Abstract
Chronic pancreatitis is characterized by inflammation, fibrosis, pain, and loss of exocrine function of the pancreas. We aimed to identify differentially-expressed proteins in the ePFT-collected pancreatic fluid from individuals with chronic pancreatitis (CP; n=9) and controls with chronic abdominal pain not associated with the pancreas (NP; n=9). Using GeLC-MS/MS techniques, we identified a total of 1391 different proteins in 18 pancreatic fluid samples. Of these proteins, 257 and 413 were identified exclusively in the control and chronic pancreatitis cohorts, respectively, and 721 were identified in both cohorts. Spectral counting and statistical analysis thereof revealed an additional 38 and 77 proteins that were up- or down-regulated, respectively, in the pancreatic fluid from individuals with chronic pancreatitis. As expected, gene ontology analysis illustrated that the largest percentage of differentially-regulated proteins was secreted/extracellular in origin. In addition, proteins that were down-regulated with statistical significance in the chronic pancreatitis cohort were determined to have biological function of proteases, corresponding to the canonical pancreatic insufficiency associated with chronic pancreatitis. Proteins enriched in pancreatic fluid from chronic pancreatitis patients had roles in fibrosis, inflammation, and pain, whereas digestive enzymes were significantly less abundant. Our workflow provided a mass spectrometry-based approach for the further study of the pancreatic fluid proteome which may lead to the discovery potential biomarkers of chronic pancreatitis.
Keywords: pancreas, pancreas juice, pancreatic function test, biomarkers, label-free quantification
1. Introduction
Chronic pancreatitis (CP) is a disease manifested by severe inflammation, progressive fibrosis, intense pain, and eventually loss of exocrine (i.e., fat and protein malabsorption) and endocrine (i.e., diabetes) function of the pancreas. Disorders of the exocrine pancreas affect over 1 million persons in the United States and cost nearly $3 billion annually. During the past decade, diseases of the exocrine pancreas have resulted in 277,000 hospitalizations and 475,000 ambulatory care visits per year. Nearly 25 % of these hospitalizations and outpatient visits are due to chronic pancreatitis. In addition, patients with chronic pancreatitis are at greater risk of developing pancreatic cancer, which results in more than 35,000 deaths annually and is the 4th leading cause of cancer death in the United States1.
Clinical diagnosis of chronic pancreatitis is based currently on identifying advanced functional, morphological, and histological features. However, the tissue damage and fibrosis are often irreversible by the time that objective diagnostic features appear. Pancreatic biopsy for histologic diagnosis is not recommended due to potential complications, such as bleeding and fistulae formation, as well as the possibility of sampling error. Currently, pancreas function testing is the non-histological “surrogate” gold standard that is used to diagnose moderate to late stage chronic pancreatitis2. The degree of pancreatic dysfunction is determined by measuring specific concentrations of cellular secretory components, typically bicarbonate, in pancreatic fluid following hormone stimulation3. The ability to diagnose chronic pancreatitis prior to irreversible organ dysfunction would revolutionize treatment and potentially lead to therapies designed to retard or modify disease progression before irreversible damages to the pancreas become apparent.
Pancreatic fluid is an excellent source of potential protein biomarkers for the diagnosis of early chronic pancreatitis. As a proximal body fluid, pancreatic juice is rich in proteins that are shed directly from the pancreas during the cell necrosis which is prevalent in the course of chronic pancreatitis. Changes in the protein expression patterns specific to pancreatic fluid collected from diseased patients can be identified using high-throughput proteomic technologies, such as mass spectrometry. Using mass spectrometric techniques, qualitative and quantitative changes in pancreatic fluid protein profiles can be determined. Proteomic alterations may reflect the underlying mechanisms of disease that have yet to progress to fibrosis or pancreatic insufficiency and may offer a means for amelioration or retardation of the disease.
To date, several mass spectrometry-based proteomic investigations of pancreatic fluid have been performed using specimens collected surgically or via endoscopic retrograde cholangiopancreatography (ERCP)4–12. In contrast, we use the well-established secretin-stimulated ePFT (endoscopic pancreatic function test) collection method, which is less invasive compared to ERCP and surgery. ePFT permits the safe collection of 10-fold larger volumes of pancreatic fluid without cannulation of the pancreatic duct, thus reducing the risk of post-procedure acute pancreatitis. Secretin stimulates bicarbonate secretion from pancreatic duct cells13, 14 and essentially flushes out the proteins, and potentially protein plugs, present in the pancreatic acini and duct. These advantages support ePFT as an exceptional fluid collection method for the comprehensive analysis of the pancreatic fluid proteome14–19.
We present a comparative mass spectrometry-based proteomic analysis of pancreatic fluid from patients with advanced chronic pancreatitis and non-pancreatitis controls. Using our previously established protocol20, 21, we 1) collected pancreatic fluid from the two cohorts via the ePFT method, 2) inactivated proteases and extracted proteins via trichloroacetic acid (TCA) precipitation, 3) fractionated proteins via one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), 4) tryptically digested proteins in-gel, 5) analyzed resulting peptides via liquid chromatography-tandem mass spectrometry (LC-MS/MS), and 6) performed bioinformatic data processing which included: a) database searching, b) label-free quantification (spectral counting and statistical analysis thereof) and c) gene ontology and pathway analysis. We have compiled a list of statistically-significant differentially-expressed proteins between our two cohorts, which can be validated further by orthogonal methodologies, such as western blotting, ELISA and protein microarrays.
2. Materials and Methods
Study Population
This protocol was approved by the Institutional Review Board at Brigham and Women's Hospital (BWH) (IRB # 2007-P-002480/1). The study population included adult patients evaluated for abdominal pain in the Center for Pancreatic Disease at BWH. Subjects were referred to BWH to eliminate a pancreatic etiology for their gastrointestinal symptoms. All subjects underwent the following: 1) comprehensive review of history and physical examination, 2) review of radiologic and endoscopic data, and 3) upper endoscopy with ePFT followed by a gastric and duodenal mucosal biopsy.
Materials
ChiRhoStim® synthetic human secretin was from ChiRhoClin (Burtonsville MD). SeeBluePlus2 Pre-Stained standard (LC5925), LDS (lithium dodecyl sulfate) sample buffer (NP0008), NuPAGE 4–12% Bis-Tris polyacrylamide gels (NP0335), SimplyBlue Coomassie stain (LC0665), and MES-SDS (2-(N-morpholino)ethanesulfonic acid-sodium dodecyl sulfate) electrophoresis buffer (NP002) were from Invitrogen (Carlsbad, CA). Other reagents and solvents were from Sigma-Aldrich and Burdick & Jackson, respectively.
Experimental Workflow
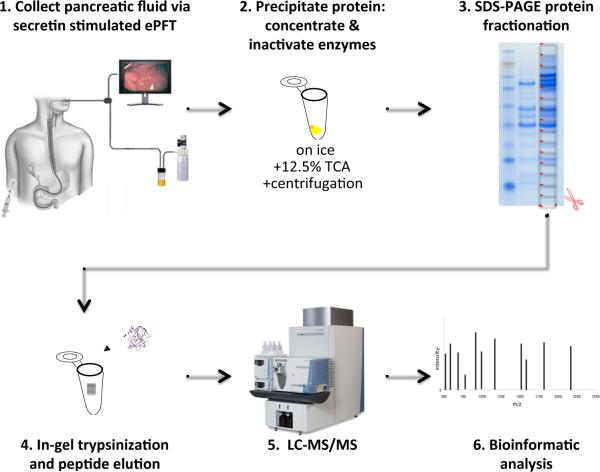
Figure 1 illustrates the general workflow for the overall analysis as follows: 1) ePFT sample collection, 2) protein precipitation, 3) SDS-PAGE, 4) in-gel tryptic digestion, 5) LC-MS/MS peptide mass determination, and 6) bioinformatic data processing.
Figure 1.
Experimental workflow. The general workflow for the overall analysis is as follows: 1) ePFT sample collection, 2) protein extraction with tricholoracetic acid (TCA), 3) SDS-PAGE protein fractionation, 4) in-gel tryptic digestion, 5) LC-MS/MS peptide mass determination, and 6) bioinformatic data processing.
Pancreatic Fluid Collection (ePFT method)
The ePFT procedure was performed in three steps:
Pre-procedural assessment. Prior to upper endoscopy, all study subjects underwent a history and physical examination recording allergies, medications, substance use/abuse, and vital signs. Pre-procedural sedation review included airway assessment based on the Mallampati airway scale and the American Society of Anesthesiologists Physical Status Classification (ASA Class). All study subjects in this protocol had a Mallampati score of B, Class 2, or better, and ASA Class II, or better.
-
Endoscopic procedure. Endoscopic collection was performed in a stepwise manner as follows: 1) The patient was placed in the left lateral decubitus position with slight head elevation. 2) The posterior pharynx was sprayed with topical cetacaine spray. 3) A sedation and analgesia bolus was administered. 4) Further sedation doses were administered, if necessary, for patient comfort. 5) After the sedation bolus, a bite-block was placed. 6) Esophagogastroduodenoscopy (EGD) was performed using a standard (10 mm) gastroscope for visualization of the esophagus, stomach, and duodenum. 7) Gastroduodenal fluid was aspirated as completely as possible through the gastroscope. 8) A test dose of synthetic human hormone secretin was administered and patients were monitored for anaphylaxis or adverse reaction. Subsequently a standard weight-based intravenous bolus (0.2 μg/kg) was given over 1 min. 9) Pancreatic fluid was aspirated from the descending duodenum at specific timed intervals following hormonal stimulation and stored on ice.
The duodenal aspirates were collected at 0, 5, 10, 15, 20, 30, 45, and 60 min after stimulation. Based on previously published pancreatic output patterns, only the 30-min time point was used for the ensuing analysis21. Biopsies of the stomach and duodenum were obtained to eliminate microscopic gastrointestinal disease, such as Helicobacter pylori or celiac sprue as a cause of dyspepsia.
Post-procedural Assessment / Recovery. Study participants were discharged from the endoscopy unit based on hospital procedural sedation guidelines assessing levels of consciousness, vital signs, oxygen saturation, alertness, gag reflex, degree of nausea, and ability to ambulate.
Pancreatic fluid biochemical analysis
All samples underwent biochemical analysis within 24 hrs. Samples were passed through a serum filter (ML0550, MarketLab, Caledonia, MI) to remove particulates and fibrin microthrombi prior to analysis. All measurements were conducted in the CLIA-certified Brigham and Women's Hospital Clinical Chemistry Laboratory, under the standard operating procedures on an AU640 (Olympus America, Center Valley, PA) automated chemistry analyzer. Sodium, potassium, and chloride were measured by indirect ion-selective electrodes, and total bicarbonate was measured by the two-step phosphoenolpyruvate carboxylase-malate dehydrogenase enzymatic-photometric method22. Samples with results greater than the upper assay limit were diluted into the linear range. The mean peak bicarbonate concentration from previously published studies in secretin-stimulated pancreatic fluid was 103 ± 11 meq/L15. A threshold limit of 80 meq/l was two standard deviations below the mean and considered abnormal14, 19. Excess pancreatic fluid samples were frozen at −80°C and stored until proteomic analysis.
Pancreatic Fluid Proteomic Analysis
Pancreatic fluid sample preparation
As described above, aliquots of pancreatic fluid samples for proteomic analysis were collected on ice, centrifuged at 4°C at 20,000 × g for 15 min to remove cellular debris, and aliquoted (500 μL) prior to storage at −80°C. Protein concentration was determined using the BioRAD protein assay according to the manufacturer's instructions. In preparation for SDS-PAGE analysis, the proteins from pancreatic fluid specimens were isolated by precipitation with the addition of 12.5% trichloroacetic acid (TCA), as described previously20, 21, 23. This process i) limited protein degradation by instantaneously deactivating enzymes and ii) removed salts that will interfere with the subsequent electrophoretic mobility-based fractionation by SDS-PAGE. The precipitated protein pellets were re-dissolved in 50 μL of reducing Laemmli buffer24 (with 10 mM DTT) for 1 hr at 56°C and alkylated with 1% acrylamide at room temperature for 30 min for subsequent GeLC-MS/MS analysis.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) prefractionation and liquid chromatography-tandem mass spectrometry (GeLC-MS/MS) of pancreatic fluid specimens
The proteins were fractionated using 4–12% NuPAGE pre-cast SDS-PAGE gels at 175V for 45 min using MES-SDS running buffer. Gels were incubated with SimplyBlue Coomassie stain for 3 hrs and afterward destained in water overnight. Subsequently, entire gel lanes were divided into 15 sections. Proteins in each gel section were digested, in-gel, with trypsin25, 26. The extracted peptides from each gel section were subjected to peptide fractionation using reversed-phase high performance liquid chromatography (HPLC; Thermo Scientific, Waltham, MA) and the gradient-eluted peptides were analyzed by a hyphenated linear trap quadrupole-Fourier Transform ion cyclotron resonance (LTQ-FTICR) mass spectrometer (Thermo Scientific, Waltham, MA). The liquid chromatography columns (15 cm × 100 μm ID) were packed in-house (Magic C18, 5 μm, 100Å beads, Michrom BioResources) into PicoTips (New Objective, Woburn, MA). Samples were analyzed with a 90 minute linear gradient (0–35% acetonitrile with 0.2% formic acid) and data were acquired in a data dependent manner, in which MS/MS fragmentation was performed using the 6 most intense peaks of every full MS scan.
Bioinformatics and Data Analysis
Database searching
All data generated from the gel sections were searched against the IPI-human database (v3.56) using the Mascot search engine (v.2.204; Matrix Science). One miscleavage per peptide was allowed and mass tolerances of ± 10 ppm for precursor and ± 0.8 Da for fragment ions were used. Amino acid modifications: fixed: propionamide (Cys); variable: deamidation (Asn/Gln), pyro-glutamate (N-terminal Glu/Gln), and oxidation (Met). Mascot search results were combined using in-house-developed software. In compliance with recommendations27–29 proposed by the major proteomic journals, we presented the following protein identification validation method that minimizes false positives and reports only high confidence identifications. Our false discovery rate (FDR) was 1% at the protein level as determined by searching the same dataset against the target database and a decoy database; the latter featured the reversed amino acid sequences of all the entries in the IPI-human database (v3.56)30, 31.
Spectral counting
Relative protein quantification was accomplished using a label-free technique, spectral counting, which compared the number of identified tandem mass spectra for the same protein across multiple data sets. To search for differences in the protein profile among data sets, spectral counts were normalized based on the total spectral counts, as previously outlined32. Specifically, spectral counts of each protein were first divided by the total spectral counts of all proteins from the same sample, and then multiplied by the total spectral counts of the sample with the maximum total number of spectral counts. Significance analysis of our normalized spectral count data was performed using QSPEC, a recently published algorithm for determining the statistical significance of differences in spectral counting data from different cohorts33. This algorithm used the Bayes Factor in lieu of the p-value, as a measure of evidential strength34, 35. By convention, a Bayes factor greater than 10 suggested strong evidence that a particular protein was differentially expressed between the two cohorts, thus a value of 10 was used as our significance threshold36.
Gene ontology analysis
Gene ontology analysis37 was performed, using the GoFact online tool38, 39, for those proteins common to, exclusive to, or significantly (statistically) different between the cohorts. GoSlim Annotation categories were chosen so as to avoid overlapping categories as follows: cellular component: cytoskeleton, cytosol, endoplasmic reticulum (ER), endosome, extracellular matrix, extracellular region, Golgi apparatus, lysosomes, membrane, mitochondrion, nucleus, peroxisome, ribonucleoprotein (RNP) complex, and vacuole; molecular functions: enzyme regulator activity, ion binding, kinase activity, lipid binding, nucleic acid binding, nucleotide binding, oxygen binding, peptidase, protein binding, signal transducer, structural molecule, transcription regulator, translation regulator, and transporter.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis
KEGG pathway analysis integrates knowledge about molecular interaction networks, including that generated by genome mapping projects, with information about biochemical compounds and reactions. This allows the classification of a protein, or a group of proteins to specific biological pathways40–42. We used the DAVID (Database for Annotation, Visualization and Integrated Discovery) Bioinformatics Database (http://david.abcc.ncifcrf.gov/) interface43, 44 to analyze our protein lists with KEGG pathway analysis.
BioBase ExPlain 3.0 for comparison of proteomics data with gene expression data for pancreatic disease
ExPlain integrates genomic information with biological knowledge databases and computational analysis methods45–47. This database was manually compiled from microarray, miRNA and ChIP-chip/Seq experiments. ExPlain applied an upstream analysis approach, based on the implementation of machine learning and graph topological analysis algorithms, to identify causality key-nodes in the network of pancreatic disease. We used ExPlain 3.0 to compare our proteomics data to that of previously identified biomarkers of pancreatic disease, including pancreatitis and pancreatic neoplasms.
Statistical analysis
Wilcoxon rank-sum non-parametric tests were performed using SAS 9.2 (Cary, NC). P-value < 0.05 was considered statistically significant.
3. Results
Sample collection
Patient medical histories and case report forms were extensively reviewed prior to initiation of our proteomics study. Table 1 summarizes the two patient cohorts in terms of their demographic, radiologic, endoscopic, histologic, and function test data. The chronic pancreatitis cohort had a mean age of 53.7±10.0 years and 5 of 9 individuals were female. The non-pancreatitis control cohort had a mean age of 45.3±13.6 years and 4 of 9 were female. The mean secretin-stimulated pancreatic fluid peak bicarbonate concentration was 93.4±13.6, meq/L in control subjects, consistent with normal pancreatic function, and 37.7±13.3 meq/L in the chronic pancreatitis patients, consistent with severe pancreatic insufficiency. The control subjects had no history of alcohol abuse, acute recurrent pancreatitis or therapeutic endoscopic pancreaticobiliary procedures involving instrumentation of the pancreas or pancreatic duct. In addition, all imaging studies, including computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) were not indicative of acute or chronic pancreatic disease. Endoscopic biopsies of the stomach and duodenum were normal in all patients and without evidence of Helicobacter pylori infection or celiac sprue. Careful review of all clinical, laboratory, and radiologic data eliminated pancreatic disease as a cause of chronic abdominal pain in the control cohort.
Table 1.
Patient charact en sties
| NPl | NP2 | NP3 | NP4 | NP5 | NP6 | NP7 | NP8 | NP9 | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | CP7 | CP8 | CP9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | male | male | male | female | female | female | male | female | male | male | female | male | female | male | male | female | female | female |
| Age (years) | 55 | 53 | 48 | 30 | 32 | 30 | 23 | 55 | 56 | 42 | 44 | 41 | 49 | 58 | 68 | 64 | 61 | 53 |
| Race | white | white | white | white | white | white | white | white | Hispanic | white | white | Hispanic | white | white | white | white | white | white |
| Smoker | − | + | + | − | − | − | − | − | − | − | + | + | + | + | + | + | − | + |
| Alcohol | + | − | + | − | − | − | − | − | − | − | − | + | + | − | + | + | – | + |
| TIGAR-O | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | G | G | T | T | I | T | T | I | T |
| Symptoms | pain | pain | pain, weight loss | Pain, Diarrhea | pain | pain | pain | diarrhea | pain | pain, recurrent pancreat it is | pain, recurrent pancreat it is | pain | pain | pain | pain | none | weight loss, pain | pain |
| CT Scan Findings | none | none | none | none | none | none | none | none | none | calcifications | calcifications, dilated main duct | calcifications | n/a | calcifications | calcifications, dilated main duct, atrophy | dilated duct, calcifications, atrophy | calcifications, dilated duct, dilated | calcifications, cyst |
| CT Grade | normal | n/a | normal | n/a | normal | n/a | normal | normal | normal | definite | definite | definite | n/a | definite | definite | definite | definite | definite |
| EUS Score | 1 | 4 | 2 | n/a | 4 | 2 | 3 | 1 | n/a | 5 | 5 | n/a | 7 | n/a | 5 | n/a | 8 | 6 |
| ERCP findings | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | dilated ducts & side branch, stones | n/a | n/a | n/a | n/a | n/a | n/a |
| ERCP grade | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | IV | n/a | n/a | n/a | n/a | n/a | n/a |
| MRI grade | n/a | 0 | n/a | 0 | 0 | 0 | 0 | 0 | 0 | IV | IV | IV | III | IV | IV | IV | IV | IV |
| CP based on imaging | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + |
| Peak HCO3− | 80 | 84 | 101 | 115 | 81 | 90 | 84 | 92 | 114 | 60 | 26 | 22 | 41 | 22 | 39 | 37 | 54 | 38 |
| Secretory dysfunction | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + |
| Classification | A | A | A | A | A | A | A | A | A | D | D | D | D | D | D | D | D | D |
TIGAR-O: T, Toxic-metabolic; I, Idiopathic; G, Genetic; A, Autoimmune; R, Recurrent and severe acute pancreatitis; O, Obstructive. EUS scores: 0–2 is normal 3–4 is equivocal, >5 is definite CP. ERCP Grade (Cambridge classification): normal=0, equivocal=I, mild=II, moderate=III, severe=IV. MRI/MRCP Grade (Cambridge classification: normal=0, equivocal=I, mild=II, moderate=III, severe=IV). Secretory dysfunction: yes (+) <80, no (−) ≥80. Classification of patient: A=healthy, B=Equivocal (Cambridge II, EUS 0–2), C=moderate (Cambridge III, EUS 3–4),D=severe (Cambridge IV, EUS ≥5), E=acute recurrent. n/a: not available; CP, chronic pancreatitis; NP, non-pancreatitis controls.
GeLC-MS/MS identified differentially expressed proteins
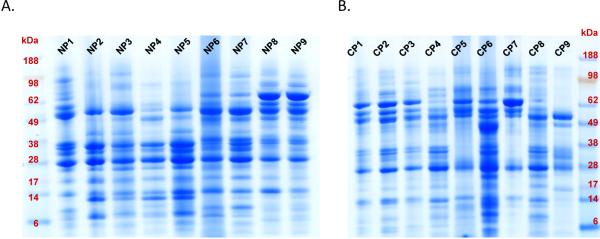
A total of 3 to 5 mL of pancreatic fluid with a protein concentration ranging from 1–2 mg/mL was collected at the 30 minute time point and used for analysis. Pancreatic fluid was cleared via centrifugation and protein was extracted by TCA precipitation -using our previously published optimized protocol20, 21- in preparation for SDS-PAGE analysis. SDS-PAGE revealed relatively similar, yet distinct, protein patterns within and between cohorts (Figure 2). We performed gel densitometry, using the ImageJ software48. Upon performing the Wilcoxon rank sum test on the densitometry measurements between the two cohorts, there is no statistically significant difference the cohorts (p-value = 0.0811). Although there is patient-to-patient variability within cohorts, there is no overall difference in protein concentration between cohorts. Each gel lane was sectioned into 15 slices, which were individually in-gel tryptically digested, after which peptides were analyzed using liquid chromatography coupled to tandem mass spectrometry (GeLC-MS/MS).
Figure 2.
SDS-PAGE protein fractionation. Each gel lane represents approximately 100 μg of ePFT-collected pancreatic fluid that has been TCA precipitated from a particular patient (9 individual patients per gel). A) NP (non-pancreatitis) controls samples. B) CP (chronic pancreatitis) samples.
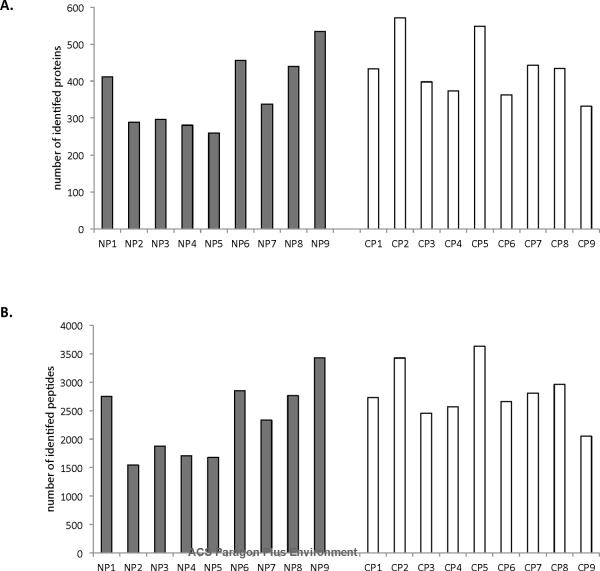
In total, 1391 non-redundant proteins were identified from the 18 samples. “Non-redundant” implies that the same protein is not counted more than once if it appears in multiple samples in a specified comparison. An average of 367 proteins was identified from individual control samples and an average of 433 proteins from individual chronic pancreatitis samples (Figure 3A). These proteins corresponded to an average of 2324 and 2808 peptides identified per control and chronic pancreatitis sample, respectively (Figure 3B). Wilcoxon rank sum tests revealed no statistically significant difference (95% confidence interval) between the controls and the chronic pancreatitis patients in terms of either the number of proteins (p-value = 0.2029) or peptides (p-value=0.2092), agreeing with the statistical analysis of protein concentrations as determined by densitometry of the SDS-PAGE results, as described above.
Figure 3.
The number of unique A) proteins and B) peptides identified in each pancreatic fluid sample analyzed. Grey bars represent NP (non-pancreatitis) controls samples and white bars represent CP (chronic pancreatitis) samples.
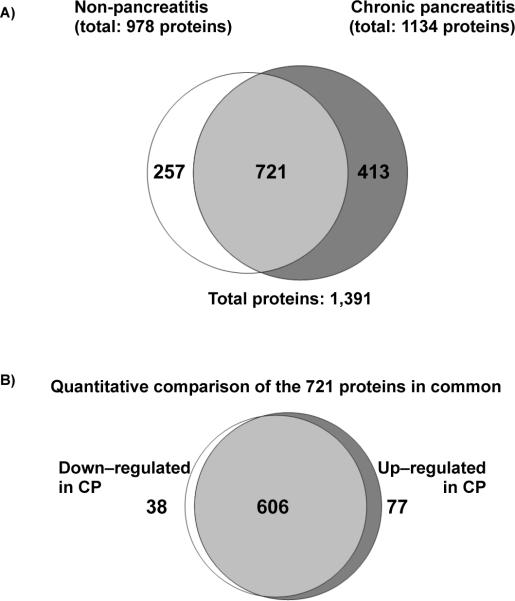
Examination of the total non-redundant proteins in each of the two cohorts identified 978 proteins in the control cohort and 1134 proteins in the chronic pancreatitis cohort. Seven hundred and twenty-one (721) proteins were common to both cohorts, 257 (Supplemental Table 1) proteins were unique to the control cohort and 413 (Supplemental Table 2) proteins were unique to the chronic pancreatitis cohort (Figure 4). Of the total 1391 non-redundant proteins, 69 proteins appeared in all 18 samples (Table 2), representing a core set of proteins identified from ePFT-collected pancreatic fluid. These proteins included various isoforms of pancreatic enzymes amylase, aminopeptidase, carboxypeptidase, chymotrypsin, elastase, lipase, and trypsin, as well as several protease inhibitors of the serpin family. When examining these common proteins, it was noted that several proteins did not show significant difference in abundance in the chronic pancreatitis cohort when compared to controls. These proteins include alpha-2-macroglobulin, alpha-1-acid glycoprotein 1, Xaa-Pro dipeptidase, enteropeptidase, as well as several serpins (A1, A3, C1, and G1). As such, one or more of these proteins may be used as normalization standards for proteomic analysis of pancreatic fluid; in a manner analogous to how actin or glyceraldehyde-3-phosphate dehydrogenase is generally used for various types of cell culture. In addition, we have included, in the supplementary material, the core proteomes for the chronic pancreatitis (Supplementary Table 3) and control cohorts (Supplementary Table 4) which are comprised of proteins identified in all 9 samples of each cohort. Further studies with much larger cohorts and targeted assays will be needed to assess the utility of these proteins for sample normalization.
Figure 4.
Venn diagrams comparing proteins identified in the NP (non-pancreatitis) and CP (chronic pancreatitis) cohorts. A) Qualitative assessment of proteins in each cohort, depicting those exclusive to either cohort. B) Quantitative assessment of proteins in common between the two cohorts. Bayes factor ≥ 10 indicates a statistically significant difference of the protein between the two cohorts.
Table 2.
Proteins identified in all 18 samples.
| spectral counts |
||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | IPI # | total NP | total CP | avg. NP | avg. CP | Bayes Factor | Fold Change | Statistically significant difference |
| A2M Alpha-2-macroglobulin | IPI00478003.1 | 2548 | 3075 | 283.11 | 341.67 | 0.00 | 1.18 | no |
| ALB Isoform 1 of Serum albumin | IPI00745872.2 | 10452 | 13709 | 1161.33 | 1523.22 | 3.88 | 1.62 | no |
| AMBP Protein AMBP | IPI00022426.1 | 70 | 194 | 7.78 | 21.56 | 468.36 | 2.48 | |
| AMY1C;AMY1B;AMY1A;AMY2A Alpha-amylase 1 | IPI00300786.1 | 5353 | 2529 | 594.78 | 281.00 | 284.76 | 2.07 | |
| AMY1C;AMY1B;AMY1A;AMY2A Pancreatic alpha-amylase | IPI00025476.1 | 9488 | 4003 | 1054.22 | 444.78 | 2.67E+06 | 2.33 | |
| AMY2B Alpha-amylase 2B | IPI00021447.1 | 8215 | 3424 | 912.78 | 380.44 | 6231.48 | 2.44 | |
| ANPEP Aminopeptidase N | IPI00221224.6 | 834 | 1417 | 92.67 | 157.44 | 6.58 | 2.17 | no |
| Anti-(ED-B)scFV | IPI00915411.4 | 239 | 461 | 26.56 | 51.22 | 25.34 | 1.88 | |
| C3 Complement C3 | IPI00783987.2 | 1339 | 2757 | 148.78 | 306.33 | 8.35E+07 | 4.34 | |
| cDNA FLJ78387 | IPI00876888.1 | 1252 | 1542 | 139.11 | 171.33 | 0.38 | 1.35 | no |
| CEL carboxyl ester lipase precursor | IPI00099670.2 | 1898 | 1202 | 210.89 | 133.56 | 2237.61 | 2.38 | |
| CLCA1 Calcium-activated chloride channel regulator 1 | IPI00014625.3 | 438 | 664 | 48.67 | 73.78 | 12.92 | 1.66 | |
| CPA1 Carboxypeptidase A1 | IPI00009823.3 | 8474 | 3610 | 941.56 | 401.11 | 6.63E+07 | 2.28 | |
| CPB1 Carboxypeptidase B | IPI00009826.2 | 5599 | 2537 | 622.11 | 281.89 | 9.38E+05 | 2.37 | |
| CTRB1 Chymotrypsinogen B | IPI00015133.1 | 2323 | 1149 | 258.11 | 127.67 | 3.01E+04 | 2.33 | |
| CTRB2 chymotrypsinogen B2 | IPI00515087.2 | 4055 | 1781 | 450.56 | 197.89 | 1.09E+05 | 2.14 | |
| CTRC Chymotrypsin-C | IPI00018553.1 | 2049 | 793 | 227.67 | 88.11 | 2.45E+04 | 2.71 | |
| DCD Dermcidin | IPI00027547.2 | 120 | 158 | 13.33 | 17.56 | 1.67 | 1.30 | no |
| DMBT1 Isoform 4 of Deleted in malignant brain tumors 1 protein | IPI00418512.5 | 412 | 175 | 45.78 | 19.44 | 26.37 | 1.89 | |
| ELA2A Elastase-2A | IPI00027722.1 | 5087 | 2780 | 565.22 | 308.89 | 87.38 | 1.94 | |
| ELA3A Elastase-3A | IPI00295663.1 | 3142 | 1328 | 349.11 | 147.56 | 624.24 | 2.52 | |
| ELA3B Elastase-3B | IPI00307485.3 | 1699 | 637 | 188.78 | 70.78 | 1987.17 | 3.13 | |
| FCGBP IgGFc-binding protein | IPI00242956.5 | 986 | 1323 | 109.56 | 147.00 | 8.72 | 1.45 | no |
| GP2 Isoform Alpha of Pancreatic secretory granule membrane major | IPI00914943.1 | 1678 | 438 | 186.44 | 48.67 | 5.74E+09 | 5.98 | |
| HBA1;HBA2 Hemoglobin subunit alpha | IPI00410714.5 | 597 | 480 | 66.33 | 53.33 | 0.72 | 1.31 | no |
| HBB Hemoglobin subunit beta | IPI00654755.3 | 898 | 904 | 99.78 | 100.44 | 4.59 | 1.39 | no |
| Ig kappa chain V-I region DEE | IPI00387025.1 | 73 | 104 | 8.11 | 11.56 | 3.83 | 1.41 | no |
| IGHA1;IGHV3OR16-13 IGHA1 protein | IPI00061977.1 | 1539 | 2264 | 171.00 | 251.56 | 0.96 | 1.39 | no |
| IGHA2 IGHA2 protein | IPI00783993.1 | 881 | 1698 | 97.89 | 188.67 | 41855.83 | 2.76 | |
| IGHG1 IGHG1 protein | IPI00448925.3 | 1371 | 1700 | 152.33 | 188.89 | 3.01 | 1.44 | no |
| IGHG2 IGHG2 protein | IPI00784807.1 | 447 | 496 | 49.67 | 55.11 | 4.47 | 1.22 | no |
| IGHG2 Putative uncharacterized protein DKFZp686I04196 | IPI00399007.5 | 894 | 896 | 99.33 | 99.56 | 0.23 | 1.18 | no |
| IGHM IGHM protein | IPI00472610.2 | 1801 | 2102 | 200.11 | 233.56 | 2.76 | 1.25 | no |
| IGHV4-31 Putative uncharacterized protein DKFZp686G11190 | IPI00784842.1 | 699 | 1148 | 77.67 | 127.56 | 22.10 | 1.65 | |
| IGJ immunoglobulin J chain | IPI00178926.2 | 236 | 344 | 26.22 | 38.22 | 10.86 | 1.44 | |
| IGKC IGKC protein | IPI00430847.1 | 902 | 1677 | 100.22 | 186.33 | 2.14 | 1.60 | no |
| IGKV1-5 IGKV1-5 protein | IPI00430820.1 | 290 | 1038 | 32.22 | 115.33 | 378.57 | 2.32 | |
| IGKV2-24 IGKV2-24 protein | IPI00440577.3 | 1365 | 1956 | 151.67 | 217.33 | 114.60 | 1.54 | |
| IGKV4-1 Similar to Ig kappa chain V–IV region precursor | IPI00026197.7 | 81 | 93 | 9.00 | 10.33 | 1.08 | 1.16 | no |
| Immunglobulin heavy chain variable region | IPI00783287.1 | 164 | 194 | 18.22 | 21.56 | 0.58 | 1.30 | no |
| JUP cDNA FLJ60424, highly similar to Junction plakoglobin | IPI00789324.3 | 212 | 260 | 23.56 | 28.89 | 1.54 | 1.18 | no |
| LOC100126583 cDNA FLJ41981 fis, clone SMINT2011888 | IPI00784830.1 | 1650 | 2420 | 183.33 | 268.89 | 384.12 | 1.56 | |
| LOC100133739 Putative uncharacterized protein DKFZp686C15213 | IPI00426051.3 | 428 | 843 | 47.56 | 93.67 | 498.98 | 1.93 | |
| LOC401847 similar to hCG1793095 | IPI00888191.1 | 250 | 304 | 27.78 | 33.78 | 0.62 | 1.31 | no |
| LOC642131 similar to hCG1812074 | IPI00887113.1 | 55 | 80 | 6.11 | 8.89 | 2.31 | 1.36 | no |
| Myosin-reactive immunoglobulin heavy chain variable region | IPI00783024.1 | 200 | 322 | 22.22 | 35.78 | 93.95 | 1.63 | |
| ORM1 Alpha-1-acid glycoprotein 1 | IPI00022429.3 | 405 | 476 | 45.00 | 52.89 | 0.85 | 1.19 | no |
| ORM2 Alpha-1-acid glycoprotein 2 | IPI00020091.1 | 184 | 231 | 20.44 | 25.67 | 0.88 | 1.37 | no |
| PEPD Xaa-Pro dipeptidase | IPI00257882.7 | 140 | 176 | 15.56 | 19.56 | 1.02 | 1.06 | no |
| PIGR Polymeric immunoglobulin receptor | IPI00004573.2 | 1807 | 2364 | 200.78 | 262.67 | 1.96 | 1.24 | no |
| PNLIP Pancreatic triacylglycerol lipase | IPI00027720.1 | 5785 | 2729 | 642.78 | 303.22 | 1.44E+09 | 2.25 | |
| PRSS1 Trypsin-1 | IPI00011694.1 | 2464 | 1533 | 273.78 | 170.33 | 20.90 | 1.77 | |
| PRSS2 Protease serine 2 isoform B | IPI00011695.8 | 1300 | 566 | 144.44 | 62.89 | 54.10 | 1.79 | |
| PRSS3 Isoform A of Trypsin-3 | IPI00015614.4 | 1336 | 804 | 148.44 | 89.33 | 2175.75 | 2.15 | |
| PRSS7 Enteropeptidase | IPI00023788.1 | 422 | 585 | 46.89 | 65.00 | 2.94 | 1.33 | no |
| Putative uncharacterized protein | IPI00807428.1 | 1289 | 1230 | 143.22 | 136.67 | 0.77 | 1.04 | no |
| REG1A Lithostathine-1-alpha | IPI00009027.1 | 645 | 284 | 71.67 | 31.56 | 58.20 | 2.03 | |
| Rheumatoid factor C6 light chain | IPI00829956.1 | 77 | 100 | 8.56 | 11.11 | 1.40 | 1.29 | no |
| SCFV Single-chain Fv | IPI00748998.1 | 331 | 464 | 36.78 | 51.56 | 3.95 | 1.34 | no |
| SERPINA1 Isoform 1 of Alpha-1-antitrypsin | IPI00553177.1 | 3075 | 3021 | 341.67 | 335.67 | 4.51 | 1.00 | no |
| SERPINA3 cDNA FLJ35730 fis | IPI00550991.3 | 544 | 479 | 60.44 | 53.22 | 1.46 | 1.01 | no |
| SERPINC1 Antithrombin III variant | IPI00032179.2 | 290 | 257 | 32.22 | 28.56 | 0.63 | 1.04 | no |
| SERPING1 Plasma protease C1 inhibitor | IPI00291866.5 | 260 | 366 | 28.89 | 40.67 | 1.72 | 1.43 | no |
| SI Sucrase-isomaltase, intestinal | IPI00221101.3 | 671 | 849 | 74.56 | 94.33 | 15.02 | 1.30 | |
| Similar to Elastase-3A precursor | IPI00921065.1 | 1609 | 665 | 178.78 | 73.89 | 2997.68 | 2.98 | |
| Single-chain Fv | IPI00470652.1 | 361 | 401 | 40.11 | 44.56 | 0.91 | 1.16 | no |
| TF Serotransferrin | IPI00022463.1 | 2806 | 3503 | 311.78 | 389.22 | 214.72 | 1.73 | |
| TTR Transthyretin | IPI00022432.1 | 284 | 354 | 31.56 | 39.33 | 0.70 | 1.02 | no |
| UGa8H | IPI00828099.1 | 275 | 282 | 30.56 | 31.33 | 0.37 | 1.08 | no |
IPI #, international protein index number; avg., average; CP, chronic pancreatitis; NP, non-pancreatitis controls.
Spectral counting analysis revealed statistically significant differences in the relative abundance of certain proteins between the two cohorts
Spectral counting analysis was performed using the QSPEC statistical tool49. We applied a strict filtering strategy, in which the threshold for statistically significant differences was determined by a Bayes factor greater than 10. Utilizing this threshold, we identified 77 proteins significantly up-regulated (Supplemental Table 5) and 38 significantly down-regulated (Supplemental Table 6) in the chronic pancreatitis cohort. The remaining 606 proteins were not significantly different in abundance in chronic pancreatitis compared to control samples (Supplemental Table 7).
Gene ontology analysis revealed differences in protein function between the two cohorts
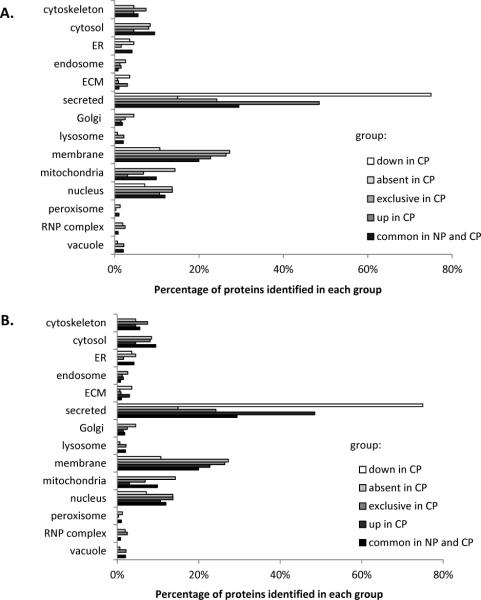
We performed gene ontology (GO) analysis of subcellular localization (Figure 5A) and biological function (Figure 5B) using the GOfact online interface (http://61.50.138.118/gofact) for proteins which we identified to be exclusive to a particular cohort, differentially expressed (with statistical significance), as well as common to both cohorts. We divided the analysis into 5 groups of proteins that were a) down-regulated in CP, b) absent in CP (i.e., exclusive to controls), c) exclusive to CP, d) up-regulated in CP and e) common to both NP and CP. GO analysis of subcellular localization of proteins in these distinct groups revealed that the highest percentage of identified proteins in 3 of the 5 comparison groups originated from the secreted category (e.g., 47% and 76% of proteins up- and down-regulated in chronic pancreatitis, respectively). As for biological function, the highest percentage of proteins in 4 of the 5 groups were of the protein binding category, with the exception of proteins up-regulated in chronic pancreatitis, was classified as having peptidase activity.
Figure 5.
Gene ontology (GO) analysis of subcellular localization and molecular function that were statistically up- or down- regulated in chronic pancreatitis. GO characterization using the categories listed for proteins with statistically significant differences was performed manually with the UniProt85 database or using the GoFact online tool38, 39. A) Subcellular localization and B) Molecular function of proteins down-regulated in CP (white bars), absent in CP (light grey). exclusive to CP (medium grey), up-regulated in CP (darker grey bars), and identified in both NP and CP (black bars). CP, chronic pancreatitis; NP, non-pancreatitis controls.
KEGG pathway analysis revealed differences in biological pathways between the two cohorts
Using the DAVID interface43, 44, we surveyed the biomolecular pathways of proteins that we determined to be differentially expressed in the pancreatic fluid of the chronic pancreatitis cohort when compared to controls. Tables 3 and 4 list the pathways, the number of proteins, and the percentage of differentially expressed proteins which were present in that particular pathway. Among the pathways identified for proteins unique to or up-regulated in chronic pancreatitis (Table 3) were the regulation of the cytoskeleton, complement and coagulation cascades, and type 1 diabetes mellitus pathophysiological pathways, as well as other disease-specific pathways. For those proteins that were not identified or were down-regulated in chronic pancreatitis (Table 4), most of the pathways identified were specific to metabolism (e.g., glycolysis, TCA cycle, fatty acid, and glycerolipid metabolism). The identification of these metabolic pathways may be expected, as cellular processes are generally diminished in diseased pancreata.
Table 3.
KEGG pathways analysis of proteins that were exclusive to or up-regulated in chronic pancreatitis.
| Pathway | # of proteins | % of total up-regulated proteins |
|---|---|---|
| Regulation of actin cytoskeleton | 12 | 3.65 |
| Complement and coagulation cascades | 11 | 3.34 |
| Alzheimer's disease | 11 | 3.34 |
| Cell adhesion molecules (CAMs) | 10 | 3.04 |
| Tight junction | 10 | 3.04 |
| Viral myocarditis | 9 | 2.74 |
| Lysosome | 9 | 2.74 |
| Pathogenic Escherichia coli infection | 8 | 2.43 |
| Leukocyte transendothelial migration | 8 | 2.43 |
| Starch and sucrose metabolism | 7 | 2.13 |
| Glycolysis / Gluconeogenesis | 7 | 2.13 |
| Antigen processing and presentation | 7 | 2.13 |
| Hematopoietic cell lineage | 7 | 2.13 |
| Prion diseases | 6 | 1.82 |
| PPAR signaling pathway | 6 | 1.82 |
| Allograft rejection | 5 | 1.52 |
| Graft-versus-host disease | 5 | 1.52 |
| Type I diabetes mellitus | 5 | 1.52 |
| Autoimmune thyroid disease | 5 | 1.52 |
| Renin-angiotensin system | 4 | 1.22 |
| Nitrogen metabolism | 4 | 1.22 |
Table 4.
KEGG pathways analysis of proteins that were not identified in or down-regulated in chronic pancreatitis.
| Pathway | # of proteins | % of total down-regulated proteins |
|---|---|---|
| Glycolysis / Gluconeogenesis | 11 | 5.47 |
| Metabolism of xenobiotics by cytochrome P450 | 7 | 3.48 |
| Leukocyte transendothelial migration | 7 | 3.48 |
| Citrate cycle (TCA cycle) | 6 | 2.99 |
| Fatty acid metabolism | 5 | 2.49 |
| Proteasome | 5 | 2.49 |
| Drug metabolism | 5 | 2.49 |
| Complement and coagulation cascades | 5 | 2.49 |
| Propanoate metabolism | 4 | 1.99 |
| Pyruvate metabolism | 4 | 1.99 |
| Amino sugar and nucleotide sugar metabolism | 4 | 1.99 |
| Valine, leucine and isoleucine degradation | 4 | 1.99 |
| Glycerolipid metabolism | 4 | 1.99 |
| Glutathione metabolism | 4 | 1.99 |
| Glyoxylate and dicarboxylate metabolism | 3 | 1.49 |
| Pentose phosphate pathway | 3 | 1.49 |
4. Discussion
We have identified differentially-expressed proteins in ePFT-collected pancreatic fluid of chronic pancreatitis patients, using a GeLC-MS/MS strategy. In total, 1391 non-redundant proteins are determined to be present among 18 samples. Of these, 257 and 413 proteins are identified exclusively in the controls and chronic pancreatitis samples, respectively. In addition, label-free quantification followed by statistical evaluation determined that of the 606 common proteins, 77 are up-regulated and 38 are down-regulated in chronic pancreatitis when compared to controls. Moreover, GO analysis of these differentially expressed proteins from each cohort reveals that most of these proteins are extracellular. In terms of biological function classification, proteins with peptidase activity comprised the largest proportion (43%) of the proteins that were down-regulated in chronic pancreatitis. This result is expected as we would anticipate that a healthy pancreas would secrete digestive enzymes into the duodenum upon stimulation, however this function would be diminished in the case of a diseased fibrotic pancreas.
We classified further the identified proteins according to the pathways in which they have been previously identified. The KEGG pathway analysis of our differentially expressed proteins reveals that those up-regulated in chronic pancreatitis are identified in disease-related pathways, such as Alzheimer's disease (11 proteins), and prion disease (6 proteins). The identification of pathways indicative of diseased states unrelated to the pancreas may be a result of analogous deregulation at the biomolecular / subcellular level. Specifically, diseases for which given pathways are better understood, compared to chronic pancreatitis, have more entries in the KEGG database. In addition, those proteins that are down-regulated in chronic pancreatitis are involved in cellular homeostasis, and as such may be indicative of cellular dysfunction. Therefore, our bioinformatics analysis agrees with expected findings for chronic pancreatitis: abrogated cellular function in a diseased state, resulting in down-regulation of pancreatic proteases (enzyme insufficiency).
To expand on the KEGG pathway results, we used BioBase to search for proteins which are involved in the four main manifestations of chronic pancreatitis related to exocrine function: fibrosis, inflammation, pain, and exocrine insufficiency. The proteins found in these processes merit further investigation, and may have roles in the pathogenesis and pathophysiology of the disease. Below we highlight several proteins from each of the four categories. Understanding the function of these proteins in relation to chronic pancreatitis may further the knowledge of the molecular mechanisms resulting in the development and progression of the disease.
Several proteins involved in fibrosis-related pathways were determined to be exclusive to or up-regulated in chronic pancreatitis (Table 5). Proteins in this category include alpha-smooth muscle actin, haptoglobin, and serpin A5. Alpha-smooth muscle actin (α-SMA, actin alpha 2) is an isoform of the highly conserved actin family of proteins that is involved in cell motility, structure and integrity. The alpha actin subfamily is a major constituent of the contractile apparatus, and has been shown to be highly expressed in activated pancreatic stellate cells50, 51 and may be the direct link to the development of chronic pancreatitis51, 52. Therefore up-regulation of this protein in chronic pancreatitis patients is consistent with pancreatic stellate cell activation.
Table 5.
Proteins exclusive to or up-regulated in chronic pancreatitis that are involved in fibrosis.
| Protein | IPI | #NP | #CP | Bayes Factor | Fold Change higher in CP |
|---|---|---|---|---|---|
| ACTA2 Actin, smooth muscle | IPI00008603.1 | 0 | 4 | -- | -- |
| APOA2 Apolipoprotein A-II | IPI00021854.1 | 0 | 2 | -- | -- |
| C5 Complement C5 | IPI00032291.2 | 2 | 6 | 11.20 | 2.73 |
| HP HP protein | IPI00431645.1 | 6 | 9 | 45.66 | 3.45 |
| HPX Hemopexin | IPI00022488.1 | 6 | 8 | 6871.66 | 3.23 |
| SERPINA5 Plasma serine protease inhibitor | IPI00007221.1 | 0 | 8 | -- | -- |
| TF Serotransferrin | IPI00022463.1 | 9 | 9 | 214.72 | 1.73 |
IPI, international protein index; #NP, number of the non-pancreatitis cohort samples in which the specified protein was present (maximum = 9); #CP, number of chronic pancreatitis cohort samples in which specified protein was present (maximum = 9).
Haptoglobin (HP protein) is identified in all 9 chronic pancreatitis patients and only 6 controls (fold change: 3.45 higher in chronic pancreatitis with a Bayes Factor of 45.66). Haptoglobin is commonly associated with liver fibrosis53 and may also play a role in the pancreatic disease, as altered post-translational modifications of this protein are involved in pathways related to pancreatic cancer54. In fact, a mass spectrometry-based assay, which analyzes the fucosylation of this protein, has been developed for pancreatic cancer. The role of this protein in chronic pancreatitis has not been determined.
Serpin A5 is identified in 8 of the 9 chronic pancreatitis specimens and none of the control specimens. Serpin A5 inhibits activated protein C55,56 and plasminogen activators57 and has been shown to interact with prostate specific antigen58, and PLAU (urokinase-type plasminogen activator)59. In chronic pancreatitis, specific changes in protein C expression in the stroma of the pancreas have been shown to modulate the intracellular signaling pathways that control homeostatic mechanisms, however the specific role of Serpin A5 has not been investigated60.
In addition to these fibrosis-related proteins, several proteins involved in inflammation-related pathways were determined to be exclusive to or up-regulated in chronic pancreatitis (Table 6). Proteins in this category include: annexin A5, defensin 5, and neprilysin. Annexin A5 was identified in 5 of the 9 chronic pancreatitis specimens and none of the control specimens. Annexin A5 is involved in the blood coagulation cascade and inhibits the activity of protein kinase C61, 62. A previous study has identified annexin A5 in the pancreatic fluid of pancreatic cancer patients9. Interestingly, annexin A5 also inhibits the secretion of phospholipase63, 64, which is one of several pancreatic enzymes that our data shows to have a lower abundance in chronic pancreatitis verses control pancreatic fluid.
Table 6.
Proteins exclusive to or up-regulated in chronic pancreatitis that are involved in inflammation.
| Protein | IPI | #NP | #CP | Bayes Factor | Fold Change higher in CP |
|---|---|---|---|---|---|
| AHSG cDNA FLJ55606, similar to Alpha-2-HS-glycoprotein | IPI00022431.2 | 0 | 2 | -- | -- |
| AMBP Protein AMBP | IPI00022426.1 | 9 | 9 | 468.36 | 2.48 |
| ANXA5 Annexin A5 | IPI00329801.12 | 0 | 5 | -- | -- |
| APOA2 Apolipoprotein A-II | IPI00021854.1 | 0 | 2 | -- | -- |
| C3 Complement C3 | IPI00783987.2 | 9 | 9 | 8.4E+07 | 4.34 |
| C4B complement component 4B preproprotein | IPI00418163.3 | 3 | 5 | 4.64 | 1.95 |
| C5 Complement C5 | IPI00032291.2 | 2 | 6 | 11.20 | 2.73 |
| C7 protein | IPI00642632.1 | 5 | 6 | 0.07 | 1.58 |
| CFHR1 Complement factor H-related protein 1 | IPI00011264.2 | 0 | 3 | -- | -- |
| DEFA5 Defensin-5 | IPI00008298.1 | 2 | 7 | 149.78 | 5.24 |
| DMBT1 Putative uncharacterized protein DMBT1 | IPI00412044.4 | 8 | 8 | 0.33 | 1.66 |
| DNASE1 Deoxyribonuclease-1 | IPI00031065.1 | 3 | 8 | 58.91 | 4.31 |
| F11R Junctional adhesion molecule A | IPI00001754.1 | 2 | 6 | 52.35 | 3.67 |
| F2 36 kDa protein | IPI00877967.1 | 0 | 3 | -- | -- |
| GC Vitamin D-binding protein | IPI00555812.4 | 3 | 7 | 71.05 | 4.45 |
| HLA-B HLA class I histocompatibility antigen, B-73 alpha | IPI00472943.1 | 0 | 2 | -- | -- |
| HLA-DRA HLA class II histocompatibility antigen, DR alpha | IPI00005171.1 | 0 | 3 | -- | -- |
| HP HP protein | IPI00431645.1 | 6 | 9 | 45.66 | 3.45 |
| HPX Hemopexin | IPI00022488.1 | 6 | 8 | 6871.66 | 3.23 |
| ICAM1 Intercellular adhesion molecule 1 | IPI00008494.4 | 0 | 2 | -- | -- |
| LTF Growth-inhibiting protein 12 | IPI00298860.5 | 4 | 7 | 20.80 | 2.28 |
| MME Neprilysin | IPI00247063.3 | 3 | 9 | 1178.29 | 5.18 |
| MUC1 Isoform 1 of Mucin-1 | IPI00013955.1 | 0 | 2 | -- | -- |
| NT5E 5'-nucleotidase | IPI00009456.1 | 0 | 3 | -- | -- |
| S100A9 Protein S100-A9 | IPI00027462.1 | 4 | 9 | 52.74 | 4.09 |
| SCGB1A1 Uteroglobin | IPI00006705.1 | 0 | 2 | -- | -- |
| SERPINB3 Isoform 1 of Serpin B3 | IPI00022204.2 | 1 | 4 | 20.58 | 3.91 |
| SIRPA signal-regulatory protein alpha precursor | IPI00332887.5 | 0 | 2 | -- | -- |
| TF Serotransferrin | IPI00022463.1 | 9 | 9 | 214.72 | 1.73 |
| TXN Thioredoxin | IPI00216298.6 | 2 | 6 | 13.30 | 3.23 |
| VTN Vitronectin | IPI00298971.1 | 4 | 7 | 13.38 | 2.49 |
IPI, international protein index; #NP, number of the non-pancreatitis cohort samples in which the specified protein was present (maximum = 9); #CP, number of chronic pancreatitis cohort samples in which specified protein was present (maximum = 9).
Defensin 5 is identified in 7 of the 9 chronic pancreatitis specimens and only 2 of the 9 control specimens (fold change: 5.24 higher in chronic pancreatitis with a Bayes Factor of 149.78). In addition, pro-defensin 5 has been shown to be processed to mature defensin 5 in the human intestinal lumen by trypsin, in a complex in which chymotrypsinogen is also cleaved and activated. The increase in defensin 5 may be due to persistence of this complex resulting from decreased levels of pancreatic enzymes or increased levels of protease inhibitors, such as alpha1-antitrypsin65. However, further studies are needed to clarify the roles of both proteins in possible mechanisms regulating the development and progression of chronic pancreatitis.
Neprilysin (MME) is identified in 8 of the 9 chronic pancreatitis specimens and only 2 of the 9 control specimens (fold change: 5.15 higher in chronic pancreatitis with a Bayes Factor of 1176.29). MME - also known as neutral endopeptidase (NEP), CD10, and common acute lymphoblastic leukemia antigen (CALLA) - (is a zinc-dependent metalloprotease enzyme that degrades a number of small secreted polypeptides of up to 30 amino acids66. In addition, MME cleaves various isoforms of angiotensin and is involved in the degradation of atrial natriuretic factor67, 68 which has been shown to stimulate pancreatic exocrine secretion by interacting with hormones that regulate pancreatic function69, 70. As such, the increase in MME is associated with an decrease in atrial natriuretic factor which in turn decreases the pancreatic exocrine secretion of digestive enzymes. In agreement with our data, the overexpression of neprilysin has been linked previously to pancreatitis and pancreatic cancer71–73.
Several proteins involved in pain-related pathways are determined to be exclusive to or up-regulated in chronic pancreatitis (Table 7). Proteins in this category include: complement C3 (fold change: 4.35 higher in chronic pancreatitis with a Bayes Factor of 8.35E7) and complement C5 (fold change: 2.73 higher in chronic pancreatitis with a Bayes Factor of 11.20), both of which are involved in the adaptive immune system74, 75, in addition to neuropathic pain76. Complement C3 may be involved in a variety of pathophysiological processes in chronic pancreatitis, including deposition at the basement membrane and inhibition by TGF-beta77. Significantly, C5 has been shown to have a role in edema formation in a mouse acute pancreatitis model78. In addition to their involvement in the pain pathway, both proteins have roles in fibrosis-related pathways, while complement C5 is also involved in inflammation pathways. However, the immediate function of either protein in chronic pancreatitis has yet to be defined clearly.
Table 7.
Proteins exclusive to or up-regulated in chronic pancreatitis that are involved in pain.
| Protein | IPI | #NP | #CP | Bayes Factor | Fold Change higher in CP |
|---|---|---|---|---|---|
| C3 Complement C3 | IPI00783987.2 | 9 | 9 | 8.35E+07 | 4.34 |
| C5 Complement C5 | IPI00032291.2 | 2 | 6 | 11.20 | 2.73 |
| CAMP Cathelicidin antimicrobial peptide | IPI00292532.6 | 0 | 1 | -- | -- |
| ICAM1 Intercellular adhesion molecule 1 | IPI00008494.4 | 0 | 2 | -- | -- |
| LTF Growth-inhibiting protein 12 | IPI00298860.5 | 4 | 7 | 20.80 | 2.28 |
| SIRPA signal-regulatory protein alpha precursor | IPI00332887.5 | 0 | 2 | -- | -- |
| TXN Thioredoxin | IPI00216298.6 | 2 | 6 | 13.30 | 3.23 |
IPI, international protein index; #NP, number of the non-pancreatitis cohort samples in which the specified protein was present (maximum = 9); #CP, number of chronic pancreatitis cohort samples in which specified protein was present (maximum = 9).
While proteins that are involved in fibrosis, inflammation, and pain are identified exclusively in, or determined to be up-regulated in chronic pancreatitis, proteins having a role in digestion are down-regulated in chronic pancreatitis. We have shown that several common pancreatic enzymes, including trypsin, chymotrypsin, lipase, and aminopeptidase are down-regulated in chronic pancreatitis (Table 8). Such a finding is expected as pancreatic enzyme deficiency, manifested by malabsorption of proteins and fat, is generally associated with late stage chronic pancreatitis79–81. These findings not only support our study, but demonstrate that quantitative analysis of these enzymes from pancreatic fluid collected using the ePFT method, may provide criteria for diagnosing chronic pancreatitis as well as determining disease severity.
Table 8.
Common pancreatic enzymes that are down-regulated in chronic pancreatitis.
| Protein | IPI | #NP | #CP | Bayes Factor | Fold Change higher in NP |
|---|---|---|---|---|---|
| AMY1C;AMY1B;AMY1A;AMY2A Alpha-amylase 1 | IPI00300786.1 | 9 | 9 | 284.76 | 2.07 |
| AMY1C;AMY1B;AMY1A;AMY2A Pancreatic alpha-amylase | IPI00025476.1 | 9 | 9 | 2.67E+06 | 2.33 |
| CTRB1 Chymotrypsinogen B | IPI00015133.1 | 9 | 9 | 3.01E+04 | 2.33 |
| CTRB2 chymotrypsinogen B2 | IPI00515087.2 | 9 | 9 | 1.09E+05 | 2.14 |
| CTRC Chymotrypsin-C | IPI00018553.1 | 9 | 9 | 2.45E+04 | 2.71 |
| ELA2A Elastase-2A | IPI00027722.1 | 9 | 9 | 87.38 | 1.94 |
| ELA3A Elastase-3A | IPI00295663.1 | 9 | 9 | 624.24 | 2.52 |
| ELA3B Elastase-3B | IPI00307485.3 | 9 | 9 | 1987.17 | 3.13 |
| PLA2G1B Phospholipase A2 | IPI00021792.1 | 9 | 8 | 7961.39 | 3.19 |
| PNLIP Pancreatic triacylglycerol lipase | IPI00027720.1 | 9 | 9 | 1.44E+09 | 2.25 |
| PNLIPRP2 pancreatic lipase-related protein 2 | IPI00005924.4 | 9 | 8 | 39.47 | 3.43 |
| PRSS1 Putative trypsin-6 | IPI00169276.2 | 9 | 8 | 3690.34 | 2.12 |
| PRSS2 Protease serine 2 isoform B | IPI00011695.8 | 9 | 9 | 54.10 | 1.79 |
| PRSS3 Isoform A of Trypsin-3 | IPI00015614.4 | 9 | 9 | 2175.75 | 2.15 |
| RP11-265F14.2 Elastase-2B | IPI00027723.2 | 9 | 8 | 34.73 | 2.00 |
| RP11-265F14.2 Pancreatic elastase IIB | IPI00746692.3 | 5 | 0 | -- | -- |
| Similar to Elastase-3A precursor | IPI00921065.1 | 9 | 9 | 2997.68 | 2.98 |
IPI, international protein index; #NP, number of the non-pancreatitis cohort samples in which the specified protein was present (maximum = 9); #CP, number of chronic pancreatitis cohort samples in which specified protein was present (maximum = 9).
To obtain a quantitative estimate of the abundance of identified proteins, we used spectral counting – a frequently-used label-free approach for quantification82. This method compares the number of MS/MS spectra for the same protein among several data sets (in the present case, 18 individuals in two cohorts). We chose to analyze our data using spectral counting, as studies have shown a strong linear correlation between relative protein abundance and sequence coverage, with a dynamic range of over two orders of magnitude83. Furthermore, spectral counting quantification has been shown to be more reproducible and have a higher dynamic range than peptide ion chromatogram-based quantification84, and is particularly useful if no labeling has been performed a priori.
In our study, we investigate proteins in pancreatic fluid collected from patients with advanced chronic pancreatitis for comparison to chronic abdominal pain controls. We did not, however, aim to investigate differences in etiology. Such analysis may reveal proteins that differ significantly in abundance among the various specific causes of chronic pancreatitis. Further comparative proteomic analysis is certainly warranted, as the molecular mechanisms directing disease pathogenesis and progression may be different among etiologies. If early detection of chronic pancreatitis is to be investigated, data for etiology-specific protein differences will be valuable. However, with our current data, we do not have the statistical power to perform such etiology-specific pancreatic fluid proteome analysis. Future investigations will require increased number of patients for both the biomarker discovery and validation phases.
Moreover, a clinically useful diagnostic test must be able to discriminate between individuals with early pancreatic disease and those whose symptoms are of non-pancreatic origin. As such, future proteomic analysis must include a wider spectrum of patients, specifically those with mild and moderate chronic pancreatitis to ensure that identified biomarkers are sensitive and are not solely markers of advanced disease. In addition, longitudinal studies that investigate the progression of chronic pancreatitis in patients at different stages of disease may lend insight into the potential for tracking disease progression, complementing current radiologic and imaging techniques and may have the additional benefit of each patient serving as his/her own control.
Although we have successfully identified a set of pancreatic proteins with statistically significant differences in abundance between cohorts of severe chronic pancreatitis patients and controls, the molecular mechanisms underlying these differences remain unresolved. Protein target-based assays with animal models and/or cell culture must be performed to understand more clearly the mechanisms by which changes in protein expression occur. Such model systems allow the modulation of specific proteins, i.e. by overexpression or transcriptional knockdowns, and the subsequent analysis of cellular and/or physiological changes, in a controlled environment. Analogous experiments would be difficult or impossible to perform with human subjects, thus studies in animal models and cell culture, including that of pancreatic duct and stellate cells, will be necessary to elucidate the molecular mechanisms of chronic pancreatitis and the role of the identified proteins at the cellular level.
In summary, we have identified successfully proteins that are differentially secreted in the ePFT-collected pancreatic fluid of chronic pancreatitis patients compared to non-pancreatitis controls using GeLC-MS/MS. An orthogonal methodology, such as western blotting, ELISA or targeted mass-spectrometry-based assays, may be performed to validate our findings at an individual protein level, using much larger cohorts. The use of the ePFT collection technique coupled with GeLC-MS/MS analysis of proteins extracted from pancreatic fluid has significant potential in the study of the exocrine pancreas. In fact, our study has identified the largest number of proteins from pancreatic fluid to date. Once the molecular mechanisms underlying the differential expression of these proteins have been determined, cell culture systems or animal models, such are mouse, rat, and zebrafish, may be developed to investigate further these proteins of interest in a well-controlled system. In conclusion, we have identified potential biomarkers of chronic pancreatitis, establishing a workflow which may also be applied to related proteomic studies of diseases of the exocrine pancreas.
Supplementary Material
Acknowledgments
Funds were provided by the following NIH grants: 1 F32 DK085835-01A1) (JP), 1 R21 DK081703-01A2 (DC) and 5 P30 DK034854-24 (Harvard Digestive Diseases Center; DC). In addition, we would like to thank the Burrill family for their generous support through the Burrill Research Grant. We would also like to thank members of the Steen Laboratory at Children's Hospital Boston, in particular John FK Sauld and Dominic Winter for their technical assistance and critical reading of the manuscript. We are also grateful to Richard Lee from Children's Hospital Boston for sharing his idea of the core proteome of pancreatic fluid. In addition, we thank members of the Center for Pancreatic Disease at Brigham and Women's Hospital, particularly Bechien Wu, Katherine Repas, Emily Webster, and Scott A. Brizard for their technical assistance.
Abbreviations
- ePFT
endoscopic pancreatic function test
- GeLC-MS/MS
in-gel tryptic digestion followed by liquid chromatography-tandem mass spectrometry
- LTQ-FTICR
linear trap quadrupole- Fourier transform ion cyclotron resonance mass spectrometry
- TCA
trichloroacetic acid
Footnotes
Conflicts of interests The authors declare no competing interests.
Author contributions JP carried out the experiments and drafted the original manuscript. JP, PB, HS, and DC conceived of the study, and participated in its design and coordination. LL and VK were involved in to collection and categorization of the pancreatic fluid samples. All authors helped to draft the manuscript and approved the final manuscript.
References
- 1.James S. Opportunities and challenges at NIDDK in digestive diseases research. Gastroenterology. 2007;132:1219–1220. doi: 10.1053/j.gastro.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31(1):63–8. doi: 10.1097/01.mpa.0000164451.69265.80. [DOI] [PubMed] [Google Scholar]
- 3.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme ouputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288(16):813–5. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, Lane Z, Crispin DA, Goodlett DR, Aebersold R, Bronner MP. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics. 2007;6(8):1331–42. doi: 10.1074/mcp.M700072-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Pan S, Cooke K, Moyes KW, Bronner MP, Goodlett DR, Aebersold R, Brentnall TA. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas. 2007;34(1):70–9. doi: 10.1097/01.mpa.0000240615.20474.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Yi EC, Donohoe S, Pan S, Eng J, Cooke K, Crispin DA, Lane Z, Goodlett DR, Bronner MP, Aebersold R, Brentnall TA. Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology. 2005;129(4):1187–97. doi: 10.1053/j.gastro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Cui Y, Tian M, Zong M, Teng M, Chen Y, Lu J, Jiang J, Liu X, Han J. Proteomic Analysis of Pancreatic Ductal Adenocarcinoma Compared with Normal Adjacent Pancreatic Tissue and Pancreatic Benign Cystadenoma. Pancreatology. 2008;9(1–2):89–98. doi: 10.1159/000178879. [DOI] [PubMed] [Google Scholar]
- 8.Gronborg M, Bunkenborg J, Kristiansen TZ, Jensen ON, Yeo CJ, Hruban RH, Maitra A, Goggins MG, Pandey A. Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res. 2004;3(5):1042–55. doi: 10.1021/pr0499085. [DOI] [PubMed] [Google Scholar]
- 9.Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, Pandey A. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157–71. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Ke E, Patel BB, Liu T, Li XM, Haluszka O, Hoffman JP, Ehya H, Young NA, Watson JC, Weinberg DS, Nguyen MT, Cohen SJ, Meropol NJ, Litwin S, Tokar JL, Yeung AT. Proteomic Analyses of Pancreatic Cyst Fluids. Pancreas. 2009;38(2):33–42. doi: 10.1097/MPA.0b013e318193a08f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Simeone DM, Brenner DE, Anderson MA, Shedden KA, Ruffin MT, Lubman DM. Pancreatic Cancer Serum Detection Using a Lectin/Glyco-Antibody Array Method. J Proteome Res. 2008;8(2):483–92. doi: 10.1021/pr8007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Lee WN, Lim S, Go VL, Xiao J, Cao R, Zhang H, Recker RR, Xiao GG. Quantitative proteomics: measuring protein synthesis using 15N amino acid labeling in pancreatic cancer cells. Anal Chem. 2009;81(2):764–71. doi: 10.1021/ac801905g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conwell DL, Zuccaro G, Jr., Vargo JJ, Dumot JA, VanLente F, Khandwala F, Trolli PA, O'Laughlin C. Comparison of the secretin stimulated endoscopic pancreatic function test to retrograde pancreatogram. Dig Dis Sci. 2007;52(4):1076–81. doi: 10.1007/s10620-006-9600-8. [DOI] [PubMed] [Google Scholar]
- 14.Stevens T, Conwell DL, Zuccaro G, Van Lente F, Khandwala F, Purich E, Vargo JJ, Fein S, Dumot JA, Trolli P, O'Laughlin C. Electrolyte composition of endoscopically collected duodenal drainage fluid after synthetic porcine secretin stimulation in healthy subjects. Gastrointest Endosc. 2004;60(3):351–5. doi: 10.1016/s0016-5107(04)01809-7. [DOI] [PubMed] [Google Scholar]
- 15.Stevens T, Conwell D, Zuccaro G, Van Lente F, Khandwala F, Hanaway P, Vargo JJ, Dumot JA. Analysis of pancreatic elastase-1 concentrations in duodenal aspirates from healthy subjects and patients with chronic pancreatitis. Dig Dis Sci. 2004;49(9):1405–11. doi: 10.1023/b:ddas.0000042238.80040.cc. [DOI] [PubMed] [Google Scholar]
- 16.Conwell DL, Zuccaro G, Jr., Vargo JJ, Morrow JB, Obuchowski N, Dumot JA, Trolli PA, Burton A, O'Laughlin C, Van Lente F. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2003;1(3):189–94. doi: 10.1053/cgh.2003.50028. [DOI] [PubMed] [Google Scholar]
- 17.Conwell DL, Zuccaro G, Jr., Vargo JJ, Trolli PA, Vanlente F, Obuchowski N, Dumot JA, O'Laughlin C. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57(1):37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- 18.Conwell DL, Zuccaro G, Morrow JB, Van Lente F, O'Laughlin C, Vargo JJ, Dumot JA. Analysis of duodenal drainage fluid after cholecystokinin (CCK) stimulation in healthy volunteers. Pancreas. 2002;25(4):350–4. doi: 10.1097/00006676-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. 2009;104(10):2381–3. doi: 10.1038/ajg.2008.181. [DOI] [PubMed] [Google Scholar]
- 20.Paulo JA, Lee LS, Wu B, Repas K, Banks PA, Conwell DL, Steen H. Optimized sample preparation of endoscopic collected pancreatic fluid for SDS-PAGE analysis. Electrophoresis. 2010;31(14):2377–87. doi: 10.1002/elps.200900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulo JA, Lee LS, Wu B, Repas K, Mortele KJ, Banks PA, Steen H, Conwell DL. Identification of pancreas-specific proteins in endoscopically (endoscopic pancreatic function test) collected pancreatic fluid with liquid chromatography--tandem mass spectrometry. Pancreas. 2010;39(6):889–96. doi: 10.1097/MPA.0b013e3181cf16f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester RL, Wataji LJ, Silverman DA, Pierre KJ. Enzymatic method for determination of CO2 in serum. Clin Chem. 1976;22(2):243–5. [PubMed] [Google Scholar]
- 23.Paulo J, Lee LS, Banks PA, Steen H, Conwell D. Proteomic analysis of endoscopically (ePFT) collected gastroduodenal fluid using in-gel tryptic digestion followed by liquid chromatography-tandem mass spectrometry. Proteomics - Clinical Applications. 2010 doi: 10.1002/prca.201000018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Neubauer G, Mann M. Mapping of phosphorylation sites of gel-isolated proteins by nanoelectrospray tandem mass spectrometry: potentials and limitations. Anal Chem. 1999;71(1):235–42. doi: 10.1021/ac9804902. [DOI] [PubMed] [Google Scholar]
- 26.Steen H, Kuster B, Fernandez M, Pandey A, Mann M. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal Chem. 2001;73(7):1440–8. doi: 10.1021/ac001318c. [DOI] [PubMed] [Google Scholar]
- 27.Carr S, Aebersold R, Baldwin M, Burlingame A, Clauser K, Nesvizhskii A. The need for guidelines in publication of peptide and protein identification data: Working Group on Publication Guidelines for Peptide and Protein Identification Data. Mol Cell Proteomics. 2004;3(6):531–3. doi: 10.1074/mcp.T400006-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Taylor GK, Goodlett DR. Rules governing protein identification by mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(23):3420. doi: 10.1002/rcm.2225. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins MR, Appel RD, Van Eyk JE, Chung MC, Gorg A, Hecker M, Huber LA, Langen H, Link AJ, Paik YK, Patterson SD, Pennington SR, Rabilloud T, Simpson RJ, Weiss W, Dunn MJ. Guidelines for the next 10 years of proteomics. Proteomics. 2006;6(1):4–8. doi: 10.1002/pmic.200500856. [DOI] [PubMed] [Google Scholar]
- 30.Elias JE, Gibbons FD, King OD, Roth FP, Gygi SP. Intensity-based protein identification by machine learning from a library of tandem mass spectra. Nat Biotechnol. 2004;22(2):214–9. doi: 10.1038/nbt930. [DOI] [PubMed] [Google Scholar]
- 31.Moore RE, Young MK, Lee TD. Method for screening peptide fragment ion mass spectra prior to database searching. J Am Soc Mass Spectrom. 2000;11(5):422–6. doi: 10.1016/S1044-0305(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 32.Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317(5838):660–3. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- 33.Choi H, Nesvizhskii AI. False discovery rates and related statistical concepts in mass spectrometry-based proteomics. J Proteome Res. 2008;7(1):47–50. doi: 10.1021/pr700747q. [DOI] [PubMed] [Google Scholar]
- 34.Goodman SN. Toward evidence-based medical statistics. 1: The P value fallacy. Ann Intern Med. 1999;130(12):995–1004. doi: 10.7326/0003-4819-130-12-199906150-00008. [DOI] [PubMed] [Google Scholar]
- 35.Goodman SN. Toward evidence-based medical statistics. 2: The Bayes factor. Ann Intern Med. 1999;130(12):1005–13. doi: 10.7326/0003-4819-130-12-199906150-00019. [DOI] [PubMed] [Google Scholar]
- 36.Jeffreys H. Theory of probability. 3d ed. Clarendon Press; Oxford: 1961. p. 447. [Google Scholar]
- 37.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Li JQ, Ouyang SG, Wu SF, Wang J, Xu XJ, Zhu YP, He FC. An integrated strategy for functional analysis in large-scale proteomic research by gene ontology. Progress in Biochemistry and Biophysics. 2005;32(11):1026–1029. [Google Scholar]
- 39.Dong L, Jianqi L, Shuguang O, Songfeng W, Jian W, Yunping Z, Fuchu H. An integrated strategy for functional analysis in large scale proteomic research by gene ontology. Molecular & Cellular Proteomics. 2005;4(8):S34–S34. [Google Scholar]
- 40.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wixon J, Kell D. The Kyoto encyclopedia of genes and genomes--KEGG. Yeast. 2000;17(1):48–55. doi: 10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 44.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 45.Kel A, Konovalova T, Waleev T, Cheremushkin E, Kel-Margoulis O, Wingender E. Composite Module Analyst: a fitness-based tool for identification of transcription factor binding site combinations. Bioinformatics. 2006;22(10):1190–7. doi: 10.1093/bioinformatics/btl041. [DOI] [PubMed] [Google Scholar]
- 46.Kel A, Voss N, Jauregui R, Kel-Margoulis O, Wingender E. Beyond microarrays: find key transcription factors controlling signal transduction pathways. BMC Bioinformatics. 2006;7(Suppl 2):S13. doi: 10.1186/1471-2105-7-S2-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kel A, Voss N, Valeev T, Stegmaier P, Kel-Margoulis O, Wingender E. ExPlain: finding upstream drug targets in disease gene regulatory networks. SAR QSAR Environ Res. 2008;19(5-6):481–94. doi: 10.1080/10629360802083806. [DOI] [PubMed] [Google Scholar]
- 48.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 49.Choi H, Fermin D, Nesvizhskii AI. Significance analysis of spectral count data in label-free shotgun proteomics. Mol Cell Proteomics. 2008;7(12):2373–85. doi: 10.1074/mcp.M800203-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Apte M, Pirola R, Wilson J. The Fibrosis of Chronic Pancreatitis: New Insights into the Role of Pancreatic Stellate Cells. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 51.Apte MV, Wilson JS. Mechanisms of pancreatic fibrosis. Dig Dis. 2004;22(3):273–9. doi: 10.1159/000082799. [DOI] [PubMed] [Google Scholar]
- 52.Ellenrieder V, Schneiderhan W, Bachem M, Adler G. Fibrogenesis in the pancreas. Rocz Akad Med Bialymst. 2004;49:40–6. [PubMed] [Google Scholar]
- 53.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. The Lancet. 2001;357(9262):1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 54.Miyoshi E, Shinzaki S, Moriwaki K, Matsumoto H. Identification of fucosylated haptoglobin as a novel tumor marker for pancreatic cancer and its possible application for a clinical diagnostic test. Methods Enzymol. 2010;478:153–64. doi: 10.1016/S0076-6879(10)78006-X. [DOI] [PubMed] [Google Scholar]
- 55.Espana F, Berrettini M, Griffin JH. Purification and characterization of plasma protein C inhibitor. Thromb Res. 1989;55(3):369–84. doi: 10.1016/0049-3848(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 56.Strandberg K, Kjellberg M, Erb EM, Persson U, Mosher DF, Villoutreix BO, Stenflo J. Activated protein C-protein C inhibitor complex formation: characterization of a neoepitope provides evidence for extensive insertion of the reactive center loop. Biochemistry. 2000;39(51):15713–20. doi: 10.1021/bi001640h. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Hayashi T. cis-elements required for expression of human protein C inhibitor gene in HepG2 cells and its androgen-dependent expression in rat reproductive organs. Semin Thromb Hemost. 2000;26(1):75–83. doi: 10.1055/s-2000-9807. [DOI] [PubMed] [Google Scholar]
- 58.Kise H, Nishioka J, Kawamura J, Suzuki K. Characterization of semenogelin II and its molecular interaction with prostate-specific antigen and protein C inhibitor. Eur J Biochem. 1996;238(1):88–96. doi: 10.1111/j.1432-1033.1996.0088q.x. [DOI] [PubMed] [Google Scholar]
- 59.Geiger M, Huber K, Wojta J, Stingl L, Espana F, Griffin JH, Binder BR. Complex formation between urokinase and plasma protein C inhibitor in vitro and in vivo. Blood. 1989;74(2):722–8. [PubMed] [Google Scholar]
- 60.Evans JD, Cornford PA, Dodson A, Neoptolemos JP, Foster CS. Expression patterns of protein kinase C isoenzymes are characteristically modulated in chronic pancreatitis and pancreatic cancer. Am J Clin Pathol. 2003;119(3):392–402. doi: 10.1309/bkpc9dx98r781b87. [DOI] [PubMed] [Google Scholar]
- 61.Raynal P, Hullin F, Ragab-Thomas JM, Fauvel J, Chap H. Annexin 5 as a potential regulator of annexin 1 phosphorylation by protein kinase C. In vitro inhibition compared with quantitative data on annexin distribution in human endothelial cells. Biochem J. 1993;292(Pt 3):759–65. doi: 10.1042/bj2920759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlaepfer DD, Jones J, Haigler HT. Inhibition of protein kinase C by annexin V. Biochemistry. 1992;31(6):1886–91. doi: 10.1021/bi00121a043. [DOI] [PubMed] [Google Scholar]
- 63.Buckland AG, Wilton DC. Inhibition of secreted phospholipases A2 by annexin V. Competition for anionic phospholipid interfaces allows an assessment of the relative interfacial affinities of secreted phospholipases A2. Biochim Biophys Acta. 1998;1391(3):367–76. doi: 10.1016/s0005-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 64.Speijer H, Jans SW, Reutelingsperger CP, Hack CE, van der Vusse GJ, Hermens WT. Partial coverage of phospholipid model membranes with annexin V may completely inhibit their degradation by phospholipase A2. FEBS Lett. 1997;402(2-3):193–7. doi: 10.1016/s0014-5793(96)01527-x. [DOI] [PubMed] [Google Scholar]
- 65.Elphick D, Liddell S, Mahida YR. Impaired luminal processing of human defensin-5 in Crohn's disease: persistence in a complex with chymotrypsinogen and trypsin. Am J Pathol. 2008;172(3):702–13. doi: 10.2353/ajpath.2008.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oefner C, Roques BP, Fournie-Zaluski MC, Dale GE. Structural analysis of neprilysin with various specific and potent inhibitors. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 2):392–6. doi: 10.1107/S0907444903027410. [DOI] [PubMed] [Google Scholar]
- 67.Pankow K, Wang Y, Gembardt F, Krause E, Sun X, Krause G, Schultheiss HP, Siems WE, Walther T. Successive action of meprin A and neprilysin catabolizes B-type natriuretic peptide. Circ Res. 2007;101(9):875–82. doi: 10.1161/CIRCRESAHA.107.153585. [DOI] [PubMed] [Google Scholar]
- 68.Oefner C, Pierau S, Schulz H, Dale GE. Structural studies of a bifunctional inhibitor of neprilysin and DPPIV. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 9):975–81. doi: 10.1107/S0907444907036281. [DOI] [PubMed] [Google Scholar]
- 69.Sabbatini ME, Rodriguez MR, Dabas P, Vatta MS, Bianciotti LG. C-type natriuretic peptide stimulates pancreatic exocrine secretion in the rat: role of vagal afferent and efferent pathways. Eur J Pharmacol. 2007;577(1–3):192–202. doi: 10.1016/j.ejphar.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Sabbatini ME, Villagra A, Davio CA, Vatta MS, Fernandez BE, Bianciotti LG. Atrial natriuretic factor stimulates exocrine pancreatic secretion in the rat through NPR-C receptors. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G929–37. doi: 10.1152/ajpgi.00010.2003. [DOI] [PubMed] [Google Scholar]
- 71.Erhuma M, Kobel M, Mustafa T, Wulfanger J, Dralle H, Hoang-Vu C, Langner J, Seliger B, Kehlen A. Expression of neutral endopeptidase (NEP/CD10) on pancreatic tumor cell lines, pancreatitis and pancreatic tumor tissues. Int J Cancer. 2007;120(11):2393–400. doi: 10.1002/ijc.22252. [DOI] [PubMed] [Google Scholar]
- 72.Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24(10):1361–71. doi: 10.1097/00000478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Deschamps L, Handra-Luca A, O'Toole D, Sauvanet A, Ruszniewski P, Belghiti J, Bedossa P, Couvelard A. CD10 expression in pancreatic endocrine tumors: correlation with prognostic factors and survival. Hum Pathol. 2006;37(7):802–8. doi: 10.1016/j.humpath.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 74.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–35. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain. 2008;137(1):182–201. doi: 10.1016/j.pain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Andoh A, Shimada M, Takaya H, Hata K, Fujiyama Y, Bamba T. Transforming growth factor-beta1 acts as a potent inhibitor of complement C3 biosynthesis in human pancreatic cancer cell lines. Pancreas. 2000;20(2):138–45. doi: 10.1097/00006676-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Merriam LT, Webster C, Joehl RJ. Complement component C5 deficiency reduces edema formation in murine ligation-induced acute pancreatitis. J Surg Res. 1997;67(1):40–5. doi: 10.1006/jsre.1996.4916. [DOI] [PubMed] [Google Scholar]
- 79.Sikkens EC, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24(3):337–47. doi: 10.1016/j.bpg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Dominguez Munoz JE. Diagnosis of chronic pancreatitis: Functional testing. Best Pract Res Clin Gastroenterol. 2010;24(3):233–41. doi: 10.1016/j.bpg.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 81.Whitcomb DC, Lehman GA, Vasileva G, Malecka-Panas E, Gubergrits N, Shen Y, Sander-Struckmeier S, Caras S. Pancrelipase Delayed-Release Capsules (CREON) for Exocrine Pancreatic Insufficiency due to Chronic Pancreatitis or Pancreatic Surgery: A Double-Blind Randomized Trial. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.201. [DOI] [PubMed] [Google Scholar]
- 82.Lundgren DH, Hwang SI, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert Rev Proteomics. 2010;7(1):39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 83.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 84.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5(9):2339–47. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 85.Bairoch A, Apweiler R, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Natale DA, O'Donovan C, Redaschi N, Yeh L-SL. The Universal Protein Resource (UniProt) Nucl. Acids Res. 2005;33(suppl_1):D154–159. doi: 10.1093/nar/gki070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.