Abstract
Rats exposed to a high binge-like dose of alcohol over postnatal days (PD) 4-9 show reductions in CA1 pyramidal cells and impairments on behavioral tasks that depend on the hippocampus. We first examined hippocampal c-Fos expression as a marker of neuronal activity in normally developing rats following different phases of the context preexposure facilitation effect (CPFE) paradigm (Exp. 1). During the CPFE, preexposure to the training context facilitates contextual conditioning to an immediate shock given on a subsequent occasion. We then examined the relationship between CPFE impairment, hippocampal cell loss and c-Fos expression in rats exposed to alcohol over PD 4-9 (Exp. 2). Normally developing (Exp. 1), sham-intubated control (SI), and PD 4-9 alcohol-exposed (4.00g and 5.25g/kg/day; Exp. 2) juvenile male rats were trained on the CPFE. The CPFE occurs over three phases separated by 24h. Starting on PD 31, rats were preexposed to Context A or Context B for five minutes. 24h later, all rats received an immediate, 1.5 mA foot shock in Context A. Finally, rats were tested for contextual conditioning in Context A on PD 33. Normally developing and SI rats preexposed to Context A showed enhanced contextual fear compared to those preexposed to Context B (Exp. 1) or alcohol-exposed rats preexposed to Context A (Exp. 2). Rats were sacrificed 2h following different phases of the CPFE and processed for c-Fos immunohistochemistry (Exp. 1 and 2) and CA1 pyramidal cell quantification (Exp. 2). In Exp. 1, c-Fos+ cells in the DG were consistently high among rats preexposed to Context A (Pre), Context B (No Pre), or sacrificed directly from their home cage (Home) and did not differ across CPFE phases. CA3 and CA1 c-Fos+ cells were highest during preexposure and decreased across training phases, with Group No Pre showing greater numbers of c-Fos+ cells during training than Group Pre and Controls. In Exp. 2, SI rats had greater numbers of CA1 c-Fos+ cells compared alcohol-exposed rats, differing significantly from rats exposed to the high alcohol dose (5.25g) over PD4-9. Exp. 2 also revealed a linear decline in CA1 pyramidal cells across treatment groups, again with rats from the high alcohol dose group showing significantly fewer CA1 pyramidal cells compared to SI. Our results reveal that context novelty may be a significant contributor to differential hippocampal c-Fos expression following different phases of the CPFE. In addition, lower levels of c-Fos+ cells in alcohol-exposed rats following preexposure may be related to general reductions in the number of CA1 pyramidal cells in these rats. The significant CPFE impairments in rats exposed to the lower alcohol dose (4.00g), who show a 15% reduction in CA1 pyramidal cells compared to SI rats, highlights the sensitivity of the CPFE to hippocampal insult.
INTRODUCTION
The developing hippocampus is susceptible to the teratogenic effects of alcohol. Children and young adults with Fetal Alcohol Spectrum Disorders (FASD) show significant impairments on a number of behavioral tasks that recruit the hippocampus (Uecker and Nadel, 1996, Hamilton, 2003, Willoughby et al., 2008, Bissiere et al., 2011); and structural imaging techniques reveal volumetric alterations of the hippocampal formation that are associated with learning impairments (Willoughby et al., 2008, Coles et al., 2010). The behavioral and anatomical abnormalities present in FASD have been largely reproduced using animal models of developmental alcohol exposure (Driscoll et al., 1990, Cudd, 2005, Gil-Mohapel et al., 2010). The rat hippocampus is particularly vulnerable to the effects of alcohol when exposure occurs over postnatal days (PD) 4-9 (Gil-Mohapel et al., 2010), a period of brain development that corresponds to the third-trimester in human development (Dobbing and Sands, 1979). For example, rats exposed to high levels of alcohol over the neonatal period show significant reductions in CA1 pyramidal cells (Maier and West, 2001, Livy et al., 2003, Tran and Kelly, 2003), impaired induction of hippocampal LTP (Bellinger et al., 1999, Puglia and Valenzuela, 2010b), and behavioral deficits on tasks disrupted by hippocampal lesions or inactivation (Goodlett and Johnson, 1997, Hunt et al., 2009, Thomas and Tran, 2011). We recently demonstrated significant impairments in neonatal alcohol-exposed rats on a variant of contextual fear conditioning called the context preexposure facilitation effect (CPFE), a task that is particularly sensitive both to neonatal alcohol and to hippocampal injury (Murawski and Stanton, 2010, Murawski and Stanton, 2011). Less is known about how hippocampal function supports the CPFE or how hippocampal dysfunction in alcohol-exposed rats impairs the CPFE.
If a normally developing rat receives a foot shock immediately upon placement into a context it will show little contextual fear conditioning - this is known as the immediate shock deficit (Fanselow, 1990). Preexposure to the context 24h prior to receiving an immediate shock overcomes this deficit – i.e., context preexposure facilitates contextual conditioning to an immediate shock (Fanselow, 1990). The CPFE paradigm consists of three phases (preexposure, training [immediate-shock], testing) run at 24h intervals. Context conditioning in this paradigm depends on hippocampal processing during each phase (for reviews see O’Reilly and Rudy, 2001, Rudy et al., 2004). For example, the CPFE can be disrupted by lesions, inactivation, or N-Methyl-d-aspartate (NMDA) receptor blockade of the dorsal hippocampus prior to preexposure, or by blocking protein synthesis following preexposure (Barrientos et al., 2002, Rudy et al., 2002, Matus-Amat, 2004, Matus-Amat et al., 2007, Schiffino et al., 2011). The CPFE is also disrupted by pre-training and pre-testing dorsal hippocampus inactivation (Matus-Amat, 2004), with similar disruptions occurring through inactivation of the ventral hippocampus (Rudy and Matus-Amat, 2005, cf., Fanselow and Dong, 2010). Juvenile rats exposed to alcohol over PD 4-9 or 7-9 fail to show the CPFE, demonstrating an immediate shock deficit regardless of preexposure context (Murawski and Stanton, 2010, Murawski and Stanton, 2011). The CPFE impairment in alcohol-exposed rats may reflect a failure to encode a representation of the context during the preexposure phase. Because the hippocampus is involved in encoding a representation of context during preexposure (Rudy and O’Reilly, 2001, Rudy et al., 2004), we sought to determine if hippocampal activity during preexposure is altered in alcohol-exposed rats compared to controls by examining behaviorally induced expression of the immediate early gene product c-Fos.
The CPFE paradigm allows for the independent examination of hippocampal contributions during 1) acquisition of the context representation, 2) acquisition of the context-shock association, and 3) the retrieval/expression of the contextual fear memory because these aspects of the learning experience occur during different phases of the CPFE. Using c-Fos as a delayed, indirect marker of neural activity (Herrera and Robertson, 1996), we first examined the functional contributions of hippocampal subregions (dentate gyrus [DG], CA3, CA1) during the different phases of the CPFE in normally developing rats (Experiment 1). Rats were either preexposed to the testing context or an alternate context during preexposure. The following day, all rats received a foot shock in the testing context immediately upon context placement. Finally, rats were tested for the CPFE 24h later in the testing context. Subsets of rats were sacrificed following different phases of the CPFE and processed for hippocampal c-Fos immunohistochemistry. We predicted enhanced CA1 hippocampal c-Fos expression during periods of active context encoding (preexposure) and retrieval (testing). Because the CA3 is hypothesized to engage pattern completion processes during the training phase of the CPFE (O’Reilly and Rudy, 2001, Rudy and O’Reilly, 2001), we also hypothesized elevated CA3 c-Fos expression in rats preexposed to the training context during immediate shock. Behaviorally, we predicted rats preexposed to the testing context would show the CPFE, whereas those preexposed to the alternate context would show the immediate shock deficit.
We then exposed rats to varying levels of alcohol over postnatal days 4-9 and examined CA1 pyramidal cell counts and c-Fos expression following the preexposure phase and behavior (the CPFE; Experiment 2). We have previously reported a complete absence of the CPFE, but normal cued fear conditioning, in rats exposed to alcohol over PD 4-9 or 7-9, suggestive of hippocampal dysfunction. (Murawski and Stanton, 2010, Murawski and Stanton, 2011). We predicted lower levels of behaviorally-induced CA1 c-Fos expression and pyramidal cell counts, as well as CPFE deficits to appear in alcohol-exposed rats in a dose-dependent manner compared to controls.
MATERIALS AND METHODS
Subjects
The subjects were 159 juvenile male Long Evans rats derived from 19 litters as described in previous reports except where noted (Murawski & Stanton, 2010; 2011). Of these, 81 were used in Experiment 1 and 78 were used in Experiment 2. Briefly, they were the offspring of time-mated females housed with breeder males overnight at the animal housing colony of the Office of Laboratory Animal Medicine at the University of Delaware. Females were inspected for the presence of an ejaculatory plug the following morning and, if found, that day was designated gestational day (GD) 0. The colony was maintained on a 12:12 hour light/dark cycle with lights on at 7:00 am. The date of birth was designated as postnatal day (PD) 0 (all births occurred on GD 22). On PD 3, litters were culled to 9 pups per litter (all male). Males (up to 4) from separate litters were fostered into experimental litters as needed. Pups received subcutaneous injections of a non-toxic black ink into one or more paws to aid in identification on PD 3. On PD 21, pups were weaned and housed in groups of 4 or 5 littermates in 45 × 24 × 17 cm cages. Starting on PD 29, pups were individually housed in small white polypropylene cages (24 × 18 × 13 cm) with ad lib access to food and water for the remainder of the study. All subjects were treated in accord with National Institutes of Health guidelines following protocols approved by the Institutional Animal Care and Use Committee at the University of Delaware.
Neonatal Alcohol Exposure
Seventy-eight rats in Experiment 2 were exposed to alcohol via intragastric intubations or sham-intubated over PD 4-9 following procedures previously described (Kelly and Lawrence, 2008, Murawski and Stanton, 2011). Rat pups from each litter were assigned to receive an alcohol-intubation at either 4.00 or 5.25 g/kg/day or to be sham intubated (3 pups per litter per dosing condition). No more than one pup per litter was assigned to any behavioral or anatomical group.
Neonatal alcohol exposure was as described in previous reports (Murawski and Stanton, 2010, Murawski and Stanton, 2011). On PD 4, pups were briefly separated from their mother and placed as a litter into one of three clear Lexan containers that sat atop a heating pad (GE model #12107) set to the lowest setting, which provided warmth during separation. Intubations involved passing PE10 tubing lubricated with corn oil down the pup’s esophagus and into the stomach. Alcohol was administered in a single binge dose at 18.19% (Group 4.00g) or 23.94% (Group 5.25g) v/v in a costume milk formula solution in a volume of 0.02778 ml/g body weight via a 1 mL syringe attached to the other end of the tubing. Sham intubated controls (Group SI) received an intubation in the absence of any milk or alcohol infusion. Approximately 2 hours (±5 min; Kelly et al., 1987) following the alcohol dose, pups received a small tail-clip and a 20-μL blood sample was collected in heparinized capillary tubes. Blood samples from Group SI were discarded and those from Groups 4.00g and 5.25g were saved for blood alcohol concentration (BAC) analysis (see below). Alcohol-exposed pups received additional milk-only intubations at 2 and 4 hours post-alcohol administration, while sham intubated pups received comparable sham intubations. Dosing continued in this manner over PD 5-9 except that blood samples were not collected and alcohol-exposed pups received only one additional milk-only intubation (Kelly and Lawrence, 2008).
Blood Alcohol Concentration Analysis
Blood samples collected from alcohol-exposed pups on PD 4 were centrifuged and the plasma was collected and stored at −20°C. Blood alcohol concentrations of plasma samples were analyzed using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described (Brown et al., 2007). The rate of oxidation of alcohol in each sample was compared to a known value of an alcohol standard solution. BACs are reported in mg/dl.
Apparatus and Stimuli
The apparatus has been described previously (Murawski and Stanton, 2010, Murawski and Stanton, 2011). Contextual fear conditioning was run over three phases (preexposure, training, and testing; see below). Rats were preexposed to one of two distinct contexts (Context A or Context B). Context A was a clear Plexiglas chamber (16.5 × 21.1 × 21.6 cm) with a stainless steel bar floor (11.5 cm from top of chamber) composed of 9 grid bars (0.5 cm in diameter and placed 1.25 cm apart). The grid floor was connected to a shock scrambler (Med Associates, Georgia, VT ENV-414S) and delivered a foot shock unconditioned stimulus (US). Four chambers of Context A were positioned on a Plexiglas stand and arranged (2 × 2) within a fume hood that provided ambient light and background noise. The sides and floors of two adjacent chambers were made opaque. A video camera recorded activity from all four chambers simultaneously and fed into a Dell computer running FreezeFrame2 software (Actimetrics, Wilmette, IL). The program determined movement by measuring changes in pixel luminance over a set time frame. Freezing was defined as a bout of 0.75 s or longer without changes in pixel luminance. Context B involved modifications to Context A, including a wire mesh insert that covered the floor and back wall, which protruded into the chamber altering the spatial extent of the chamber. Additionally, a paper drape covered three of the four chamber walls, leaving only the wall facing the camera unobstructed.
Contextual Fear Conditioning
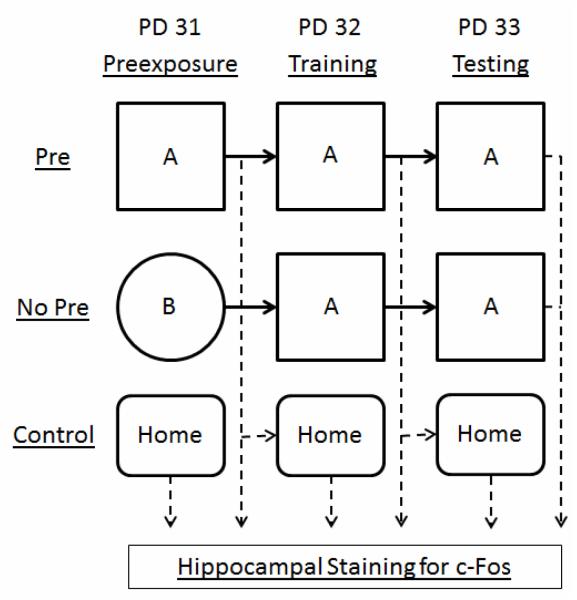
The procedure has been described previously (Murawski and Stanton, 2010, Murawski and Stanton, 2011). Rats were placed into one of three preexposure groups. Rats in Group Pre were preexposed to Context A while rats in Group No Pre were preexposed to Context B. Home-caged controls (Group Control) remained in their home cage. The context preexposure facilitation effect design occurred over three phases starting on PD 31, with each phase separated by 24h (Figure 1). During each of the three phases, rats were weighed, individually placed into a distinctive clear lexan transport cage (11 × 11 × 18 cm) with orange construction paper surrounding four sides, and transported in groups of four to a room adjacent to the conditioning room. The experimenter wiped down each of the four chambers with a 5% ammonium solution before animal placement. During preexposure (PD 31), rats were brought into the conditioning room and placed into either Context A or Context B and allowed to freely explore the context for a 5 minute preexposure period. During training (PD 32), rats were individually brought into the conditioning room and received a 1.5 mA, 2 s foot shock immediately upon placement into Context A. Following training, rats were immediately returned to their home cage. During testing (PD 33), rats were placed into Context A and monitored for freezing behavior over a 5 minute testing phase. In Experiment 1, rats were preexposed to either Context A (Pre) or Context B (No Pre), or sacrificed from their home cage (Control). In Experiment 2, all rats were preexposed to Context A, except for rats sacrificed directly from their home cage.
Figure 1.
Schematic of the three phases (preexposure, training, testing) of the context preexposure facilitation effect (CPFE) behavioral paradigm. Rats are preexposed to one of two contexts (A or B) for 5 minutes. During training, all rats receive an immediate foot shock in Context A. Finally, rats are tested for contextual fear in Context A over a 5 minute testing period. Each phase of the CPFE is separated by 24h. Subsets of rats are sacrificed 2h following different phases of the CPFE (dashed arrows) and processed from c-Fos immunohistochemistry, with additional rats sacrificed directly from their home cage (Control). Pre=preexposed to Context A. No Pre=preexposed to Context B. PD=postnatal day.
Tissue Preparation
Rats from different preexposure groups (Pre, No Pre, Control) were sacrificed 2h following different phases of the CPFE (phase: preexposure, training, testing) when experience-dependent c-Fos expression is high (Morgan and Curran, 1991). Controls were yoked to rats in their Pre or No Pre sampling conditions and were sacrificed prior to particular phases of the CPFE: controls sacrificed prior to preexposure were behaviorally naïve; those sacrificed prior to training received context preexposed to either Context A or B 24h earlier; and those sacrificed prior to testing received an immediate shock 24h and context preexposure 48h earlier. Rats were deeply anesthetized with an injection (ip) of sodium pentobarbital and perfused transcardially with 50 mL of heparinized 0.1 M phosphate-buffered saline (PBS, pH 7.2) followed by 200 mL of cold 4% paraformaldehyde in 0.1 M PBS. The brains were carefully removed, postfixed overnight in 4% paraformaldehyde and transferred to 30% sucrose in 4% paraformaldehyde the next day, and again a week later. Serial horizontal cryostat sections (40 μm) through the entire dorsal-ventral extent of the brain were stored at −20° in cryoprotectant (30% sucrose and ethylene glycol in 0.1M PBS) and processed as free-floating sections for c-Fos.
c-Fos Immunohistochemistry
Every 16th consecutive section (640 μm apart) of the entire hippocampus was chosen in a systematic random manner (the first section was randomly chosen from the first 16th consecutive sections containing the hippocampus) and processed for c-Fos immunohistochemistry. Sections were pre-incubated in 1% hydrogen peroxide for 20 min, washed in Tris-buffered saline (TBS), and transferred to blocking solution (10% normal goat serum [NGS] and 0.5% Triton X-100 in 0.1M TBS). Sections were then incubated in the primary anti-Fos antibody (rabbit polyclonal, raised against a peptide corresponding to amino acids 3-16 of human Fos, Santa Cruz Biotechnology, Santa Cruz, CA; 1:500) for 36h. Following 3 × 10 min rinses in TBS, sections were incubated in corresponding secondary antibody solution (Vector, anti-rabbit, 1:1000) for 1 h and placed into ABC solution for an additional hour (VectaStain ABC kit, Vector Laboratories). All antibodies as well as ABC solutions were diluted in 10% NGS and 0.1M TBS. Immunoreactions were developed in diaminobenzidine (DAB) peroxidase substrate (Sigma) for 5-20 min. Control sections received the same treatment in the absence of the primary antibody. After extensive rinsing in TBS, free-floating sections were mounted onto gelatin-coated slides and cover-slipped.
Cresyl Violet Staining
Every 10th consecutive section of the hippocampus was stained with 0.1% cresyl violet for CA1 pyramidal cell quantification. Briefly, sections were mounted in dH2O onto gelatin-coated slides, stained in 0.1% cresyl violet solution for 25 minutes, run through a series of alcohol baths at increasing concentrations, and cover slipped using DPX mounting medium.
Definitions of Hippocampal Cell Layers
Processing of horizontal sections allows for the examination of all three areas of interest (DG, CA3, and CA1) within the same section of the hippocampus proper between ~−3.0 and −8.1 mm Bregma (Paxinos and Watson, 2005). Definitions follow those as described by Tran and Kelly (Tran and Kelly, 2003). The granule cell layer of the DG is composed of tightly packed granule cells with a clearly defined, roughly U-shaped appearance. Pyramidal cells of CA3 are relatively large and less densely packed together. CA1 is distinguished from CA3 by smaller, tightly packed pyramidal cells along the CA3/CA1 border. CA1 ends at the border with the subiculum, where cell packing become non-contiguous.
Cell Quantification
c-Fos positive (c-Fos+) cells were quantified within a known volume of tissue using one of the unbiased stereology approaches, the optical fractionator probe (StereoInvestigator, Micro Bright Field Inc., Williston, VT). Briefly, StereoInvestigator software calculates the total volume of an outlined brain region (DG, CA2/3, CA1) and calculates an unbiased estimate of cell counts based upon the number of cell counts within a randomized systematic sampling of the outlined brain region and the serial position of a given section (see Tran and Kelly, 2003). c-Fos+ cells were counted within a counting frame set to 100 × 100 μm and a grid size of 200 × 200 μm. The dissector height was 20 μm with a 2 μm guard zone. c-Fos+ cell estimates derive from between 6-8 sections per animal. CA1 neurons stained with cresyl violet were quantified within a counting frame set to 20 × 15 μm and a grid size of 130 × 130 μm (ibid). The dissector height was 6 μm with a 2 μm guard zone. CA1 pyramidal cell estimates derive from between 9-12 sections per rat.
Data Analysis
Data were collected from 159 rats run in two separate experiments. In Experiment 1, 81 normally developing rats provided data for hippocampal c-Fos analysis, including nine rats per group (Pre vs. No Pre vs. Control) per training phase (preexposure vs. training vs. testing); behavioral data from 18 of the 27 rats sacrificed following testing (Pre=9; No Pre=9; excluding 9 Control) were also analyzed. In Experiment 2,a total of 78 rats were exposed to alcohol (4.00 [n=28] or 5.25g [n=23]/kg/day) or sham intubated (SI; n=27) over PD 4-9. Twenty-eight rats (SI=10; 4.00g=10; 5.25g=8) were sacrificed following preexposure provided data for CA1 c-Fos analysis and pyramidal cell counts. CA1 c-Fos analysis also included an additional nine rats (SI=3, 4.00g=3, 5.25g=3) sacrificed directly from their home cage (Control). Thirty-one rats were tested for contextual freezing. One rat from each of the dosing conditions was excluded from behavioral analysis for meeting the criterion of an outlier (± 2 SD from group mean). Behavioral analysis was conducted on the remaining 28 rats (SI=10; 4.00g=9; 5.25g=9).
Behavioral data were analyzed using FreezeFrame software (Actimetrics, Wilmette IL) as previously described (Murawski and Stanton, 2010, Murawski and Stanton, 2011). The bout length was set a 0.75 s and the freezing threshold (change in pixels/frame) was initially set as described in the instructions. A human observer blind to treatment groups verified the setting by watching the session and adjusting the threshold to ensure that small movements were not recorded as freezing (with freezing defined as the absence of movement except that needed for respiration). Freezing behavior was measured as the percentage of time spent freezing during the 5 min testing session.
The data were imported into Statistica 10 data analysis software. Behavioral analysis of percent freezing involved a t-test comparing preexposure groups (Pre vs. No Pre) for normally developing rats (Exp. 1) or a one way ANOVA between dosing conditions (SI vs. 4.00g vs. 5.25g) for alcohol-exposed rats (Exp. 2). We compared group changes in c-Fos+ cell number across the three phases of the CPFE by using litter as the unit of analysis, i.e., both phase and preexposure factors were treated as repeated-measures on the same litter (Exp. 1). Additional repeated measures ANOVA examined group c-Fos+ cell differences within different regions of the hippocampus across training phases [Preexposure vs. Training vs. Testing; Bonferroni-corrected alpha set to p(0.05/3)<0.0167; see (Gordi and Khamis, 2004)]. Additional CA1 c-Fos+ cells and CA1 pyramidal cell counts following preexposure in alcohol-exposed rats were analyzed via a one way ANOVA involving the factor of dosing condition (Exp. 2; SI vs. 4.00g vs. 5.25g).
RESULTS
Hippocampal c-Fos Expression in Normally Developing Rats (Experiment 1)
Home cage Controls had a minimum of 24 h without any behavioral experience prior to being sacrificed from their home cages. Because Control rats differed on the amount and type of previous exposure (Pre vs. No Pre; Preexposure vs. Preexposure plus Training; see Figure 1), we initially compared hippocampal c-Fos+ cells in Controls across different phases of the CPFE and found no significant differences (Fs<1.0). In subsequent analyses, Pre and No Pre rats are compared to Controls sacrificed during the same phase. Initial analyses also failed to show any main effect or interactions with the hemisphere in which cell estimates were obtained (Left or Right) (Fs<2.0), and thus subsequent analyses are collapsed across this factor.
Dentate Gyrus
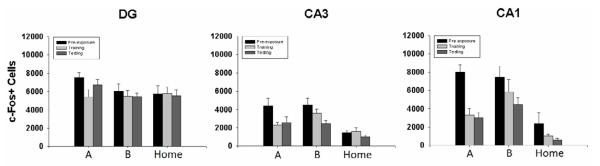
c-Fos+ cell number within the DG was marginally increased in Group Pre compared to Groups No Pre and Control across training phases (ps=0.05) as illustrated in Figure 2. Additional one-way ANOVAs performed on c-Fos+ cells within DG following different phases, however, demonstrated no group differences following preexposure, training, or testing (Fs<1.7).
Figure 2.
Mean (±SE) of c-Fos+ cell counts in the dentate gyrus (DG), CA3, and CA1 2h following different phases of the CPFE (Preexposure, Training, Testing). Rats were either preexposed to Context A (A) or Context B (B). Preexposure involved 5 minutes of Context A or B exploration. Training involved an immediate 1.5 mA foot shock in Context A. During Testing, rats were placed back into Context A for a 5 minute context test. Additional rats were sacrificed directly from their home cage (Home) to assess basal levels of c-Fos expression.
CA3
c-Fos+ cell number in CA3 for Groups Pre and No Pre was highest during preexposure and continued to fall over training and testing; home-cage controls showed low levels of c-Fos+ cells in CA3 over all training phases (Figure 2). This was supported by a significant main effect of preexposure group [F(2,16)=16.01, p<0.01] and of training phase [F(2,16)=5.84, p<0.01] without an interaction (F<1.9). We further examined CA3 c-Fos+ cell number by running a number of one-way ANOVAs during different phases of the CPFE. Following preexposure, significant group differences in CA3 c-Fos+ cell number were present [F(2,24)=6.40, p<0.01], with Groups Pre (4387.2 ± 840.2) and No Pre (4473.1 ± 768.4) not differing from one another (p>0.92), but both groups showing significantly greater numbers of CA3 c-Fos+ cells than Controls (1490.3 ± 233.6; ps<0.02). Group differences were also evident following training [F(2,24)=7.11, p<0.01]; Group No Pre showed significantly greater numbers of c-Fos+ cells (3615.1 ± 459.4) compared to Controls (1634.4 ± 342.9; p<0.01), but differed only marginally from Group Pre (2302.0 ± 315.7; p<0.022). Group Pre and Controls did not did not differ from one another (p>0.2). Following testing, there were only marginal group differences present [F(2,24)=4.29, p<0.03], with Group Pre (2604.6 ± 626.0) and No Pre (2464.3 ± 344.2) showing similar numbers of c-Fos+ cells (p>0.8) that were slightly elevated compared to Controls (1010.8 ± 184.4; ps<0.03).
CA1
Group differences in CA1 c-Fos+ cell number across phases of the CPFE are shown in Figure 2. Groups Pre and No Pre showed high numbers of CA1 c-Fos+ cells during preexposure that decreased during training and testing; controls showed consistently lower c-Fos+ cells than Groups Pre and No Pre. This was supported by a main effect of preexposure group [F(2,16)=26.83, p<0.01] and of training phase [F(2,16)=14.59, p<0.01] without an interaction (F<2.0). Post-hoc analysis showed that Group Pre CA1 c-Fos+ cell number following preexposure was significantly higher than both training and testing (ps<0.01), which did not differ from one another (p>0.8). Additional one-way ANOVAs revealed significant group differences in CA1 c-Fos+ cell number following different phases of the CPFE. Following preexposure, group differences were evident [F(2,24)=8.56, p<0.01], where the number of c-Fos+ cells were significantly increased in both Groups Pre (8034.3 ± 733.4) and No Pre (7424 ± 1197.4) compared to Controls (2393.0 ± 1177.2). Analysis of CA1 c-Fos+ cell number following training revealed a significant main effect of preexposure group [F(2,24)=7.31, p<0.01], with Group No Pre (5836.3 ± 1330.0) showing significantly higher numbers of c-Fos+ cells compared to Controls (1034.9 ± 213.9; p<0.01); group differences between Group Pre (3291.0 ± 743.6) and No Pre rats, however, did not reach significance (p<0.06). The total number of CA1 c-Fos+ cells in Group Pre did not differ from Controls (p>0.08). Finally, group differences were also present in CA1 c-Fos+ cells following testing [F(2,24)=12.12, p<0.01]; c-Fos+ cell number in Groups Pre (2999.4 ± 612.3) and No Pre (2999.4 ± 612.3) were both significantly higher than in Controls (615.0 ± 139.3; ps<0.01). CA1 c-Fos expression in Group No Pre showed non-significant increase compared to Group Pre (p>0.07).
Hippocampal c-Fos Expression and cell counts in Alcohol-Exposed Rats (Experiment 2)
Body weights and BACs of Alcohol-Exposed Rats
Body weight averages from each of the three dosing conditions (Groups SI, 4.00g, and 5.25g) appear in Table 1. All groups gained a significant amount of weight over the dosing period (PD 4-9) and up to the age of testing (PD 31). A 3 (dosing condition) × 2 (days) ANOVA on PD 4 and PD 9 body weights revealed significant main effects of dosing condition [F(2,75)=9.8, p<0.01] and days [F(1,75)=1158.8, p<0.01] with a dosing condition × days interaction [F(2,75)=64.3, p<0.01]. Newman-Keuls posthoc test revealed that although body weights did not differ among groups at PD 4 (ps>0.66), at PD 9 Group 5.25g body weights were significantly lower than either Groups SI or 4.00g (ps<0.01), which did not differ from one another (p>0.60). Analysis of PD 31 body weights revealed significant differences between dosing conditions (p<0.02), with Group 5.25g weighing significantly less than Group 4.00g (p< 0.01; 86.6g ± 3.3 vs. 100.5g ± 2.4); neither alcohol-exposed group differed significantly from Group SI (ps>0.06; 95.1g ± 3.9). Significant body weight reductions following neonatal alcohol exposure are commonly reported (e.g., Hunt et al., 2009, Murawski and Stanton, 2010, Thomas and Tran, 2011); these studies report impairments in alcohol-exposed rats on hippocampus-dependent tasks but not during control tasks suggesting that initial body weight reductions in alcohol-exposed rats did not produce global impairments.
Table 1.
Average body weights (in grams; ± SE) are given from the three dosing conditions of Experiment 2 (SI=sham intubated; 4.00g=4.00g/kg/day over PD4-9; 5.25g=5.25g/kg/day over PD4-9) during the first (PD4) and last (PD9) day of dosing as well as the first day of behavioral training (PD31). Average blood alcohol concentrations (BACs) obtained from blood samples collected on PD4 from alcohol-exposed rats are given in mg/dl. “*” indicates significant differences (p<0.05) from Group SI.
| Dosing Condition |
Body Weight | BACs (mg/dl) |
||
|---|---|---|---|---|
| PD 4 | PD 9 | PD 31 | ||
| SI | 10.7 ± .4 | 18.7 ± .6 | 95.1 ± 3.9 | N/A |
| 4.00g | 11.3 ± .2 | 19.0 ± .5 | 100.5 ± 2.4 | 342.4 ± 10.9 |
| 5.25g | 11.1 ± .3 | 14.3 ± .5* | 86.6 ± 3.3* | 435.8 ± 13.0 |
BACs were obtained from blood samples taken on PD 4 from alcohol-exposed rats (Table 1). BACs from Group 4.00g and Group 5.25g differed significantly from one another [F(1,49)=30.5, p<0.01], averaging 342.4 ± 10.9 mg/dl for Group 4.00g and 435.8 ± 13.0 mg/dl for Group 5.25g. Group BACs are similar to those we have previously reported at these alcohol doses (Murawski and Stanton, 2011).
CA1 Fos+ Cells in Alcohol-Exposed Rats
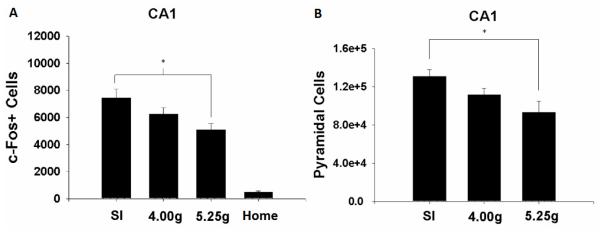
CA1 c-Fos+ cells following preexposure from rats from the three dosing conditions and Home caged controls are shown in Figure 3a. An initial analysis revealed no effects of the hemisphere in which CA1 c-Fos+ cell number was counted (ps>0.3); subsequent analyses are collapsed across this variable. A one-way ANOVA examining total CA1 c-Fos+ cells across dosing conditions revealed significant group differences [F(3,33)=40.0, p<0.01]. Preexposure to Context A resulted in significant increases in CA1 c-Fos+ cells among all dosing conditions compared to Controls (ps<0.01). CA1 c-Fos+ cell number was highest in Group SI (7446.5 ± 672.2), lower in Group 4.00g (6248.7 ± 462.7), and lowest in Group 5.25g (5110.1 ±434.2). Group 5.25g had significantly fewer CA1 c-Fos+ cells compared to Group SI (p<0.01); Group 4.00g did not differ significantly from either group (ps>0.09). Across the two alcohol doses, the negative correlation between BACs in alcohol-exposed rats and CA1 c-Fos+ cells (r=−0.17) was non-significant (p>0.5). Figure 4 shows CA1 c-Fos+ cells of representative rats from each group.
Figure 3.
A. Mean estimates of CA1 c-Fos+ cells (±SE) 2h following preexposure (5 minutes of context exploration) in Groups SI, 4.00g, and 5.25g. Home rats were sacrificed from their home cage. B. Mean (±SE) CA1 pyramidal cells in rats from Groups SI, 4.00g, and 5.25g. “*” indicates significant group differences (p<0.05).
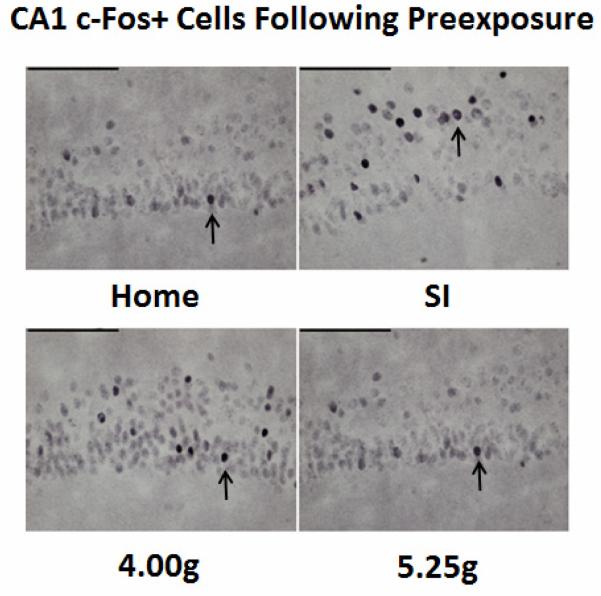
Figure 4.
Representative samples of c-Fos+ cells (arrow) within the CA1 (40x) from Groups Home, SI, 4.00g, and 5.25g following 5 minutes of Preexposure to Context A. Home (caged controls) were sacrificed directly from their home cage. Scale bar= 100 μm.
CA1 Pyramidal Cell Counts in Alcohol-Exposed Rats
Estimates of CA1 pyramidal cells from Groups SI, 4.00g, and 5.25g are shown in Figure 3b. As with CA1 c-Fos+ cells, there was no effect of hemisphere on cell counts (Fs<3.0) and subsequent analyses are thus collapsed across this variable. There were general decreases in CA1 pyramidal cell counts with increases in alcohol dose. A one-way ANOVA examining total CA1 pyramidal cells across dosing conditions revealed a significant main effect of dosing condition [F(2,25)=4.5, p<0.03]. Estimates of CA1 pyramidal cells were significantly reduced in Group 5.25g compared to Group SI (93159.9 ± 11715.5 vs. 130592.3 ±7593.4; p<0.02). Group 4.00g did not significantly differ from either Group SI or Group 5.25g, with cell counts falling between the two groups (111616.9 ± 6948.1). We further examined the relationship between CA1 pyramidal cell number and dosing conditioning by running a trend analysis (planned orthogonal comparisons) using a one-way ANOVA (dosing condition) on CA1 pyramidal cell number. The only significant trend was the linear relationship [F(1,25)=9.04, p<0.01]. Analysis of total CA1 volume (in μm3) did not differ among dosing conditions (F<0.7; data not shown). We found no significant correlation between BACs and CA1 pyramidal cell counts in alcohol-exposed rats (r = −0.09, n = 18, p>0.7).
Relationship of CA1 Pyramidal Cell Number and c-Fos after Preexposure
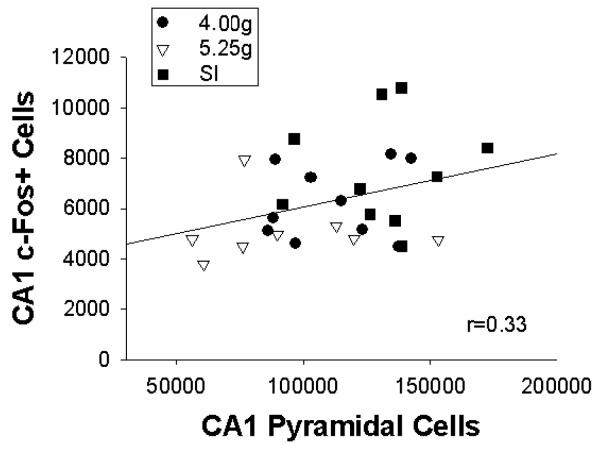
We further examined the percentage of CA1 pyramidal cells expressing c-Fos protein following Preexposure within the same rat among dosing conditions (total CA1 c-Fos+ cells/total CA1 pyramidal cells). About 6% of CA1 pyramidal cells expressed c-Fos protein following preexposure, which did not differ significantly across dosing conditions (F<0.06; SI=5.8% ± 0.6; 4.00g=5.8% ± 0.4; 5.25g=6.1% ± 0.8). The correlation between CA1 pyramidal cells and the number of c-Fos+ cells among dosing conditions was only marginally significant (r=0.33, p>0.08; Figure 6).
Figure 6.
Scatter plot showing total number of CA1 pyramidal cells (X-axis) and CA1 c-Fos+ cells (Y-axis) in rats from Experiment 2. Sham-intubated (SI=solid square); 4.00g (4.00 g/kg/day over PD4-9= solid circle); 5.25g (5.25 g/kg/day over PD4-9= clear triangle).
Behavioral measures
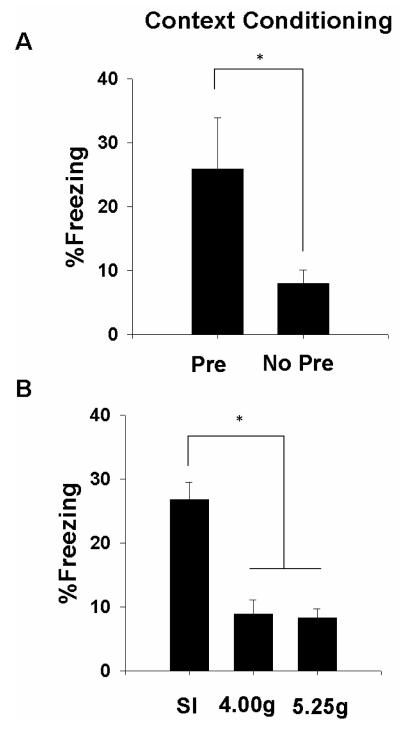
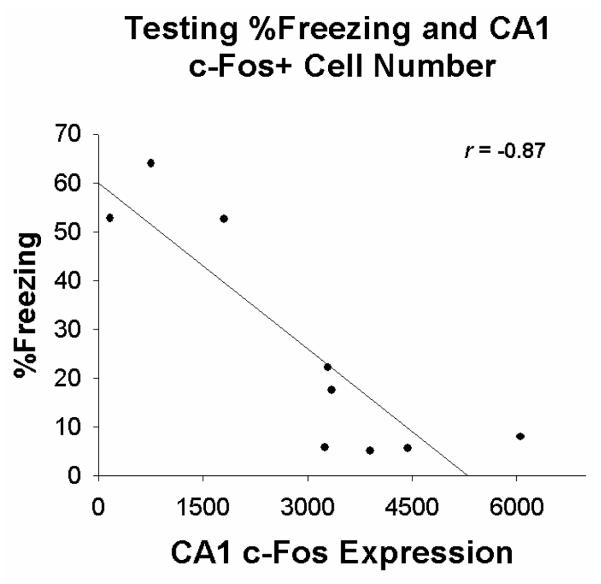
In the first experiment, normally developing rats were preexposed to either Context A (Pre) or Context B (No Pre), trained in Context A, and tested for the CPFE in Context A. The amount of freezing during preexposure in rats from Groups Pre and No Pre were very low (2.71 ± 0.6 vs. 1.76 ± 0.4%) and did not differ from one another (p>0.2). As shown in Figure 5a, rats from Group Pre froze significantly more during testing than rats from Group No Pre (26.0 ± 7.9 vs. 8.1 ± 2.0%). A t-test revealed significant group differences in contextual freezing, t(16)=−2.19, p<0.05. We performed correlational analysis on Group Pre and No Pre freezing behavior and hippocampal c-Fos+ cell number on the day of testing. Although correlations were limited to groups of nine animals and thus were underpowered, we did find a significant correlation between Group Pre freezing and the number of CA1 c-Fos+ cells, where freezing was negatively correlated with CA1 c-Fos+ cell number (Figure 7; r=−.87, p<0.05).
Figure 5.
Mean (±SE) percent freezing during the 5 minute test of contextual fear conditioning in normally developing (A) and alcohol-exposed (B) rats. A. Rats preexposed to Context A (Pre) show significantly higher levels of contextual freezing compared to rats preexposed to Context B (No Pre). The significant increase in contextual freezing in Groups Pre compared to Group No Pre defines the context preexposure facilitation effect. B. Rats from three dosing conditions (SI=sham intubated; 4.00g=4.00g/kg/day; 5.25g=5.25g/kg/day over PD4-9) were preexposed, trained, and tested in Context A. Group SI shows enhanced contextual freezing compared to both alcohol-exposed groups. “*” indicates significant group differences.
Figure 7.
Scatterplot showing the relationship between %Freezing during testing (X-axis) and the number of CA1 c-Fos+ cells following testing in rats preexposed to the testing context in Experiment 1.
In the second experiment, rats from each dosing condition showed low levels of freezing during preexposure (SI=5.0 ± 1.1; 4.00g=3.6 ± 0.7; 5.25g=3.1 ±0.5%; F<1.3). The percentage of freezing during testing for alcohol-exposed rats is shown in Figure 5b. Rats in Groups 4.00g and 5.25g froze significantly less than rats in Group SI [F(2,25)=22.9, p<0.01]. Post hoc tests confirmed that Group SI showed significantly higher levels of freezing (26.8 ± 2.7 %) compared to both Groups 4.00g and 5.25g, which did not differ from one another (8.9 ± 2.2 vs. 8.3 ± 1.4 %, respectively).
DISCUSSION
We first demonstrate experience-dependent changes in hippocampal c-Fos expression following different phases of the context preexposure facilitation effect (CPFE) paradigm in normally developing juvenile male rats. Rats preexposed to the testing context show the CPFE through enhanced contextual fear conditioning to an immediate shock relative to rats preexposed to an alternate context. Five minutes of preexposure to either context significantly increases the number of c-Fos+ cells in CA1 and CA3 pyramidal neurons. Following training to an immediate shock, rats preexposed to the testing context show lower numbers of CA1 and CA3 c-Fos+ cells compared to rats preexposed to the alternate context. Following testing, both preexposure groups showed low numbers of CA3 c-Fos+ cells, whereas group differences were evident in CA1, with higher numbers of c-Fos+ cells in those rats preexposed to the alternate context. We did not find differences in the number of c-Fos+ DG cells among groups (including home caged controls) or across training phases.
Second, we furthered examined the number of CA1 c-Fos+ cells following preexposure in male rats exposed during the neonatal period (PD 4-9) to different levels of alcohol (4.00 & 5.25 g/kg/day) compared to sham-intubated (SI) controls. Experience-dependent increases in the number of CA1 c-Fos+ cells were lower in alcohol-exposed rats compared to controls. Alcohol-exposed rats also showed reductions in the total number of CA1 pyramidal cells relative to sham-intubated rats, with greater reductions in rats exposed to the higher alcohol dose (i.e., 5.25 g). Finally, during contextual fear testing, sham-intubated rats showed significantly higher levels of contextual freezing compared to alcohol-exposed rats, with both alcohol-exposed groups showing an immediate shock deficit.
In both experiments, non-treated or sham-intubated controls preexposed to the testing context demonstrated contextual fear conditioning to an immediate shock (i.e., the CPFE), with ~25% freezing during testing; non-treated rats preexposed to the alternate context or alcohol-treated rats showed an immediate shock deficit (Fanselow, 1990), freezing less than 10% during the context test. These levels of CPFE freezing are consistant with some (Matus-Amat et al., 2007, Kenney and Gould, 2008, Lee, 2010, Murawski and Stanton, 2010, Schiffino et al., 2011), but not other reports of the CPFE (Biedenkapp and Rudy, 2004, Matus-Amat et al., 2007, Lee, 2010), with freezing levels at testing ranging from 20% to greater than 80%. One factor contributing to this variability in the CPFE literature may involve how well the transport experience becomes associated with the preexposure context (see Rudy et al., 2004). Rudy and colleagues (Biedenkapp and Rudy, 2004, Matus-Amat et al., 2007) employ a method of multiple, brief preexposure episodes to facilitate the transport cage-preexposure context association and report a robust CPFE (test freezing > 60%). Although multiple preexposure episodes results in higher levels of context freezing during testing than a single episode (unpublished observation; Lee, 2010), it was important in the current experiments to equate the transport experience across the three phases of the CPFE - multiple preexposure episodes would be expected to influence hippocampal c-Fos expression. As we demonstrate, a single preexposure episode is sufficient to produce the CPFE.
Hippocampal c-Fos Expression During the CPFE in Normally Developing Rats
The context preexposure facilitation effect provides a behavioral paradigm in which to examine the contributions of the hippocampus during 1) acquisition of the context representation (during preexposure); 2) acquisition of the context-shock association (during training to an immediate shock); and 3) the retrieval/expression of the contextual fear memory (during testing). Five minutes of context preexposure significantly elevated the number of CA1 and CA3 c-Fos+ cells in rats exposed to either the testing or an alternate context compared to rats sacrificed directly from their home cages. These findings agree with a number of other studies in both juvenile and adult rats that show significant CA1 and CA3 c-Fos expression following context exploration (Milanovic et al., 1998, Huff and Rudy, 2004, Huff et al., 2006, Raineki et al., 2010, Bissiere et al., 2011). Although c-Fos expression may follow activation of multiple pathways (e.g., Mitsikostas and Sanchez del Rio, 2001), the number of c-Fos+ neurons in CA1 significantly increases following activation of NR2A-containing NMDA receptors through the ERK1/2/MSK1/c-Fos pathway (Gao et al., 2010). The ability of context preexposure to overcome the immediate shock deficit requires hippocampal NMDA receptor activity during the preexposure phase (Matus-Amat et al., 2007, Schiffino et al., 2011). The significant increases in CA1 and CA3 c-Fos+ cells following preexposure, therefore, are likely the result of NMDA receptor mediated activity.
During the training phase of the CPFE, the immediate shock becomes associated with the previously learned context (Rudy and O’Reilly, 2001); this requires NMDA receptor-independent hippocampal activity (Matus-Amat, 2004, Matus-Amat et al., 2007) and the translation of the immediate early gene Zif268/Egr1 within the hippocampus (Lee, 2010). The CA3 is thought to participate in the active retrieval of the context memory through the process of pattern completion, where exposure to a subset of context features activates the representation of the total set of context features (Rudy and O’Reilly, 2001, Kesner, 2007). We therefore predicted significant increases in the number of CA3 c-Fos+ cells in rats preexposed to the training context; CA1 c-Fos+ cell number was also expected to be high due to its role in the retrieval of contextual memories (Hunsaker and Kesner, 2008, Hunsaker et al., 2009). Contrary to our prediction, training failed to significantly increase the number of CA3 or CA1 c-Fos+ cells in rats preexposed to the testing context compared to home caged controls. The hippocampal activity required to associate the previously learned context with the immediate shock may not drive c-Fos expression. We additionally predicted little to no increases in the number of c-Fos+ cells in rats preexposed to an alternate context based on the many reports that exposure to immediate shock alone is insufficient to drive hippocampal c-fos expression or its protein product (Milanovic et al., 1998, Strekalova et al., 2003, Huff et al., 2006). Also contrary to our prediction, immediate shock training resulted in significant increases in both CA1 and CA3 c-Fos+ cell number in rats preexposed to an alternate context. Rudy and colleagues speculate that the distinct transport cages used in the CPFE paradigm activate a contextual representation of where the rats think they are going (Rudy et al., 2004). In rats preexposed to an alternate context, the transport cage would then activate a representation of the alternate context (presumably engaging the hippocampus), which would be incongruous with the actual context within which the rats receive an immediate shock. Both preexposure groups shared similar context preexposure, transport, and immediate shock experiences, only differing in the specific context in which preexposure occurred. The different contexts rats experienced during preexposure were sufficient to produce differences in CA3 and CA1 hippocampal c-Fos+ cell levels between groups following training. The novelty of the training context may have induced higher numbers of c-Fos+ cells in rats preexposed to the alternate context than rats preexposed to the training context (Sheth et al., 2008); the processes behind these differences will need further exploration.
The retrieval/expression of contextual fear conditioning during the testing phase of the CPFE requires hippocampal activity that does not rely on NMDA receptor activation (Matus-Amat, 2004, Matus-Amat et al., 2007) and results in significant increases in CA1 c-Fos expression (Bissiere et al., 2011). Rats from both preexposure groups show comparably elevated CA3 and CA1 c-Fos+ cell number following testing, although only rats preexposed to the testing context show significant amounts of contextual freezing (i.e., the CPFE). The different amount of c-Fos+ cells among rats preexposed to the testing context and those preexposed to the alternate context, then, may reflect different hippocampal processes, perhaps retrieval/expression in the former and further context encoding in the later. It should be noted, however, that both preexposure groups show significantly fewer CA1 c-Fos+ cells following testing than following preexposure. The general pattern of hippocampal c-Fos expression across the different phases of the CPFE suggests that context novelty may account for group differences. Re-exposure to a familiar context results in fewer hippocampal c-Fos+ cells than the original exposure produced (Radulovic et al., 1998). This might explain the greater numbers of c-Fos+ cells when training occurs in a novel context (Group No Pre) relative to a familiar context (Group Pre) and the general decrease in hippocampal c-Fos+ cells from the first context exposure (preexposure) to the last (testing) in the current experiment and in other reports (e.g., Bissiere et al., 2011).
Alcohol-Exposed Rats Show Reduced Levels of CA1 c-Fos Expression Following Preexposure
Following context preexposure, juvenile rats exposed to alcohol over PD 4-9 show reductions in the number of CA1 c-Fos+ cells, with those rats given the highest alcohol dose (5.25 g/kg/day) expressing significantly fewer c-Fos+ cells than sham-intubated controls. NMDA/AMPA receptor function and glutamate release are not altered in CA1 slice preparations taken from rats exposed to alcohol vapor over PD 2-9, although LTP induction is significantly reduced compared to controls (Puglia and Valenzuela, 2010a); this failure to potentiate CA1 synapses may reflect alterations in molecular cascades associated with plasticity in alcohol-exposed rodents. For example, adult mice prenatally exposed to alcohol show deficits in NMDA receptor-dependent ERK1/2 activation in the DG (Samudio-Ruiz et al., 2009). If neonatal alcohol exposure produced a similar effect, this may account for reductions in behaviorally induced hippocampal c-Fos expression (see Gao et al., 2010) that we report here and others report elsewhere (Clements et al., 2005). It is also possible that the timing of peak c-Fos expression in the hippocampus following preexposure in alcohol-exposed rats may also differ from sham-intubated controls. A more parsimonious explanation accounting for the reduced number of CA1 c-Fos+ cells in alcohol-exposed rats may be related to general reductions in CA1 pyramidal cells following neonatal alcohol exposure (see below).
Neonatal Alcohol Exposure Reduces CA1 Pyramidal Cells
Rats exposed to alcohol over the neonatal period show significant reductions in CA1 pyramidal cells over a range of high blood alcohol concentrations (BACs: 268-457 mg/dl; Bonthius and West, 1990, Bonthius and West, 1991, Livy et al., 2003, Tran and Kelly, 2003, Marino, 2004). We demonstrate significant reductions in CA1 pyramidal cells in rats exposed to the high alcohol dose (5.25g; BACs ~435 mg/dl) compared to sham-intubated controls. Rats exposed to the lower alcohol dose (4.00g; BACs ~ 342 mg/dl) showed a 15% reduction in CA1 pyramidal cells compared to controls, although this difference did not reach significance. These reductions agree with other reports when similar BACs are reached over the neonatal period (Tran and Kelly, 2003, Marino, 2004). Around 6% of CA1 pyramidal cells were c-Fos+ following preexposure, which did not differ among rats from the three dosing conditions. This suggests that the reductions in cell number (and not alterations in gene expression) are sufficient to account for the reductions in behaviorally-induced CA1 c-Fos expression in alcohol-exposed rats; the negative correlation between CA1 count and c-Fos+ cell number that we observed, though only marginally significant (p < .08), is consistent with this possibility (Figure 7).
Neonatal Alcohol Exposure Disrupts the Context Preexposure Facilitation Effect
Juvenile rats exposed to alcohol over the neonatal period show significant deficits during both standard contextual fear conditioning and during the CPFE, suggesting impaired hippocampal function (Murawski and Stanton, 2010, Murawski and Stanton, 2011); control-level cued-fear conditioning in alcohol-exposed rats (Wagner and Hunt, 2006, Hunt et al., 2009, Murawski and Stanton, 2010) suggests that the basic fear circuit, centered on the amygdala (reviewed in Fanselow and Poulos, 2005), and the ability to behaviorally express fear learning (i.e., freezing) is not compromised in these rats. We demonstrate significant CPFE deficits in rats exposed to either 4.00g or 5.25g of alcohol over PD4-9, whose littermate counterparts show both reductions in the number of behaviorally-induced CA1 c-Fos+ cells and CA1 pyramidal cell number. Although the lower numbers of CA1 c-Fos+ and pyramidal cell number in rats exposed to the lower alcohol dose did not differ significantly from controls, this alcohol exposure was sufficient to disrupt the CPFE. Group 5.25g showed a 29% reduction in CA1 pyramidal cells whereas Group 4.00g showed a 15% reduction compared to Group SI. This suggests that as little as a 15% reduction in total CA1 pyramidal cells can disrupt the CPFE, highlighting the sensitivity of this task to hippocampal disruption (Rudy et al., 2004). It is also possible that neonatal alcohol affected other brain regions or cellular functions not examined in the current study, contributing to the behavioral deficits we report.
We hypothesized that the CPFE impairments demonstrated in alcohol-exposed rats reflect a failure to learn about the context during the preexposure phase. Our findings of reductions in experience-dependent c-Fos expression and reductions in CA1 pyramidal cells in alcohol-exposed rats compared to controls provide some support for this hypothesis. Further studies examining additional IEG expression in the hippocampus and amygdala (e.g., Arc, BDNF, Egr1) over the different phases of the CPFE and their relationship to the CPFE impairments in alcohol-exposed rats are needed to determine if the impairment is due to learning about the context, associating the previously learned context with the shock, or retrieval of the context-shock association.
Highlights.
We show hippocampal c-Fos expression during different phases of context learning.
Neonatal alcohol-exposed rats show reductions in CA1 c-Fos during context learning.
Neonatal alcohol-exposed rats show reductions in CA1 pyramidal cell number.
Neonatal alcohol-exposed rats show impairments in contextual fear conditioning.
Impairments in alcohol-exposed rats follow a dose-response relationship.
ACKNOWLEDGMENTS
The authors would like to thank Lisa B. Dokovna, Gillian F. Hamilton, and Karen Boschen for technical assistance.
Support: NIH 1F31AA019594-01 to NJM; NIH R01-AA9838-14 to AYK; NIH R01-AA0-14288-05 to MES
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrientos RM, O’Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behavioural brain research. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bedi KS, Wilson P, Wilce PA. Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse. 1999;31:51–58. doi: 10.1002/(SICI)1098-2396(199901)31:1<51::AID-SYN7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behavioral neuroscience. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical synapses control hippocampal contributions to fear learning and memory. Science. 2011;331:87–91. doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcoholism, clinical and experimental research. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Developmental psychobiology. 2007;49:243–257. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Clements KM, Girard TA, Ellard CG, Wainwright PE. Short-Term Memory Impairment and Reduced Hippocampal c-Fos Expression in an Animal Model of Fetal Alcohol Syndrome. Alcoholism: Clinical & Experimental Research. 2005;29:1049–1059. doi: 10.1097/01.alc.0000171040.82077.e. [DOI] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcoholism, clinical and experimental research. 2010;34:897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Experimental biology and medicine. 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early human development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicology and teratology. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990:264–270. [Google Scholar]
- Fanselow MS, Dong H-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual review of psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20:1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain research reviews. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicology and teratology. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Gordi T, Khamis H. Simple solution to a common statistical problem: interpreting multiple tests. Clinical therapeutics. 2004;26:780–786. doi: 10.1016/s0149-2918(04)90078-1. [DOI] [PubMed] [Google Scholar]
- Hamilton D. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Progress in neurobiology. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Rudy JW. The amygdala modulates hippocampus-dependent context memory formation and stores cue-shock associations. Behavioral neuroscience. 2004;118:53–62. doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiology of learning and memory. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Tran GT, Kesner RP. A behavioral analysis of the role of CA3 and CA1 subcortical efferents during classical fear conditioning. Behavioral neuroscience. 2009;123:624–630. doi: 10.1037/a0015455. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol. 2009;43:465–474. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental-Changes in Alcohol Pharmacokinetics in Rats. Alcoholism-Clinical and Experimental Research. 1987;11:281, 286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods in molecular biology. 2008;447:101, 110. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behavioral neuroscience. 2008;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learning & memory. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Frontiers in behavioral neuroscience. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicology and teratology. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Marino M. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. International Journal of Developmental Neuroscience. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P. The Role of the Dorsal Hippocampus in the Acquisition and Retrieval of Context Memory Representations. Journal of Neuroscience. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behavioral neuroscience. 2007;121:721, 731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain research. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M. Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain research Brain research reviews. 2001;35:20–35. doi: 10.1016/s0165-0173(00)00048-5. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annual review of neuroscience. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4-9. Behavioural Brain Research. 2010;212:133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of Dose and Period of Neonatal Alcohol Exposure on the Context Preexposure Facilitation Effect. Alcoholism, clinical and experimental research. 2011 doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; San Diego: 2005. [Google Scholar]
- Puglia MP, Valenzuela CF. Ethanol acutely inhibits ionotropic glutamate receptor-mediated responses and long-term potentiation in the developing CA1 hippocampus. Alcoholism, clinical and experimental research. 2010a;34:594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010b;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between Fos production and classical fear conditioning: Effects of novelty, latent inhibition, and unconditioned stimulus preexposure. Journal of Neuroscience. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20:1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and biobehavioral reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral neuroscience. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, affective & behavioral neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Valenzuela CF, Perrone-Bizzozero NI, Caldwell KK. Prenatal ethanol exposure persistently impairs NMDA receptor-dependent activation of extracellular signal-regulated kinase in the mouse dentate gyrus. Journal of neurochemistry. 2009;109:1311, 1323. doi: 10.1111/j.1471-4159.2009.06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of learning and memory. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth A, Berretta S, Lange N, Eichenbaum H. The amygdala modulates neuronal activation in the hippocampus in response to spatial novelty. Hippocampus. 2008;18:169–181. doi: 10.1002/hipo.20380. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Zorner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes, brain, and behavior. 2003;2:3–10. doi: 10.1034/j.1601-183x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2011 doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicology and teratology. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behavioral neuroscience. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society : JINS. 2008;14:1022, 1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]