Abstract
Previous research has suggested that sensory areas may play a role in adaptation to repeated stress. The auditory cortex was the target of the present studies because it is a major projection area of the auditory thalamus, where functional inactivation disrupts stress habituation to repeated loud noise. Large bilateral excitotoxic lesions of the auditory cortex were made in male rats 2 weeks prior to (Exp 1) or a few days after (Exp 2) a 5 day 30 min repeated 95 dBA noise or no noise regimen. Blood was collected immediately after exposure on days 1, 3, and 5. Two weeks after the 5th exposure, the rats were retested with 30 min noise or no noise to determine retention of the habituated responses. Animals were killed immediately after the retest and trunk blood and brains collected for lesion verification. Plasma adrenocorticotropic hormone (ACTH) and corticosterone levels were determined. In both experiments, significant between-subjects effects were found for noise (95 dBA or no noise) but not for surgery (lesion, sham, or no surgery control rats), with lesion groups exhibiting similar levels of ACTH and corticosterone across days as the sham and no surgery control groups. All noise exposed groups displayed similar habituation rates and retention levels. A third experiment indicated that similar auditory cortex lesions significantly disrupted background noise gap detection in an acoustic startle paradigm. Overall, these data suggest that the information mediating hypothalamic-pituitary-adrenal axis response habituation to repeated loud noise exposures is not derived from the auditory cortex.
Keywords: adaptation, adrenocorticotropin hormone, corticosterone, NeuN, startle
1. Introduction
Hypothalamic-pituitary-adrenal (HPA) axis response habituation can be characterized as a reduction in stress-induced hormone release (plasma ACTH and corticosterone) after repeatedly experiencing the same stress situation. HPA axis habituation to specific stimuli, including loud noise exposures, usually develops relatively quickly (Armario et al. 1984, Campeau et al., 2002, De Boer et al., 1990, Masini et al., 2008, Natelson et al., 1988), and lasts for weeks (Bhatnagar et al., 2002, Nyhuis et al., 2010). The concept of habituation was originally based on reflexive and behavioral responses to a variety of stimuli (see Groves and Thompson, 1970, Thompson and Spencer, 1966, for reviews), and habituation of HPA axis responses have often been discussed to involve similar neural processes (De Boer et al., 1990, Natelson et al., 1988, Pitman et al., 1988). The observed decrements in responding in reflexive behavior are not mediated by motor fatigue or adaptation at the level of sensory receptors (Carew and Kandel, 1973, Christoffersen, 1997). HPA axis habituation likewise cannot be easily explained by proximal sensory and/or motor modulation because a heterotypic (novel) stressor presented after habituation to a homotypic stressor will induce a normal, or even sensitized, HPA axis response (Bhatnagar et al., 1995, Marti and Armario, 1998, Masini et al., 2012). Studies in several invertebrate organisms have suggested that reflex habituation is mediated by long-term synaptic depression at sensory-motor synapses (Christoffersen, 1997, Frost et al., 1997, Gover and Abrams, 2009), a possibility that may also apply to HPA axis response habituation. However the putative site of the sensory-motor interface mediating habituation to various stressful situations has not been characterized in mammals and may involve more complex mechanisms, as recognized in invertebrate habituation models (Christoffersen, 1997, Frost et al., 1997, Gover and Abrams, 2009).
The results of immediate early gene studies have suggested that the plasticity associated with HPA axis habituation to repeated homotypic stress exposures may occur in central sensory afferents that provide inputs to more central stress-responsive brain regions. For example, repeated restraint stress led to decreased c-fos mRNA expression in the ventroposteromedial and dorsolateral geniculate nuclei (Girotti et al., 2006) and the barrel field of the primary somatosensory cortex (Masini et al., 2012) compared to acute restraint. Likewise, reduced levels of c-fos mRNA in the medial geniculate nucleus (MGN) of the thalamus were observed after repeated exposure to loud noise. However, Campeau et al. (2002) found that repeated exposure to loud noise stress did not reduce c-fos mRNA expression in lower auditory sensory areas like the cochlear nuclei, nucleus of the trapezoid bodies, superior olivary complex, nuclei of the lateral lemniscus, and inferior colliculus compared to single acute noise exposure. It should be noted that lower somatosensory processing regions such as the dorsal column nuclei (nuclei gracile and cuneate) have not been assessed following acute or repeated restraint. It is conceivable that for both restraint and loud noise stimulation, thalamic sensory information processing areas might provide an important site where the plasticity associated with habituation is mediated.
Interestingly, reversible inactivation of the auditory thalamus (MGN) by muscimol led to disrupted HPA axis habituation to repeated loud noise (Day et al., 2009). Inactivation of the MGN also disrupted the acute response to loud noise. Bhatnagar et al. (2002) found that ibotenic acid lesions of the posterior paraventricular nucleus of the thalamus disrupted HPA axis habituation to repeated restraint, without significantly disrupting the acute responses to restraint. These studies then support the hypothesis that habituation-induced plasticity may be mediated at the level, or targets, of the thalamus. One of the main projection areas of the MGN (auditory thalamus) is the auditory cortex (Ryugo & Killackey, 1974). Therefore, the present study tested the role of the auditory cortex (both primary and secondary), a target of the auditory thalamus, in HPA axis habituation to repeated loud noise stress exposures. Both pre-habituation and post-habituation lesions were produced to determine the role of the auditory cortex in acquisition, expression, and retention of habituated HPA axis responses, as measured by ACTH and corticosterone release. To verify that the bilateral auditory cortex lesions were functionally effective, an acoustic startle reflex gap detection test was employed. Auditory cortex lesions have been reported to increase the threshold detection of a short quiet period (gap) during a background noise exposure (Bowen et al., 2003, Kelly et al., 1996). Our findings reveal that large bilateral auditory cortex lesions that significantly attenuated gap detection do not disrupt the acquisition or the expression of HPA axis response habituation to repeated loud noise exposures.
2. Results
2.1 Experiment 1
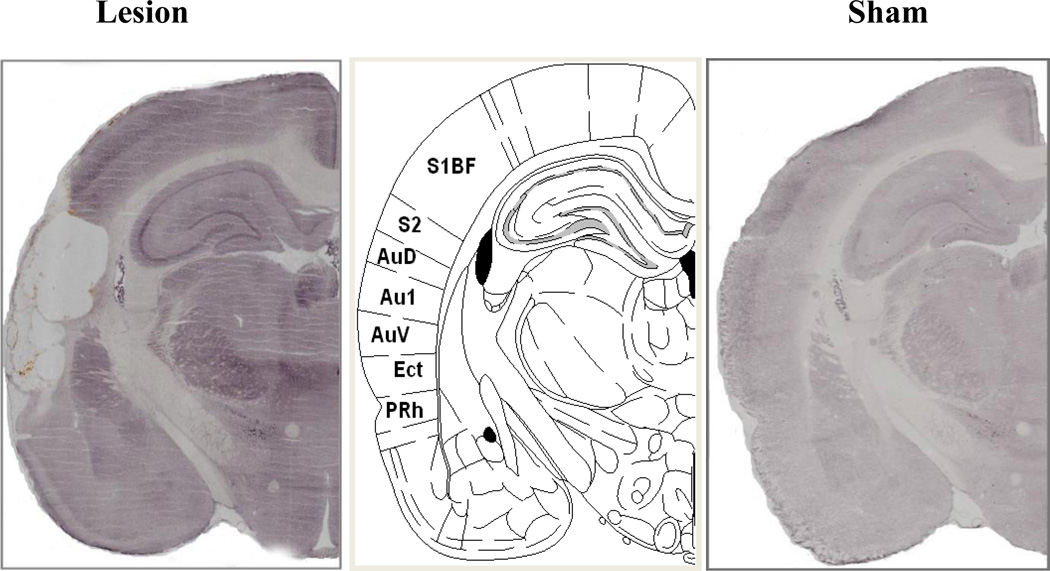
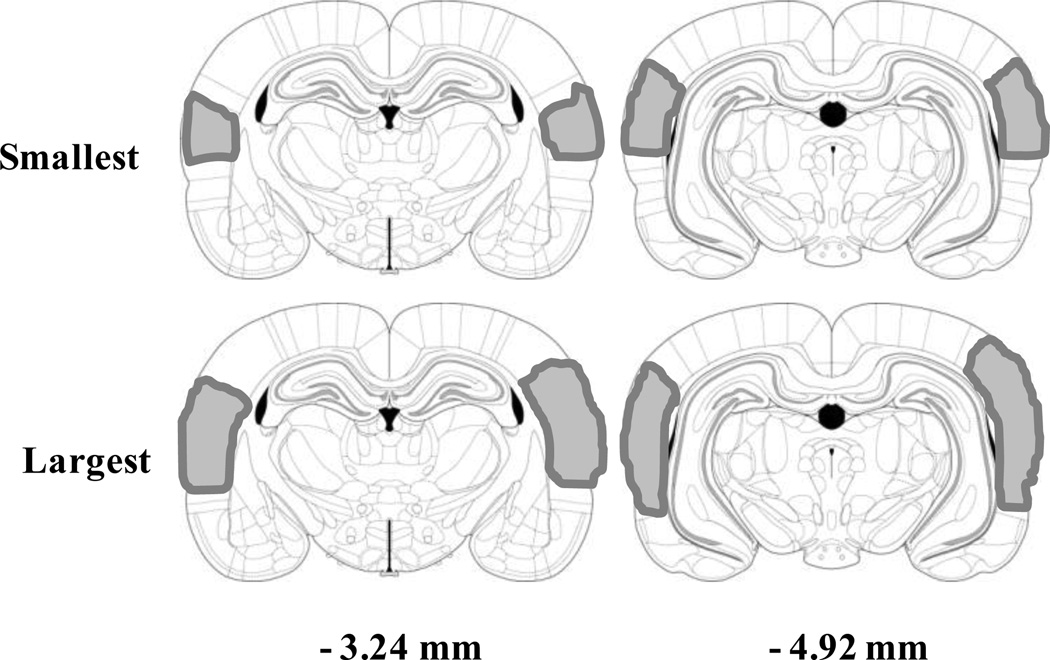
A total of 14 of the 32 lesions attempted were assessed as complete and used in the data analysis. Complete lesions included damage to all of the auditory cortical areas including the primary and secondary auditory cortices. Additionally, the larger lesions encroached to a variable extent into the motor cortex, ectorhinal cortex and perirhinal cortex. Rats with incomplete or unilateral lesions were excluded from the analyses. A representative example of a complete lesion is shown in Figure 1, while representations of the largest and smallest lesions that were determined as complete are displayed in Figure 2.
Figure 1.
Representative examples of NeuN immunohistochemistry of a complete auditory cortex lesion or sham-operated control section at the same level of the middle image of Figure 61 (−3.36 mm from bregma) from Paxinos and Watson’s The Rat Brain in Stereotaxic Coordinates, 5th Edition. Au1: primary auditory cortex; AuD: secondary auditory cortex, dorsal part; AuV: secondary auditory cortex, ventral part; Ect: ectorhinal cortex; PRh: perirhinal cortex; S1BF: primary somatosensory cortex, barrel field; S2: secondary somatosensory cortex.
Figure 2.
Lesion locations on coronal sections at −3.24, and −4.92 mm from bregma, with representative smallest (top) and largest (bottom) lesions shown. Shaded areas represent loss of NeuN cells.
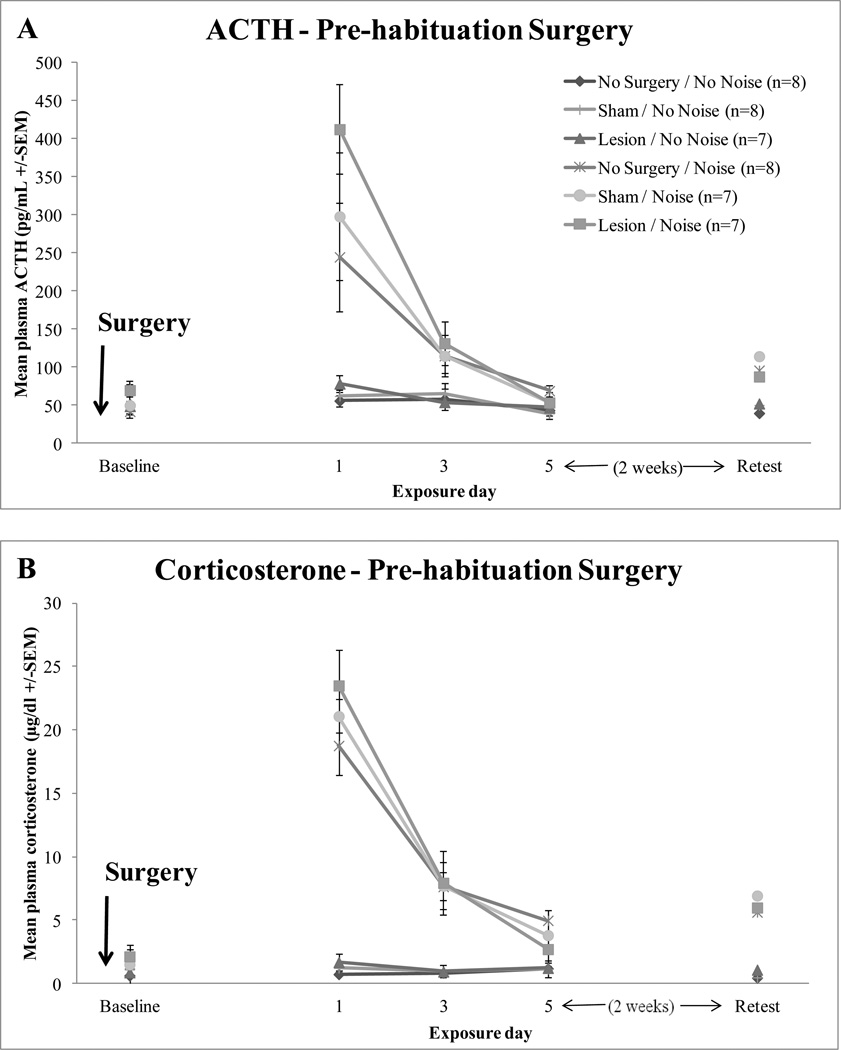
Auditory cortex lesions did not modify the rate or extent of HPA axis habituation to repeated loud noise exposures, as depicted in Figure 3. A significant effect of day (1, 3, and 5) was found for both ACTH and corticosterone, F(2,76) = 30.556, p < 0.0001 and F(2,78) = 108.190, p < 0.0001), suggesting that the responses habituated over time. A significant day x noise interaction effect was also found for ACTH, F(2,76) = 22.905, p < 0.0001 and corticosterone, F(2,78) = 105.179, p < 0.0001) indicating a reduction of both responses over days but only in the noise condition. The day x surgery interaction was not significant for ACTH, F(4,76) = 1.383, p = 0.262 or corticosterone, F(4,78) = 1.763, p = 0.167. The day x surgery x noise interactions were not found to be significant for either ACTH: F(4,76) = 0.810, p = 0.466 or corticosterone: F(4,78) = 1.019, p = 0.338. Overall significant main between-subject effects were found for noise, ACTH: F(1,38) = 53.793, p < 0.0001 and corticosterone: F(1,39) = 169.193, p < 0.0001. However, surgery did not significantly modify ACTH or corticosterone levels, F(2,38) = 1.304, p = 0.283 and F(2,39) = 0.242, p = 0.786, respectively. The surgery x noise interaction was similarly not significant for ACTH, F(2,38) = 0.835, p = 0.442 and corticosterone, F(2,39) = 0.047, p = 0.954).
Figure 3.
Mean hormonal responses of no surgery control, sham-operated control, and lesioned rats post-surgery. Baseline measurement, days 1, 3, and 5 of 30 min 95 dBA white noise or no noise exposure and an additional retest of the 30 min noise or no noise exposure. Panel A depicts mean plasma ACTH (+/−SEM). Panel B depicts mean plasma corticosterone (+/−SEM) responses.
Pre-habituation auditory cortex lesions also did not disrupt retention of habituated HPA axis responses when retested two weeks after the 5th exposure. Univariate ANOVAs revealed a significant effect of noise on plasma ACTH, F(1,40) = 8.848, p < 0.006, and corticosterone levels, F(1,40) = 22.528, p < 0.0001. However, no significant effects of surgery were found for ACTH, F(2,40) = 0.277, p = 0.760 or corticosterone, F(2,40) = 0.066, p = 0.936 or their interaction, F(2,40) = 0.232, p = 0.794 and F(2,40) = 0.049, p = 0.952 for ACTH and corticosterone, respectively.
Plasma ACTH and corticosterone levels were additionally determined before noise exposures to determine if auditory cortex lesions or sham surgery produced any changes in basal levels of these hormones. ANOVA revealed that there were no basal differences in ACTH or corticosterone between lesion, sham-operated, and no surgery control rats, F(2,44) = 1.084, p = 0.348 and F(2,44) = 0.460, p = 0.635, respectively.
2.2 Experiment 2
A total of 14 of the 30 lesions attempted were assessed as complete and used in the data analysis. Complete lesions were assessed as described in Experiment 1.
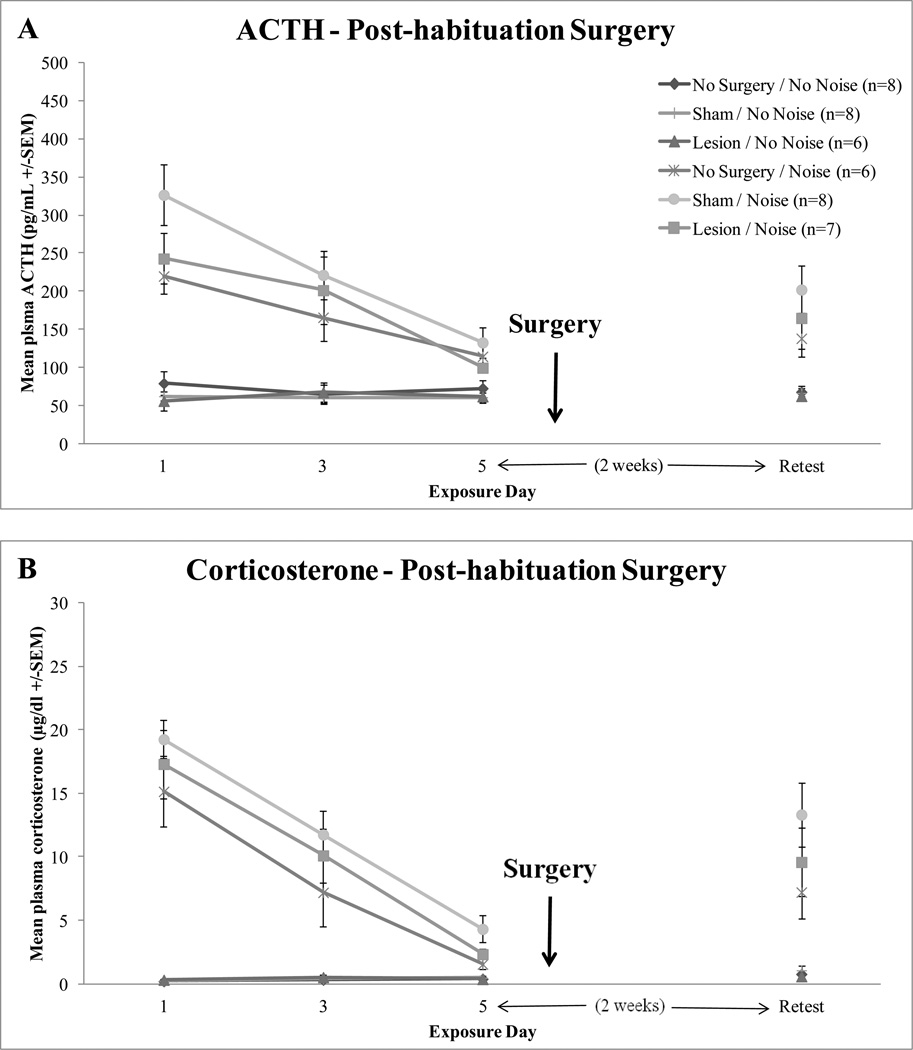
Post-acquisition auditory cortex lesions did not affect retention of habituated HPA axis responses when retested two weeks after the 5th consecutive exposure, as shown in Figure 4. Univariate ANOVAs revealed a significant effect of noise for ACTH, F(1,40) = 23.256, p < 0.0001, and corticosterone, F(1,40) = 37.367, p < 0.0001. Importantly, no significant effect of surgery on ACTH (F(2,40) = 0.887, p = 0.420) or corticosterone levels (F(2,40) = 1.626, p = 0.209) or surgery x noise interactions were found on either ACTH or corticosterone, F(2,40) = 0.907, p = 0.412 and F(2,40) = 1.309, p = 0.281, respectively.
Figure 4.
Mean hormonal responses of no surgery control, sham-operated control, and lesioned rats before surgery. Days 1, 3, and 5 of 30 min 95 dBA white noise or no noise exposure are depicted. An additional retest of the 30 min noise or no noise exposure post-surgery is also shown. Panel A depicts mean plasma ACTH (+/−SEM) responses. Panel B depicts mean plasma corticosterone (+/−SEM) responses.
Pre-lesion habituation on days 1, 3, 5 went as expected. A significant effect of day was found for both ACTH and corticosterone, F(2,78) = 18.811, p < 0.0001 and F(2,80) = 58.172, p < 0.0001 respectively, suggesting that the responses habituated over time. Prior to the surgeries, all the groups displayed equivalent habituation to repeated loud noise exposures (ACTH: F(2,39) = 1.382, p = 0.263, corticosterone: F(2,40) = 1.619, p = 0.211).
2.3 Experiment 3
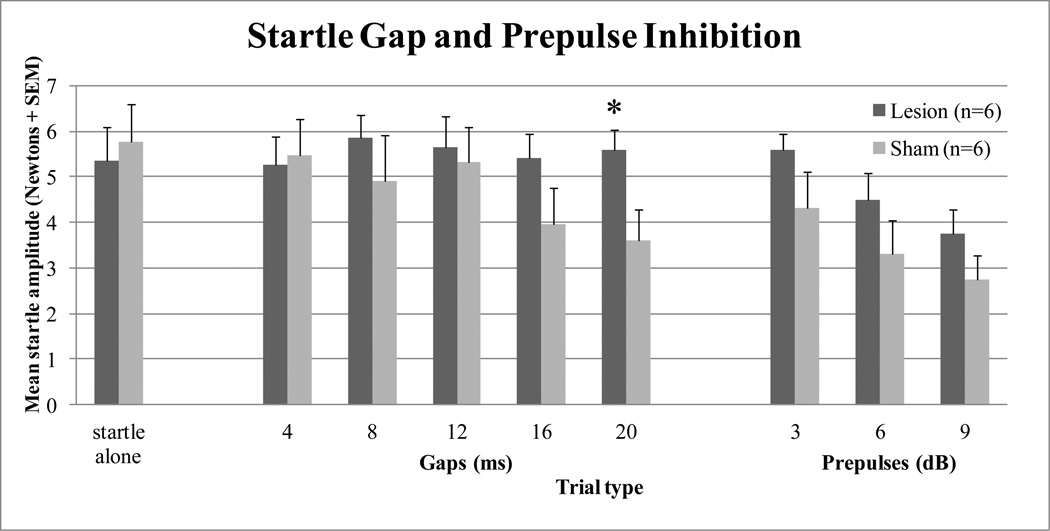
A total of 6 out of 10 lesions attempted were assessed as complete and used in the data analysis. Complete lesions were assessed as described in Experiment 1. The initial startle alone trials were employed to produce reflex habituation to reduce response variability and were therefore excluded from analyses. The startle alone trials that were embedded within the main prepulse/gap test were assessed to determine overall startle response differences between the different surgical groups. No significant difference was found between the sham-operated and auditory cortex lesioned groups, F(1,10) = 0.143, p = 0.713. Significant within-subject differences were found for gap duration, F(4,40) = 8.361, p < 0.001, suggesting that the noise gaps were detected. Additionally, a significant gap duration x surgery interaction was revealed, F(2,40) = 8.652, p < 0.001. However, no significant main effect of surgery was revealed, F(1,10) = 0.885, p = 0.369. Additional analyses of the different gap duration trials indicated that the interaction effect was due to a significant difference between surgery groups at the 20 ms gap duration, F(1,10) = 5.861, p = 0.036, without significant differences at any other gap durations, as shown in Figure 5. Significant within-subject differences were found for prepulse intensity, F(2,20) = 26.596, p < 0.0001, suggesting that the noise prepulses were detected. But there was no significant prepulse intensity x surgery interaction, F(2,20) = 0.162, p = 0.802, nor was there a significant main effect of surgery, F(1,10) = 2.041, p = 0.184, suggesting that auditory cortex lesions did not significantly disrupt prepulse inhibition, as shown in Figure 5.
Figure 5.
Graph showing mean amplitude startle (Newtons +SEM) for sham-operated control and auditory cortex lesioned rats by trial type. Depicted are startle alone (105 dBA noise) trials, noise gap inhibition trials (4, 8, 12, 16, and 20 ms gaps), and prepulse inhibition trials (3, 6, and 9 dB above background noise levels). * Auditory cortex lesioned group significantly different from sham-operated group, p = 0.036.
3. Discussion
The present studies provide evidence that the auditory cortex does not modulate the acquisition, expression, or retention of habituated HPA axis responses to repeated loud noise stress. Large bilateral auditory cortex lesions did not affect basal levels or the rate or extent of plasma ACTH or corticosterone habituation over 5 consecutive daily exposures to loud noise stress compared to sham-operated and no surgery control rats. Likewise, auditory cortex lesions produced before or after the 5 days of stress exposures did not affect the retention of habituated ACTH or corticosterone levels when an additional noise exposure was given 2 weeks after the 5th noise exposure.
The final experiment was conducted to provide some evidence of the functional effects of the lesions on a task previously reported to be sensitive to auditory decortications, which was important because of the lack of effect of the lesions on habituation. Auditory cortex lesions have been shown to impair the ability of rats to discriminate gaps in noise (Bowen et al., 2003, Kelly et al., 1996), whereas the lesions were not reported to significantly modify the acoustic startle reflex (Bowen et al., 2003), or pre-pulse inhibition of acoustic startle (Floody et al., 2010). The results of the current study found no differences in mean startle amplitude between groups (large auditory cortex lesion rats, 5.4 Newtons; and sham-operated rats, 5.8 Newtons). Auditory cortex lesioned rats also did not significantly differ in their ability to detect pre-pulses of 3, 6, or 9 dBA above background noise levels compared to sham-operated control rats. A significant gap duration x surgery group difference was found which was due to the auditory cortex lesioned rats showing a significant deficit at detecting gaps in noise, but only at the longest gap interval (20 ms). It appears that only at the longer gap intervals do the sham rats detect the gaps. This was surprising as previous studies found effects of auditory cortex lesions at gap durations of 2 – 10 ms, but this may be due to methodological differences including stimulus noise intensity and other employed trial types (Bowen et al., 2003).
The results of the present study strongly suggest that the auditory cortex does not mediate the acquisition, expression, or retention of HPA axis response habituation to repeated loud noise exposures. These results are similar to those found for fear conditioning, in which pre- and post-training primary auditory cortex lesions do not reliably disrupt fear-potentiated startle or conditioned emotional responses (Campeau and Davis, 1995, LeDoux et al., 1983). Combined with the findings that the auditory thalamus is necessary for the acquisition of habituated HPA axis responses to repeated loud noise exposures (Day et al., 2009), the evidence points to a subcortical projection from the auditory thalamus in the mediation of habituation to repeated loud noise. The recent demonstration that the basolateral complex of the amygdala is an important substrate for habituation of HPA axis responses to repeated restraint (Grissom and Bhatnagar, 2011) suggest this site as a putative target of the auditory thalamus for habituation to repeated loud noise exposures. There are, however, a number of additional subcortical auditory targets, such as the posterior hypothalamus, the preoptic area, and regions of the bed nucleus of the stria terminalis that may provide important substrates for the acquisition and retention of habituated HPA axis responses to stress (Campeau and Watson, 2000). In conclusion, despite the fact that the auditory cortex is a major projection target for the MGN, which has previously been shown to be necessary for acquisition of HPA axis response habituation to loud noise stress (Day et al., 2009), this regions does not appear to be important for the acquisition, expression or retention of HPA axis response habituation to loud noise. More likely, one or more subcortical targets of the MGN are required for this particular stress adaptation.
4. Experimental Procedure
4.1 Experiment 1
4.1.1 Subjects
Sixty-three male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225 – 250 g were used. They were housed in a dedicated colony facility and initially grouped four to five in clear polycarbonate cages (48 × 27 × 20 cm) containing wood shavings, and covered with wire lids providing food (rat chow) and water ad libitum. Animals were housed for a period of 7 days after arrival from the supplier, before any experimental manipulations were conducted. They were kept on a controlled light/dark cycle (lights on 7:00 am – off at 7:00 pm), under constant humidity and temperature conditions. All procedures were performed between 8:00 am and 2:00 pm to reduce variability due to normal circadian hormonal variations. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the United States of America National Institute of Health Guide for the Care and Use of Laboratory Animals.
4.1.2 Surgery
Rats were anesthetized with halothane and placed in a Kopf stereotaxic apparatus. The skin overlying the skull was disinfected (Betadine), an incision made, and small burr holes drilled through the skull bone to allow penetration of the injector (Hamilton 1 µl syringe). Bilateral excitotoxic lesions (two per side) were produced by injections of 0.25 µl ibotenic acid (10 µg/µl in 0.1 M sodium phosphate buffer, pH: 7.4; Ascent Scientific, Princeton, NJ). The rate of infusion was 0.05 µl/min. The injector was lowered in the brain and left in place 3 min before and 5 min after each injection. The flat skull coordinate system of Paxinos and Watson (2005) was used to determine coordinates for the auditory cortex lesions: AP −3.6, ML +/− 6.4, DV −5.6 and AP −5.5, ML +/− 6.8, DV −5.3. Following the injections, the scalp incision was closed with surgical stainless steel wound clips, and rats were kept warm until recovery from anesthesia. The same procedure was followed for the sham-operated rats except that only the vehicle solution (0.1 M sodium phosphate buffer) was injected. No surgery control rats were left in the animal colony room and were weighed and handled. All rats were single housed and kept under close observation for any indications of weight loss or other debilitating signs post-surgery and allowed at least 7 days to recover before testing.
4.1.3 Noise Apparatus
The acoustic chambers used in this experiment consisted of ventilated double wooden (2.54 cm plywood board) chambers, with the outer chamber lined internally with 2.54 cm insulation (CelotexTM). The internal dimensions of the inner box were 59.69 cm (w) × 38.10 cm (d) × 38.10 cm (h), which allows placement of a polycarbonate rat home cage. Each chamber was fitted with a single 15.24 cm × 22.86 cm Optimus speaker (#12-1769 - 120 W RMS) in the middle of the ceiling. Lighting was provided by a fluorescent lamp (15W) located in the upper left corner of the chamber. Noise was produced by a General Radio (#1381) solid-state random-noise generator with the bandwidth set at 2 Hz-50 kHz. The output of the noise generator was amplified (Pyramid Studio Pro #PA-600X), and fed to the speakers. The speaker characteristics allowed relatively flat delivery between 20 and 27,000 Hz, rolling off quickly (20 dB/octave) at both ends of the spectrum. Noise intensity was measured by placing a Radio Shack Realistic Sound Level Meter (A scale; #33-2050) in the rat's home cage at several locations and taking an average of the different readings. The noise intensity was checked daily before and after each session. The intensity level was set at 95 dBA. The ambient/quiet (no noise) level inside the chamber is approximately 60 dBA, and approximately 55 dBA in the rat colony.
4.1.4 Procedure
Seven days after lesion or sham surgery, all the rats were acclimated to the noise chambers 30 min / day for 3 consecutive days. The rats in their home cages were always placed in the same chamber. After the 3 day acclimation period, the rats were placed in the chambers for 30 min / day for 5 days and then an additional test day 2 weeks after the last exposure (retest day) and exposed to noise (95 dBA) or no noise. Groups were no surgery / no noise (n=8), no surgery / noise (n=8), sham surgery / no noise (n=8), sham surgery / noise (n=7), lesion / no noise (n=7 after verification), and lesion / noise (n=7 after verification). Blood was collected on days 1, 3, and 5 by restraining the rats gently after the 30 min noise or no noise exposure and making a small incision in the lateral tail vein with the corner of a razor blade. Additionally, three days before the 5 days of noise or no noise exposures, blood was collected to determine if there were basal differences between groups. Approximately 400 µl of blood was collected, using hematocrit capillaries, and deposited into 1.5 mL microcentrifuge tubes containing EDTA on ice. The whole procedure lasted less than 3 min from removal to return of the rat to its home cage. The choice of the 30 min noise or no noise exposure period was dictated by our previous findings that indicated peak ACTH and corticosterone release in response to loud noise 30 min after the onset of the stressor (Patz et al., 2006). On the retest day, the rats were re-exposed to the noise condition they had previously been tested with to determine retention of habituated responses. After the 30 min exposure, the rats were rapidly decapitated and blood collected in ice-chilled tubes containing EDTA (20 mg/ml) and brains collected for lesion verification.
4.1.5 Corticosterone ELISA
The corticosterone enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s instructions (kit #ADI-901-097 – Enzo Life Sciences, Ann Arbor, MI), except for an adaptation for using a smaller volume of plasma, as follows. The steroid displacement reagent (provided with the kit) was added to the assay buffer at a concentration of 0.5 µl/ml. Plasma (10 µl) was diluted 1:50 with the amended assay buffer. The diluted plasma sample (100 µl) was then processed as described in the kit directions. This method used equivalent final concentrations of steroid displacement reagent, and was determined to result in equivalent assayed corticosterone levels as the standard method, in which 2.5 µl steroid displacement reagent was added to 97.5 µl plasma, and the plasma diluted with standard assay buffer (data not shown). All samples were run in the same assay. Levels were then quantified on a BioTek Elx808 microplate reader and calculated against a standard curve generated concurrently.
4.1.6 ACTH Radioimmunoassay
The ACTH radioimmunoassay was performed according to the manufacturer’s instructions (ACTH IRMA Ref 27130 - Diasorin, Stillwater, MN). Fifty to 200 µl of plasma was used for this assay. The plasma was incubated overnight with an 125I-labeled monoclonal antibody specific for ACTH 1–17, a goat polyclonal antibody specific for ACTH 26–39, and a polystyrene bead coated with a mouse anti-goat antibody. Only ACTH 1–39 in the sample bound both antibodies to form an antibody complex. Beads were washed to remove unbound radioactivity, counted with a gamma counter, and the concentrations of ACTH values were quantified and calculated against a standard curve generated concurrently. The sensitivity of the assay ranged from 1.5 to 1400 pg/ml. All samples from this experiment were run in the same assay.
4.1.7 NeuN Immunohistochemistry
To determine the areas of neuronal loss after the ibotenic acid lesions, neuronal nuclei (NeuN) immmunoreactivity was examined. Frozen brains were sectioned through the auditory cortex with a cyrostat (Leica 1850) at a thickness of 30 µm in the coronal plane, and thaw mounted onto microfrost plus glass slides (Fisher, cat #12-550-19). All of the incubations were carried out with gentle agitation at room temperature and the sections were washed in 0.1 M phosphate buffer saline (PBS) between incubations in different solutions. The sections were first fixed in 4% paraformaldehyde for 60 minutes. Sections were washed and incubated in a 0.1 M PBS solution containing 0.3% hydrogen peroxide for 20 minutes. Sections were then blocked for 20 minutes each in avidin and biotin blocking solutions (blocking kit #SP-2001, Vector Laboratories, Burlingame, CA). After a 1-hr incubation in the immunohistochemical diluent (0.1 M PBS containing 0.25% carageenan lambda and 0.5% Triton X-100), sections were incubated in the immunohistochemical diluent containing a mouse anti-NeuN (1:1,000; Chemicon International, Temecula, CA) 40 – 50 hours at 4° C. Sections were then washed and incubated in a solution containing a biotinylated anti-mouse secondary antibody raised in horse (1:500) for 2 hours, then washed and incubated for an additional 2 hours in the ABC complex (Vectastain Elite ABC peroxidase kit; Vector Laboratories, Burlingame, CA). Sections were again washed before a peroxidase reaction was performed using the chromagen 3,3’-diaminobenzidene (DAB) enhanced with nickel chloride and hydrogen peroxide (Vector kit SK-4100, carried out according to manufacturer’s instructions). The slides were then dehydrated, coverslipped, and examined for complete neuronal loss in the primary and secondary auditory cortices. Eighteen rats were excluded from this experiment due to incomplete / missed lesions.
4.1.8 Data Analysis
Plasma ACTH and corticosterone data from basal samples were analyzed using one way analyses of variance (ANOVA’s) with surgery (lesion, sham, or no surgery control) as the between groups variable. Plasma ACTH and corticosterone data from days 1, 3, and 5 of noise or no noise exposures were analyzed using a multivariate design. Days (1, 3, and 5) were used as within-subject factors (Greenhouse-Geisser test) and surgery (lesion, sham-operated, or no surgery) and noise (95 dBA or no noise) were used as between-subject factors. Additionally, plasma ACTH and corticosterone data from the retest day were examined using a univariate ANOVA with surgery and noise set as between-subject factors to examine the responses upon re-exposure after the two week delay.
4.2 Experiment 2
4.2.1 Procedure
Sixty-two male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 225 g were used. These rats were housed and treated identically as the rats in Experiment 1 with the following exceptions. Seven days after arrival to the animal colony, all the rats were acclimated to the noise chambers 30 min / day for 3 consecutive days. After the 3 day acclimation period, the rats were placed in the chambers for 30 min / day for 5 days, and exposed to 95 dBA or no noise as described for Experiment 1. After the 5 days of noise / no noise manipulations, the rats were divided into groups and surgery was performed as described in Experiment 1. The groups were no surgery / no noise (n=8), no surgery / noise (n=8), sham surgery / no noise (n=8), sham surgery / noise (n=7), lesion / no noise (n=6 after verification), and lesion / noise (n=8 after verification), depending on previous noise exposure conditions. Approximately a week after surgery (and 2 weeks after the last noise / no noise exposure, the rats were given an additional test day and exposed to noise (95 dBA) or no noise. Blood was collected on days 1, 3, 5, and retest day as described in Experiment 1. Basal blood sampling was not conducted because Experiment 1 revealed no significant differences in basal ACTH or corticosterone levels in surgery vs. no surgery rats. Plasma ACTH and corticosterone level determination, use of NeuN for lesion verification, and data analysis were conducted as described in Experiment 1. Seventeen rats were excluded from this experiment due to incomplete / missed lesions.
4.3 Experiment 3
4.3.1 Subjects
Sixteen male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 225 g were used. These rats were housed as described in Experiments 1 and 2.
4.3.2 Startle Testing
Startle testing was performed in eight identical ventilated isolation chambers (Hamilton-Kinder LLC, San Diego,CA), and illuminated by 8W bulbs located in the ceiling of the chambers. Each startle chamber contained a rat holder (19.05 × 9.14 × 10.67 cm acrylic cage) held in place on a platform load cell that detects cage displacement. Startle amplitude was defined as the maximum load cell output (amplified and digitized) during the first 200 ms after the startle stimulus onset. The startle stimuli (pulses) were 50 ms (rise-decay: 1 ms) bursts of white noise at intensities of 105 dBA (sound pressure level, SPL; A scale). Background noise (white noise) of 60 dBA was delivered throughout the startle test. The auditory prepulse stimuli consisted of 20 ms bursts of white noise (rise-decay: 1 ms) at intensities of 63, 66, or 69 dB, presented 50 ms before the pulse stimuli (auditory prepulse trials). The auditory gap stimuli consisted of 4, 8, 12, 16, or 20 ms gaps in the background white noise (reduction of noise from 60 to 52 dBA, which was the ambient noise intensity in the acoustic chamber) presented 75 ms before the pulse stimuli (auditory gap trials). These parameters were derived from Bowen, Lin, Taylor, & Ison (2003). For the startle test, the rats were placed in the holders, and after a 5 min acclimation period, the first of 20, 105 dBA pulses were presented at a fixed 20-s interstimulus interval (ISI). These initial startle pulses produce some startle habituation, which reduce variability and stabilize baseline startle amplitudes, but they were not used in the estimation of startle modification. Twenty seconds later, the first of 100 additional test trials were presented, at a 20 sec ISI, consisting of pulse alone stimuli (20 trials), intermixed with prepulse + pulse trials (10 trials at each prepulse intensity level), or auditory gap + pulse trials (10 trials at each gap duration). There were 10 occurrences of each trial type in a quasi-random sequence.
4.3.3 Procedure
Seven days after arrival to the animal colony, all the rats were given an initial startle test as described above. Approximately one week after testing, the rats were divided into groups and surgery was performed as described in Experiment 1. The groups were sham surgery (n=6) or bilateral auditory cortex lesions (n=6 after verification). Approximately two weeks post-surgery, the rats were given the startle test as described above.
4.3.4 Data Analysis
Only data from the post-surgery startle test were examined. Startle alone data was analyzed with a one-way ANOVA with surgery as the between-subjects factor. Gap detection was analyzed using multivariate ANOVA with the gap duration (4, 8, 12, 16, and 20) as the within-subject factors and surgery (lesion or sham) as the between-subjects factor. Prepulse inhibition was analyzed using multivariate ANOVA with prepulse intensity (3, 6, and 9 dB above background) as the within-subjects factor and surgery (lesion or sham) as the between-subjects factor.
Highlights.
-
-
Auditory cortex lesions disrupt noise gap detection
-
-
Auditory cortex lesions do not disrupt HPA axis habituation
-
-
Acquisition, expression and retention of habituation retained after AC lesions
Acknowledgements
This research was supported by NIMH R01 MH077152 (SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behav Neural Biol. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Mitchell J, Betito P, Meaney M. Effects of chronic intermittent cold stress on pituitary adrenocortical and sympathetic adrenomedullary functioning. Physio Behav. 1995;57:633–639. doi: 10.1016/0031-9384(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Carew TJ, Kandel ER. Acquisition and retention of long-term habituation in Aplysia. Science. 1973;182:1158–1160. doi: 10.1126/science.182.4117.1158. [DOI] [PubMed] [Google Scholar]
- Christoffersen GRJ. Habituation: Events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Day EW, Masini CV, Campeau S. Reversible inactivation of the auditory thalamus disrupts HPA axis habituation to repeated loud noise stress exposures. Brain Res. 2009;1276:123–130. doi: 10.1016/j.brainres.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van Der Gugten J. Plasma catecholamine, corticosterone, and glucose responses to repeated stress in rats: Effect of interstressor interval length. Physiol Behav. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- Floody OR, Ouda L, Orter BA, Kilgard MP. Effects of damage to auditory cortex on the discrimination of speech sounds by rats. Physiol Behav. 2010;101:260–268. doi: 10.1016/j.physbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L, Kaplan SW, Cohen TE, Henzi V, Kandel ER, Hawkins RD. A simplified preparation for relating cellular events to behavior: contribution of LE and unidentified siphon sensory neurons to mediation and habituation of the Aplysia gill- and siphon-withdrawal reflex. J Neurosci. 1997;17:2900–2913. doi: 10.1523/JNEUROSCI.17-08-02900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neurosci. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gover TD, Abrams TW. Insights into a molecular switch that gates sensory neurons synapses during habituation in Aplysia. Neurobiol Learn Mem. 2009;92:155–165. doi: 10.1016/j.nlm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Bhatnagar S. The basolateral amygdala regulates adaptation to stress via β-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinases. Neurosci. 2011;178:108–122. doi: 10.1016/j.neuroscience.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behav Neurosci. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1983;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Masini CV, Day HE, Campeau S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behav Neurosci. 2008;122(1):210–223. doi: 10.1037/0735-7044.122.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Day HE, Gray T, Crema LM, Nyhuis TJ, Babb JA, Campeau S. Evidence for a lack of phasic inhibitory properties of habituated stressors on HPA axis responses in rats. Physiol Behav. 2012;105(2):568–575. doi: 10.1016/j.physbeh.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav. 1988;43:41–46. doi: 10.1016/0031-9384(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Nyhuis TJ, Sasse SK, Masini CV, Day HEW, Campeau S. Lack of contextual modulation of habituated neuroendocrine responses to repeated audiogenic stress. Behav Neurosci. 2010;124(6):810–820. doi: 10.1037/a0021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz MD, Day HE, Burow A, Campeau S. Modulation of the hypothalamo-pituitary-adrenocortical axis by caffeine. Psychoneuroendocrinol. 2006;31(4):493–500. doi: 10.1016/j.psyneuen.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Vol. 5th Edition. San Diego, CA: 2005. [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during presentation of two intensities of restraint stress: chronic stress and habituation. Physio Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Killackey HP. Differential telencephalic projections of the medial and ventral divisions of the medial geniculate body of the rat. Brain Res. 1974;82:173–177. doi: 10.1016/0006-8993(74)90903-2. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]