Abstract
Molecular targeting agents play important roles in non-small-cell lung cancer (NSCLC) therapy. Published studies have investigated new drugs categorized as molecular targeting agents that inhibit the mammalian target of rapamycin (mTOR). We focused on a small interfering RNA (siRNA) that specifically inhibits mTOR and has fewer side effects. To evaluate the antitumor effects of the siRNA, cell proliferation, apoptosis, and migration were assessed. In the study group, the siRNA was transfected into NSCLC cells. The number of cells present after 6 days of culture was counted to determine changes in cell proliferation. The level of apoptosis was evaluated by the detection of DNA-histone complexes in the cytoplasmic fraction using an absorption spectrometer. Changes in migration were evaluated by calculating the number of cells that passed through a specific filter using a commercial chemotaxis assay kit. mTOR-siRNA transfection inhibited cell proliferation as indicated by 37.3% (p = 0.034) decrease in the number of cells compared with the control cells. Analysis of the level of apoptosis in NSCLC cells revealed 16.7% (p = 0.016) increase following mTOR-siRNA transfection, and mTOR-siRNA transfection significantly inhibited cell migration by 39.2% (p = 0.0001). We confirmed that mTOR-siRNA induces apoptosis and inhibits the proliferation and migration of NSCLC cells in vitro. Further studies using mTOR-siRNA may aid in the development of an alternative therapy that maximizes the antineoplastic effect of mTOR inhibition.
Keywords: mTOR, siRNA, non-small-cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide and accounts for 1.3 million deaths each year. Non-small-cell lung carcinoma (NSCLC) represents 80% of all cases of lung cancer and is primarily treated by surgical resection because of its insensitivity to drugs. Despite advances in systemic chemotherapy, only small improvements in the quality of life and overall survival have been achieved for patients with advanced NSCLC.
Efforts are now being directed at developing molecular targeting agents. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that belongs to the phosphati-dylinositol 3-kinase (PI3K)-related kinase family and acts a key regulator of cell growth, proliferation and survival. Excessive mTOR activity through the PI3K/Akt/mTOR pathway is a prominent feature of many neoplasms, permitting unrestricted cancer cell growth that contributes to tumorigenesis. Therefore, pharmacological inhibitors of mTOR should directly target the pathogenesis of neoplasms. Indeed, novel analogs of the mTOR inhibitor rapamycin (temsirolimus, everolimus, and deforolimus) have been designed as cancer treatments, and clinical trials of these compounds have validated the importance of mTOR inhibition as a profound treatment strategy for several malignancies. Similarly, various studies have shown that mTOR activity is important in lung cancer growth through Akt activation [1], pAkt overex-pression, phosphatase and tensin homolog (PTEN) loss [2-5], EGFR amplification or mutations [6], PI3KCA mutations [7], LKBI mutations or silencing [8], and K-ras mutations [9]. However, the efficacy of mTOR inhibitors requires further investigation in relation to NSCLC, and the adverse effects of these inhibitors (such as liver damage, anemia, and non-infectious pneu-monitis) remain current problems.
Small interfering RNA (siRNA) technology promises greater advantages over drugs for therapeutic application because of the ability of siRNAs to target the expression of a specific gene. siRNAs also have low toxicity, as they are metabolized into natural nucleotide components by endogenous cell systems [10]. Therefore, if the effects of targeting mTOR with siRNAs are optimal, this strategy may provide a good alternative to the use of rapamycin.
The aim of this study was to evaluate the potency of siRNA to transiently inhibit mTOR expression in NSCLC cells and examine its effects on cell proliferation, apoptosis, and migration.
Materials and methods
Cell culture
Human NSCLC cells and squamous lung carcinoma (RERF-LC-AI) cells were obtained from the RIKEN BioResource Center (Tsukuba, Japan) and grown as recommended at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY) supplemented with 4 mM glutamine, 4.5 g/L glucose, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, 10% fetal bovine serum (FBS) and 100 μg/mL penicillin-streptomycin (Sigma, St. Louis, MO).
siRNA transfection
mTOR-siRNA was purchased from Cell Signaling (Danvers, MA). The cells were transfected with 10 nM of siRNA using Lipofectamine 2000 (Invitrogen, Grand Island, NY) according to the manufacturer's protocol. Following transfection for 48 hours, the cells were enriched with 10% FBS, incubated for another 48 hours and collected for analysis. The transfection reagent alone was employed as a control.
Real-Time PCR
Total RNA was isolated as described above. TaqMan quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis was performed using the standard protocol provided by Applied Biosystems (Austin, TX). Briefly, samples were reverse-transcribed into cDNA using random hexamer primers, and RT-PCR was performed with the ABI Prism 7900 HT sequence detector. The primer pairs and probes for the GAPDH housekeeping gene and the mTOR gene were purchased from Applied Biosystems. The level of mTOR expression of each sample was standardized to the level of GAPDH expression.
Proliferation assay
NSCLC cells were seeded onto 6-well plates at a density of 10,000 cells/well in 10% FBS-DMEM (day 0) and treated with transfection reagent with or without mTOR-siRNA. After 48 hours of incubation, 2.5 mL of 10% media was added to each well, and the cells were incubated for another 48 hours. On day 6, cell proliferation was assessed by counting the number of cells in five independent high-powered fields (magnification, x 200). Each experiment was repeated three times.
Apoptosis assay
DNA fragmentation was determined using the Cell Death Detection ELISA system (Roche Applied Science, Indianapolis, IN), which is an assay based on the principle of a quantitative sandwich-enzyme immunoassay, using mouse monoclonal antibodies directed against the DNA and histones. NSCLC cells that were treated with transfection reagent with or without mTOR siRNA were harvested. The cellular extracts were incubated in 96-well plates coated with anti-histone antibodies. The plates were incubated with anti-DNA antibodies conjugated to peroxidase for 2 hours, and then the absorbance was measured at 405 nm.
Chemotaxis assay
Chemotaxis assays were performed at 37 °C using a 24-transwell plate containing a polycarbonate filter with 8-μm pores (Poretics, Livermore, CA). NSCLC cells that were treated with transfection reagent alone or transfected with mTOR siRNA were harvested using 0.05% tryp-sin/ethylenediaminetetraacetic acid and seeded onto the upper wells in 0.5% FBS media at a density of 40,000 cells/well. Platelet-derived growth factor-BB (PDGF-BB), which was diluted in 0.5% FBS-DMEM to 5 ng/mL, was placed into the lower chamber. The cells were incubated in a 5% CO2 incubator at 37°C for 6 hours. Following the completion of the assay, the membranes were fixed in 70% ethanol at 20°C for 30 minutes and stained with hematoxylin at room temperature for 30 minutes. The upper sides of the membranes were scraped using a cotton swab to remove cells that had attached but had not migrated. The membranes were mounted onto glass slides. Chemotaxis in each well was assessed by counting the number of cells from five independent high-powered fields (magnification, x 200).
The values were expressed as the fold-increase (mean ± SE) from at least three independent experiments. The paired data were evaluated using Student's t-tests, and one-way ANOVA was used for multiple comparisons. Values p < 0.05 were considered statistically significant.
Results
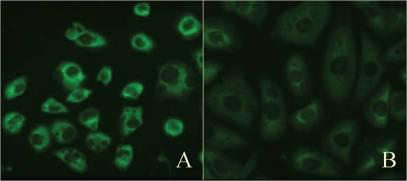
Initially, we investigated the efficacy of mTOR-siRNA by evaluating the mTOR protein levels and mRNA levels in NSCLC cells transfected with mTOR-siRNA or treated with the transfection reagent alone. Immunohistological analysis showed a marked decrease in the number of mTOR-positive cells among those transfected with mTOR-siRNA (Figure 1). Quantitative realtime PCR was performed to confirm the effects of transfection on mTOR mRNA levels. Treatment with mTOR-siRNA led 42.5% reduction (Control: 1.60 ± 0.06 vs. mTOR: 0.92 ± 0.06, p = 0.012) in gene expression (Figure 2).
Figure 1.
Evaluation of transfection efficiency. Representative photomicrographs of immunofluorescence staining for mTOR (green) in (A) cells treated with the transfection reagent alone or (B) with mTOR-siRNA.
Figure 2.
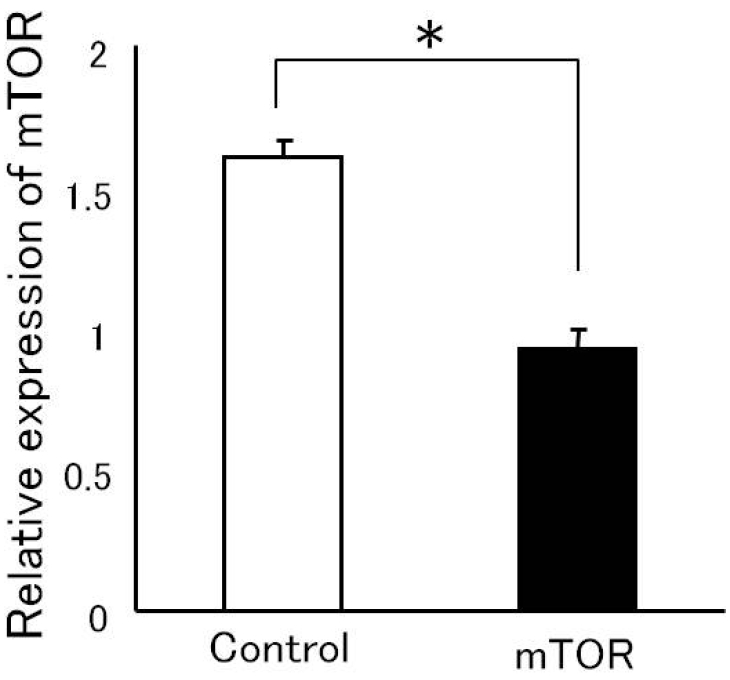
The levels of mTOR-RNA were confirmed by RT-PCR. NSCLC cells were treated with the transfection reagent alone (control) or were transfected with mTOR-siRNA and harvested 96 hours later. Total RNA was isolated, and quantitative real-time PCR was performed. The values of mTOR-mRNA expression were normalized to GAPDH mRNA expression (*p = 0.012).
Cell proliferation was used to monitor how the inhibition of mTOR gene expression affects the growth rate of NSCLC cells. NSCLC cells were seeded at a low density following treatment with the transfection reagent alone or transfected with mTOR-siRNA and allowed to grow in the presence of 10% serum. Transfection with mTOR-siRNA inhibited cell proliferation, which was indicated by 37.3% decrease (Control: 151.1 ± 8.6 vs. mTOR: 94.7 ± 3.0, p = 0.034) in the number of viable cells at day 6 after seeding compared with the transfection with the reagent alone (Figure 3).
Figure 3.
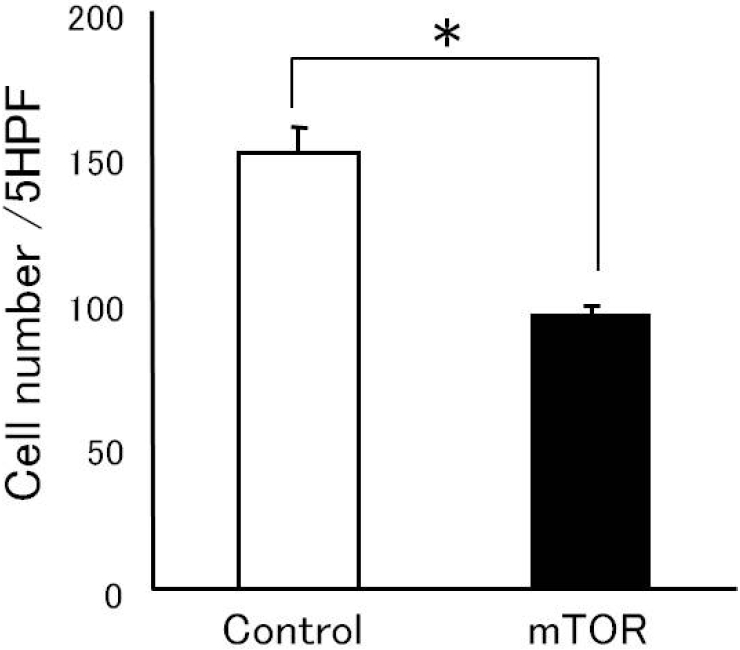
Effect of mTOR-siRNA on cell proliferation. NSCLC cells were seeded onto 6-well plates at a density of 10,000 cells/wells and treated with the transfection reagent alone or with mTOR-siRNA. After 48 hours of incubation, 2.5 mL of 10% media was added to each well, and the cells were incubated for an additional 48 hours. Cell proliferation was measured by counting the number of cells from five independent high-powered fields (x 200) (*p = 0.034).
The disruption of programmed cell death induces uncontrolled tumor growth, and one of the major factors determining the efficacy of a chemotherapeutic drug is its ability to induce apoptosis of tumor cells. Therefore, we evaluated the effect of mTOR-siRNA on apoptosis. DNA fragmentation significantly increased in NSCLC cells in which mTOR expression was inhibited compared with that in control cells (Control: 0.24 ± 0.01 vs. mTOR: 0.28 ± 0.01, p = 0.016; Figure 4).
Figure 4.

Effect of mTOR-siRNA on apoptosis. NSCLC cells were treated with the transfection reagent alone (control) or with mTOR-siRNA and harvested 96 hours later. Apoptosis was evaluated by ELISA to measure the level of DNA fragmentation (*p = 0.016).
Because migration is a key factor for the dispersion and dissemination of tumor cells, we evaluated the role of mTOR in NSCLC cell migration. NSCLC cells transfected with mTOR-siRNA demonstrated a reduction of 39.2% in PDGF-stimulated chemotaxis compared with control cells (Control: 158.0 ± 7.4 vs. mTOR: 96.0 ± 3.4, p = 0.0001; Figure 5).
Figure 5.

Effect of mTOR-siRNA on cell migration. NSCLC cell migration was examined using a Boyden microchemotaxis chamber. NSCLC cells were treated with the transfection reagent alone (control) or with mTOR-siRNA, plated in the upper compartment and allowed to migrate through 8-μm-sized pores. After 6 hours, migrated cells were fixed and stained with hematoxylin and eosin. Chemotaxis in each well was assessed by counting the number of cells from five independent high-powered fields (x 200) (*p = 0.0001).
Discussion
In this study, we revealed that mTOR-siRNA mediated silencing of mTOR gene expression effectively controlled cell growth by attenuating cell proliferation, increasing cell apoptosis, and inhibiting the migration of NSCLC cells in vitro.
Through its downstream effectors, mTOR is involved in the initiation of the ribosomal translation of mRNA into proteins necessary for cell growth, cell cycle progression, and cell metabolism. The mTOR protein is known to sense and integrate signals that are initiated by nutrient intake, growth factors, and other cellular stimuli to regulate downstream signaling and protein synthesis. This regulation can prevent cells from responding to growth and proliferation.
Pivotal clinical trials of mTOR inhibitors for the treatment of many cancers are ongoing; these cancers include renal cell carcinomas, neuroendocrine tumors, pancreatic islet cell tumors, breast cancer, diffuse large B-cell lymphomas, hepatocellular carcinomas, and gastric cancer. Rapamycin, a chemical mTOR inhibitor, causes G1 cell cycle arrest in cancer cell lines and inhibits growth and metastatic progression in NSCLC animal models [11]. Conversely, the association between the overexpression or activation of mTOR and biological behaviors remains unclear.
We have previously reported that immunohistochemical staining of surgically-resected NSCLC tissues revealed high levels of mTOR and phosphorylated-mTOR (p-mTOR). We observed that p-mTOR is present in tissues that overexpress mTOR [12]. Furthermore, squamous cell carcinoma specimens that stained positive for p-mTOR exhibited a high frequency of lymph node metastasis. These results suggest that p-mTOR may promote cell motility, migration and subsequent metastasis. Another study with NSCLC specimens also revealed that, L-type amino acid transporter 1 (LAT 1), a glucose transporter necessary to enhance glucose uptake in cancer cells is closely associated with poor prognosis in NSCLC, and that LAT 1 expression is correlated with the EGFR/Akt/mTOR pathway [13]. Therefore, inhibitors directed against mTOR holds high potential for the treatment of advanced NSCLC.
With such intention, chemical treatments using mTOR inhibitors have been introduced, however, there are a few major issues that remain with the usage of such drugs. Reports have suggested that low- to high-grade noninfectious pneumonitis with a relationship to rapamycin treatment occurs in 25% of all patients [14].
The occurrence of these side effects of mTOR chemical inhibitors necessitates the close monitoring of patients for adverse pulmonary events and limits the use of these inhibitors in patients with low respiratory function.
Another issue is factors that inhibit the efficacy of chemical inhibitors. A study on persistent hyperinsulinemic hypoglycemia in infancy (PHHI) has shown that, in order to achieve high efficacy of mTOR inhibition, one must consider the distribution of phospholipase D1 (PLD1), which activates the mTOR pathway through its product phosphatidic acid (PA) [15]. Although rapamycin inhibits mTOR by associating with its intracellular receptor FKBP-12, PA is known to inhibit this effect. Thus, with abundant PLD1 and PA, rapamycin can be ineffective, whereas in cells lacking PLD1 as in acinar cells of PHHI, rapamycin in combination with calcium channel blockers that decrease activation of p-mTOR can be highly effective. Since the distribution of PLD1 in NSCLC is not well known, upstream effects on mTOR must be considered when using chemical inhibitors. On the other hand, by the use of siRNA targeting mTOR, such consideration is unnecessary.
Moreover, recent insights now indicate that rapamycin is only a partial inhibitor of mTOR. mTOR forms the catalytic core of at least two functionally distinct complexes, mTOR complex 1 (mTORC1) and 2 (mTORC2), of which mTORC2 is relatively resistant to rapamycin. Even more, although rapamycin possesses high inhibitory effects on mTORC1-mediated S6K1 phosphorylation, it only displays limited effects on 4EBP1 phosphorylation, protein synthesis, cell growth, and cell proliferation depending on the cell type [1, 16, 17]. In detail, mTORC1 is composed of mTOR, regulatory-associated protein of mTOR (Raptor), mammalian LST8/G-protein β-subunit like protein (mLST8/GβL) and PRAS40 and DEP-TOR, and has a role in translation, ribosome biogenesis, autophagy and hypoxic adaptation. On the other hand, mTORC2 is composed of mTOR, rapamycin-insensitive companion of mTOR (Rictor), GβL, and mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and is known to take part in growth-factor signaling and survival by activating the Akt pathway and functions as a regulator of the cytoskeleton though its stimulation of protein kinase Cα and Cdc42. Rosnerand and Hengstschlager [18] have shown that Raptor has a low affinity for mTOR in the nucleus, yielding very low amounts of mTORC1 in the nucleus, whereas mTORC2 is detected abundantly in both the cytoplasm and nucleus. Furthermore, Dhingra et.al have found overexpression and nuclear localization of p-mTORC2, and overexpression of phospholipase D1 in uterine leiomy-osarcoma and smooth muscle tumors of uncertain malignant potential [19]. These findings support the importance for inhibiting both mTORC1 and mTORC2 in cancer therapy. Due to the limited effects of pre-existing mTOR inhibitors, novel second-generation ATP-competitive mTOR inhibitors have been introduced, and have shown to inhibit mTORC1 and mTORC2 hence, inhibit 4EBP1 phosphorylation and cell proliferation [17, 20]. Similarly, we can anticipate that, by the use of siRNAs that target mTOR, inhibitory effects on mTORC1 as well as effects on mTORC2 could be achieved, inducing a highly effective and selective inhibition of cell growth.
Dicer is an enzyme that processes siRNAs, and siRNAs can also be exogenously introduced into cells using various transfection methods. Recent studies have shown that siRNA-mediated mechanisms hold substantial potential for therapeutic use due to the high efficiency and lack of nonspecific genomic effects of siRNAs. RNAi studies have demonstrated the clinical potential of synthetic siRNAs in allergic skin diseases [21], dental diseases, eye diseases, cancer, metabolic diseases [22] and neurodegenerative disorders [23].
However, therapeutic applications are limited due to the instability of double-stranded RNA and the absence of a reliable delivery method to target cells. Degradation by enzymes in blood, interaction with blood components, nonspecific uptake by cells and immune responses to siRNAs must be considered for in vivo applications. Moreover, because mTOR is ubiquitously expressed in mammalian cells, further studies to specifically target siRNAs to tumor lesions with systemic administration need to be investigated.
This is the first report showing that transfection of cells with mTOR-siRNA effectively represses cell proliferation and migration and induces apoptosis of NSCLC cells. Further studies using mTOR-siRNA may lead to the development of an alternative therapy that maximizes the antineoplastic effects of mTOR inhibition and reduces the risk of toxicities.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan. (KAKENHI: 19790966).
References
- 1.Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, Unger M, Testa JR. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 2.Tsurutani J, Fukuoka J, Tsurutani H, Shih JH, Hewitt SM, Travis WD, Jen J, Dennis PA. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 3.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Lim WT, Zhang WH, Miller CR, Watters JW, Gao F, Viswanathan A, Govindan R, McLeod HL. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer. Oncol Rep. 2007;17:853–857. [PubMed] [Google Scholar]
- 5.David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, Brody AR. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res. 2004;10:6865–6871. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 6.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 9.Friday BB, Adjei AA. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127–144. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami S, Hashida M. Targeted delivery systems of small interfering RNA by systemic administration. Drug Metab Pharmacokinet. 2007;22:142–151. doi: 10.2133/dmpk.22.142. [DOI] [PubMed] [Google Scholar]
- 11.Boffa DJ, Luan F, Thomas D, Yang H, Sharma VK, Lagman M, Suthanthiran M. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin Cancer Res. 2004;10:293–300. doi: 10.1158/1078-0432.ccr-0629-3. [DOI] [PubMed] [Google Scholar]
- 12.Dobashi Y, Suzuki S, Matsubara H, Kimura M, Endo S, Ooi A. Critical and diverse involvement of Akt/mammalian target of rapamycin signaling in human lung carcinomas. Cancer. 2009;115:107–118. doi: 10.1002/cncr.23996. [DOI] [PubMed] [Google Scholar]
- 13.Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–478. [PMC free article] [PubMed] [Google Scholar]
- 14.White DA, Schwartz LH, Dimitrijevic S, Scala LD, Hayes W, Gross SH. Characterization of pneumonitis in patients with advanced non-small cell lung cancer treated with everolimus (RAD001) J Thorac Oncol. 2009;4:1357–1363. doi: 10.1097/JTO.0b013e3181ba20b1. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrescu S, Tatevian N, Olutoye O, Brown RE. Persistent hyperinsulinemic hypoglycemia of infancy: constitutive activation of the mTOR pathway with associated exocrine-islet transdifferentiation and therapeutic implications. Int J Clin Exp Pathol. 2010;3:691–705. [PMC free article] [PubMed] [Google Scholar]
- 16.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2 doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 17.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 18.Rosner M, Hengstschläger M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 componets rictor and sin1. Hum Mol Genet. 2008;17:2934–2948. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- 19.Dhingra S, Rodriguez ME, Shen Q, Duan X, Stanton ML, Chen L, Zhang R, Brown RE. Constitutive activation with overexpression of the mTORC2-phospholipase D1 pathway in uterine leiomyosarcoma and STUMP: morphoproteomic analysis with therapeutic implications. Int J Clin Exp Pathol. 2010;4:134–146. [PMC free article] [PubMed] [Google Scholar]
- 20.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma M, Ritprajak P, Hashiguchi M. Topical application of siRNA targeting cutaneous dendritic cells in allergic skin disease. Methods Mol Biol. 2010;623:373–381. doi: 10.1007/978-1-60761-588-0_24. [DOI] [PubMed] [Google Scholar]
- 22.Rondinone CM. Therapeutic potential of RNAi in metabolic diseases. Biotechniques. 2006:31–36. doi: 10.2144/000112163. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell MM. RNAi applications in therapy development for neurodegenerative disease. Curr Pharm Des. 2009;15:3977–3991. doi: 10.2174/138161209789649295. [DOI] [PubMed] [Google Scholar]