Abstract
Children with single ventricle (SV) physiology have increased ventricular work and are at risk for heart failure (HF). However, HF diagnosis is especially difficult because there are few objective measures of HF validated in this cohort. We previously showed that plasma B-type natriuretic peptide (BNP) levels were sensitive and specific for detecting HF in a small, heterogeneous SV cohort. The aim of this study was to define the impact of SV morphology and stage of palliation on the correlation between BNP and HF. We also examined the utility of N-terminal pro-BNP (NT-proBNP), a more stable product of pre-BNP processing, as a biomarker of HF in these patients. A cross-sectional observational study of SV children 1 month–7 years was conducted. The presence of HF was defined as a Ross score >2. The association of BNP or NT-proBNP with HF was assessed using logistic regression and ROC curves. Twenty-two of 71 included children (31%) had clinical HF. A doubling of BNP was associated with an odds ratio for HF of 2.20 (95%CI 1.36–3.55, p=0.001) with a c-statistic >75%, yielding a detection threshold of ≥45 pg/ml. This threshold was preserved when patients were stratified by right ventricular morphology or stage of surgical palliation. Similarly, a doubling of NT-proBNP was associated with an odds ratio for HF of 1.92 (95% CI 1.17–3.14, p=0.009). In contrast with BNP, the threshold value of NT-proBNP for predicting HF decreased with stage of palliation. In conclusion, plasma BNP and NT-proBNP are reliable tests for clinical HF in young children with SV physiology, specifically those with right ventricular morphology, regardless of stage of palliation.
Keywords: Single ventricle, congenital heart defect, heart failure, B-type natriuretic peptide, N-terminal pro-BNP, hypoplastic left heart, atrioventricular canal defect, double-outlet right ventricle
Introduction
Toward establishing B-type natriuretic protein (BNP) as a potential biomarker of heart failure (HF) in children with single ventricle (SV) physiology, we previously demonstrated its utility in a small cohort of these patients1. We demonstrated in a sample of 29 children that plasma BNP was sensitive and specific for detecting mild HF, with a cutpoint value of ≥30 pg/mL. This threshold value was markedly lower than the value reported for HF in the adult population (>100 pg/ml) 2. In addition, a recent post hoc analysis of patients in the Pediatric Carvedilol Trial suggested a threshold of >140 pg/mL for predicting adverse outcomes in children with known HF, however, this study did not distinguish between subjects with SV versus biventricular physiology3. To determine the reliability of BNP as a predictor of HF in children with SV heart disease, we studied a larger cohort of patients and analyzed HF versus BNP based on ventricular morphology as well as stage of surgical palliation. Since BNP has a relatively short half-life in plasma, we also compared BNP to N-terminal pro-B-type natriuretic peptide (NT-proBNP) in this same population to determine whether a related biomarker with a longer half-life would prove more useful.
Methods
A single site cross-sectional observational study utilizing a secondary study base was conducted. All children 1 month–7 years old with SV physiology presenting to the UCSF Pediatric Heart Center between February 2007 and December 2010 were eligible for the study. Patients were excluded if they had renal failure, trisomy 21, an acute intercurrent illness, a congenital defect that interfered with feeding (e.g., cleft palate, esophageal atresia), or had participated in an investigational drug or device study in the last 6 months. In addition, patients for whom a valid Ross score could not be determined (e.g., those receiving nasograstric tube feedings, those with post-operative diaphragmatic paralysis) were excluded. This study was approved by the UCSF IRB, and written consent was obtained from guardians of all subjects.
Each child was assigned a Ross score 4–6 to determine the presence of clinical HF immediately prior to phlebotomy(Table 1). For this study, HF scoring was recorded independent of presumed mechanism, since the goal was to evaluate biomarkers in SV patients that would be clinically useful across etiologies. Predictor measurement occurred subsequent to outcome determination, allowing assessors to be blinded to plasma protein levels.
Table 1.
Ross Score for Infants and Children
| Infants | ||||
|---|---|---|---|---|
| Score
|
||||
| 0 | 1 | 2 | ||
| Feeding History | ||||
|
| ||||
| Volume/feed | >3.5 oz | 2.5–3.5 oz | <2.5 oz | |
|
| ||||
| Time/feed | <40 min | >40 min | ||
|
| ||||
| Physical Exam | ||||
|
| ||||
| RR | <50/min | 50–60/min | >60/min | |
|
| ||||
| HR | < 160/min | 160–170/min | >170/min | |
|
| ||||
| Respiration | Normal | Abnormal | ||
|
| ||||
| Perfusion | Normal | Decreased | ||
|
| ||||
| S3 | Absent | Present | ||
|
| ||||
| Liver size | <2 cm | 2–3 cm | >3 cm | |
| Children | |||
|---|---|---|---|
| Score
|
|||
| 0 | 1 | 2 | |
| Feeding History | |||
|
| |||
| Meals | Normal | Variable | Less |
|
| |||
| Physical Exam | |||
|
| |||
| RR | <20/min | 20–30/min | >30/min |
|
| |||
| HR | <110/min | 111–130/min | >130/min |
|
| |||
| Respiration | Dyspnea on exertion | Marked dyspnea on exertion | Dyspnea at rest |
|
| |||
| Perfusion | Normal | Decreased | |
|
| |||
| S3 | Absent | Present | |
|
| |||
| Liver size | Above costal margin | At costal margin | Below costal margin |
Total score: ≤2 = none; 3–6 = mild; 7–9 = moderate; ≥10 = severe
At the time of cardiac catheterization, pre-operative evaluation for cardiac surgery, or medical admission, 6 mL of whole blood were obtained. Plasma BNP and NT-proBNP were measured following assignment of Ross score. For BNP and NT-proBNP, plasma was collected from whole blood within 2 hours and stored at −80°C prior to assay. BNP was assayed using the Biosite Triage kit (Biosite Diagnostic, San Diego, CA) as previously described7. NT-proBNP was assayed using the Elecsys proBNP kit (Roche Diagnostics, Pleasanton, CA) according to the manufacturer’s instructions. Each sample was assayed in duplicate.
The primary outcome was HF (Ross score ≥3) versus no HF (Ross score ≤2). The relationship between raw Ross score and plasma proteins was summarized using scatter plots, and analyzed using both linear and logistic regression. Univariate logistic regression was used to assess the crude association between each of the cardiac-related plasma proteins and clinical HF. A p-value <0.05 was considered statistically significant. Receiver Operator Characteristic (ROC) curves were generated to evaluate proteins as biomarkers in the entire cohort and stratified groups. Assays were deemed useful if they carried a c-statistic ≥0.75 (graph area encompassed by the curve). For a useful test, a clinically relevant cut point was identified that would distinguish SV children in HF from those free of HF at the time of evaluation. All statistical analyses were performed using Stata 10 (StataCorp LP, College Station, TX).
Results
We approached 91 SV patients meeting inclusion criteria and presenting to the UCSF Pediatric Heart Center between February 2007 and December 2010 for enrollment. Fourteen (15%) declined. Of the 77 remaining subjects, 6 (8%) were subsequently excluded because of missing data. Thus, 71 children were studied (Table 2). Of the single RV patients, 28 had hypoplastic left heart (10 with mitral/aortic atresia, 8 with mitral stenosis and aortic atresia, and 10 with mitral/aortic stenosis), 13 had a right dominant atrioventricular canal defect, 6 had double-outlet RV, 2 had interrupted aortic arch with ventricular septal defect and small LV, and one patient each had aortic stenosis and severe LV dysfunction or L-transposition of the great arteries with severe pulmonary stenosis and small LV. Of the single LV patients, 10 had tricuspid atresia, 3 had pulmonary atresia with intact ventricular septum, 2 had double-inlet LV, 2 had D-transposition of the great arteries with straddling mitral valve and small RV, and one had critical pulmonary stenosis with small RV. The patients with indeterminate ventricular morphology had primitive ventricles with heterotaxy syndrome.
Table 2.
Patient Data
| Ventricular Morphology | Stage* | No. Patients | No. Female/Male | Median Age, mos (Range) | No. Ross Score ≤2 | No. Ross Score >2 | Median Ross Score (Range) |
|---|---|---|---|---|---|---|---|
| Right | Total | 51 | 22/29 | 16 (2–80) | 36 | 15 | 2 (0–9) |
| Stage I | 19 | 9/10 | 4.4 (2–8) | 13 | 6 | 2 (0–6) | |
| Stage II | 24 | 11/13 | 35 (5–72) | 17 | 7 | 2 (0–9) | |
| Stage III | 8 | 2/6 | 66 (34–80) | 6 | 2 | 1.5 (0–4) | |
| Left | Total | 18 | 4/14 | 44 (3–78) | 11 | 7 | 1.5 (0–8) |
| Stage I | 4 | 1/3 | 5 (3–11) | 4 | 0 | 0 | |
| Stage II | 1 | 4/7 | 45 (17–69) | 5 | 6 | 3 (1–8) | |
| Stage III | 3 | 0/3 | 73 (69–78) | 2 | 1 | 1 (0–3) | |
| Indeterminate | Total | 2 | 1/1 | 60 (56–66) | 2 | 0 | 1.5 (1–2) |
| Stage I | 0 | 0 | 0 | 0 | 0 | - | |
| Stage II | 2 | 1/1 | 60 (56–66) | 2 | 0 | 1.5 (1–2) | |
| Stage III | 0 | 0 | 0 | 0 | 0 | - |
Stage I: stabilization of aortic and pulmonary blood flow, e.g., Norwood, Sano, pulmonary artery band, Blalock-Taussig shunt, central shunt; Stage II: establishment of partial cavopulmonary circulation between the superior vena cava and pulmonary arteries, e.g., Glenn shunt, Kawashima; Stage III: completion of cavopulmonary circulation, e.g., Fontan.
Patients were assigned Ross scores as a measure of clinical HF as previously described1(Table 1) and the surgical stage of palliation was noted (Table 2). Of the 49 children free of clinical HF, the median Ross score was 1±0.8. For the 22 children with clinical HF, the median Ross score was 4±1.7. The overall prevalence of HF in the included sample was 31%, with morphology-specific prevalence of 30% in those with single RV and 39% in those with single LV.
A scatter plot of BNP versus Ross score was used to assess the relationship between the biomarker and clinical status. After log2 transformation, BNP demonstrated a roughly linear relationship with Ross score (Figure 1A). A doubling of BNP was associated with an odds ratio for HF of 2.20 (95% CI 1.36–3.55, p=0.001) when side and stage were held constant. Utilizing univariate logistic regression, and stratifying the subjects into morphology and stage, there was a statistically significant correlation between BNP and HF in the single RV group with an odds ratio for HF of 2.03 (95% CI 1.24–3.31, p=0.005), and in all patients at stage II with an odds ratio of 1.5 (95% CI 1.03–2.16, p=0.03). In SV patients at stage I, the correlation between BNP and HF approached statistical significance with an odds ratio of 2.92 (95% CI 0.90–9.51 p=0.08) as did the correlation between BNP and HF among patients at stage III (odds ratio 3.51 [95% CI 0.75–16.5, p=0.11]) We cannot preclude the possibility that our failure to detect a significant association in these two groups may be due to insufficient power. There was no statistically significant correlation between BNP and HF in the single LV group (odds ratio 1.94 [95% CI 0.89–4.22, p=0.095]) although this too was suggestive of an association.
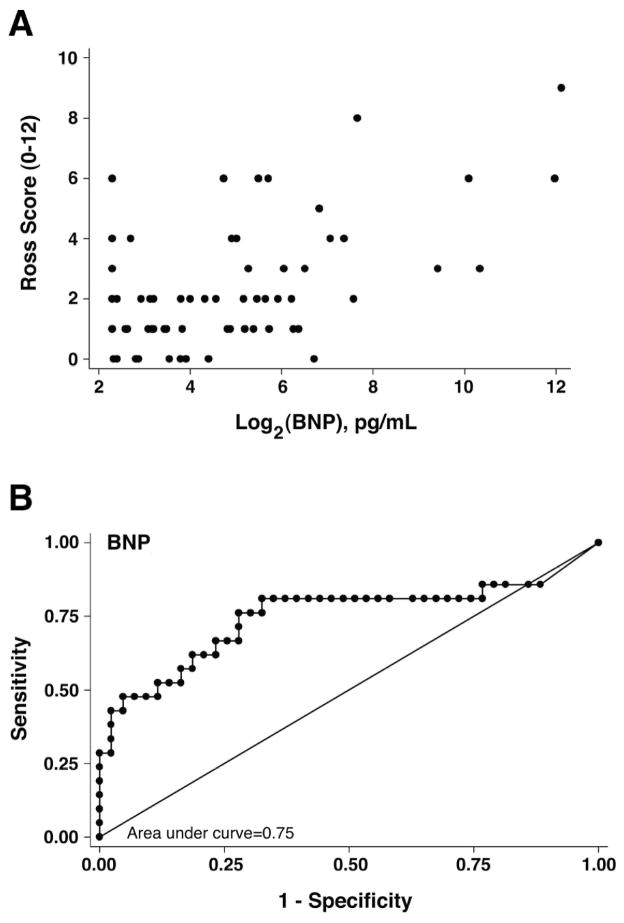
Figure 1. Evaluation of BNP versus Ross scores.
(A) Scatter plot of plasma BNP concentration versus raw Ross scores in 71 SV patients. Log2 transformation of BNP revealed a roughly linear relationship with raw Ross score. (B) False positives (1-Specificity) were plotted against true positives (Sensitivity) for all patients. The area under the curve (c-statistic) was 0.75, which was significant.
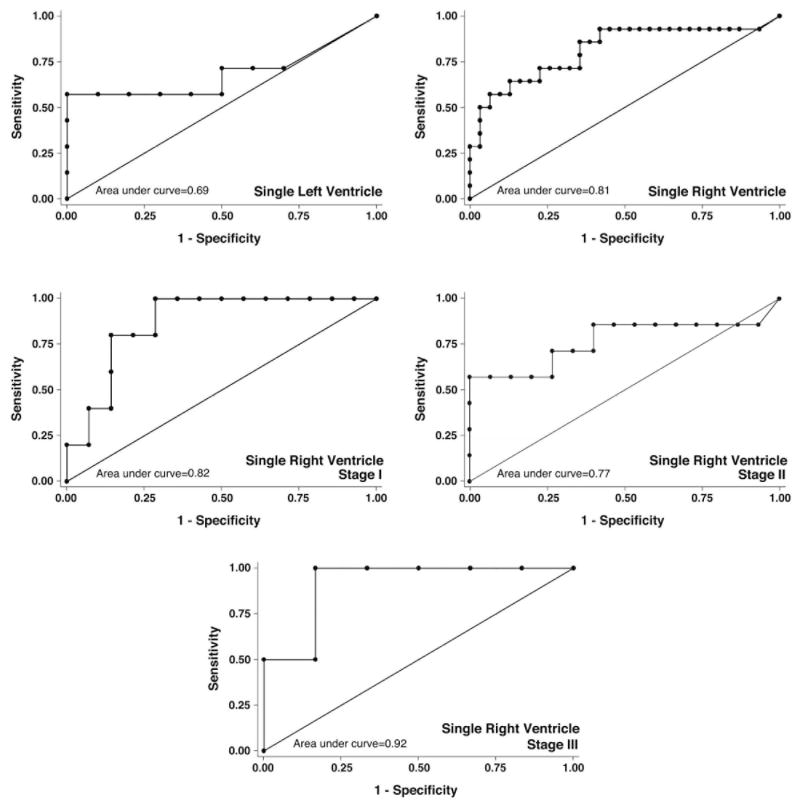
ROC curve analysis of BNP data for the entire cohort met our pre-specified threshold of ≥75% area contained by the curve (Figure 1B). ROC analysis stratified by ventricular morphology demonstrated that the right SV group exceeded the threshold of ≥75% (c-statistic= 81%), however, left SV patients did not (c-statistic=69%) (Figure 2). ROC analysis of right SV patients stratified by stage of surgical palliation demonstrated that patients at all 3 stages of palliation exceeded the usefulness threshold (c-statistic=82%, 77%, and 92%, for stage I, II, and III, respectively) (Figure 2). Together, logistic regression and ROC curve analysis supported the conclusion that BNP is a useful biomarker of HF (Ross score ≥3) for single RV patients at all stages of palliation.
Figure 2. Receiver Operator Characteristic curves for BNP by Patient Group.
False positives (1-Specificity) were plotted against true positives (Sensitivity) for patients with single left or right ventricle, and for patients with single right ventricles who had undergone Stage I (Norwood, Sano, pulmonary artery band, Blalock-Taussig shunt, or central shunt), Stage II (partial cavopulmonary shunt, i.e., Glenn or Kawashima), or Stage III (complete cavopulmonary shunt, i.e., Fontan) surgeries. Patient groups with a single right ventricle, regardless of stage of palliation, demonstrated an area under the curve (c-statistic) ≥0.75, indicating a useful test.
We determined sensitivity and specificity for various BNP levels as markers of HF for the entire cohort and groups stratified by ventricular morphology and stage of surgical palliation that demonstrated c-statistic values ≥75%. A cut point of ≥45 pg/ml correctly classified 75% of SV patients for all patients considered in total, with both sensitivity (62%) and specificity (81%). Right SV morphology also demonstrated a cut point of ≥45 pg/ml that correctly classified 76% of patients (sensitivity 71%, specificity 77%). When stratified by stage, a cut point of 45 pg/ml correctly classified 73% of stage I patients (sensitivity 100%, specificity 60%), 73% of stage II patients (sensitivity 57%, specificity 80%), and 88% of stage III patients (sensitivity 100%, specificity 83%). This analysis supports a cut point of 45 pg/ml for predicting HF in single RV patients, regardless of stage of palliation.
BNP is rapidly cleared from the circulation through a process of cell surface binding and internalization, with a half-life of 20 minutes8. The BNP threshold for predicting HF in this and our previous pilot study was significantly lower than for predicting HF in both pediatric and adult patients with biventricular hearts. Since it is possible that SV patients produce less BNP precursor (pre-BNP), we wanted to determine whether NT-proBNP, another product of pre-BNP processing cleared by renal excretion with a longer half-life (1–2 hours)8, might be more useful for predicting HF in SV patients. In addition, NT-proBNP is stable at room temperature for 72 hours, while BNP is stable for 24 hours, making NT-proBNP a more stable biomarker target9.
Scatter plot analysis of log2 NT-proBNP versus Ross score demonstrated a roughly linear relationship (Figure 3A). Multivariate logistic regression of plasma NT-proBNP levels demonstrated that a doubling of NT-proBNP was associated with an odds ratio for HF of 1.92 (95% CI 1.17–3.14, p=0.009) when side and stage are held constant. Univariate logistic regression of groups stratified by ventricular morphology and stage showed a statistically significant correlation between NT-proBNP and HF in the single RV group, with an odds ratio for HF of 1.77 (95% CI 1.08–2.88, p=0.02). In SV patients at stage I, the correlation between NT-proBNP and HF approached statistical significance with an odds ratio of 2.63 (95% CI 0.96–7.26 p=0.06). The correlations between BNP and HF in patients at stages II and III were not significant, with few patients at stage III. There was no statistically significant correlation between NT-proBNP and HF in the single LV group (odds ratio 1.19 [95% CI 0.69–2.05, p=0.53]).
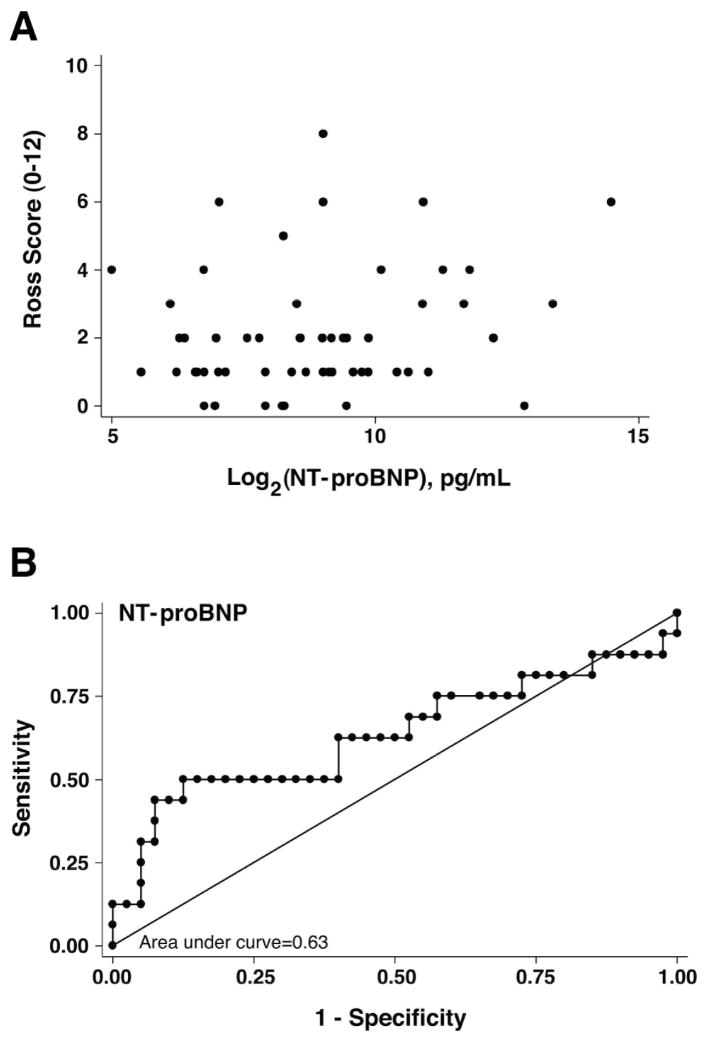
Figure 3. Evaluation of NT-proBNP versus Ross scores.
(A) Scatter plot of plasma NT-proBNP concentration versus raw Ross scores in 71 SV patients. Log2 transformation of NT-proBNP failed to reveal a linear relationship with raw Ross score. (B) False positives (1-Specificity) were plotted against true positives (Sensitivity) for all patients. The area under the curve (c-statistic) was 0.63, which was not significant.
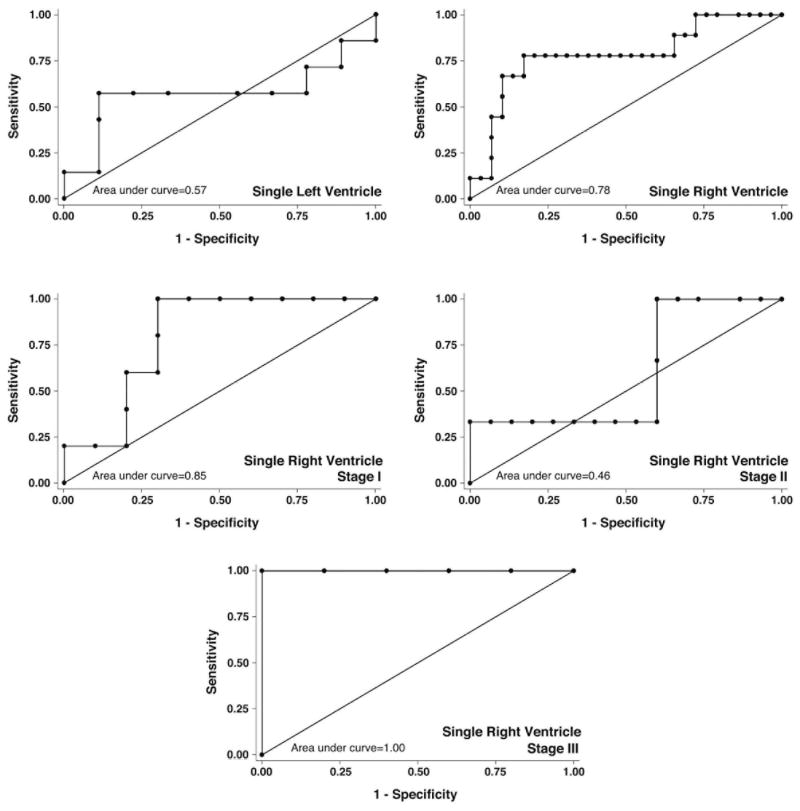
ROC curve analysis of NT-proBNP data for the entire study cohort gave a c-statistic of 63%, which did not exceed our pre-specified threshold of ≥75% area contained by the ROC curve (Figure 3B). ROC analysis stratified by ventricular morphology demonstrated that the right SV group exceeded the threshold of ≥75% (c-statistic=78%), however, left SV group did not (c-statistic=57%) (Figure 4). ROC analysis stratified by stage of palliation demonstrated that patients at stages I and III exceeded the usefulness threshold (c-statistic=85% and 100%, respectively), however, those at stage II did not (c-statistic=46%) (Figure 4).
Figure 4. Receiver Operator Characteristic curves for NT-proBNP by Patient Group.
False positives (1-Specificity) were plotted against true positives (Sensitivity) for patients with single left or right ventricle, and for patients with single right ventricles who had undergone Stage I, Stage II, or Stage III surgeries. Patients with a single right ventricle, or who had undergone either Stage I or Stage III surgeries demonstrated an area under the curve (c-statistic) ≥0.75, indicating a useful test.
The test characteristics for various plasma levels of NT-proBNP as markers of HF were examined for the single RV group and for those at stages I and III (those that demonstrated c-statistic values ≥75%). For all patients with single RV, a cut point of ≥1100 pg/ml showed both sensitivity (78%) and specificity (83%), thus correctly classifying 82% of patients. Interestingly, when single RV patients were stratified by stage, the threshold for classifying HF decreased with stage. A cut point of 1900 pg/ml was needed to correctly classify 80% of patients at stage I, with sensitivity 100% and specificity 70%, while this decreased to 1100 pg/ml for stage II patients, and 300 pg/ml for stage III patients (sensitivity 100%, specificity 75%). Logistic regression, ROC curve analysis, and evaluation of test characteristics suggest that NT-proBNP can be a useful biomarker of HF (Ross score ≥3) in single RV patients at all stages of palliation.
Discussion
This study confirms a direct relationship between clinical HF and BNP level in young children with SV physiology. We propose a cut point of ≥45 pg/ml for determining HF in this patient population. This threshold is distinct from the cut point of 100 pg/ml established for adults2. However, 45 pg/ml is also at least double the previously published normal range observed in similarly aged children with structurally normal hearts (5.1–12.1 pg/ml)10. Interestingly, in one small study of patients undergoing cavopulmonary shunt surgery, two patients undergoing stage III palliation with pre-operative BNP values over 50 pg/ml were the only patients in the study with adverse outcomes7.
Apost hoc analysis of the Pediatric Carvedilol Trial suggested that a BNP level of ≥140 was predictive of adverse outcomes in children with known HF3. The original trial included all children with HF regardless of their congenital defect, and included those with normal anatomy and cardiomyopathy as well. As we had previously proposed1,11, children with univentricular heart disease have distinct physiology, and HF in this group deserves special consideration. The current study supports a specific cut point for HF in this patient group that is distinct from, and significantly lower than, those with other congenital and non-congenital causes of HF.
The cohort enrolled in this study allowed us to stratify patients according to primary ventricular morphology and stage of surgical palliation. Stratification by right ventricular morphology regardless of stage of palliation indicated a cut point of 45 pg/ml for predicting HF that was both highly sensitive and specific. The diagnosis of HF in patients with univentricular heart disease relies mainly on clinical signs and observed or reported symptoms, since echocardiographic quantitation of single ventricular function, especially of right-sided morphology, has not been universally standardized. Our results show that BNP can be a reliable tool for predicting even mild HF in the subgroup of patients with SV of right morphology independent of stage of palliation. Effective monitoring for HF in SV patients as they undergo staged palliation is particularly relevant, since most children undergo the first palliative procedure during the neonatal period, the second between 4–6 months of age, and the third surgery at 3–4 years. BNP levels will help the clinician distinguish between HF and the various respiratory infections to which infants and young children are exposed during the first several years of life. The detection of early HF by plasma BNP assay may also be important in determining the timing of staged palliation to unload the SV.
Within the single right ventricle group, the threshold for detecting HF was 45 pg/ml regardless of stage of palliation, while our original pilot study of 18 single right ventricle, 9 single left ventricle, and 2 SV patients of indeterminate morphology implicated a lower cut point (i.e., 30 pg/ml 1). Holmogren et al. measured plasma concentrations of BNP after the three palliative steps in children with univentricular heart defects and good ventricular function as assessed by an arbitrary function scale12. They noted a slightly higher BNP value in those children with a systemic right ventricle compared to those with systemic left ventricle. The higher relative number of single right ventricle patients in the current study likely explains this discrepancy, and supports the observation of Holmgren et al. that baseline BNP values in SV patients are higher in those with right morphology.
Because the NT-proBNP peptide has a longer circulating half-life than BNP, we evaluated whether this might be a more reliable biomarker of ongoing HF in SV patients. We observed that NT-proBNP similarly served as a sensitive and specific biomarker in patients with single right ventricular physiology, and that the threshold for detecting HF decreased with subsequent stage of palliation and state of unloading of the SV. In a prospective study following SV patients through the staged palliation protocol, Eerola et al. evaluated 19 patients (16 with single right and 3 with single left ventricle), without reference to HF status. They found a decrease in NT-proBNP levels when the SV was unloaded following cavopulmonary shunt (stage II) 13, consistent with our observation that the NT-proBNP threshold value for detecting HF decreases with each stage of palliation.
Our study was limited by our clinical assessment tool, i.e., the Ross score has not been specifically validated in the single-ventricle population. However, a generalized HF grading system based on the specific criteria of the Ross score, the Ross classification of HF, has been used in several previous studies of HF, including recent multicenter trials of carvedilol and enalapril therapy for pediatric HF that included SV patients 14,15. As such, we considered the combined criteria included in the Ross score the most relevant outcome measure for this study.
Based on our findings, we propose that a BNP threshold of 45 pg/ml or NT-proBNP threshold of 300, 1100, or 1900 pg/ml, depending on stage of palliation, should be used in monitoring the onset of HF in patients with SV physiology. We suggest that measurement of BNP or NT-proBNP become an integral part of the diagnostic workup in the acute setting, and as a monitoring tool for these patients outside of the tertiary care setting.
Acknowledgments
This work was supported by funds from Biosite Diagnostic to J.R.F., and funds from the Lorraine Newman Fund of the UCSF Division of Pediatric Cardiology, a Strategic Opportunity Award from the UCSF Clinical and Translational Science Institute (NIH/NCRR UL RR024131), and funds from Roche Diagnostics Corporation to H.S.B.
The authors thank the UCSF pediatric cardiology fellows, the UCSF Congenital Cardiac Catheterization Laboratory staff, and Kirk Sujishi of the UCSF Clinical Chemistry Laboratory for their invaluable assistance with the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah A, Feraco AM, Harmon C, Tacy T, Fineman JR, Bernstein HS. Usefulness of various plasma biomarkers for diagnosis of heart failure in children with single ventricle physiology. Am J Cardiol. 2009;104:1280–1284. doi: 10.1016/j.amjcard.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisel A. B-type natriuretic peptide measurements in diagnosing congestive heart failure in the dyspneic emergency department patient. Rev Cardiovasc Med. 2002;3 (Suppl 4):S10–17. [PubMed] [Google Scholar]
- 3.Auerbach SR, Richmond ME, Lamour JM, Blume ED, Addonizio LJ, Shaddy RE, Mahony L, Pahl E, Hsu DT. BNP Levels Predict Outcome in Pediatric Heart Failure Patients: Post-hoc Analysis of the Pediatric Carvedilol Trial. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.906875. [DOI] [PubMed] [Google Scholar]
- 4.Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–75. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone DE, Abdulla A, Arnold JM, Bernstein V, Bourassa M, Brophy J, Davies R, Gardner M, Hoeschen R, Mickleborough L, et al. Diagnosis and management of heart failure. Canadian Cardiovascular Society. Can J Cardiol. 1994;10:613–631. 635–654. [PubMed] [Google Scholar]
- 6.Rosenthal D, Chrisant MR, Edens E, Mahony L, Canter C, Colan S, Dubin A, Lamour J, Ross R, Shaddy R, Addonizio L, Beerman L, Berger S, Bernstein D, Blume E, Boucek M, Checchia P, Dipchand A, Drummond-Webb J, Fricker J, Friedman R, Hallowell S, Jaquiss R, Mital S, Pahl E, Pearce B, Rhodes L, Rotondo K, Rusconi P, Scheel J, Pal Singh T, Towbin J. International Society for Heart and Lung Transplantation: Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Hsu JH, Oishi PE, Keller RL, Chikovani O, Karl TR, Azakie A, Adatia I, Fineman JR. Perioperative B-type natriuretic peptide levels predict outcome after bidirectional cavopulmonary anastomosis and total cavopulmonary connection. J Thorac Cardiovasc Surg. 2008;135:746–753. doi: 10.1016/j.jtcvs.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Mair J, Gerda F, Renate H, Ulmer H, Andrea G, Pachinger O. Head-to-head comparison of Btype natriuretic peptide (BNP) and NT-proBNP in daily clinical practice. Int J Cardiol. 2008;124:244–246. doi: 10.1016/j.ijcard.2006.11.230. [DOI] [PubMed] [Google Scholar]
- 9.Das BB. Plasma B-type natriuretic peptides in children with cardiovascular diseases. Pediatr Cardiol. 2010;31:1135–1145. doi: 10.1007/s00246-010-9758-x. [DOI] [PubMed] [Google Scholar]
- 10.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenthal A, Shah A, Bernstein HS. Letter by Lowenthal et al regarding article, “BNP levels predict outcome in pediatric heart failure patients: post hoc analysis of the Pediatric Carvedilol Trial”. Circ Heart Fail. 2010;3:e32. doi: 10.1161/CIRCHEARTFAILURE.110.958470. author reply e33. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren D, Westerlind A, Berggren H, Lundberg PA, Wahlander H. Increased natriuretic peptide type B level after the second palliative step in children with univentricular hearts with right ventricular morphology but not left ventricular morphology. Pediatr Cardiol. 2008;29:786–792. doi: 10.1007/s00246-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 13.Eerola A, Jokinen E, Sairanen H, Pihkala J. During treatment protocol for univentricular heart serum levels of natriuretic peptides decrease. Eur J Cardiothorac Surg. 2010;38:735–740. doi: 10.1016/j.ejcts.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 15.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]