Abstract
Cell surface proteins Hfe, Tfr2, hemojuvelin and Tmprss6 play key roles in iron homeostasis. Hfe and Tfr2 induce transcription of hepcidin, a small peptide that promotes the degradation of the iron transporter ferroportin. Hemojuvelin, a co-receptor for bone morphogenic proteins, induces hepcidin transcription through a Smad signaling pathway. Tmprss6 (also known as matriptase-2), a membrane serine protease that has been found to bind and degrade hemojuvelin in vitro, is a potent suppressor of hepcidin expression. In order to examine if Hfe and Tfr2 are substrates for Tmprss6, we generated mice lacking functional Hfe or Tfr2 and Tmprss6. We found that double mutant mice lacking functional Hfe or Tfr2 and Tmprss6 exhibited a severe iron deficiency microcytic anemia phenotype mimicking the phenotype of single mutant mice lacking functional Tmprss6 (Tmprss6 msk/msk mutant) demonstrating that Hfe and Tfr2 are not substrates for Tmprss6. Nevertheless, the phenotype of the mice lacking Hfe or Tfr2 and Tmprss6 differed from Tmprss6 deficient mice alone, in that the double mutant mice exhibited much greater erythropoiesis. Hfe and Tfr2 have been shown to play important roles in the erythron, independent of their role in regulating liver hepcidin transcription. We demonstrate that lack of functional Tfr2 and Hfe allow for increased erythropoiesis even in the presence of high hepcidin expression, but the high levels of hepcidin levels significantly limit the availability of iron to the erythron, resulting in ineffective erythropoiesis. Furthermore, repression of hepcidin expression was unaffected by loss of functional Hfe, Tfr2 and Tmprss6.
Keywords: hepcidin, iron, TMPRSS6, hemochromatosis, anemia, HFE, TFR2, matriptase
Introduction
In the past few years, the understanding of the roles of hepcidin, ferroportin, Hfe, transferring receptor 2 (Tfr2), hemojuvelin, and Tmprss6 (matriptase-2) in iron regulation has been rapidly growing. Hepcidin is a 25 amino acid peptide that promotes internalization and degradation of ferroportin, an iron transporter molecule [18]. Hfe, Tfr2 and hemojuvelin are positive regulators for transcriptional expression of the hepcidin gene, Hamp. In the current model, transferring receptor 1 (Tfr1) sequesters Hfe under low levels of holo-transferrin [13;14;23]. At high levels of holo-transferrin, the Hfe Tfr1 complex is destabilized and Hfe interacts with Tfr2 and holo-transferrin. The Hfe Tfr2 holo-transferrin complex promotes hepcidin expression through a currently unknown pathway [13;14;23]. Hemojuvelin is a co-receptor for Bmps, particularly Bmp6, the Bmp induced by iron [1;2]. Hemojuvelin upregulates hepcidin expression through activation of the Smad1/5/8 and Smad4 signaling pathway [1;2]. Tmprss6, a membrane associated serine protease was shown to be a potent repressor of Hamp expression [7]. In vitro, Tmprss6 was found to bind and promote the cleavage of hemojuvelin [24]. Nevertheless, Tmprss6 protease activity has been demonstrated to be promiscuous in vitro, cleaving a variety of proteins including fibronectin, type I collagen, fibrinogen, pro-urokinase plasminogen activator and artificial peptides corresponding to cleavage sites predicted in filaggrin, Cub-Domain Containing Protein 1 (CDCP1) and αEβ7 integrin [3;27]. Furthermore, Krijt et al have demonstrated that mice lacking Tmprss6 do not express elevated levels of hemojuvelin protein, which would have been expected if hemojuvelin was indeed the major substrate for Tmprss6 [16]. This raised the question as to whether Tmprss6 might cleave Hfe or Tfr2, the other two positive regulators of Hamp expression. We previously reported that mice lacking functional hemojuvelin and Tmprss6 exhibited repression of the Hamp gene and severe iron overload mimicking mice lacking hemojuvelin alone [26]. The phenotype of these mice provided in vivo evidence that hemojuvelin or a protein upstream of hemojuvelin (possibly Hfe or Tfr2) was the major substrate for the serine protease Tmprss6.
It remains unclear how the Hfe/Tfr2 regulates Hamp gene expression. Hfe deficient mice exhibit inappropriately low levels of Smad1/5/8 phosphorylation despite high levels of endogenous Bmp6 suggesting that Hfe is required for Bmp6 signaling [15]. In that hemojuvelin is the putative endogenous receptor for Bmp6, this would suggest that Hfe and Tfr2 merge with the hemojuvelin/Bmp/Smad pathway in regulating the expression of Hamp. Hfe and Tfr2 were also found to regulate levels of furin through phosphorylation of erk1/2 [21]. Upregulation of furin would increase the maturation of hepcidin, and Bmps leading to increased hepcidin expression, as well as promote the generation of the soluble form of hemojuvelin to act as a feedback inhibitor of hepcidin expression.
Evidence is also emerging that Hfe and Tfr2 might have functional roles in the erythron independent of the hepatocyte. Ramos et al demonstrated that Hfe modulates transferrin receptor mediated iron uptake in erythroid precursor cells. They suggested that this contributes to the increased hemoglobin levels, mean corpuscular volume and mean cell hemoglobin levels observed in patients and mice lacking functional Hfe, since the magnitude of red cell changes were not observed in age matched Hamp deficient mice exhibiting higher liver iron content [22]. Forejtnikova et al identified Tfr2 by mass spectroscopy to be a component of the erythropoietin receptor complex [12]. Tfr2 was shown to be required for efficient cell surface expression of the erythropoietin receptor. Erythroid progenitor cells from mice lacking functional Tfr2 exhibited diminished responsiveness to erythropoietin and higher levels of serum erythropoietin. Silencing of Tfr2 in human erythroid progenitor cells resulted in a delay, but not inhibition of hemoglobinization resulting in a slight increase in total cell number [12]. In addition, genome wide association meta analysis identified Hfe, Tfr2 and Tmprss6 to play a role in MCV, red cell number, and mean cell hemoglobin, respectively [25].
In this report, we describe the phenotype of mice lacking Hfe or Tfr2 and Tmprss6 and their response to normobaric chronic hypoxia.
Methods
Mice
Tfr2 Y245X mutant mice (Tfr2 tm1slu/tm1slu) and the AKR control strain were a generous gift from Dr. Robert Fleming at Saint Louis University Medical School [10]. Hfe−/− mice in a C57BL/6 background (Hfe tm1sly/tm1sly) were a generous gift from Dr. William Sly of Saint Louis University [11]. Hfe−/− mice were backcrossed for 10 generations into a 129SvJ background. Tmprss6 Mask mutant mice (Tmprss6 msk/msk) were previously described [7]. All mice were used in accordance with the IACUC regulations and with Institutional Department of Animal Resource approval.
Male Tmprss6 msk/msk mice were bred with female Hfe−/− mice or Tfr2 Y245X/Y245X mice maintained on a standard mouse chow diet. Double heterozygous offspring were mated to each other and their progeny genotyped to identify Hfe−/−;Tmprss6msk/msk offspring and wildtype offspring 129SvJ × C57 BL/6J (Hfe+/+;Tmprss6+/+) or Tfr2 Y245X/Y245X; Tmprss6msk/msk offspring and wildtype AKR × C57BL/6 (Tfr2+/+ Tmprss6+/+). Double mutant or double wildtype mice were used for the current studies. Since iron deficient double mutant female mice do not breed well, the mice strains were maintained by breeding double knockout males with Tfr2 Y245X/Y245X;Tmprss6+/msk or Hfe−/−;Tmprss6+/msk females.
Serum iron and transferrin saturation determinations were made according to the method described by Fielding [8]. Hemoglobin, mean corpuscular volume (MCV), hematocrit, mean corpuscular hemoglobin (MCH), red blood cell distribution width (RDW), and red blood cell count (RBC) were determined using the Drew Scientific Hemavet® 950 Hematology Analyzer System (Oxford, CT). Tissue iron levels and Hamp mRNA expression in isolated livers from the various mouse strains was determined as previously described [26].
Chronic hypoxia was induced by placing mice in a hypoxia chamber at 8% oxygen for up to 8 consecutive days. The oxygen level was regulated by nitrogen gas infusion and monitored with a Biosperix (Lacona, NY) oxycycler controller (Model A84XOV). Control mice were exposed to similar environmental conditions within the same room except that they were kept at normoxia for the length of the experiment.
Results
We generated mice lacking functional Tfr2 and Tmprss6/matriptase-2 as well as mice lacking functional Hfe and Tmprss6 to determine if Tfr2 and/or Hfe might also be endogenous substrates for Tmprss6 proteolytic activity since Tmprss6 has been shown to be a promiscuous protease in vitro [3;27]. We found that mice lacking functional Hfe or Tfr2 and Tmprss6 exhibited severe iron deficiency microcytic anemia compared to controls (Table 1). Both double mutant mice had reduced hemoglobin (Hg) levels, lower mean corpuscular volume (MCV), lower serum iron levels, reduced transferrin saturation, and higher expression of Hamp mRNA. In some respects, the microcytic anemia (as indicated by hemoglobin levels, MCV, mean corpuscular hemoglobin (MCH)) of the double mutant mice, appeared to be more severe than in the single mutant mice lacking only functional Tmprss6 (Tmprss6 msk/msk mutant). The iron deficient phenotypes of the mice lacking functional Hfe or Tfr2 and Tmprss6 is in stark contrast to the iron overload phenotype exhibited by mice lacking both functional hemojuvelin and Tmprss6 [26]. Although Bmp6 levels have been reported to be elevated in Hfe and Tfr2 single mutant mice, we found that Bmp6 levels were not elevated in Tfr2/Tmprss6 and Hfe/Tmprss6 double mutant mice and therefore Bmp6 does not contribute to the elevated expression of hepcidin.
Table 1.
Characteristics of Tfr2Y245X/Y245X/Tmprss6msk/msk and Hfe−/− /Tmprss6msk/msk mice.
| strain | Mean ± SE (n) | P value | |
|---|---|---|---|
| Hemoglobin (g/dL) | AKRB6 | 13.3 ± 0.3 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 9.9 ± 0.3 (20) | <0.0001 | |
| 129SvJB6 | 15.8 ± 0.5 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 9.8 ± 0.6 (13) | <0.0001 | |
| C57BL/6 | 12.4 ± 0.2 (8) | ||
| Tmprss6 msk/msk | 9.3 ± 0.3 (8) | <0.0001 | |
| MCV (fL) | AKRB6 | 44.0 ± 0.5 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 28.4 ± 0.3 (20) | <0.0001 | |
| 129SvJB6 | 49.6 ± 0.7 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 32.8 ± 1.6 (13) | <0.0001 | |
| C57BL/6 | 47.2 ± 0.2 (8) | ||
| Tmprss6 msk/msk | 31.7 ± 0.9 (8) | <0.0001 | |
| Hematocrit (%) | AKRB6 | 42.2 ± 0.9 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 40.8 ± 0.8 (20) | 0.439 | |
| 129SvJB6 | 52.6 ± 1.9 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 37.0 ± 1.7 (13) | <0.0001 | |
| C57BL/6 | 39.6 ± 0.7 (8) | ||
| Tmprss6 msk/msk | 32.7 ± 1.8 (8) | 0.0086 | |
| MCH (pg) | AKRB6 | 13.8 ± 0.2 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 7.0 ± 0.2 (20) | <0.0001 | |
| 129SvJB6 | 14.8 ± 0.1 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 8.9 ± 0.6 (13) | <0.0001 | |
| C57BL/6 | 15.3 ± 0.2 (8) | ||
| Tmprss6 msk/msk | 9.6 ± 0.4 (8) | 0.0009 | |
| RDW (%) | AKRB6 | 18.0 ± 0.1 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 25.8 ± 0.4 (20) | <0.0001 | |
| 129SvJB6 | 17.0 ± 0.2 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 25.2 ± 0.7 (13) | <0.0001 | |
| C57BL/6 | 17.9 ± 0.1 (8) | ||
| Tmprss6 msk/msk | 22.6 ± 0.6 (8) | 0.0009 | |
| RBC (M/µl) | AKRB6 | 9.7 ± 0.2 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 14.4 ± 0.4 (20) | <0.0001 | |
| 129SvJB6 | 10.6 ± 0.4 (21) | ||
| Hfe−/− /Tmprss6msk/msk | 11.5 ± 0.5 (13) | 0.1099 | |
| C57BL/6 | 8.3 ± 0.1 (8) | ||
| Tmprss6 msk/msk | 9.8 ± 0.4 (8) | 0.0281 | |
| Plasma Iron (µ/dL) | AKRB6 | 88.9 ± 4.7 (29) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 24.7 ± 3.2 (19) | <0.0001 | |
| 129SvJB6 | 88.1 ± 8.8 (18) | ||
| Hfe−/− /Tmprss6msk/msk | 29.9 ± 5.7 (17) | <0.0001 | |
| C57BL/6 | 94.6 ± 5.0 (6) | ||
| Tmprss6 msk/msk | 21.5 ± 4.9 (10) | 0.0002 | |
| Tf Saturation (%) | AKRB6 | 39.6 ± 2.5 (28) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 8.2 ± 1.2 (16) | <0.0001 | |
| 129SvJB6 | 42.2 ± 3.6 (18) | ||
| Hfe−/− /Tmprss6msk/msk | 13.2 ± 3.3 (17) | <0.0001 | |
| C57BL/6 | 31.9 ± 3.1 (6) | ||
| Tmprss6 msk/msk | 6.6 ± 2.2 (6) | 0.0022 | |
| spleen iron (mg/g) | AKRB6 | 1.0 ± 0.2 (7) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 1.2 ± 0.2 (6) | 0.4452 | |
| 129SvJB6 | 2.1 ± 0.3 (18) | ||
| Hfe−/− /Tmprss6msk/msk | 2.1 ± 0.2 (16) | 0.9039 | |
| C57BL/6 | 0.8 ± 0.2 (8) | ||
| Tmprss6 msk/msk | 2.3 ± 0.2 (8) | 0.0006 | |
| liver iron (mg/g) | AKRB6 | 0.4 ± 0.0 (32) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 0.3 ± 0.0 (30) | 0.0008 | |
| 129SvJB6 | 0.7 ± 0.1 (29) | ||
| Hfe−/− /Tmprss6msk/msk | 0.6 ± 0.1 (28) | 0.2671 | |
| C57BL/6 | 0.6 ± 0.1 (10) | ||
| Tmprss6 msk/msk | 0.2 ± 0.1 (9) | 0.0057 | |
| Hamp/Rps18 ratio | AKRB6 | 4.7 ± 0.4 (8) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 8.2 ± 0.5 (10) | 0.0002 | |
| 129SvJB6 | 6.8 ± 0.8 (10) | ||
| Hfe−/− /Tmprss6msk/msk | 11.8 ± 1.3 (10) | 0.0011 | |
| C57BL/6 | 5.9 ± 0.7 (10) | ||
| Tmprss6 msk/msk | 10.5 ± 1.5 (10) | 0.0021 | |
| Bmp6/Rps18 ratio | AKRB6 | 0.004 ± 0.00 (16) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 0.006 ± 0.00 (18) | 0.0943 | |
| 129SvJB6 | 0.006 ± 0.00 (10) | ||
| Hfe−/− /Tmprss6msk/msk | 0.006 ± 0.00 (10) | 0.7959 | |
| C57BL/6 | 0.001 ± 0.00 (10) | ||
| Tmprss6 msk/msk | 0.001 ± 0.00 (10) | 0.0524 | |
Hematological values were measured using a Hemavet® 950 Hematology Analyzer System (Drew Scientific). Plasma iron, transferrin saturation, spleen and liver iron content were measured as previously described [26]. Hamp, Bmp6 and Rps18 mRNA expression were measured as described previously [26]. Data include approximately equal numbers of male and female mice between 8–14 weeks old. Mean and standard error are shown, along with the number of mice (n) used for each analysis. Statistical significance was determined using Mann Whitney test (GRAPHPAD Prism 5.0).
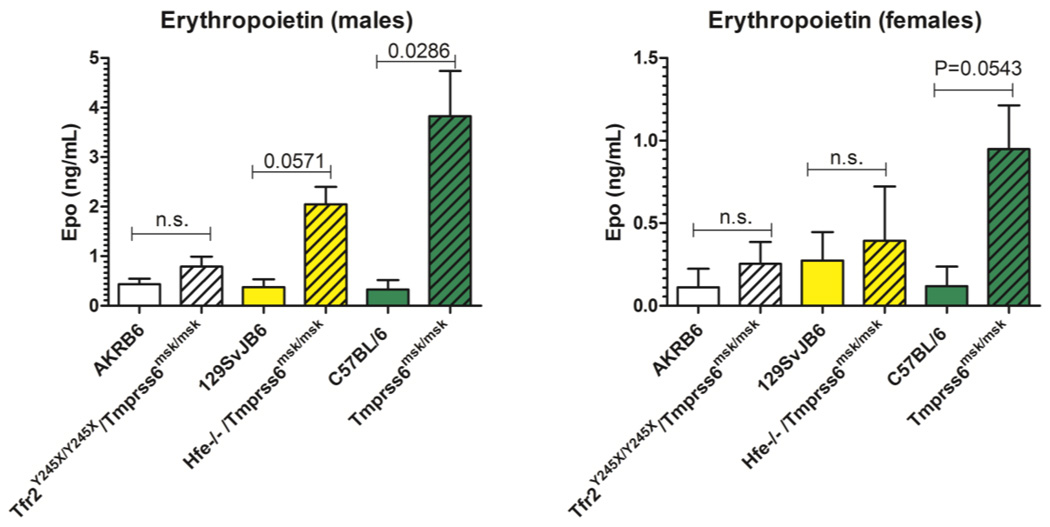
There were several notable differences between the double mutant and single mutant Tmprss6 mice. Tfr2/Tmprss6 double mutant mice exhibited high red blood cell count that essentially normalized the hematocrit (Table 1). Hfe/Tmprss6 double mutant mice, like Tmprss6 single mutant mice did not exhibit nearly as high red cell counts and had lower hematocrits. Increased erythropoiesis in the double mutant mice was indicated by the reticulocyte percent. Tfr2/Tmprss6 and Hfe/Tmprss6 double mutant mice (males +females) had a mean reticulocyte % of 7.25±0.5 and 6.25±1.2, respectively, as compared to wild type controls of 4.55±0.2 and 4.05±0.4 % respectively. In contrast, Tmprss6 single mutant mice exhibited 4.86±0.2% reticulocytes compared to control mice with 5.19±0.1%. Only the difference between Tfr2/Tmprss6 double mutant mice and their control wildtype strain reached statistical significance (P=0.0011). Spleen iron levels in the double mutant strains were the same as wildtype controls but the Tmprss6 single mutant mice exhibited significantly higher spleen iron content than control mice (P=0.0006). Liver iron content was lower in Hfe/Tmprrs6 double mutant and Tmprss6 single mutant mice as compared to wildtype controls, but the liver iron content of Tfr2/Tmprss6 double mutant mice was not significantly different from wildtype mice (P=0.2671) (Table 1). Erythropoietin levels were also significantly different between the Tmprss6 double and single mutant mice. We found that Tmprss6 single mutant mice exhibited significantly higher levels of erythropoietin than either of the double mutant strains (Figure 1).
Figure 1.
Endogenous plasma erythropoietin levels in wildtype and mutant mice. Plasma erythropoietin levels were measured by ELISA as described in Methods. Each group represents n=4 mice. Means and standard errors are shown. Statistical significance was determined using Mann Whitney test (GRAPHPAD PRISM 5.0).
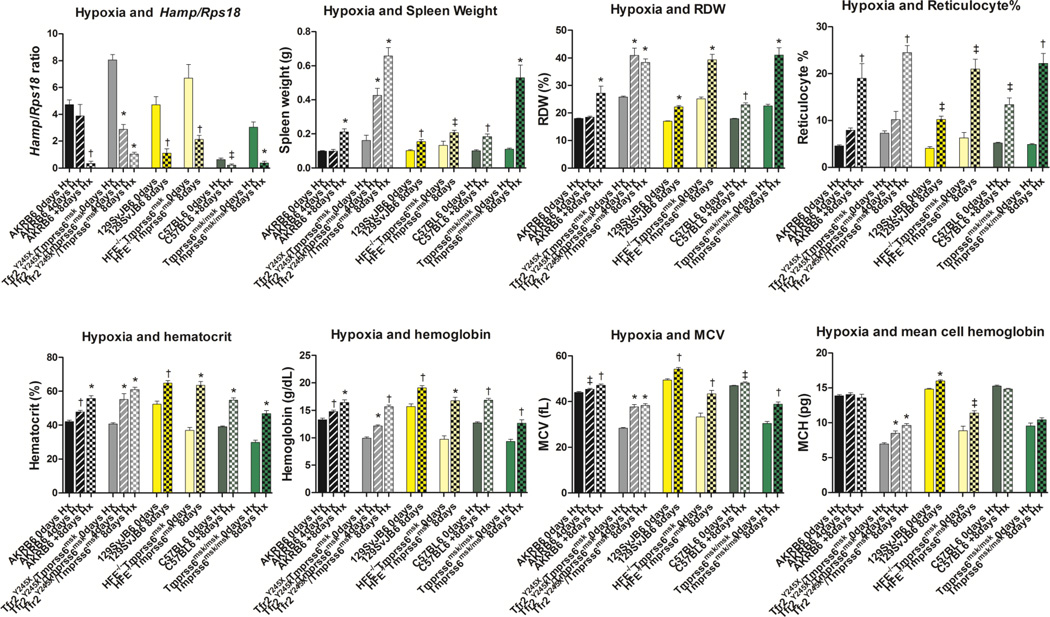
We used normal barometric pressure hypoxia (8% oxygen) for 4 or 8 days in order to examine if hepcidin could be effectively repressed in these mutant strains. Hypoxia significantly reduced Hamp expression in single and both double mutant mice and controls demonstrating that the responsiveness of Hamp to hypoxia did not require functional Hfe, Tfr2, or Tmprss6 (Figure 2). Furthermore, hypoxia significantly increased the % reticulocytes, spleen weight, RDW and RBC count of Tfr2/Tmprss6, Hfe/Tmprss6 double mutant mice, and Tmprss6 single mutant mice indicating substantial erythropoiesis (Table 2, Figure 2). Hypoxia was effective in correcting the hematocrit and hemoglobin concentration in both double mutant and the single mutant Tmprss6 mice, however, the mice remained hypochromic and microcytic as indicated by low mean corpuscular volume (MCV) and mean cell hemoglobin (MCH) (Figure 2). Subjecting Tfr2/Tmprss6 double mutant mice to three consecutive 8 day exposures of hypoxia separated by 2 week recovery times in normoxia was insufficient to correct the hypochromia and microcytosis (Table 3).
Figure 2.
Response to hypoxia by control and mutant mice. Homozygous Tfr2 Y245XTmprss6 msk double mutant, Hfe −/−Tmprss6 msk double mutant, Tmprss6 msk single mutant mice and their respective control strains were exposed to 4 or 8 days of 8% oxygen under normal pressure. For each group, n≥4, approximately equal numbers of males and females. The effect on hepcidin gene (Hamp) expression, spleen weight and hematological indices were determined as previously described [26]. Means and standard errors are shown. Statistical significance was determined using Mann Whitney test (GRAPHPAD Prism 5.0). * P≤0.0005; † P≤0.005; ‡ P≤0.05.
Table 2.
Reticulocytosis following hypoxia for 4 or 8 days
| % reticulocytes | |||
|---|---|---|---|
| normoxia | 4 days hypoxia | 8 days h ypoxia | |
| AKRB6 | 4.32 (females) | 7.62 (females*) | 14.20 (females*) |
| 5.20 (males) | 8.13 (males*) | 23.85 (males*) | |
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 7.18 (females) | 8.34 (females*) | 26.95 (females*) |
| 9.00 (males) | 12.05 (males*) | 22.00 (males*) | |
| 129SvJB6 | 4.65 (females) | nd | 9.77 (females) |
| 3.45 (males) | nd | 10.84 (males) | |
| Hfe−/− /Tmprss6msk/msk | 6.52 (females) | nd | 19.13 (females) |
| 5.98 (males) | nd | 23.53 (males) | |
| C57BL6 | 5.21 (females) | nd | 10.21 (females) |
| 5.25 (males) | nd | 16.5 (males) | |
| Tmprss6msk/msk | 4.76 (females) | nd | 26.2 (females) |
| 4.94 (males) | nd | 18.2 (males) | |
For each value, n≥4, unless indicated with *, where n=2. Statistics are shown with means and standard errors (males+females) in Figure 2 (panel 4).
Table 3.
Response to repeated hypoxia by control and Tfr2/Tmprss6 double mutant mice.
| strain | Normoxia Mean ± SE (n) |
3 × Hypoxia Mean ± SE (n) |
P value no hpx (Cont vs mut) |
P value 3x Hpx (Cont vs mut) |
P value control (no vs 3x Hpx) |
P value mutant (no vs 3x Hpx) |
|
|---|---|---|---|---|---|---|---|
|
Hemoglobin (g/dL) |
AKRB6 |
12.1 ± 0.7 (8) |
14.3 ± 0.6 (8) |
||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 10.4 ± 0.1 (8) | 10.6 ± 0.5 (8) | 0.0133 | 0.002 | 0.0356 | 0.4913 | |
| MCV (fL) | AKRB6 | 45.6 ± 2.5 (8) | 49.3 ± 1.3 (8) | ||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 28.8 ± 0.5 (8) | 37.9 ± 0.6 (8) | 0.0027 | 0.0009 | 0.1557 | 0.0009 | |
| Hematocrit (%) | AKRB6 | 39.6 ± 2.1 (8) | 47.4 ± 2.3 (8) | ||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 39.0 ± 0.9 (8) | 41.1 ± 1.5 (8) | 0.3823 | 0.0585 | 0.0458 | 0.1949 | |
| MCH (pg) | AKRB6 | 13.9 ± 0.8 (8) | 14.9 ± 0.4 (8) | ||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 7.7 ± 0.4 (8) | 9.7 ± 0.3 (8) | 0.0013 | 0.0009 | 0.0806 | 0.0073 | |
| RDW (%) | AKRB6 | 17.9 ± 0.5 (8) | 21.7 ± 1.3 (8) | ||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 24.5 ± 0.4 (8) | 33.5 ± 0.7 (8) | 0.0009 | 0.0009 | 0.0051 | 0.0002 | |
| RBC (M/µl) | AKRB6 | 8.8 ± 0.3 (8) | 9.7 ± 0.6 (8) | ||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 13.6 ± 0.5 (8) | 10.9 ± 0.4 (8) | 0.0002 | 0.1049 | 0.5737 | 0.003 | |
|
Liver Iron (mg/g) |
AKRB6 |
0.39 ± 0.1 (8) |
0.74 ± 0.1 (8) |
||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 0.29 ± 0.0 (8) | 0.78 ± 0.1 (8) | 0.3823 | 0.7209 | 0.0281 | 0.0006 | |
|
Spleen Iron (mg/g) |
AKRB6 |
4.3 ± 0.8 (8) |
7.2 ± 1.0 (8) |
||||
| Tfr2Y245X/Y245X/Tmprss6msk/msk | 2.9 ± 0.3 (8) | 3.6 ± 0.5 (8) | 0.1275 | 0.0104 | 0.0379 | 0.1560 | |
Mice were exposed to 8% oxygen under normal pressure three times for 8 consecutive days separated by 2 weeks of normoxia. Means and standard errors are shown. Eight mice were used in each group. Statistical significance was determined using Mann Whitney test (Graphpad Prism 5.0).
Discussion
Silvestri et al demonstrated in vitro that hemojuvelin is a substrate for Tmprss6/matriptase-2 [24]. These data suggested that Tmprss6 mutant and knockout mice and humans should be associated with increased hemojuvelin expression, causing elevated hepcidin expression leading to microcytic anemia. Recently, Krijt et al demonstrated that this was not the case. Tmprss6 defective mice do not express elevated levels of hemojuvelin [16]. In vivo studies in our lab demonstrated that mice lacking functional hemojuvelin and Tmprss6 exhibit an iron overload phenotype highly similar to hemojuvelin deficient mice supporting that hemojuvelin or some substrate upstream of hemojuvelin was the substrate for Tmprss6 [26]. To determine if Tfr2 or Hfe might be substrates of Tmprss6, we bred double mutant mice defective in Tfr2 or Hfe and Tmprss6.
We reasoned that if either Hfe or Tfr2 were substrates for Tmprss6 proteolytic activity, we would expect the double mutant strain to be iron overloaded in the same manner as the double mutant mice lacking functional hemojuvelin and Tmprss6 [26]. The phenotype of the Tfr2/Tmprss6mskmsk double mutant or Hfe −/− /Tmprss6mskmsk strain would be more similar to the single TfR2 mutant or HFE −/− strain alone. Alternatively, if Hfe and Tfr2 were independent of the Tmprss6 and hemojuvelin pathway, we would expect mice to have a near normal phenotype since the suppression of Hamp by loss of Tfr2 or Hfe might be partially or totally rescued by upregulation of Hamp from increased hemojuvelin. In fact, we found a severe iron deficient phenotype in both Tfr2/Tmprss6 and Hfe/Tmprss6 double mutant mice, demonstrating that Hfe and Tfr2 are not substrates for Tmprss6 protease activity, and that Hfe and Tfr2 are upstream of hemojuvelin in the hemojuvelin/BMP/SMAD pathway regulating expression of hepcidin.
While these studies were in progress, Finberg et al reported that Hfe/Tmprss6 double mutant mice were iron deficient and indistinguishable from the Tmprss6 single mutant mice [9]. Our work described here is the first description of the Tfr2/Tmprss6 double mutant mice. Our datademonstrate differences between the double mutant mice strains, particularly in the extent of ineffective erythropoiesis, and the Tmprss6 single mutant strain.
Tfr2 has been demonstrated to play a role in erythropoiesis since it modulates cell surface localization of the erythropoietin receptor [12]. Loss of Tfr2 is associated with decreased responsiveness to EPO and a compensatory increase in plasma EPO levels. Genome wide association studies have identified Tfr2 to be significantly associated with red cell number [25]. We found that functional loss of both Tfr2 and Tmprss6 was associated with increased erythropoiesis (indicated by increased reticulocytosis, increased red cell number, and increased hematocrit). These data support that Tfr2 plays an important role in the erythron that is independent of its role in regulating hepcidin expression in the hepatocyte.
Mutations in Hfe have been shown to be associated with increased mean corpuscular volume and mean hemoglobin levels [4;25]. We found that loss of functional Hfe and Tmprss6 was not associated with a significant increase in MCV or hemoglobin levels as compared to loss of Tmprss6 alone. These data suggest that the affect of Hfe on MCV and hemoglobin levels is not able to manifest under iron deficiency conditions or high hepcidin levels caused by loss of Tmprss6. Nevertheless, loss of both Hfe and Tmprss6 resulted in an increase in reticulocytosis but not to the extent as loss of Tfr2 and Tmprss6 as indicated by a smaller basal level elevation of spleen weight and red cell number.
Nicolas et al was the first to demonstrate that long-term hypoxia in vivo was able to repress hepcidin expression [19]. In vitro, hypoxia has been inconsistent in demonstrating direct repression of hepcidin expression [5;6;17;20;28]. Our data clearly demonstrated that in vivo chronic hypoxia mediated repression of hepcidin expression did not require functional Tmprss6, Hfe or Tfr2. Since expression levels of EPO did not correlate with the degree of erythropoiesis, the repression of hepcidin expression was likely mediated through erythropoietin-independent hypoxia induced erythropoiesis.
Genome wide studies indicated an association of TMPRSS6 polymorphisms and mean cell hemoglobin levels [25]. In this study, we find that loss of functional Tmprss6 was associated with a significant reduction in mean cell hemoglobin. Furthermore, while hypoxia was able to restore hemoglobin and hematocrit levels of both double and single Tmprss6 mutant mice, mean cell hemoglobin levels and mean corpuscular volume improved but remained significantly lower than normal.
In conclusion, we found that upregulation of hepcidin expression by Tmprss6 deficiency supercedes the down regulation of hepcidin expression associated with lack of Hfe or Tfr2, demonstrating that Hfe and Tfr2 are not substrates for Tmprss6 and are likely upstream of hemojuvelin in regulating hepcidin expression. Mice lacking functional Hfe or Tfr2 and Tmprss6 exhibited increased ineffective erythropoiesis despite iron deficiency. Furthermore, we demonstrate that loss of Tmprss6, Hfe and Tfr2 does not affect the repression of hepcidin expression in response to hypoxia.
Acknowledgements
This is manuscript 20421-MEM from the Scripps Research Institute. This work was supported by a grant from the National Institutes of Health DK53505-12, and The Beutler Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors
H. Peng and M-H Hsu performed the experiments and helped write the manuscript. J. Welser-Alves assisted with design and implementation of the hypoxia experiments. P. Lee conceived and designed the experiments, and wrote the manuscript.
Disclosure
The authors have no conflicts of interest to declare.
Reference List
- 1.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat.Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat.Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 3.Beliveau F, Desilets A, Leduc R. Probing the substrate specificities of matriptase, matriptase-2, hepsin and DESC1 with internally quenched fluorescent peptides. FEBS J. 2009;276:2213–2226. doi: 10.1111/j.1742-4658.2009.06950.x. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, Felitti V, Gelbart T, Ho T. The effect of HFE genotypes in patients attending a health appraisal clinic. Ann.Intern.Med. 2000;133:329–337. doi: 10.7326/0003-4819-133-5-200009050-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chaston TB, Matak P, Pourvali K, et al. Hypoxia inhibits hepcidin expression in HuH7 hepatoma cells via decreased SMAD4 signaling. Am.J.Physiol Cell Physiol. 2011;300:C888–C895. doi: 10.1152/ajpcell.00121.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem.Biophys.Res.Commun. 2007;356:312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- 7.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding J. Iron. In: Cook JD, editor. Methods in Hematology. New York: Churchill Livingstone; 1980. pp. 15–43. [Google Scholar]
- 9.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117:4590–4599. doi: 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming RE, Ahmann JR, Migas MC, et al. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc.Natl.Acad.Sci.USA. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming RE, Holden CC, Tomatsu S, et al. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc.Natl.Acad.Sci.USA. 2001;98:2707–2711. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forejtnikova H, Vieillevoye M, Zermati Y, et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010;116:5357–5367. doi: 10.1182/blood-2010-04-281360. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T. Iron homeostasis: fitting the puzzle pieces together. Cell Metab. 2008;7:288–290. doi: 10.1016/j.cmet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Chen J, Kramer M, et al. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114:2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 16.Krijt J, Fujikura Y, Ramsay AJ, et al. Liver hemojuvelin protein levels in mice deficient in matriptase-2 (Tmprss6) Blood Cells Mol.Dis. 2011;47:133–137. doi: 10.1016/j.bcmd.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Mastrogiannaki M, Matak P, Keith B, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J.Clin.Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 19.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J.Clin.Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J.Clin.Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poli M, Luscieti S, Gandini V, et al. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica. 2010;95:1832–1840. doi: 10.3324/haematol.2010.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos P, Guy E, Chen N, et al. Enhanced erythropoiesis in Hfe-KO mice indicates a role for Hfe in the modulation of erythroid iron homeostasis. Blood. 2011;117:1379–1389. doi: 10.1182/blood-2010-09-307462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt PJ, Toran PT, Giannetti AM, et al. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvestri L, Pagani A, Nai A, et al. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soranzo N, Spector TD, Mangino M, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat.Genet. 2009;41:1182–1190. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truksa J, Gelbart T, Peng H, et al. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br.J.Haematol. 2009;147:571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- 27.Velasco G, Cal S, Quesada V, et al. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J.Biol.Chem. 2002;277:37637–37646. doi: 10.1074/jbc.M203007200. [DOI] [PubMed] [Google Scholar]
- 28.Volke M, Gale DP, Maegdefrau U, et al. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PLoS.ONE. 2009;4:e7875. doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]