Abstract
Adipocytes are insulin-sensitive cells that play a major role in energy homeostasis. Obesity is the primary disease of fat cells and a major risk factor for the development of Type 2 diabetes, cardiovascular disease, and metabolic syndrome. The use of botanicals in the treatment of metabolic diseases is an emerging area of research. In previous studies, we screened over 425 botanical extracts for their ability to modulate adipogenesis and insulin sensitivity. We identified St. John’s Wort (SJW) extracts as inhibitors of adipogenesis of 3T3-L1 cells and demonstrated that these extracts also inhibited insulin-sensitive glucose uptake in mature fat cells. In these follow-up studies we have further characterized the effects of SJW on insulin action in both murine and human fat cells. We have shown that SJW also attenuates insulin-sensitive glucose uptake in human adipocytes. Moreover, SJW inhibits IRS-1 tyrosine phosphorylation in both murine and human fat cells. Botanical extracts are complex mixtures. Many bioactive compounds have been identified in SJW, including hypericin (HI) and hyperforin (HF). We have examined the ability of HI and HF, purified from SJW, to modulate adipocyte development and insulin action in mature adipocytes. Our novel studies indicate that the profound effects of SJW on adipogenesis, IRS-1 activation, and insulin-stimulated glucose uptake are not mediated by HI and/or HF. Nonetheless, we propose that extracts of SJW may contribute to adipocyte related diseases by limiting differentiation of preadipocytes and significantly inducing insulin resistance in mature fat cells.
Keywords: fat cell, insulin action, botanicals, hypericin, hyperforin
1. Introduction
Herbala supplements, prepared from various plant parts (e.g. flowers, leaves, and roots) have been used as traditional medicines to promote general good health and to treat disease for thousands of years. In the U.S., there has been an increase in botanical supplement use among consumers to treat many chronic disease states, such as obesity, insulin resistance, and other risk factors of the metabolic syndrome. According to the American Botanical Council, U.S. consumers spent over $5 billion in 2009 on herbal supplements, up 17% over the previous 5 year period. Often the safety and efficacy of these herbal remedies has not been thoroughly investigated. In response to these concerns, the federal government and national funding agencies have amplified their efforts to subject botanicals to rigorous modern scientific research.
Adipocytes are lipid storing, insulin-sensitive cells that play a key role in energy homeostasis. Obesity, the principal disease of adipocytes, is a major risk factor of the metabolic syndrome, and significantly contributes to the development of type 2 diabetes mellitus (T2DM), cardiovascular disease, and certain cancers. In the past, it was thought that limiting adipose tissue development might be a good way to combat obesity, and many researchers have investigated anti-adipogenic agents as potential therapeutics for decreasing or preventing obesity. However, the prevailing current hypothesis is that disruption of adipocyte differentiation limits adipose tissue expansion and is linked to insulin resistance and the development of T2DM [6,14]. Additionally, factors that interfere with the ability of adipocytes to store lipid, respond to insulin, or secrete adipokines may promote the development of the metabolic syndrome [reviewed in [3]]. Thus, we have hypothesized that the ability of a botanical extract to modulate adipocyte development and/or function in a positive or negative manner correlates with its ability to protect against or contribute to metabolic syndrome and/or T2DM.
Previous screening efforts in our laboratory resulted in the identification of two extracts of St. John’s Wort (Hypericum perforatum L) that inhibited adipogenesis and induced insulin resistance in adipocytes [1]. St. John’s Wort (SJW) is used worldwide as a treatment for depression and a variety of other conditions [4,11]. Since SJW is readily available and consumed by millions of people, and both obesity and diabetes are world-wide epidemics, further understanding the effects of SJW on adipocyte development and function is worthwhile. Our previous investigations demonstrated that leaf and flower extracts, but not root extracts, from SJW were capable of inhibiting adipocyte differentiation of 3T3-L1 cells. Moreover, these flower and leaf extracts were shown to inhibit insulin-sensitive glucose uptake in mature murine fat cells [1]. In this study, we observed that SJW can modulate IRS-1 activation in both murine and human fat cells and can also inhibit glucose uptake in human adipocytes. Moreover, these studies examined the effects of hypericin (HI) and hyperforin (HF), two well-known bioactive constituents of SJW that are associated with its antidepressant activity [5,17]. Overall, these studies identify IRS-1 as a target of SJW action on adipocytes, and this is the first study to show negative effects of this widely used botanical on human fat cells.
2. Material and Methods
2.1 Materials
Hypericin and a methanolic solution of hyperforin, both isolated from St. John’s Wort, and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Sigma-Aldrich (St. Louis, MO). Bovine and fetal bovine sera were purchased from HyClone (Thermo Scientific, Logan, UT). The SJW flower and root extracts were prepared at the Rutgers Botanical Core facility as previously described [1]. For immunoblotting, polyclonal STAT5A and IRS-1, and monoclonal PPARγ antibodies were purchased from Santa Cruz Biotechnology. The polyclonal anti-aP2 and anti-adiponectin antibodies were purchased from Abcam (Cambridge, MA) and Thermo Scientific (Rockford, IL). Anti-phospho-IRS-1 (Tyr896), which is polyclonal and highly specific for phospho-tyrosine 896 of the mouse IRS-1, was purchased from Invitrogen (Carlsbad, CA). The BCA and enhanced chemiluminescence kits were from Thermo Scientific. Anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Mature human subcutaneous adipocytes were purchased in a 12-well plate format from ZenBio. These human adipocytes were derived from preadipocytes collected from the subcutaneous thigh region of non-diabetic, females with a BMI of less than 25.
2.2 Cell culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days post-confluence in DMEM containing 10% bovine serum. Medium was changed every 48–72 hours. Cells were induced to differentiate by changing the medium to DMEM containing a standard MDI induction cocktail of 10% fetal bovine serum (FBS), 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1.7 μM insulin. After 48 h, this medium was replaced with DMEM supplemented with 10% FBS and 0.43 μM insulin. Each additional feeding occurred every 2–3 days in DMEM supplemented with 10% FBS, and cells were maintained in this medium until utilized for experimentation. Human adipocytes were maintained in adipocyte maintenance media (Zenbio Cat # AM-1) and used for experimentation within one week of arrival.
2.3 Whole cell extract preparation
Adipocyte monolayers were harvested in a non-denaturing buffer containing 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% Igepal CA-630, 1 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, and 2 mM sodium vanadate. The samples were either extracted on ice for 30 min or frozen. For analysis, the samples were centrifuged at 13,000 × g at 4°C for 10 min. Supernatants containing whole cell extracts were analyzed for protein content using a BCA kit according to the manufacturer’s instructions.
2.4 Gel electrophoresis and immunoblotting
Seventy-five to one-hundred-fifty micrograms of protein from whole cell extracts were separated on 7.5% or 15% SDS-polyacrylamide gels and transferred to nitrocellulose membrane in 25 mM Tris, 192 mM glycine, and 20% methanol. Following transfer, the membrane was blocked in 4% milk and then immunoblotted. Results were visualized with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
2.5 Oil Red O staining
An Oil Red O stock was prepared as previously described [16]. Cell monolayers were aspirated and rinsed with PBS. Following incubation in a fixative solution (10% formaldehyde in PBS) for 10–15 min, the monolayers were rinsed under tap water. The remaining water was aspirated, and the cells were incubated for 1 h in the working Oil Red O solution (0.3% in isopropanol). Following incubation, the stain was aspirated, the cells were rinsed under tap water, and then they were examined by microscopy and scanned to produce the figures in this manuscript.
2.6 Insulin-stimulated 2-[3H] deoxyglucose uptake assay
The assay of 2-[3H] deoxyglucose uptake was performed as previously described [22]. Prior to the assay, mature adipocytes were serum deprived and pre-treated with SJW flower extract, HI, or HF for various times. Next, the cells were incubated in the presence or absence of insulin (5 nM) for 7 min. Glucose uptake was initiated by addition of 2-[3H] deoxyglucose at a concentration of 0.1 mM 2-deoxyglucose in 1 mCi 2-[3H] deoxyglucose in Krebs–Ringer–Hepes buffer and incubated for 3 min at room temperature. Glucose uptake is reported as [3H] radioactivity and normalized to total protein content as determined by BCA analysis. Uptake measurements were performed in triplicate under conditions where hexose uptake was linear.
2.7 Statistics
Results were analyzed using the statistical software SAS® 9.3 (SAS Institute, Inc., Cary, NC). The general linear models procedure was employed to conduct an analysis of variance for glucose uptake and to calculate least squares means and standard deviations. Statistical significance was declared for p-values ≤ 0.05.
3. Results
3.1 Hypericin and hyperforin do not mediate the ability of SJW to inhibit adipogenesis
We previously reported that St. John’s Wort flower and leaf, but not root extracts, inhibited adipogenesis and induced insulin resistance in adipocytes [1]. Hypericin and hyperforin are two key bioactive constituents of SJW that have been reported to be responsible for its antidepressant activity [5,17]. To determine if these constituents are capable of mediating the effects of the active SJW extracts on adipocyte development and function, we examined the ability of HI and HF that were purified from SJW to modulate adipocyte differentiation and insulin sensitivity.
The following data were considered in the selection of appropriate HI and HF concentrations for our experiments. The HF content within SJW is typically ~3% but ranges from 2–5% [21]. The combined content of HI and pseudohypericin within SJW is typically ~0.3% but ranges from 0.02–2.5% [20]. Based on the active concentrations of SJW in our previous studies [1] and the typical percent composition of HI and HF within SJW, the concentrations of active extract in terms of HI and HF concentration are equivalent to 0.02–0.3 μM HI and 0.17–2.8 μM HF. Additionally, HI inhibits the action of protein kinase C with an IC50 of 3.4 μM [23], and HF activates the pregnane X receptor with a binding constant of 27 nM [18]. Thus, we initially examined the effects of HI and HF on adipogenesis using concentration ranges of 0.05–6 μM HI and 0.01–0.47 μM HF (HF was commercially available only as a 0.47 mM methanolic solution).
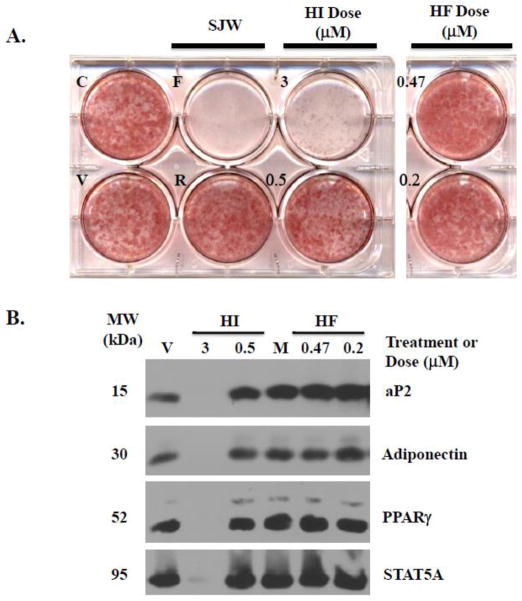
As shown in Figure 1A, neither HI nor HF was capable of inhibiting adipogenesis of 3T3-L1 cells as judged by Oil Red O staining, which is indicative of lipid accumulation. The SJW flower extract was used as a positive control and completely blocked lipid accumulation, while the root extract does not inhibit lipid accumulation and was used as a negative control. HI doses of 3 μM and higher were toxic and resulted in cell death as judged by detachment of the cell monolayer from the culture plate. Notably, there was no observable cell death with HI and HF concentrations equivalent to 0.5 μM or lower (data not shown).
Figure 1. Hypericin and Hyperforin do not inhibit adipogenesis.
3T3-L1 preadipocytes, induced to differentiate using the typical MDI cocktail, were treated at the time of induction with DMSO vehicle (V), methanol vehicle (M), 50 μg/ml flower (F) and root (R) extracts of SJW, or the indicated the doses of hypericin (HI) and hyperforin (HF). The cells were retreated following each media change every 48–72 hours. Control cells (C) received no treatment. Each panel is representative of 3–6 independently performed experiments. A: Oil Red O staining of neutral lipid content was performed 7 days post-MDI. All of the wells shown were stained at the same time with the same Oil Red O working solution. B: Western blot analysis of whole cell extracts from cells harvested four days post-MDI. Similar results were obtained at 4 and 7 days post-MDI, and panel B contains a representative subset of these blots.
Adipogenesis of 3T3-L1 cells was also assessed by examining the induction of adipocyte marker proteins. As shown in Figure 1B, HI and HF did not inhibit the induction of aP2, adiponectin, PPARγ, and STAT5A that normally accompanies adipogenesis. Both HF doses and 0.5 μM HI resulted in equivalent adipocyte marker protein levels compared to the respective vehicle treatment. Additionally, a combined treatment of approximately 0.5 μM HI and HF was also not capable of inhibiting lipid accumulation or the induction of adipocyte marker protein expression (data not shown). As a result of its toxicity, no adipocyte marker protein bands were observed for the 3 μM HI treatment. Based on its percent composition as described above, the 3 μM dose of HI was higher than the calculated concentration of HI in the active SJW extracts. Since this HI dose was toxic to differentiating 3T3-L1 preadipocytes, we focused on the 0.5 μM dose of HI and HF for our studies in mature fat cells.
3.2 Effects of SJW extract, HI, and HF on insulin-sensitive glucose uptake
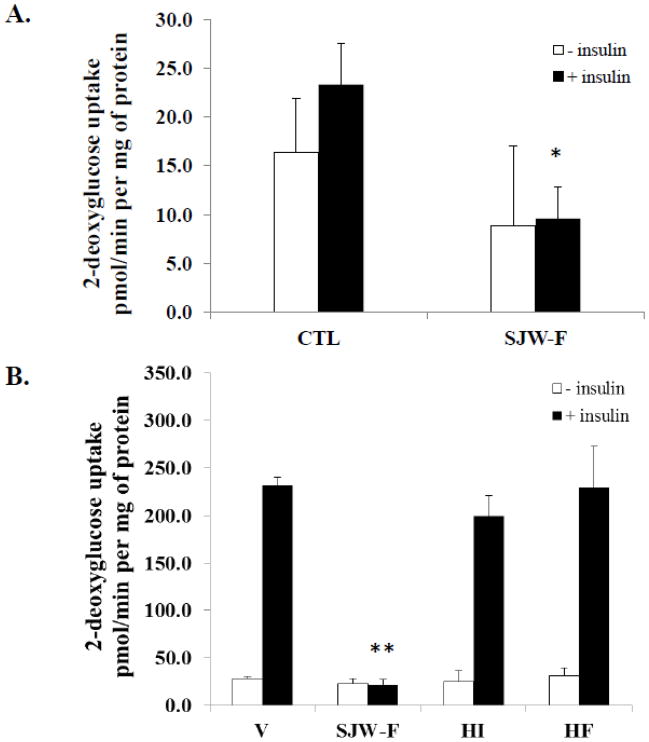
We previously observed that the SJW flower extract induced insulin resistance in fully differentiated murine 3T3-L1 adipocytes [1]. To address the relevance of this observation in human cells, we assessed the ability of the flower extract to inhibit insulin-stimulated glucose uptake in mature subcutaneous human adipocytes. As shown in Figure 2A, untreated control human adipocytes were not highly responsive to insulin treatment, which resulted in a small, not statistically significant 1.5-fold increase in glucose uptake. Nonetheless, compared to the control cells, both basal and insulin stimulated glucose uptake rates for these human adipocytes were reduced in the presence of the SJW flower extract. While the decrease in basal glucose uptake observed when the fat cells were treated with SJW-F was not statistically significant, the reduction of insulin-sensitive glucose uptake in the presence of SJW-F did reach statistical significance (p < 0.02) compared to CTL untreated, insulin-stimulated human adipocytes (Figure 2A).
Figure 2. HI and HF do not mediate insulin resistance induced by the SJW flower extract.
A: Mature subcutaneous human adipocytes were serum deprived overnight before performing glucose uptake assays. Prior to insulin administration, the cells were either untreated or pre-treated with 50 μg/ml SJW-F extract for 18 hours. B: Fully differentiated 3T3-L1 adipocytes were serum deprived and pre-treated with DMSO vehicle (V), 0.5 μM HI, and 0.47 μM HF overnight or 50 μg/ml SJW-F for 4 hours prior to performing glucose uptake assays. Similar results were obtained with an overnight treatment of SJW-F. The glucose uptake values shown represent the mean ± SEM of triplicate determinations from two independent experiments. *p < 0.02 (n = 3): SJW-F versus CTL (plus insulin); **p < 0.0001 (n = 3): SJW-F versus V, HI, and HF (plus insulin).
Our prior investigations showed that SJW flower extract inhibited insulin-sensitive glucose uptake in 3T3-L1 adipocytes [1]. Therefore, we examined the ability of HI or HF to impair the function of fully differentiated fat cells by assessing the capacity of these compounds to modulate insulin-stimulated glucose uptake in murine adipocytes. As shown in Figure 2B, vehicle-treated 3T3-L1 adipocytes were highly responsive to insulin treatment, which resulted in a 9-fold increase over the basal glucose uptake rate. Compared to the vehicle-treated cells, neither HI nor HF significantly altered basal or insulin-sensitive rates of glucose uptake. However, SJW flower extract substantially inhibits insulin-sensitive glucose uptake.
3.3 Effects of SJW extracts, HI, and HF on IRS-1 tyrosine phosphorylation
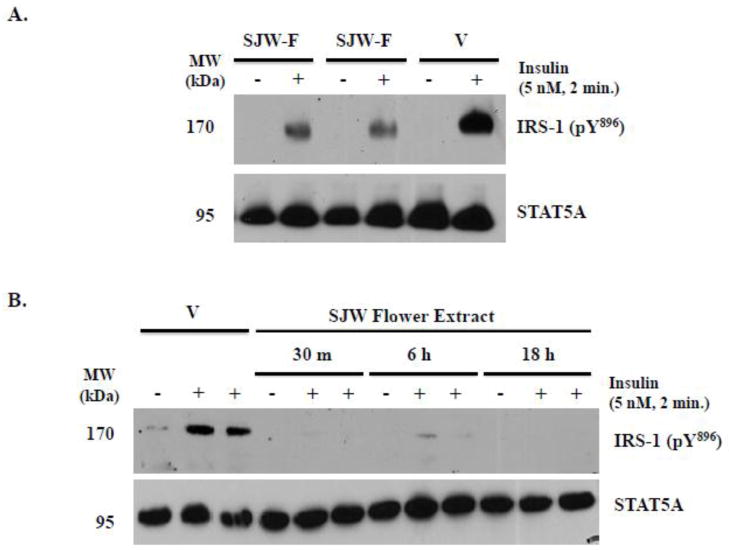
To investigate how the SJW flower extract disrupts insulin signaling in fat cells, we examined its ability to inhibit tyrosine phosphorylation of IRS-1. IRS-1 is a key protein in insulin signaling, and inhibition of its expression or tyrosine phosphorylation is often associated with insulin resistance [25]. Using a phosphospecific anti-IRS-1 pY896 antibody, we monitored the effect of the flower extract on IRS-1 phosphorylation at tyrosine896 in mature 3T3-L1 and human adipocytes following administration of an acute dose of insulin. As shown in Figure 3, we observed a robust increase in IRS-1 pY896 following insulin stimulation of DMSO vehicle-treated mouse and human adipocytes. However, an overnight pre-treatment of murine and human fat cells with the flower extract substantially inhibited insulin-induced IRS-1 tyrosine phosphorylation. Additionally, the SJW flower extract also blocked IRS-1 phosphorylation in human adipocytes following 30 minute and 6 hour pre-treatments (Figure 3B).
Figure 3. SJW flower extract modulates insulin action via attenuation of IRS-1 tyrosine896 phosphorylation.
A: Mature 3T3-L1 adipocytes were serum deprived and pre-treated with DMSO vehicle (V) or 25 μg/ml SJW flower (SJW-F) extract overnight. Two independently performed experiments with the flower extract are shown, and this experiment was independently repeated a total of 2 times. B: Fully differentiated subcutaneous human adipocytes were serum deprived and pre-treated with 25 μg/ml SJW-F for the indicated lengths of time. Cells were then stimulated with insulin prior to preparation of whole cell extracts. Proteins from the whole cell extracts were resolved and immunoblotted. For each treatment and/or time point in B, data from two separate wells treated with insulin are shown. STAT5A levels are not altered by acute insulin stimulation, and its expression was examined as a loading control.
To complete our evaluation of the capability of HI and/or HF to mediate the effects of the SJW flower extract on adipocyte function, we also examined their ability to modulate insulin-stimulated IRS-1 tyrosine phosphorylation in mouse and human adipocytes. As shown in Figure 4A, following an overnight pre-treatment of murine 3T3-L1 adipocytes with HF, IRS-1 pY896 was substantially induced by acute stimulation with insulin. The magnitude of this response was comparable to that of the untreated control and methanol vehicle-treated cells. In murine adipocytes, pre-incubation with HI for 2 hours was also unable to significantly inhibit IRS-1 pY896 in the presence of insulin (data not shown). Conversely, using adipocytes from the same group, we examined the effect of the flower extract as a positive control, and as shown in Figure 4A, we observed that it completely blocked insulin-sensitive phosphorylation of IRS-1 at tyrosine896 without decreasing total IRS-1 protein levels. For the data shown in Figure 4A, the cells were pre-treated with HF overnight and with the SJW flower extract for 2 hours. Nevertheless, we observed results comparable to those shown by pre-treating the 3T3-L1 adipocytes for 2 hours with HF (data not shown). Additionally, neither HI nor HF was capable of inhibiting IRS-1 phosphorylation in fully differentiated human adipocytes (Figure 4B). Unlike the data presented from the murine 3T3-L1 adipocytes, we observed IRS-1 pY896 under basal conditions in the human fat cells, and they were less sensitive to insulin stimulation than the mouse adipocytes (i.e. the induction of IRS-1 pY896 was less substantial than observed for the 3T3-L1 fat cells). Regardless, compared to the untreated control and vehicle treatments, IRS-1 pY896 levels were not decreased in the presence of HI or HF. Nonetheless, these are the first studies to demonstrate that an extract of SJW can inhibit IRS activation in both mouse and human fat cells.
Figure 4. HI and HF do not block IRS-1 tyrosine896 phosphorylation in mature 3T3-L1 or subcutaneous human adipocytes.
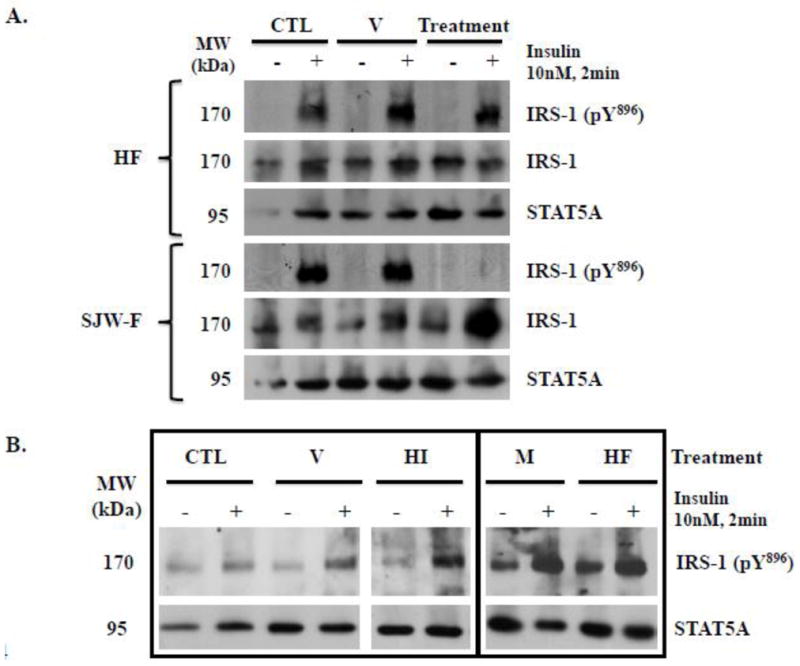
A: Mature 3T3-L1 adipocytes were serum deprived overnight. Cells were pre-treated with methanol vehicle (V) and 0.47 μM HF overnight, or with DMSO vehicle and 50 μg/ml SJW flower (SJW-F) extract for 2 hours. B: Fully differentiated subcutaneous human adipocytes were serum deprived overnight and then pre-treated with DMSO vehicle (V), 0.5 μM HI, methanol vehicle (M), or 0.47 μM HF for 2 hours. For each antibody, the results shown within the rectangle are from the same exposure of the same blot. Control cells (CTL) were untreated. Cells were stimulated with insulin prior to preparation of whole cell extracts. Proteins from the whole cell extracts were resolved and immunoblotted. STAT5A expression was probed as a loading control. Each panel is representative of an experiment independently repeated two times.
4. Discussion
Our recently published work [1] demonstrated that extracts of SJW impair adipogenesis and insulin action in murine fat cells. In our current studies, we have extended our examination of the effects of SJW and its known bioactive constituents, hypericin and hyperforin, on the development and function of murine adipocytes. Moreover, we have demonstrated that human adipocytes are also sensitive to the negative effects of SJW. However, these profound effects of SJW on mouse and human fat cells are not mediated by HI or HF. In response to insulin, human subcutaneous fat cells do not exhibit a 6–10 fold increase in glucose uptake as has been observed in mouse adipocytes. Nonetheless, we clearly observed impaired insulin-stimulated glucose uptake following treatment with the SJW flower extract in human fat cells. In both murine and human adipocytes, insulin-induced IRS-1 tyrosine phosphorylation was substantially attenuated. Since both limited expansion of adipose tissue and impaired insulin signaling in adipocytes have been associated with the development of insulin resistance [10,12], we propose that the results from our in vitro investigations suggest that SJW usage has the potential to cause insulin resistance in vivo.
Although our studies on SJW are limited to in vitro observations, there is some other data to support a role of SJW in insulin resistance. Mukerjee and colleagues recently demonstrated that an endogenous protein purified from SJW can increase MCP-1 expression via modulation of its promoter [19]. Monocyte chemoattractant protein 1 (MCP-1) regulates the migration and infiltration of immune cells into various tissues in response to cytokines or growth factors. In mice and humans, it is well known that a considerable increase in macrophage-derived inflammatory mediators accompanies obesity and T2DM [8]. Moreover, MCP-1 has been shown to enhance the infiltration of macrophages into adipose tissue and contribute to insulin resistance [13]. Since SJW has been shown to increase MCP-1 expression [19], it is reasonable to hypothesize that this popular botanical may also be capable of inducing insulin resistance in vivo, perhaps via induction of MCP-1.
To date, several bioactive substances have been identified in SJW. The greatest interest has focused on the characterization of hypericin and hyperforin. Not only are these organic compounds associated with the antidepressant effects of SJW, but they also have been studied as antiviral, antimicrobial, and anticarcinogenic agents [reviewed in [17,24]]. Of note, there is a substantial body of literature indicating that SJW interferes with the action of numerous drugs [reviewed in [15]]. The ability of SJW to lower the circulating concentrations and pharmacological efficacy of a number of drugs and oral contraceptives [7] is largely attributed to hyperforin. HF is a psychoactive constituent of SJW that potently activates the pregnane X receptor [18], which is known to turn on various sets of genes that are involved in the metabolism and excretion of drugs [9]. Since hyperforin and hypericin are considered the primary bioactive ingredients of SJW, the standardization of SJW extracts currently is based on the content of both of these phytochemicals; HI content is standardized to 0.3% and HF content to 3%. Although SJW extracts prevent fat cell differentiation, our studies demonstrate that neither HI nor HF were capable of inhibiting adipogenesis. Moreover, the ability of SJW to modulate insulin-sensitive glucose uptake and IRS-1 phosphorylation was not mediated by HI or HF.
The concentration of HF utilized in our adipogenesis and insulin sensitivity assays was limited by the concentration of the stock solution as indicated in section 3.1. The highest HF concentration tested in our experiments was 0.47 μM. However, according to the calculations presented in section 3.1, the concentration of HF within our active SJW extracts may have been 6 – fold higher. Therefore, a plausible explanation for the lack of effect of HF on adipogenesis may be the use of a sub-efficacious dose. Conversely, in our insulin-responsive glucose uptake assay, the effective dose of SJW was lower (3 μg/ml) [1] and corresponds to a maximal HF concentration of 0.28 μM, which is 1.7 – fold lower than the HF dose used in our experiments. Yet, HF was not capable of inhibiting insulin-sensitive glucose uptake in these studies. Taken together, these data suggest that neither HI nor HF would elicit the same potentially harmful metabolic side effects that we propose may be associated with SJW flower and leaf extracts as a result of their negative effects on adipocyte development and function.
SJW is comprised of many other bioactive compounds of which approximately 50–70% have been identified; these known phytochemicals belong to a variety of chemical classifications [5]. In addition to HI and HF, which represent napthodianthrones and phloroglucinols, SJW is known to contain numerous flavonoids, biflavonoids, and phenolic acids [2]. In this study, we have demonstrated that HI and HF are not capable of mediating the inhibitory effects of SJW in adipocytes. Further studies are necessary to identify the component(s) that are responsible for mediating the ability of SJW extracts to inhibit adipogenesis and induce insulin resistance in fat cells.
4.1 Concluding Remarks
In summary, our data indicate that human fat cells, like murine adipocytes, are highly sensitive to SJW and that this botanical results in a potent inhibition of IRS-1 tyrosine phosphorylation. In these novel studies, we demonstrated that the anti-adipogenic effects of SJW are not mediated by HI and/or HF. Moreover, HI and HF are not responsible for the ability of SJW to inhibit insulin action in murine or human adipocytes. Obesity and diabetes are world-wide epidemics, and SJW is a ubiquitous, commercially available herbal remedy that is consumed by millions of people globally as an over-the-counter treatment for mild depression. We hypothesize that the use of SJW could have additional health risks besides reducing drug effectiveness. Overall, our studies suggest that SJW may have a negative impact on adipocyte-related diseases by limiting differentiation of preadipocytes and significantly inducing insulin resistance in mature fat cells.
Highlights.
St. John’s Wort (SJW) induces insulin resistance in 3T3-L1 and human adipocytes.
SJW impairs IRS-1 tyrosine activation in murine and human fat cells.
Hypericin and hyperforin are considered the primary active ingredients of SJW.
Hypericin and hyperforin do not mediate effects of SJW in 3T3-L1 or human fat cells.
SJW may contribute to or exacerbate adipocyte related diseases.
Acknowledgments
This work was supported by an NCAAM and ODS funded NIH grant 2P50AT002776-06. AJR was supported by an NIH T-32 training fellowship in botanicals granted to Pennington Biomedical Research Center. We are grateful to Ursula A. White and William Johnson for technical assistance.
Footnotes
Abbreviations: SJW, St. John’s Wort; HI, hypericin; HF, hyperforin; T2DM, Type 2 diabetes mellitus; IRS-1, insulin receptor substrate 1; IRS-1 pY896, IRS-1 phosphorylated at tyrosine 896
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amini Z, Boyd B, Doucet J, Ribnicky DM, Stephens JM. St. John’s Wort inhibits adipocyte differentiation and induces insulin resistance in adipocytes. Biochem Biophys Res Commun. 2009;388:146–149. doi: 10.1016/j.bbrc.2009.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J, Anderson LA, Phillipson JD. St John’s wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53:583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 3.Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–250. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- 4.Butterweck V. Mechanism of action of St John’s wort in depression : what is known? CNS Drugs. 2003;17:539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- 5.Butterweck V, Schmidt M. St. John’s wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 2007;157:356–361. doi: 10.1007/s10354-007-0440-8. [DOI] [PubMed] [Google Scholar]
- 6.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 7.Di YM, Li CG, Xue CC, Zhou SF. Clinical drugs that interact with St. John’s wort and implication in drug development. Curr Pharm Des. 2008;14:1723–1742. doi: 10.2174/138161208784746798. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 10.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Greeson JM, Sanford B, Monti DA. St. John’s wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology (Berl) 2001;153:402–414. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 12.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kober M, Pohl K, Efferth T. Molecular mechanisms underlying St. John’s wort drug interactions. Curr Drug Metab. 2008;9:1027–1037. doi: 10.2174/138920008786927767. [DOI] [PubMed] [Google Scholar]
- 16.Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978;75:6107–6109. doi: 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75:1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukerjee R, Deshmane SL, Darbinian N, Czernik M, Khalili K, Amini S, Sawaya BE. St. John’s Wort protein, p27SJ, regulates the MCP-1 promoter. Mol Immunol. 2008;45:4028–4035. doi: 10.1016/j.molimm.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulinacci N, Bardazzi C, romani A, Pinelli P, Vincieri FF, Costantini A. HPLC-DAD and TLC-densitometry for quantification of hypericin in Hypericum perforatum L. extracts. Chromatographia. 1999;49:197–201. [Google Scholar]
- 21.Orth HC, Rentel C, Schmidt PC. Isolation, purity analysis and stability of hyperforin as a standard material from Hypericum perforatum L. J Pharm Pharmacol. 1999;51:193–200. doi: 10.1211/0022357991772132. [DOI] [PubMed] [Google Scholar]
- 22.Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- 23.Takahashi I, Nakanishi S, Kobayashi E, Nakano H, Suzuki K, Tamaoki T. Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun. 1989;165:1207–1212. doi: 10.1016/0006-291x(89)92730-7. [DOI] [PubMed] [Google Scholar]
- 24.Vacek J, Klejdus B, Kuban V. Hypericin and hyperforin: bioactive components of St. John’s Wort (Hypericum perforatum). Their isolation, analysis and study of physiological effect. Ceska Slov Farm. 2007;56:62–66. [PubMed] [Google Scholar]
- 25.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]