Abstract
The author herein reports a large cell neuroendocrine carcinoma (LCNEC) of the lung diagnosed at a brain metastasis without clinical data. A 70-year-old man underwent esophagectomy for esophageal squamous cell carcinoma, and was treated with chemotherapy. At 72 years of age, he was found to have prostatic well differentiated adenocarcinoma, and treated by estrogen. At 78 years of age, he was pointed out to have gastric advanced tumor, and the biopsy showed moderately differentiated tubular adenocarcinoma. The gastric carcinoma was treated by chemotherapy. At 79 years of age, he was shown to have right lung shadow (2 cm in diameter) and brain shadow (cerebellar vermis) of 1 cm in diameter. Multiple biopsy and cytology of the lung failed to detect carcinoma cells. Biopsy of the brain was performed. The biopsy showed medullary undifferentiated carcinoma. Immunohistochemically, the tumor cells were positive for pancytokeratin AE1/3, synaptophysin, CD56 (NCAM), p53, Ki67 (labeling 40%), KIT and TTF-1, but were negative for vimentin, chromogranin, neuron-specific enolase and PDGFRA. A pathological diagnosis of metastatic LCNEC form the lung was made. A molecular genetic analysis for KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes identified no mutations of the KIT and PDGFRA genes. The patients died of carcinomatosis one month after the diagnosis. In conclusion, careful histological and immunohistochemical examination can diagnose LCNEC of the lung at the metastatic site.
Keywords: LCNEC, lung, brain, metastasis, immunohistochemistry
Introduction
Large cell neuroendocrine carcinoma (LCNEC) of the lung is high grade neuroendocrine carcinoma composed of large cells. LCNEC shows features common to small cell carcinoma, and characterized by positive neuroendocrine markers and KIT (CD117). The author herein reports an LCNEC of the lung diagnosed in a small brain metastasis without clinical data.
Case report
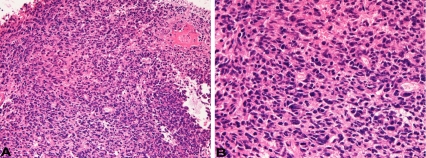
A 70-year-old man underwent esophagectomy for esophageal squamous cell carcinoma, and was treated with chemotherapy. The squamous cell carcinoma was moderately differentiated with keratinization. Two years later, he was found to have prostatic adenocarcinoma, and treated by estrogen. The prostatic adenocarcinoma was well differentiated and its Gleeson's score was 3+4. At 78 years of age, he was pointed out to have gastric advanced tumor, and the biopsy showed moderately differentiated tubular adenocarcinoma. The gastric carcinoma was treated by chemotherapy. At 79 years of age, he was shown to have right lung apex shadow (2 cm) (Figure 1) and brain shadow (cerebellar vermis) (Figure 2) of 1 cm in diameter. Metastasis of the esophageal squamous cell carcinoma, gastric adenocarcinoma or prostatic adenocarcinoma was considered, but primary lung malignancy could not be denied. Multiple biopsies and cytologies of the lung failed to detect carcinoma cells. Biopsy of the brain was performed. The biopsy showed medullary undifferentiated carcinoma (Figure 3A and 3B). The tumor cells were relatively large and had hyperchromatic nuclei and nucleoli. Necrosis and mitotic figures were scattered.
Figure 1.
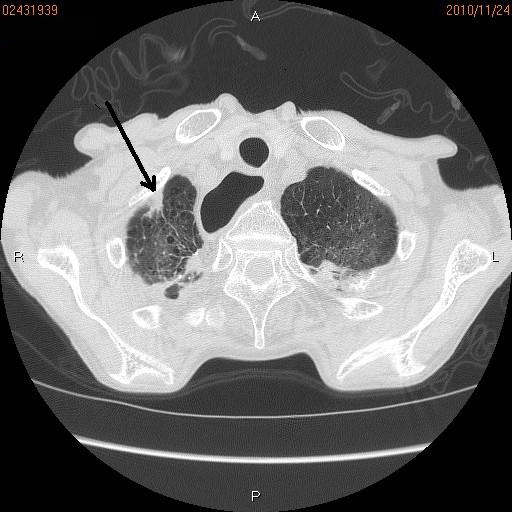
Chest CT. A small abnormal shadow (Arrow) measuring 2 cm is seen in the apex of the right lung.
Figure 2.
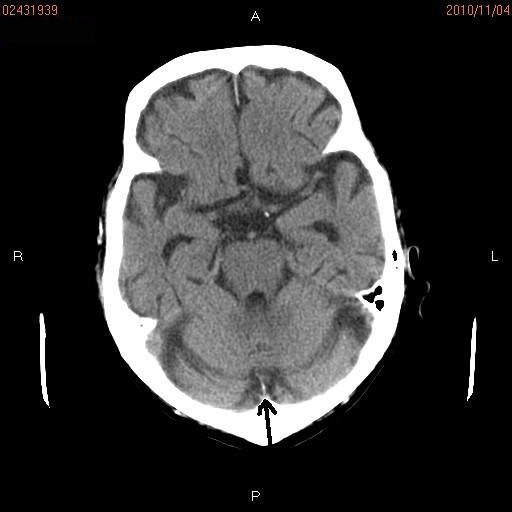
Brain CT. A small abnormal shadow (arrow) measuring 1 cm is seen in the vermis of the cerebellum.
Figure 3.
Histological features of the brain tumor. The tumor is medullary, and shows no differentiation. The tumor is relatively large and have hyperchromatic nuclei and nucleoli. Mitotic figures are scattered. HE; A. x50, B. x200.
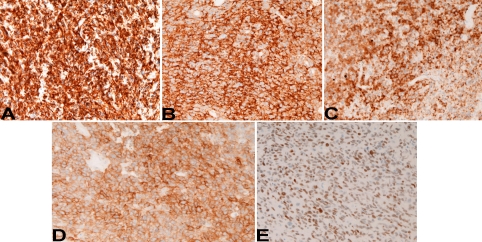
An immunohistochemical study was performed by Dako's Envision method, as previously reported [1-3]. Immunohistochemically, the tumor cells were positive for pancytokeratin AE1/3 (Figure 4A), synaptophysin (Figure 4B), CD56 (NCAM) (Figure 4C), p53, Ki67 (labeling 40%), KIT (Figure 4D) and TTF-1 (Figure 4E), but were negative for vimentin, chromogranin, neuron-specific enolase and PDGFRA. A pathological diagnosis of metastatic LCNEC form the lung was made. The patients died of carcinomatosis one month after the diagnosis. Autopsy was not performed.
Figure 4.
Immunohistochemical findings. The tumor cells are positive for pancytokeratin AE1/3 (A), synaptophysin (B), CD56 (C), KIT (D), and TTF-1 (E). Immunostaining, x200.
A molecular genetic analysis for KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes was performed, in paraffin specimens of the brain tumor, by the PCR-direct sequencing method, as previously described [4-9]. The analysis identified no mutations of the KIT and PDGFRA genes.
Discussion
The present case had quadruple carcinomas; esophageal squamous cell carcinoma, prostatic well differentiated adenocarcinoma, gastric moderately differentiated adenocarcinoma, and lung LCNEC. Such multiple carcinomas in a single patient are rare.
In the present study, the lung and brain tumors were considered as metastases from gastric, esophageal or prostatic carcinoma. The repeated biopsies and cytologies of the lung tumor failed to reveal malignant cells. The biopsy of brain showed LCNEC with TTF-1 positivity. Since TTF-1 (a marker of lung carcinoma) was positive, the lung and brain tumors were diagnosed as pulmonary LCNEC.
LCNEC is relatively rare tumor with aggressive characters, like small cell lung carcinoma (SCLC). LCNEC and SCLC are neuroendocrine malignancies of the lungs. The differences between them are cell size and cell morphologies. The present case showed apparent neuroendocrine features, and cell morphologies were those of LCNEC. It is unique that the diagnosis of LCNEC of the lung was made by pathologic examinations of one metastatic site without knowledge of clinical data.
In the present case, KIT and PDGFRA protein and genes were investigated. KIT protein is frequently expressed in SCLC [10, 11] and LCNEC [12-14], as in the present case. Rossi et al. [12] reported that KIT protein was expressed in 63% of LCNEC of the lung. Sihto et al [10] reported that 30% of SCLC expressed KIT protein. KIT mutations are extremely rare in SCLC [10, 11]. Rossi et al [12] found no mutations in exons 9 and 11 of KIT gene in 83 patients with LCNEC.
In the present case, KIT mutations in exons 9, 11, 13 and 17 were not present.
Although there have been no reports of PDGFRA protein expression in SCLC to the author's best knowledge, Sihto et al. [10] reported no PDGFRA mutations in 30 SCLCs. Rossi et al. [12] reported that PDGFRA expression was found in 80% of LCNEC of the lung. PDGFRA expression was seen in the present case. Sihto et al. [10] reported that no PDGFRA mutations were identified in 30 SCLCs. Rossi et al. [12] found no mutations in exons 12 of PDGFRA gene in 83 patients with LCNEC. In the present case, there was no PDGFRA protein expression, and PDGFRA mutations in exons 12 and 18 were not present.
In conclusion, careful histological and immunohistochemical examination can diagnose LCNEC of the lung at the metastatic site.
References
- 1.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductalendocrine 10|carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 2.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 3.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 4.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Oncol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Primary multiple extragastrointestinal stromal tumor of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T. Primary extragastrointestinal stromal tumor of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 7.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]
- 10.Sihto H, Sarlomo-Rikara M, Tynnienen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 11.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camcci T, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 12.Rossi G, Cavazza A, Marchion A, Longo L, Migaldi M, Sartori G, Gigiani N, Schirosi L, Casali C, Morandi U, Facciolongo N, Mariorana A, Barvieri M, Fabbri LM, Brambilla E. Role of chemotherapy and the receptor tyrosine kinase KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2005;23:8774–8785. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- 13.Araki K, Ishii G, Yokose T, Nagai K, Funai K, Kodama K, Nishikawa Y, Ochiai A. Frequent overexpression of the c-kit protein in large cell neuroendocrine carcinoma of the lung. Lung Cancer. 2003;40:173–180. doi: 10.1016/s0169-5002(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 14.Lapoint RJ, Bourne PA, Wang HL, Xu H. Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. App Immunohistochem Mol Morphol. 2007;15:401–406. doi: 10.1097/01.pai.0000213153.41440.7d. [DOI] [PubMed] [Google Scholar]