R/Kb positive CD8+ T cells. Numbers represent the percentage of cells with the phenotype of memory (CD44hi/CD122+). (B) Depicts the number of B8R/Kb positive CD8+ T cells in the spleen and lymph nodes of TCRα +/− and B6 mice. (C) Shows the percentage of VM cells among the B8R/Kb positive CD8+ T cells of TCRα +/− and B6 mice. A total of 3 experiments were performed, each with 3 TCRα heterozygous B6 mice and 2 wild type B6 controls.
Together with earlier studies (28), these findings suggest that the VM population is a feature of diverse foreign-antigen specific precursor pools, and that these cells are unlikely to be generated by conventional priming or as a by-product of incomplete TCRα-chain allelic exclusion.
The size of the VM pool is dependent on IL-4 reactivity
Two distinct mechanisms have been shown to induce memory-phenotype CD8 T cells in the absence of foreign antigen priming: Homeostatic memory T cells are generated during the response of naïve CD8 T cells to lymphopenic conditions in secondary lymphoid tissues, while bystander memory CD8 T cells are generated in the thymus in response to IL-4. Our studies and others (24, 37) suggested that the bystander memory mechanism was prominent in Balb/c but not B6 strain mice, which correlated with an expanded pool of PLZF-expressing NK-T cells, capable of IL-4 secretion, in Balb/c mice. Nevertheless, it was possible that such IL-4 dependent bystander memory cells were part of the VM population studied in B6 strain mice.
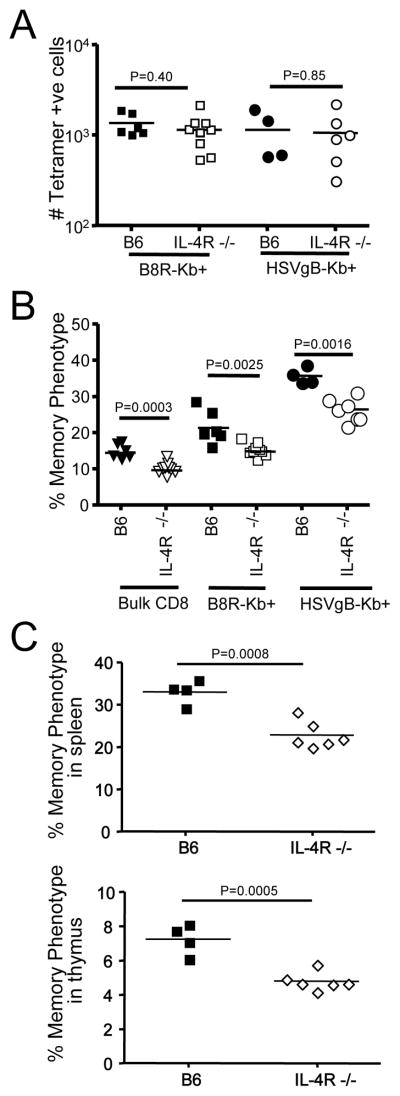
To address this hypothesis, we examined the VM population in IL-4R−/− B6 mice. The total numbers of B8R/Kb and HSVgB/Kb specific precursors was similar in WT and IL-4R−/− animals (Fig. 3A), yet frequency of VM cells within this pool was significantly reduced in IL-4R−/− animals (Fig. 3B). We also noted a reduction in the percentage of memory-phenotype cells in the bulk CD8 T cell pool (Fig. 3B/C). Together, these data suggest that IL-4Rα-deficiency leads to a reduction (but not complete loss) of the VM population in B6 mice. Previous work showed that IL-4 induced generation of bystander memory CD8 T cells in the thymus of some mouse strains (23–25) and, although this population is quite rare in B6 mice, we observed a significant reduction in the frequency of memory phenotype thymic CD8 single positive T cells in IL-4R deficient animals (Fig. 3C).
Figure 3. The size of the VM pool depends on IL-4 reactivity.
Cells from lymph nodes and spleen were obtained from 9–10-week-old IL-4R−/− B6 and wild-type B6 mice, and stained for CD8, CD44, and CD122. MHC-I tetramer enrichment was also performed using B8R/Kb and HSVgB/Kb tetramers. (A) Depicts the number of B8R/Kb and HSVgB/Kb tetramer positive CD8+ T cells in the spleen and lymph nodes of IL-4R−/− and B6 mice. (B) Shows the percentage of memory phenotype cells among total, B8R/Kb and HSVgB/Kb tetramer positive CD8+ T cells in adult IL-4R−/− and B6 mice. A total of 3 experiments were performed, each with 3 IL-4R−/− B6 and 2 wild-type B6 control mice. In (C), 4–5-week-old wild type or IL-4R−/− mice were analyzed for frequency of memory-phenotype cells among CD8 single positive T cells from spleen and thymus.
VM cells arise in the peripheral pool during the neonatal period
The data above demonstrated a role for IL-4 in production of VM cells, and raised the possibility that these memory-phenotype cells are produced during thymic development, rather than during physiological homeostatic proliferation in the periphery, as we had previously proposed (28). However, reports have indicated that homeostatic memory CD8 T cells produced in the periphery can home to the thymus (38), making it difficult to determine where the thymic memory-like CD8 T cell pool was generated.
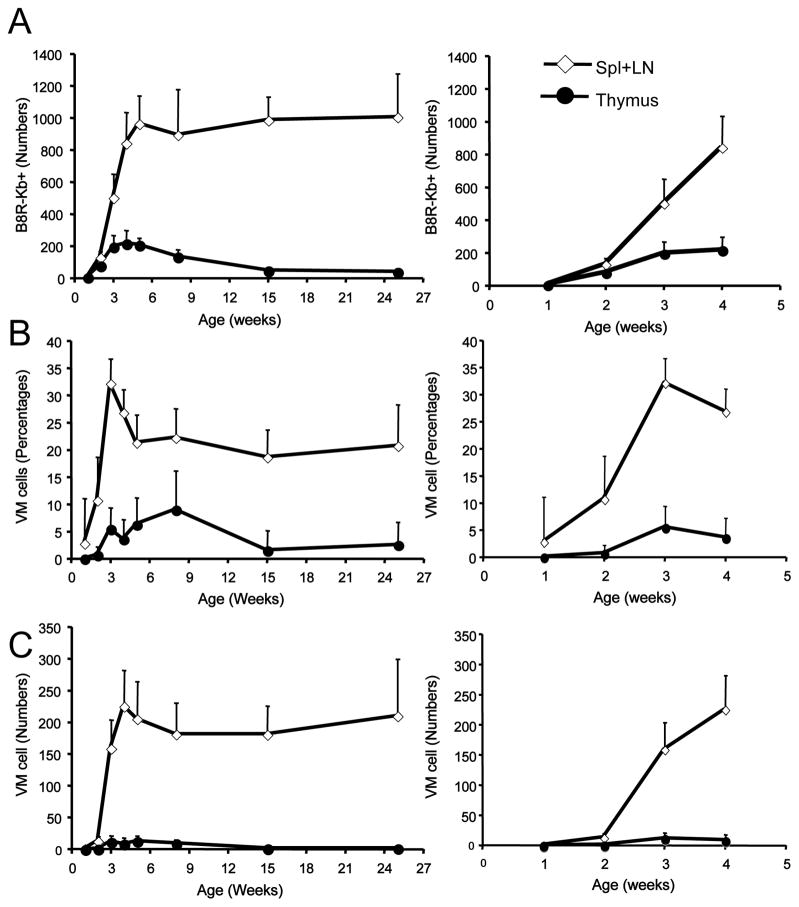
To address this issue, we studied when and where the VM population first appears, by performing tetramer enrichment assays on thymic and peripheral lymphoid tissues from mice of 1–4 weeks after birth. Peripheral B8R/Kb specific CD8 T cells accumulate over this time, and while B8R/Kb specific pool of thymic CD8 SP cells is predictably small, these cells could be detected in mice from 2 weeks of age (Fig. 4A). Strikingly, by two weeks there was clear appearance of memory-phenotype B8R/Kb specific CD8 T cells in the peripheral but not the thymic pool (Fig. 4B/C). When we extended this analysis through the neonatal period, we observed a small population of VM cells that appeared in the thymus from 3 weeks, a timepoint when the peripheral pool of B8R/Kb specific VM cells had peaked (Fig. 4B). Both the low frequency of thymic VM cells, and their appearance after the VM cells have arisen in the periphery argue against thymic generation of VM cells as a major component of their derivation in B6 mice.
Figure 4. VM cells arise first in the periphery during the neonatal period.
Thymus and pooled spleen/lymph nodes were harvested from mice ranging from 1- to 25-weeks of age and tetramer enrichment was performed using B8R/Kb tetramers. CD8 single positive/CD3+ve cells were gated on. (A) Shows the number of B8R/Kb tetramer binding CD8+ T cells, (B) the percentage of memory-phenotype cells within this population and (C) shows the absolute number of memory-phenotype B8R/Kb tetramer binding CD8+ T cells in the thymus and pooled spleen and lymph nodes. For each group, the right-hand panel highlights the data from animals of 1- to 4-week old. Each time point represents a minimum of 2 experiments with at least 4 mice per experiment.
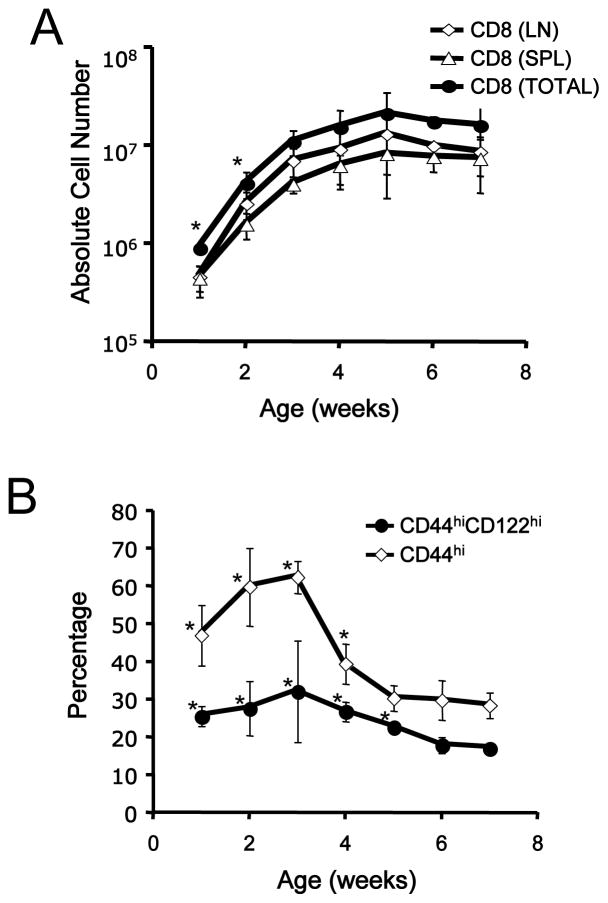
We also extended this time course to explore the maintenance of the VM pool in adult animals. The frequency of B8R/Kb specific VM cells reached a peak (>30%) at around 3 weeks of age, and then declined to the frequencies observed in adult animals (around 20%) by 5 weeks (Fig 4B). However, the absolute number of B8R/Kb specific cells (both total and memory phenotype) was maintained at a plateau from around 4–5 weeks of age into adulthood. These findings are similar to the changes observed in the bulk CD8 T cell pool, which gradually accumulates in the periphery from birth to 5 weeks of age (Fig 5A), while we found that the frequency of bulk memory-phenotype CD8 T cells peaks in younger animals (Fig 5B) – similar to the findings reported by Ichii et al. earlier (18). Previous studies showed that adoptively transferred naïve T cells proliferate in neonatal mice and acquire the phenotype of memory cells (17–20), suggesting that the neonatal environment is functionally lymphopenic. Hence, taken together, our data are most consistent with VM cells arising in the peripheral pool, in response to neonatal lymphopenia, rather than generated in the thymus.
Figure 5. The frequency of memory-phenotype CD8 T cells is elevated during establishment of the peripheral T cell pool.
Lymph node and spleen cells were obtained from B6 mice ranging from 1- to 7-week-old and stained with antibodies to CD4, CD8, CD44, and CD122. (A) shows the absolute number of bulk CD8+ T cells in the spleen, lymph nodes, and both tissues combined, while (B) shows the percentage of splenic CD8+ T cells that are of memory-phenotype (CD44hi/CD122+). These data derive from at least 2 individual experiments for each time point. An asterisk (*) indicates that a data set is significantly different (p<0.05) compared to the data from 7-week old animals.
VM cells are maintained long term under steady state conditions and during bystander immune responses
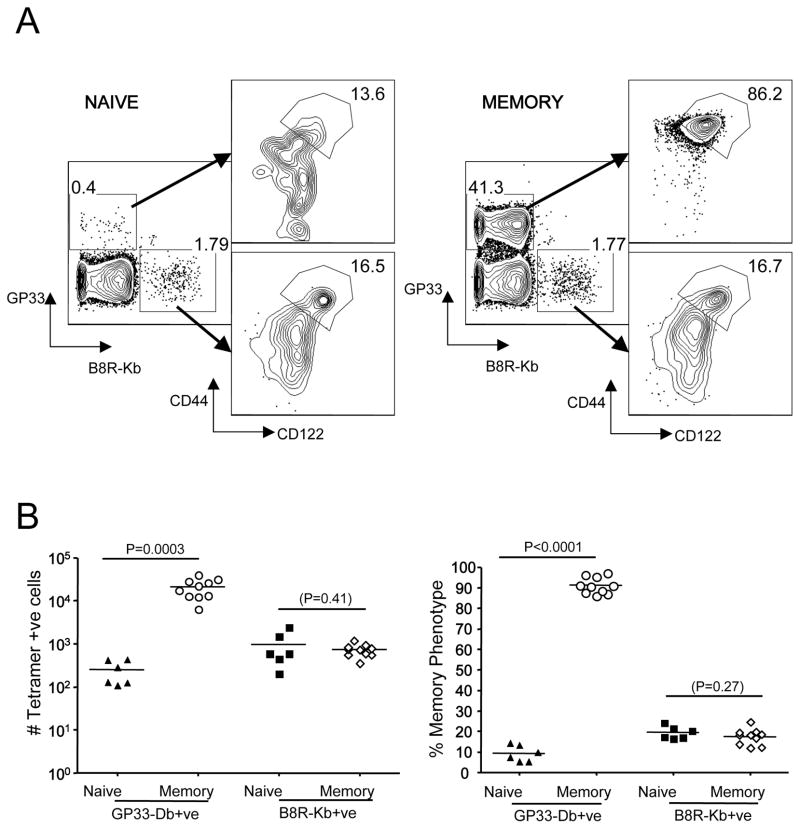
Our data show that the frequency and number of B8R/Kb binding memory-phenotype CD8 T cells was constant in mice from 5 to 25 weeks of age (Fig. 4B,C), indicating that the VM pool was stable. However, such maintenance may be a consequence of the minimal exposure to immune challenge associated with SPF housing. To test whether the VM population is affected by a vigorous CD8 T cell response, we infected B6 mice with LCMV Armstrong and tracked B8R/Kb specific precursors >30 days post-infection. Since B8R/Kb specific T cells are not expected to participate in the LCMV response, this approach allows us to test how a bystander infection influences the stability of a VM population of distinct specificity. As expected, the LCMV GP33/Db specific T cell pool was greatly expanded and was uniformly of memory phenotype (Fig. 6A). However, the B8R/Kb tetramer binding pool was of similar size (Fig. 6B) and, most importantly, contained a similar frequency of VM cells following LCMV infection (Fig. 6A). These data suggest that the persistence of the VM population is not a consequence of artificially low immune exposure, and that this population can be maintained in the presence of a greatly expanded antigen-driven memory CD8 T cell pool. Our finding suggests that preexisting memory (VM) cells are not displaced by new antigen-experienced memory cells with different specificities, but instead the memory pool expands in size with each immunological experience in order to accommodate newly generated memory cells (consistent with studies by Vezys et al (39)).
Figure 6. VM cells are maintained during bystander immunization.
Wild type B6 mice were infected with LCMV (Armstrong). Splenocytes were harvested 30–45 days later from these animals (“memory”) or uninfected controls (“naïve”) and subjected to enrichment using both B8R/Kb and GP33/Db tetramers. Numbers in the left-hand contour plots represent the percent of CD8 T cells in the bound fraction that are GP33/Db andB8R/Kb positive, while numbers in the right-hand contour plots represent the percent of VM cells for each specificity. These plots are representative of 3 experiments with a total of 10 LCMV memory mice and 6 unprimed B6 controls.
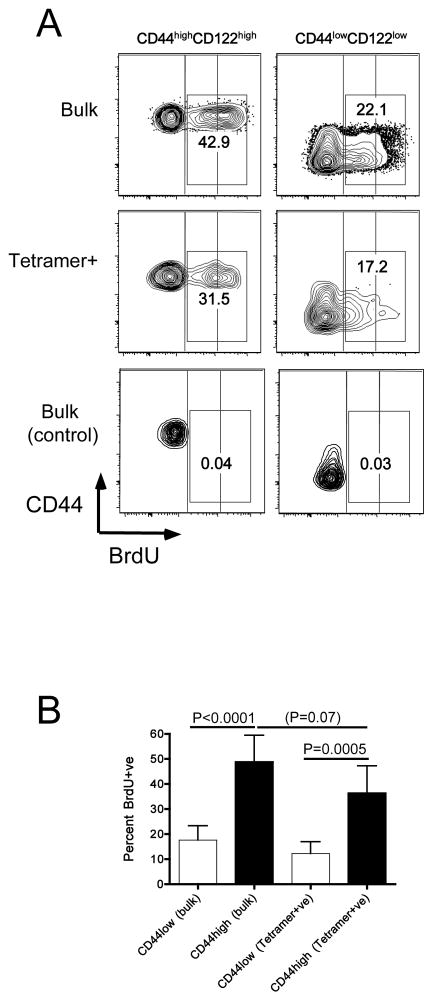
Memory CD8 T cells are characterized by basal proliferation which maintains their numbers long-term (15, 40, 41). However, it is not clear whether a similar process occurs for the VM population. To explore this issue, we measured BrdU incorporation during a 2 week labeling period in adult B6 mice. At the end of the labeling period, BrdU labeling was measured in naïve and memory phenotype CD8 T cells in the bulk population as well as cells isolated using a cocktail of peptide/MHC tetramers (comprised of B8R/Kb, M57/Kb and HSVgB/Kb). As was expected based on previous studies (42) a fraction of bulk naïve CD8 T cells incorporated a low level of BrdU (Fig 7A), which has been ascribed to labeling during thymic development (42). A larger cohort of bulk memory phenotype CD8 T cells showed BrdU incorporation, and brdU staining was of greater intensity, suggestive of active proliferation during the labeling period. Importantly, similar patterns of BrdU incorporation were observed for the tetramer-staining cells isolated by tetramer enrichment (Fig 7A,B), suggesting that VM cells undergo basal proliferation, albeit at a slightly lower rate than observed in the bulk memory-phenotype CD8 T cell pool (Fig 7B).
Figure 7. Basal proliferation of VM cells at steady state.
Normal adult B6 mice were labeled with BrdU for 14–16 days, or were maintained in parallel without BrdU exposure (control). Spleen and lymph nodes cells were isolated and stained for cell surface markers and intracellular BrdU (for the “bulk” population), or were first subjected to tetramer enrichment (using a cocktail of B8R/Kb, M57/Kb and HSVgB/Kb tetramers) prior to surface and intracellular staining with antibodies. Events were gated on bulk or tetramer+ve naïve- and memory-phenotype CD8 T cells, as indicated. (A) shows representative data. Vertical blocks are overlaid on the contour plots to highlight the different levels of BrdU staining on naïve and memory T cells. The numbers on the plots indicate the percentage of total BrdU+ve cells (regardless of staining intensity). (B) shows compiled data on the percentage of BrdU+ve cells among the naïve and memory-phenotype cells among bulk and tetramer+ve CD8 T cells. Data are from 3 experiments deriving from two independent BrdU labeling cohorts.
Overall, these data suggest VM cells, similar to conventional memory CD8 T cells, are capable of long term maintenance (involving basal proliferation).
Discussion
It is now recognized that multiple pathways can lead to the production of CD8 memory-phenotype T cells in mice: In addition to conventional immune responses against foreign antigens, there is evidence for “homeostatic memory” cells produced by lymphopenia induced proliferation and “bystander memory” induced by IL-4 in the thymus (21). In this report, we sought to determine how VM cells, being memory phenotype CD8 T cells specific for an unencountered foreign antigen, are generated and maintained.
Our data reinforce the concept that VM cells are not induced by conventional immune responses against foreign antigens: We find VM cells among CD8 T cells of multiple specificities, including immunodominant and subdominant cells responsive to MCMV epitopes, and these data are in agreement with studies finding VM cells among CD8 T cells specific for both dominant and sub-dominant epitopes in an influenza model (29). Between data presented here and elsewhere, VM cells have now been defined in more than a dozen specificities (28–31). Furthermore, our data using TCRα hemizygous mice make it unlikely that the VM pool derives from conventional memory cells bearing a second TCR specificity. These data indicate that VM cells are consistently found within CD8 T cells of various specificities from the unimmunized pool.
Of non-conventional mechanisms for memory-phenotype CD8 T cell generation, our recent findings that elevated levels of IL-4 can cause induction of thymic memory like cells prompted us to reassess this pathway in VM generation. Our published studies demonstrated that IL-4 levels in normal Balb/c mice but not B6 mice were sufficient to drive production of a substantial “bystander memory” pool of mature CD8 thymocytes (24), and similar conclusions were reached by others (37). Hence the substantial decrease in the size of the bulk memory and VM pool in IL-4Rα−/− B6 mice was not anticipated. IL-4Rα also binds to IL-13, and it is possible that this cytokine contributes to the effects we report here. However, unpublished studies in B6 background IL-4−/− mice suggest a reduction in the size of the VM population similar to that we observe in IL-4Rα deficient mice (Ross Kedl, University of Colorado, personal communication), suggesting that IL-4 is the more relevant cytokine involving in VM induction and/or maintenance.
At the same time, around half of the VM population remained in IL-4α−/− animals, suggesting some cells are IL-4 independent. Numerous studies have described the capacity of IL-7 and IL-15 to support both lymphopenia-induced proliferation and maintenance of memory CD8 T cells(15, 40), and it is likely that the residual VM pool in IL-4α deficient animals was generated in response to these cytokines. Whether there are qualitative differences between the VM cells in normal versus IL-4Rα−/− animals will require further investigation.
Since our previous work suggested IL-4 induces CD8 memory-like cell generation in the thymus (23, 24), these new findings prompted the question of whether VM cells were generated during or after thymic development. Also, although the conventional CD8 memory T cell pool is renown for its long-term maintenance, even in the face of subsequent immune responses (30, 39), the sustainability of the VM population has not been addressed. We explored these issues by tracking VM cells longitudinally across a range of mouse ages. These data showed that the VM population initially appears in peripheral tissues, while memory-phenotype cells are scarce in the thymus. At later time points, however, a small pool of VM cells was detected in the thymus: this might indicate a second “wave” of thymically generated VM cells, but interpretation of such data is complicated, due to data suggesting peripheral homeostatic memory cells can recirculate to the thymus (38). These thymic VM cells appear at a stage (3 weeks of age) where thymic function is critical for generating the T cell pool, making alternative approaches - such as thymectomy – difficult to interpret. Hence, we cannot completely exclude a contribution of thymically differentiated VM cells at this stage.
Previous studies, using adoptive transfer approaches, suggested that lymphopenia supports homeostatic proliferation of naïve CD8 T cells in very young neonatal mice, but that this effect was lost by 2 weeks after birth (20). In contrast, we observed continued accumulation of VM cells up until 4 weeks after birth (Fig 4). These disparate results may arise because the homeostatic cues differ for naïve and memory CD8 T cells: For example, by 2 weeks of age the lymphopenic space may be “filled” in its capacity to induce homeostatic proliferation of naïve CD8 T cells, yet there may still be sufficient cues to allow for continued expansion of memory-phenotype cells (including VM cells) which had already been generated. Furthermore, in our colony the CD8 T cell compartment does not reach its full size until 3–4 weeks of age (Fig 5) which approximates to the time at which the size of the VM pool plateaus.
Our data also indicate that the VM pool is remarkably stable: For the tracked B8R/Kb specificity, VM cell numbers peaked at around 4 weeks of age, and were sustained at similar numbers for the next ~5 months. Likewise, priming of a robust conventional immune response (against LCMV) did not reduce either the percentage or number of B8R/Kb specific VM cells. In addition, we observed basal proliferation (measured by steady state BrdU incorporation) in the VM pool similar to that observed in bulk memory CD8 T cells (Fig. 7), and comparable to previous reports of BrdU labeling in both memory-phenotype and antigen-primed memory CD8 T cells (42–45). Together, these data suggest that the majority of the VM pool is generated in the peripheral compartment, and maintained long term, similar to conventional memory cells. Furthermore, recent studies indicate that the VM population increases in frequency with aging (as the frequency of naïve T cells declines)(31), although it is difficult to know from that study (or our own) whether VM cells are maintained as a population or are constantly replaced by cells arising from the naïve pool. Indeed, Rudd et al also report that the TCR repertoire of Kb-restricted HSV-gB specific CD8 T cells changes with aging, such that the increasing frequency of VM cells is accompanied by increasing avidity for the specific peptide/MHC ligand (31). These studies suggest that the homeostatic “fitness” of CD8 T cells may not only favor their recruitment into the VM pool but also enhance their ability to respond to foreign antigens.
The factors which maintain the VM pool are currently unclear. Previous studies suggest both IL-7 and IL-15 contribute to maintenance of the CD44hiCD122hi memory-phenotype CD8 T cell pool, while self peptide/Class I MHC is dispensable (15, 40, 46): Whether the same parameters will apply to persistence of the foreign-antigen specific VM pool is unclear, and studies to directly address this issue are technically complex since they would necessitate adoptive transfer of extremely scarce VM cells of defined specificity into cytokine- or MHC-deficient mice.
The studies in this report reinforce the concept that VM cells are a pool of unprimed, memory-like CD8, that this population is produced during the neonatal stage, in a process enhanced by IL-4, and VM cells are maintained long term in immunologically competent animals. Given the relatively high frequency of VM cells within multiple antigen specificities studied, a large fraction of the endogenous memory pool must be comprised of VM cells, in contrast to the typical view that such cells are the result of immune responses to environmental foreign antigens. Intriguingly, a similar population may be generated in humans during gestation (a stage typically aligned with mouse neonatal immune development): Studies on the fetal spleen revealed a prominent fraction of CD8 T cells with multiple memory traits (elevated CD122 and Eomes, competence to make IFN-γ rapidly)(47). Whether this pool includes bona fide memory cells will require further studies, but given the sterile environment of the fetus, it is unlikely that this population was primed by foreign antigen exposure. Such findings underscore the importance of future studies to define how the VM population may contribute to primary immune responses, perhaps especially during the neonatal period.
Acknowledgments
We thank Jane Ding for technical support, Sara Hamilton and Kris Hogquist for critical reading of the manuscript and discussion, the Jamequist lab for input during this project and Dr. Ross Kedl (University of Colorado) for communicating data ahead of publication.
This work was supported by a Predoctoral Training GrantT32 AI07313 (to A.D.A.) and NIH grants AI075168 and AI38903 (to S.C.J.).
References
- 1.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac VP, Harty JT. CD8(+) T-cell homeostasis after infection: setting the ‘curve’. Microbes and infection/Institut Pasteur. 2002;4:441–447. doi: 10.1016/s1286-4579(02)01558-7. [DOI] [PubMed] [Google Scholar]
- 3.D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Seminars in immunology. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature immunology. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 8.Bender J, Mitchell T, Kappler J, Marrack P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. The Journal of experimental medicine. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. Differential survival of naive CD4 and CD8 T cells. J Immunol. 2000;165:3689–3694. doi: 10.4049/jimmunol.165.7.3689. [DOI] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 11.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. The Journal of experimental medicine. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18484–18489. doi: 10.1073/pnas.0806487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nature immunology. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 14.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Current opinion in immunology. 2004;16:775–779. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. Journal of leukocyte biology. 2005;78:575–584. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- 17.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nature immunology. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 19.Le Campion A, Bourgeois C, Lambolez F, Martin B, Leaument S, Dautigny N, Tanchot C, Penit C, Lucas B. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuler T, Hammerling GJ, Arnold B. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15–19. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends in immunology. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Molecular and cellular biology. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nature immunology. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. The Journal of experimental medicine. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. The Journal of clinical investigation. 2010;120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. Journal of immunology. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nature immunology. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 33.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 34.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padovan E, Casorati G, Dellabona P, Giachino C, Lanzavecchia A. Dual receptor T-cells. Implications for alloreactivity and autoimmunity. Annals of the New York Academy of Sciences. 1995;756:66–70. doi: 10.1111/j.1749-6632.1995.tb44482.x. [DOI] [PubMed] [Google Scholar]
- 36.Heath WR, Carbone FR, Bertolino P, Kelly J, Cose S, Miller JF. Expression of two T cell receptor alpha chains on the surface of normal murine T cells. European journal of immunology. 1995;25:1617–1623. doi: 10.1002/eji.1830250622. [DOI] [PubMed] [Google Scholar]
- 37.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, Krensky AM. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. The Journal of experimental medicine. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian C, Bagley J, Iacomini J. Homeostatic expansion permits T cells to re-enter the thymus and deliver antigen in a tolerogenic fashion. Am J Transplant. 2007;7:1934–1941. doi: 10.1111/j.1600-6143.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- 39.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 40.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nature reviews. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 41.Antia R, V, Ganusov V, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nature reviews. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 42.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. Journal of Experimental Medicine. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science (New York, N Y. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 44.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting Edge: Requirement for IL-15 in the Generation of Primary and Memory Antigen-Specific CD8 T Cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 45.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. The Journal of experimental medicine. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. Journal of immunology. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]