Abstract
Pyothorax-associated lymphoma (PAL) is a B-cell non-Hodgkin's lymphoma, and develops after 20-40 years of pyothorax or pleuritis including tuberculosis. An 88-year-old Japanese woman developed fever and right pleural effusion. No mycobacterium was recognized by Ziel-Neelsen stain, culture tests and PCR technique in the effusion. The patient was diagnosed as non-specific pleuritis. No tumor formations were seen in the right pleura by imaging modalities, and she was treated by antibiotics. Eight months later, she complained of severe back pain and fever. Imaging modalities revealed many tumors in the right pleura, and biopsy of the tumors was performed. The biopsy showed severe diffuse proliferation of lymphoid cells. The lymphoid cells consisted of atypical large lymphoid cells and small lymphoid cells. The large atypical cells were positive for CD45 and CD20 but negative for CD15 and CD30, thus confirming B cell neoplasm. Ki-67 labeling was 79%. The small cells were positive for CD45, CD20 and CD3, reflecting a mixture of mature B and T-cells. The small cells appeared non-neoplastic inflammatory cells. CD68-positive macrophages were also scattered. In situ hybridization for EB virus DNA showed positive signals in the large atypical B-cells and, to a lesser degree, in the small lymphocytes. The author thinks that this tumor is PAL with inflammatory reaction. The present case shows that the duration between PAL and pleuritis can be very short, and PAL may be associated with inflammatory reaction at the early neoplastic stage.
Keywords: Pyothorax-associated lymphoma, pathology, immunohistochemistry
Introduction
Pyothorax-associated lymphoma (PAL) is characterized by intrathoracic cavity B-cell non-Hodgkin's lymphoma, and develops after 20-40 years of pyothorax or tuberculous pleuritis [1-7]. PAL is a very rare tumor, and most cases of PAL have been reported from Japan [1-5]. PAL affects old individual [1-5]. PAL in western countries is rare [6, 7]. PAL is strongly associated with EB virus [1-7]. PAL is different from another intrathoracic cavity B-cell lymphoma, primary effusion lymphoma (PEL), in that PAL forms tumors in the thoracic cavity [1, 8]. PAL is thought to develop after longstanding chronic inflammation [2, 4, 5], and it is considered that longstanding inflammation lead to malignant transformation (PAL) [4]. The author herein reports a case of PAL of a Japanese woman. It was characterized by acute onset with short duration between PAL and non-specific pleuritis (8 months), and by histological inflammatory features.
Case report
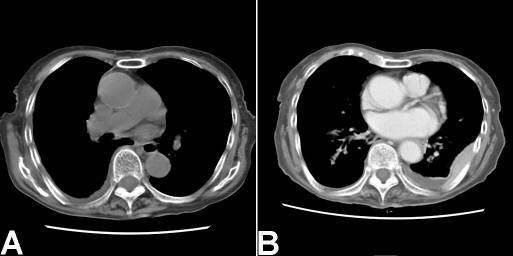
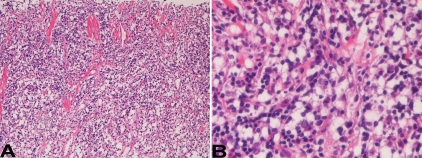
An 88-year-old Japanese woman was admitted to our hospital because of fever and chest pain. She denied past history of tuberculosis, pleuritis and pyothorax. A blood laboratory test showed leucocytosis and inflammatory reactions. Physical and imaging modalities showed right pleural effusion. No mycobacterium was recognized by Ziel-Neelsen stain, culture tests and PCR technique in the effusion. She was diagnosed as non-specific pleuritis. Tumor formations were not seen in the right pleura by imaging modalities (Figure 1A). She was treated by antibiotics. Eight months later, she complained of fever and severe back pain. Imaging modalities revealed many tumors in the right thoracic cavity (pleura) (Figure 1B), and a large biopsy of the tumors was performed. The biopsy showed severe diffuse proliferation of lymphoid cells (Figure 2A). The lymphoid cells are not monotonous, and consisted of large atypical lymphoid cells and small lymphoid cells (Figure 2B). The large cells had vesicular nuclei with nucleoli, while the small cells were very reminiscent to normal lymphocytes (Figure 2B). The ratio of the large and small lymphoid cells was 1:5.
Figure 1.
CT findings. A. CT of the first admission. No tumors are seen in the pleura. B. CT 8 months after the first manifestation. Many tumors are seen in the right pleura. In this slice, back side of the right pleura shows tumor formations.
Figure 2.
Histological features. A. Diffuse proliferation of lymphoid cell proliferation is seen. HE, x20. B. The lymphoid cells are composed of atypical large large cells and normal-appearing small lymphoid cells. The atypical large cells have hyperchromatic vesicular nuclei and nucleoli. HE, x400.
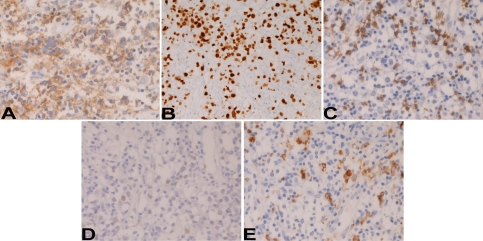
An immunohistochemical study was performed by Dako's Envision method, as previously described [9, 10]. The large lymphoid cells were positive for CD45 (Figure 3A) and CD20 (Figure 3B), but negative for various cytokeratins, EMA, CD3, CD15, CD30, and TdT. Ki-67 labeling was 80 % (Figure 3C). The small lymphoid cells were positive for CD45, CD20 (Figure 3B) and CD3 (Figure 3D), but negative for cytokeratins, EMA, CD15, CD30, and TdT. Ki-67 labeling was 34%. No light chain restriction was seen in the small lymphocytes; λ-chain and κ-chain were expressed equally. In situ hybridization for EB virus DNA showed positive signals in the large atypical B-cells and, to a lesser extent, in small lymphocytes (Figure 3E). CD68-positive macrophages were scattered (Figure 3F).
Figure 3.
Immunohistochemical features. The large atypical lymphoid cells are positive for CD20 (A), but negative for CD3 (C). The Ki-67 labeling of the large cells is very high (B). The large lymphoid cells are positive for EB virus DNA in their nuclei. The small lymphoid cells are positive for CD20 (A), and CD3 (C). CD68-positive macrophages are scattered (E). Immunostains, for CD20 (A), Ki-67 (B), CD3 (C), and CD68 (E), x400. In situ hybridization for EB virus (D), x400.
The pathological diagnosis was EB virus-associated PAL (diffuse large B- cell lymphoma) with inflammatory lymphocytes.
Discussion
The author thinks that the large lymphoid cells are true neoplastic cells, because they showed CD20 but not CD3. In addition, the Ki67 labeling was very high. The author thinks that the small lymphoid cells are normal inflammatory cells, because they expressed CD20 and CD3. In addition, the Ki67 labeling was relatively low and no light chain restriction was observed in these small cells.
The present case is different from usual PAL in two points. The first point is that the duration between the pleuritis and PAL is very short (8 months); that of usual PAL is reported to be 20-40 years [1-5]. The second point is that the present case accompanied inflammatory infiltration.
The first point is difficult to explain. However, it can be possible that malignant transformation of chronic inflammation (non-specific pleuritis) was very rapid in the present case, though the mechanism is unclear.
The second point is also difficult to explain. However, the dominant infiltration of inflammaroty lymphocytes undergoes malignant transformation. The present case may be the early phase of such malignant transformation. PAL is though to develop from chronic inflammation [1-5], supporting this hypothesis.
PAL is well known to be strongly associated with EB virus. In the present study, EB virus DNA was detected in the neoplastic large lymphocytes and, to a lesser extent, in the inflammatory small cells, suggesting that EB virus is associated with the pathogenesis of PAL in the present case.
References
- 1.Banks PM, Harris NL, Warnke RA, Gaulard PH. Pyothrax-asociated lymphoma. In: Travis WD, Brambilla E, Konrad Muller-Hermelink H, Harris CC, editors. World Health Organization Classification of Tumours. Pathology and Genetics of tumours of the lung, pleura, thymus and heart. IARC Press, Ryon; 2004. pp. 138–140. [Google Scholar]
- 2.Nakatsukasa S, Yao M, Hoshida Y, Yamamoto S, Iuchi K, Aozasa K. Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol. 2002;20:4255–4260. doi: 10.1200/JCO.2002.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Narimastu H, Ota Y, Kami M, Takeuchi K, Suzuki R, Matsuo K, Matsumura T, Yuji Y, Kishi Y, Hamaki T, Sawada U, Miyata S, Sasaki T, Tobinai K, Kawabata M, Atsuta Y, Tanaka Y, Ueda R, Nakamura S. Clinicopathological features of pyothorax associated lymphomas; a retrospective survey involving 98 patients. Ann Oncol. 2007;18:122–128. doi: 10.1093/annonc/mdl349. [DOI] [PubMed] [Google Scholar]
- 4.Aozasa K, Takakuwa T, Nakatsuka S. Pyothorax -associated lymphoma: a lymphoma developing in chronic inflammation. Adv Anat Pathol. 2005;12:324–331. doi: 10.1097/01.pap.0000194627.50878.02. [DOI] [PubMed] [Google Scholar]
- 5.Aozasa K. Pyothrax-associated lymphoma. Int J Hematol. 1996;65:9–16. doi: 10.1016/s0925-5710(96)00532-4. [DOI] [PubMed] [Google Scholar]
- 6.Androulaki A, Drakos E, Hatzianastassiou D, Vgenopoulou S, Gazouli M, Korkolopoulou P, Patsouris E, Dosios T. Pyothorax-associated lymphoma (PAL); a western case with marked angiocentricity and review of the literature. Histopathology. 2004;44:69–76. doi: 10.1111/j.1365-2559.2004.01737.x. [DOI] [PubMed] [Google Scholar]
- 7.Petitjean B, Jardin F, Joly B, Martin-Garcia N, Tilly H, Picquenot JM, Briere J, Danel C, Mehaut S, Abd-Al-Samad I, Copie-Bergman C, Delfau-Larue MH, Gaulard P. Pyothorax-associated lymphoma: clinicopathological entity derived from B cell at late stage of differentiation and with occasional aberrant dual B- and T-cell phenotype. Am J Surg Pathol. 2002;26:724–732. doi: 10.1097/00000478-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Banks PM, Harris NL, Warnke RA, Gaulard PH. Primary effusion lymphoma. In: Travis WD, Brambilla E, Konrad Muller-Hermelink H, Harris CC, editors. World Health Organization Classification of Tumours. Pathology and Genetics of tumours of the lung, pleura, thymus and heart. IARC Press, Ryon; 2004. pp. 137–138. [Google Scholar]
- 9.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductalendocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 10.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]