Abstract
Background
Abnormal brain functioning during verbal working memory tasks has been shown in individuals with alcohol use disorders (AUDs). Since adolescents with a familial history of alcoholism (FHP) are at high risk for developing an AUD, it is important to consider whether atypical brain activity during verbal working memory may help to explain FHP vulnerability toward developing alcoholism.
Methods
To that end, using functional magnetic resonance imaging, we examined brain response during a verbal working memory 2-back task in 19 FHP adolescents and 16 age and gender-matched family history negative (FHN) controls.
Results
Despite no group differences in task accuracy, FHP youth had significantly slower average reaction time when making correct responses during the 2-back condition than FHN youth. In contrast to a vigilance control condition, while covarying for reaction time, FHP adolescents showed less activation during verbal working memory than FHN youth in multiple areas of the prefrontal cortex (PFC) – a brain region crucial to intact working memory skills.
Conclusions
These results suggest that even prior to heavy alcohol use, FHP adolescents show atypical executive brain functioning during verbal working memory, and that these differences are independent of slower working memory reaction time in FHP youth. Given the importance of working memory in numerous areas of day-to-day functioning, such as adaptive decision-making, these abnormalities may contribute to FHP youth vulnerability toward developing AUDs.
Keywords: Family History, Alcoholism, Verbal Working Memory, fMRI, Adolescence
1. Introduction
Adolescents with a family history of alcoholism (FHP) are at greater risk for developing an alcohol use disorder (AUD) than their family history negative (FHN) peers (Dawson et al., 1992). Previous investigations have found that in the absence of heavy alcohol use, FHP adolescents have atypical brain structure (Hill et al., 2001; Hill et al., 2007), as well as aberrant brain functioning (Heitzeg et al., 2008; Heitzeg et al., 2010; Herting et al., 2011; Schweinsburg et al., 2004; Silveri et al., 2011; Spadoni et al., 2008) and behavior (Corral et al., 2003; Corral et al., 1999; Harden and Pihl, 1995; Nigg et al., 2004; Tapert and Brown, 2000) compared to FHN youth. These neurobiological and behavioral phenotypes may help to explain an increased vulnerability for developing an AUD in FHP youth.
Interestingly, deficits in executive functioning have been reported in both adults with AUDs and FHP individuals on measures assessing decision-making (Bechara et al., 2001; Lovallo et al., 2006), working memory (Ambrose et al., 2001; Harden and Pihl, 1995; Lovallo et al., 2006; Noel et al., 2001), response inhibition (Lawrence et al., 2009; Nigg et al., 2004; Noel et al., 2007), and attention (Ahveninen et al., 2000; Corral et al., 1999; Tapert and Brown, 2000). Thus, atypical executive functioning may not only be a consequence of alcohol abuse, but could also be a pre-morbid marker for future alcohol dependence (Hesselbrock et al., 1991; Peterson et al., 1992). In order to develop effective prevention strategies for FHP youth, it is important to better characterize specific brain and behavior deficits related to executive functions in this population.
Working memory, or the temporary manipulation and maintenance of information (Baddeley and Hitch, 1974), is an important skill for adaptive decision-making and successful day-to-day functioning. Neuroimaging has been used to characterize the neural substrates of working memory, which broadly involve a network of brain activity including the premotor cortex, dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex, frontal poles, inferior and posterior parietal cortex, and cerebellum (Owen et al., 2005). Prior investigations have shown performance deficits during working memory in both alcoholics and FHP adults (Ambrose et al., 2001; Lovallo et al., 2006; Noel et al., 2001), which may contribute to deficits in decision-making (Finn, 2002). Consequently, sub-optimal decision-making may lead to poor choices with regards to alcohol consumption. Furthermore, investigations using functional magnetic resonance imaging (fMRI) have found atypical brain activity in alcoholics during working memory tasks. Studies in adults with AUDs have shown largely weaker, but in some cases greater brain activity during both verbal (Desmond et al., 2003; Park et al., 2010) and spatial (Tapert et al., 2001) working memory tasks. These differences have been reported in the prefrontal cortex (PFC) (DLPFC, inferior frontal gyrus (IFG), anterior PFC (APFC), medial frontal gyrus (MEFG), premotor cortex) and parietal lobe (inferior parietal lobule (IPL), superior parietal lobule (SPL)), brain regions that undergo substantial maturation over the course of development (Crone et al., 2006). Interestingly, risk for alcoholism itself may also be associated with brain activity on tasks involving working memory. For example, non-alcohol abusing FHP young adults show weaker fronto-parietal brain response compared to their peers during a visual oddball task (Rangaswamy et al., 2004) in the IFG and IPL. Since behavioral and fMRI studies in both alcoholics and FHP individuals have reported atypical working memory performance and brain activity, this makes it difficult to disentangle the effects of alcohol abuse versus risk for alcoholism on poor working memory functioning.
It is possible that fronto-parietal dysfunction may be present in FHP individuals at much earlier stages of development, long before the initiation of heavy alcohol use and thus may be a neurobiological marker of risk for the development of future AUDs. To date, there has been only one neuroimaging study of working memory in largely alcohol and substance naïve FHP youth, which found atypical default mode network activity during the vigilance control condition in FHP versus FHN youth (Spadoni et al., 2008). While no group differences in spatial working memory brain activity were present in this study, other types of working memory functioning, that may rely on different brain regions (D'Esposito et al., 1998), have not been investigated in FHP youth. While information on verbal working memory performance and brain activity in FHP youth has been absent from the adolescent literature, a recent study in adults with AUDs (Park et al., 2010) found weaker brain activity when comparing alcoholics and control subjects in both frontal (DLPFC, IFG, MEFG) and parietal lobes (SPL, paracentral lobule) during a 2-back verbal working memory task. Notably, regions of the PFC may be specifically important for the maintenance of verbal information (Wager and Smith, 2003), which relies on phonological processing and rehearsal (Baddeley and Hitch, 1974). Aberrant brain activity during working memory in prefrontal cortical areas may also affect decision-making skills (Suhr and Hammers, 2010). Thus, it is important to examine if verbal working memory brain response is altered in FHP youth, in the absence of heavy alcohol use, to understand whether these pathways may be atypical and thus contribute to the higher rates of AUDs seen in this population.
The goal of the current study was to investigate verbal working memory brain activity and behavior in FHP and FHN adolescents to better understand neural and behavioral phenotypes that may predict AUD risk in FHP youth. To this end, we used fMRI to examine brain response during a verbal working memory 2-back task. Based on previous findings of weaker prefrontal and parietal brain response in adults with AUD (Park et al., 2010; Tapert et al., 2001), we hypothesized that even in the absence of heavy alcohol use, FHP youth would show weaker prefrontal activity in the DLPFC and IFG, as well as weaker BOLD response in the parietal lobes in both the SPL and IPL during verbal working memory than their FHN peers.
2. Materials and Methods
2.1 Participants
Participants included 19 FHP (6 females, 13 males) and 16 FHN (8 females, 8 males) youth, ages 12 to 15 years. All youth had an absence of heavy alcohol and substance use, as defined by our criteria (see below and Table 1). Participants were recruited through advertisements and mailings distributed throughout the community as part of an ongoing study focused on adolescent neurodevelopment in at-risk youth. Briefly, following written consent and assent, separate structured telephone interviews were conducted with both the youth and one of their parents. Interviews consisted of the Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b; Hoven et al., 2005; Lucas et al., 2001), the Family History Assessment Module (FHAM; Rice et al., 1995), the Brief Lifetime version of the Customary Drinking and Drug Use Record (Brown et al., 1998), and the Structured Clinical Interview (SCI; Brown et al., 1994). Exclusionary criteria for adolescents included left-handedness (Edinburgh Handedness Inventory (Oldfield, 1971)), lifetime history of a diagnosed DSM-IV psychiatric disorder, absence of family history information, significant alcohol and/or substance use (> 10 lifetime alcoholic drinks or > 2 drinks/occasion, > 5 uses of marijuana, any other drug use, or > 4 cigarettes per day), neurological illness, significant head trauma (loss of consciousness > 2 minutes), serious medical problems, learning disability, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents, irremovable metal, and pregnancy. All procedures were in accordance with the ethical standards of the Oregon Health and Science University (OHSU) Institutional Review Board.
Table 1.
| FHP5 | FHN | Statistic | |
|---|---|---|---|
| N | 19 | 16 | |
| Female | 6 | 8 | Χ21,35 = 1.23 |
| Age | 14.01 (.90) | 14.18 (.70) | t33 = .64 |
| Puberty1 | 2.55 (.60) | 2.73 (.57) | t33 = .89 |
| IQ2 | 110.16 (11.69) | 111.25 (12.41) | t33 = .27 |
| GPA3 | 3.38 (.57) | 3.66 (.41) | U31 = .88, Z = −1.73 |
| Socioeconomic status4 | 36.68 (15.72) | 29.94 (12.10) | U33 = 108.5, Z = −1.45 |
| Ethnicity (% Caucasian) | 89.47 | 93.75 | Χ21,35 = .20 |
| Verbal Working Memory | |||
| Accuracy (%) | 96.63 (2.91) | 97.07 (2.85) | U33 = 138.5, Z = −.46 |
| Mean Reaction Time (ms) | 627.61 (117.71) | 534.76 (98.49) | t33 = −2.50* |
| Hits | 18.95 (1.08) | 18.69 (1.62) | U33 = 146.5, Z = −.19 |
| Hit Rate | .95 (.05) | .93 (.08) | U33 = 146.5, Z = −.19 |
| False Alarms | 1.11 (1.29) | .56 (.89) | U33 = 110, Z = −1.5 |
| False Alarm Rate | .03 (.03) | .01 (.02) | U33 = 110, Z = −1.5 |
| D-Prime | 3.62 (.53) | 3.70 (.50) | U33 = 136.5, Z = −.52 |
| Vigilance | |||
| Accuracy (%) | 98.19 (3.35) | 97.46 (3.65) | U33 = 132.5, Z = −.75 |
| Mean Reaction Time (ms) | 504.67 (63.32) | 501.50 (71.27) | t33 = .14 |
| Hits | 11.89 (.46) | 11.81 (.54) | U33 = 141.5, Z = −.72 |
| Hit Rate | .99 (.04) | .98 (.05) | U33 = 141.5, Z = −.72 |
| False Alarms | .47 (.77) | .63 (.89) | U33 = 140, Z = −.47 |
| False Alarm Rate | .02 (.04) | .03 (.04) | U33 = 140, Z = −.47 |
| D-Prime | 3.49 (.38) | 3.41 (.41) | U33 = 131, Z = −.81 |
| Alcohol Use | N = 2 | N = 0 | Χ21,35 = 1.79 |
| Number of Occasions | 2.5 (.71) | 0 | |
| Days Since Last Use | 420 (424) | 0 | |
| Number of Drinks/Occasion | 2 | 0 | |
| Marijuana Use | N = 2 | N = 0 | Χ21,35 = 1.79 |
| Number of Occasions | 3.5 (2.12) | 0 | |
| Days Since Last Use | 397.5 (456) | 0 | |
| Cigarette Use | N = 2 | N = 0 | Χ21,35 = 1.79 |
| Number of Occasions | 1.5 (.71) | 0 | |
| Days Since Last Use | 1085 (502) | 0 |
Pubertal Developmental Scale; scores range 1–5, with higher scores reflecting greater maturity (Petersen et al., 1988)
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Grade Point Average. Total N = 33; Two subjects (1 FHP and 1 FHN) did not have GPA data, as they were homeschooled.
Hollingshead Index of Social Position; higher scores indicate lower socioeconomic status; mean scores here are commensurate with middle (FHP) and upper-middle class (FHN) status (Hollingshead, 1975)
Three FHP youth had used alcohol and/or substances in the current study, and use for these youth was below the limited amount permitted for eligibility as described in the Methods. No FHN youth had ever used alcohol or substances in the present sample.
No significant group differences were present on any of these variables (p > 0.05) except Verbal Working Memory Mean Reaction.
denotes p < 0.01.
2.1.1. Family History of Alcohol and Substance Use Disorders
Dichotomizing individuals based on first, or first and second degree relatives with a AUD, has been shown to be a valid predictor of alcohol use vulnerability and future dependence (Stoltenberg et al., 1998). Thus, the FHAM was administered during the structured telephone interview with both the youth and their biological parent to assess DSM-IV criteria for substance abuse and dependence of first and second degree relatives. Both youth and parent were administered the FHAM in order to examine any instances in which youth reported parental use that met criteria for an AUD, but the biological parent did not. No discrepancies in reporting existed for the current sample. Based on the information provided on the FHAM, youth were considered FHP if a history of alcohol abuse and/or dependence was reported for at least one biological parent or two or more second degree relatives on either the maternal or paternal side of the family; youth with a complete absence of substance abuse/dependence among relatives were considered FHN. In the FHP group, a family history density (FHD) score was calculated for each participant based on the youth’s familial relatedness to the relative(s) with an AUD. Biological parents received a score of 0.5, grandparents a score of 0.25, while aunts and uncles with an AUD received a weighted ratio of 0.25 divided by the total number of aunts and uncles on the maternal or paternal side of the family in which the AUD was reported. FHD scores in the FHP youth ranged from 0.06 to 1.00 with mean = 0.49 and standard deviation (SD) = 0.26.
2.2 Imaging Procedures
Images were acquired on a 3.0 Tesla Siemens Magnetom Tim Trio system at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900ms, Flip Angle = 10 degrees, TE = 3.58ms, TR = 2300ms, acquisition matrix = 256×240, resolution = 1mm × 1mm × 1.1mm). Whole-brain functional images were collected in the axial plane oblique to the AC-PC, using a T2*-weighted echo planar blood oxygen level dependent (BOLD) sequence (TR = 2000ms, TE = 30ms, FOV= 240mm, flip-angle = 90°, 33 slices no gap, resolution = 3.75mm × 3.75mm × 3.8mm).
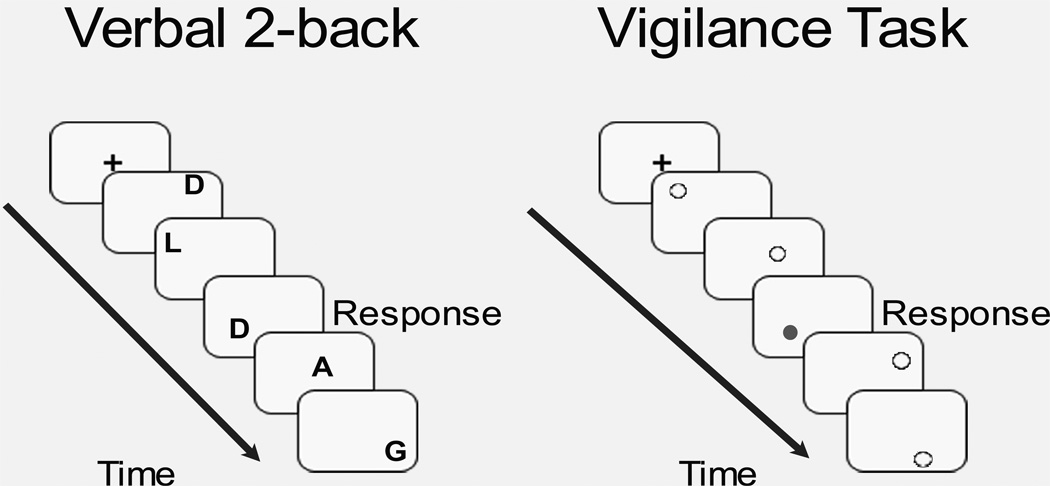
A modified block design fMRI task was used to assess verbal working memory (Nagel et al., 2007). The task included 8 blocks of an alternating experimental verbal working memory 2- back (VWM) condition and a control, vigilance condition, with brief presentations of fixation between block conditions. In the VWM condition, white alphabetical letters were presented in various locations on a black screen, and participants were told to “Press for the same LETTER as 2 screens prior” (See Figure 1). For each block, 5 out of the 16 trials was a 2-back verbal letter repeat. In the vigilance condition, gray and white dots appeared in random locations on the screen, and subjects were told to “Press the button when a gray dot appears” (See Figure 1). Each block of the vigilance condition had 8 trials, with 3 out of 8 trials requiring a button press. The purpose of the vigilance condition was to control for attentional and simple motor processes involved during the VWM condition. In each condition, stimuli were presented on the screen for 500 ms, with an inter-trial stimulus interval and stimulus response window of 1500 ms.
Figure 1.
Verbal working memory fMRI task. In the verbal 2-back condition shown on the left, participants use a button press to respond when they see the same letter as they saw two screens before. In the vigilance condition shown on the right, participants use a button press to respond when they see a grey dot. “Response” in the figure indicates when a correct response would have been made in these examples. Each block of verbal 2-back or vigilance is initiated by a fixation cross-hair, as seen in the figure.
2.3 Image Processing
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI; Cox, 1996). Preprocessing included slice timing correction, motion correction, co-registration of functional to anatomical images, and spatial smoothing using a Gaussian filter (full-width half maximum = 6 mm kernel). Time repetitions that showed > 2.5 mm or 2.5° in any of six displacement or rotational parameters were removed from the subsequent analyses. In addition, analysis of root mean square indicated no differences in movement between FHP and FHN youth (U33 = 120, Z = −1.06, p = 0.29). Next, functional masks were created to mask out non-brain areas, and time series data were normalized to its mean, resulting in images scaled by percent signal change. Time series data were then correlated with a vector representing the task design, in light of the delay of the hemodynamic response, while covarying for motion and linear trends (Cohen, 1997). The fit coefficients derived from fitting the time series data to the model represented the blood oxygen-level dependent (BOLD) response, which was contrasted between the VWM and vigilance, VWM and baseline, and vigilance and baseline conditions. The estimated baseline model in this analysis was AFNI’s intrinsic baseline, which is comprised of the mean BOLD signal from the entire timecourse of the task, linear drift, unmodeled fixation periods between task blocks, and regressors of no interest (e.g., motion parameters) (Cox, 1996). Functional data sets were resampled into 3 mm3 voxels and transformed into standard Talairach coordinates (Talairach and Tournoux, 1988) for anatomical localization and between-subject comparisons.
2.4 Group Analyses
2.4.1 Demographic and Behavioral Data
Statistical analyses were performed in PASW Statistics 18 (PASW, Chicago, Illinois). Data normality was assessed using Shapiro-Wilk. Demographic and fMRI task performance data were analyzed using independent-sample t-tests and Χ2. Linear regressions examined FHD scores in relation to task measures. When data were in violation of normality, Mann-Whitney U tests were performed. No outliers greater than 2.5 SD were present in any of the analyses.
2.4.2 Imaging Data
To examine verbal working memory BOLD response, one-sample t-tests were performed for whole-brain BOLD response for VWM (versus vigilance) for each group. In order to best represent task-related activity for both FHP and FHN participants, individual group maps were thresholded at p < 0.05 and then combined to form a map of task-related brain activity for the entire sample. Significant activation between FHP and FHN during verbal working memory was then assessed in this voxel thresholded task-related activity map using an analysis of covariance (ANCOVA), controlling for VWM reaction time. To control for Type I error, Monte Carlo simulation was performed using both a voxel and cluster threshold (Forman et al., 1995) with the input mask being the voxel thresholded, binary task-related activity mask. Only clusters with a voxel threshold of p < .01 exceeding 810 microliters, equal to 30 contiguous significant (α < .05) 3 mm3 voxels were considered significant. Additionally, regressions examining FHD in relation to BOLD response were performed in clusters which showed group differences in brain activity.
3. Results
3.1 Demographic and Behavioral Data
Participant demographics are presented in Table 1. Participants were not significantly different on any demographic variables, including number of alcohol and substance users. Percent accuracy for each condition was calculated by adding number of hits on target stimuli to number of correct rejections on non-target stimuli and dividing this value by the total number of trials, which was multiplied by 100. In terms of accuracy, FHP and FHN youth performed similarly on both the VWM [U(33) = 138.5, Z = −.46, p = .65] and vigilance conditions of the task [U(33) = 132.5, Z = −.75, p = .45]. Furthermore, group differences were not seen on mean reaction time during the vigilance task [t(33) = .12, p = .85], but FHP youth were significantly slower on average during the VWM trials compared to FHN youth [t(33) = −2.50, p = .02]. Additionally, no group differences on hits, false alarms, hit rate, false alarm rate, and d-prime were found for either VWM or vigilance (see Table 1). FHD did not relate to task performance in FHP youth.
3.2 Verbal Working Memory fMRI Results
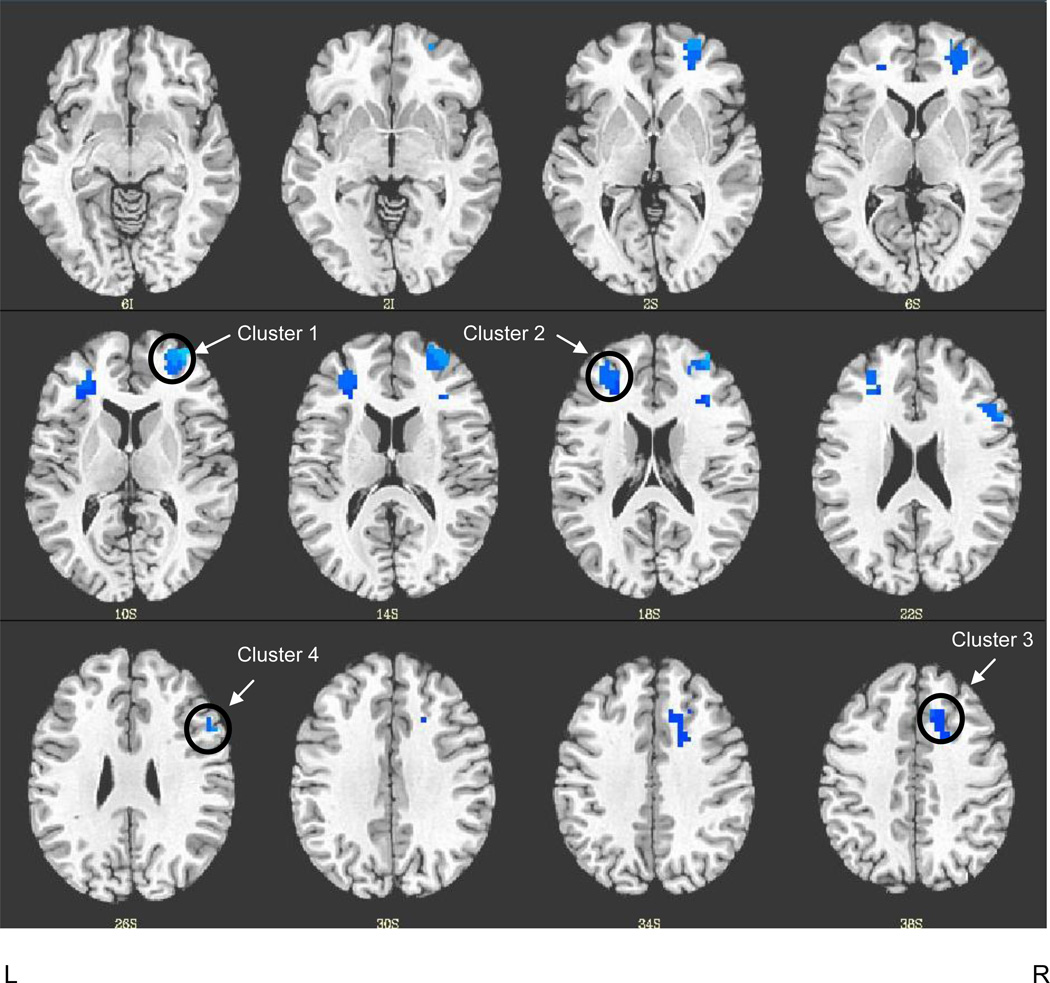
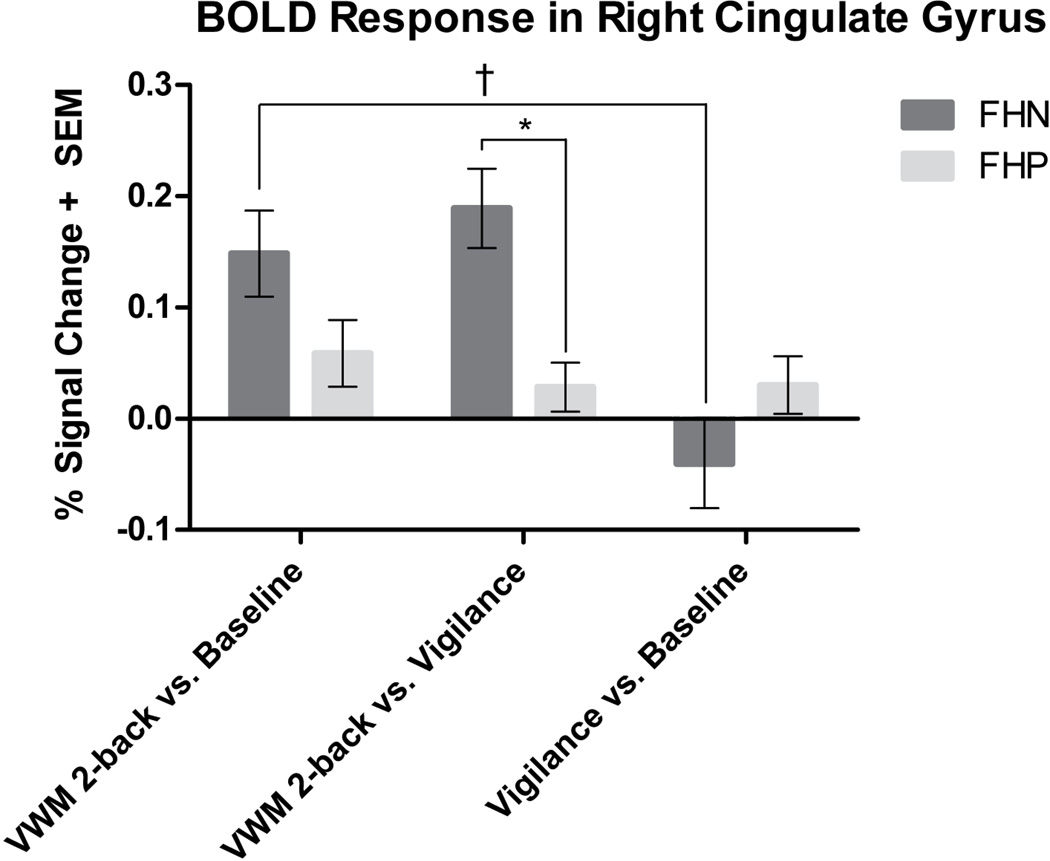
FHP and FHN youth showed comparable BOLD response during verbal working memory in widespread regions of the frontal and parietal cortices including superior, middle, and inferior frontal gyri, superior and inferior parietal lobules, precuneus, and cingulate gyrus. Table 2 presents detailed results of task-related activity for each group. To ensure relevance to verbal working memory, areas of group difference were examined only in verbal working memory-related (2-back versus vigilance) areas of activation. Controlling for group differences in VWM reaction time, this analysis revealed that FHP youth had significantly weaker verbal working memory brain response in four clusters of the PFC, including the right anterior PFC (2916 µl), left DLPFC (2430 µl), right cingulate gyrus (1431 µl), and right IFG (1134 µl) (Figure 2). To further explore whether group differences in verbal working memory functioning were due to brain response during the VWM or vigilance condition, we examined BOLD response in VWM versus baseline and vigilance versus baseline contrasts in the four clusters of group difference. There was a significant interaction between verbal working memory BOLD signal and group in all of the clusters [right anterior PFC: F(1, 33) = 17.34, MSE = 0.04, p < 0.001, partial η2 = 0.34; left DLPFC: F(1, 33) = 17.57, MSE = 0.02, p < 0.001, partial η2 = 0.35; right cingulate gyrus: F(1, 33) = 15.79, MSE = 0.01, p < 0.001, partial η2 = 0.32; right IFG: F(1, 33) = 15.97, MSE = 0.03, p < 0.001, partial η2 = 0.33]. Paired sample t-tests indicated that FHN youth showed significantly greater mean BOLD response in the VWM condition compared to vigilance [right anterior PFC: t(15) = 6.76, p < 0.001; left DLPFC: t(15) = 5.16, p < 0.001; right cingulate gyrus: t(15) = 5.32, p < 0.001; right IFG: t(15) = 4.26 p < 0.005]. FHP youth displayed no significant differences in brain activity between VWM and vigilance selection types in any of the clusters [left DLPFC: t(18) = .28, p = 0.78; right cingulate gyrus: t(18) = 1.34, p = 0.20; right IFG: t(18) = −.67 p = 0.51] except for greater VWM vs. vigilance BOLD response in the right anterior PFC: t(18) = 1.94, p = 0.04. A representative bar graph of brain activity in all four clusters is illustrated by BOLD signal change in the cingulate gyrus seen in Figure 3. FHD was not related to neural response during verbal working memory in any of the clusters of significant group difference.
Table 2.
Whole-brain patterns of verbal working memory 2-back vs. vigilance brain activity in each group and significant group differences in brain response. For each cluster, the anatomic location of significant voxels, the coordinates of the peak voxels of the cluster in Talaraich space, the size of the cluster, as well as the corresponding t-value and the effect size (Cohen’s d) are listed. One sample t-tests for verbal working memory 2-back vs. vigilance whole-brain analyses for each group were evaluated at p < 0.05 with a minimum cluster size of 200 voxels to illustrate overall patterns of activation in each group. Significant group differences are corrected for multiple comparisons, voxel/clusterwise correction p < 0.01; minimum cluster volume: 30 voxels. R = right; L = left; ACC = anterior cingulate cortex; AG = angular gyrus; AMYG = amygdala; CG = cingulate gyrus; FG = fusiform gyrus; HIPP = hippocampus; IFG = inferior frontal gyrus; IPL = inferior parietal lobule; LG = lingual gyrus; MFG = middle frontal gyrus; MOG = middle occipital gyrus; MTG = middle temporal gyrus; MeFG = medial frontal gyrus; PCG = precentral gyrus; PG = parahippocampal gyrus; PoC=posterior cingulate; PoCG = postcentral gyrus; SFG = superior frontal gyrus; SMG = supra marginal gyrus; SOG = superior occipital gyrus; SPL = superior parietal lobule; STG = superior temporal gyrus.
| Anatomic Location | x | y | z | Volume (voxels) |
t- value |
Cohen’s d |
|---|---|---|---|---|---|---|
| FHP | ||||||
| Cluster 1: Bilateral SMG, IPL, SPL, Precuneus, AG, PoCG | −8 | −65 | 57 | 1963 | 4.33 | 0.99 |
| Cluster 2: Bilateral MFG, SFG, MeFG, CG, PCG, L IFG | 29 | 5 | 63 | 1841 | 4.38 | 1.00 |
| Cluster 3: Right MTG, PG, FG, HIPP, AMYG, STG, LG, Insula, PCG, PoCG, IPL, SMG | 26 | −53 | −7 | 1708 | −3.79 | 0.87 |
| Cluster 4: Left STG, MTG, PG, HIPP, IFG, LG, Insula, Bilateral PoC, PoCG, PCG, IPL | −2 | −50 | 12 | 1404 | −3.81 | 0.87 |
| Cluster 5: Bilateral LG, Cuneus, MTG, MOG, R Precuneus | 11 | −86 | 36 | 1253 | −4.26 | 0.98 |
| Cluster 6: Bilateral MeFG, ACC, SFG | −2 | 56 | 9 | 679 | −3.74 | 0.86 |
| Cluster 7: Right MFG, MeFG, SFG | 38 | 59 | −4 | 228 | 3.99 | 0.92 |
| Cluster 8: Bilateral CG, MeFG | −2 | −14 | 42 | 218 | −3.64 | 0.84 |
| FHN | ||||||
| Cluster 1: Bilateral MFG, IFG, MeFG, SFG, ACC, R Insula, CG, L PG | 32 | 65 | −1 | 3457 | 3.84 | 0.96 |
| Cluster 2: Bilateral PoCG, SPL, IPL, Precuneus, AG, SMG | −5 | −56 | 60 | 1763 | 3.73 | 0.93 |
| Cluster 3: Right Cuneus, SOG, MOG, MTG | 14 | −89 | 36 | 229 | −4.07 | 1.02 |
| FHP < FHN | ||||||
| Cluster 1: Right SFG, MFG, MeFG | 38 | 56 | 12 | 108 | −3.11 | 1.08 |
| Cluster 2: Left MeFG, SFG, ACC, MFG | −32 | 47 | 18 | 90 | −3.23 | 1.12 |
| Cluster 3: Right CG, MeFG | 11 | 26 | 39 | 53 | −3.12 | 1.09 |
| Cluster 4: Right MFG, IFG | 53 | 14 | 27 | 42 | −3.06 | 1.07 |
Figure 2.
FHP youth show less BOLD response in verbal 2-back versus vigilance than FHN youth. Results are displayed in the axial view on a T1 anatomical template in Talairach space. The four clusters of group difference are in the frontal lobe: Cluster 1 = R anterior prefrontal cortex, Cluster 2 = L dorsolateral prefrontal cortex, Cluster 3 = R cingulate gyrus, Cluster 4 = R inferior frontal gyrus (multiple comparison corrected, p < 0.01). (See Table 2 for details of cluster locations). L = left, R = right.
Figure 3.
Representative bar graph showing blood oxygen level-dependent response in the right cingulate gyrus in FHP and FHN youth in VWM versus vigilance, VWM versus baseline, and vigilance versus baseline contrasts. This bar graph represents the pattern of brain activity for each group seen in three of four clusters of group difference. *Indicates significant group difference in VWM versus vigilance brain activity (multiple comparison corrected, p < 0.01, †Indicates significant at p < 0.001 in FHN youth. FHP youth show no significant differences between VWM versus baseline and vigilance versus baseline brain activity. VWM = verbal working memory.
4. Discussion
The current study investigated verbal working memory behavior and brain response in FHP and FHN youth. The groups did not differ on accuracy during the VWM or the vigilance control condition, but FHP youth showed significantly slower mean reaction time on accurate responses on VWM than FHN youth. After controlling for this reaction time difference, FHP youth showed significantly weaker brain response during verbal working memory in the right anterior PFC, right cingulate gyrus, right IFG, and left DLPFC.
Interestingly, while not hypothesized, FHP youth were significantly slower on average when making correct responses during the VWM condition compared to FHN youth. A previous study from our lab also found reaction time differences on decision-making during the delay discounting task (Herting et al., 2010), suggesting that slower reaction time in FHP youth may be present across a variety of tasks that assess cognitive functioning. Further support to the current findings is given by Nigg et al. (2004), who found significant differences in reaction time between FHP adolescents and controls in a task measuring response inhibition. Lack of attention may not explain the slower reaction time seen during working memory in the current study, since no difference in reaction time was present during the vigilance condition. Rather, behavioral deficits in executive functioning appear to be present, resulting in slower processing speed during a variety of tasks that rely on cognitive control. A previous study indicated that alcoholics show similar performance to controls on a short-term memory task, but display slower reaction time on the task (Mohs et al., 1978). Thus, it is possible that slower processing speed in FHP youth, even in the absence of heavy alcohol use, could result in less efficient decision-making or poor response inhibition (Nigg et al., 2004), which may increase their vulnerability for future alcohol abuse.
In the current study, we did not find any group differences in accuracy for either the VWM or vigilance conditions. Previously, neuropsychological assessment of FHP youth showed that they performed significantly poorer on both attentional and visuospatial tasks compared to FHN youth (Corral et al., 1999). Additionally, at follow-up, these same individuals also performed worse compared to their peers on tasks of executive functioning, such as the Wisconsin Card Sorting Task (Corral et al., 2003). However, none of these studies reported reaction time during these tests, so it is unknown whether any group differences in performance may have been due to reaction time differences between FHP and FHN youth. The comparable performance seen during the VWM condition in the current study may have been due to the relatively easy nature of the 2-back task design (average accuracy in both groups >96%). It is possible that accuracy differences would have been present if a condition requiring greater working memory load was used, such as a 3-back task, since previous studies examining verbal working memory load have found significant differences in accuracy across different load conditions (Nagel et al., 2007; O'Hare et al., 2008).
As hypothesized, FHP youth showed significantly weaker brain response in prefrontal cortical regions during verbal working memory compared to FHN youth. Because the DLPFC, and cingulate are important for verbal working memory functions in n-back tasks (Owen et al., 2005; Rodriguez-Jimenez et al., 2009), weaker brain response observed in these regions may suggest the VWM 2-back condition was more taxing for FHP youth than for their FHN peers. This idea is supported by the simple effects analysis, which indicated that in three of four areas of group difference in the frontal lobe, FHN youth showed significantly greater brain response in VWM versus vigilance, while FHP youth showed no difference in brain activity between these conditions. This suggests that FHP youth were not activating these working memory-related brain regions to the same extent as FHN youth during the VWM condition. Further, these results are consistent with the largely weaker frontal lobe response found in tasks involving working memory in both alcoholics and FHP adults in the DLPFC and IFG (Park et al., 2010; Rangaswamy et al., 2004). This indicates that even during adolescence, before the heavy use of any alcohol or substances, FHP youth show weaker patterns of frontal lobe activity during working memory similar to FHP adults and those with AUDs, suggesting that these neurobiological phenotypes may be premorbid markers of risk for future alcohol abuse. Further, binge drinking adolescents also show weaker BOLD response than controls in DLPFC during verbal encoding (Schweinsburg et al., 2011), similar to the current findings of weaker VWM brain activity seen in this region in FHP youth. Thus, a family history of alcoholism may be associated with premorbid deficits in verbal processing that may later be followed by heavy drinking. Again, perhaps due to the relative ease of this task for all participants, group differences in accuracy did not emerge, even in the presence of weaker prefrontal brain response. However, since working memory is essential for day-to-day functioning and decision-making (Finn, 2002), persistent hypoactivity in the PFC in FHP youth could lead them to make poorer decisions with regards to alcohol and/or substance use in more challenging situations and could increase their vulnerability for developing AUDs.
Contrary to our hypothesis, no significant differences in parietal BOLD response were present between FHP and FHN youth. While parietal brain regions have been shown to be important for working memory functioning (Carlson et al., 1998), and each group showed VWM-related parietal brain response, it is possible that group differences in brain activity in these regions may only emerge in more demanding tasks, or that they may be a result of alcohol abuse itself, as seen in the weaker parietal brain response previously reported in alcoholics (Tapert et al., 2001). Thus, it is possible that premorbid differences in PFC brain response, as shown in the current study, may increase risk for alcoholism in FHP youth and are also compromised following heavy alcohol use (Park et al., 2010), while parietal lobe brain activity during working memory might only be affected by heavy alcohol use itself (Tapert et al., 2001).
While this study was the first to find differences in brain activity during verbal working memory in FHP and FHN youth, limitations and future directions should be discussed. First, due to the relatively small sample size of the study, sex differences in brain response could not be analyzed, but should be explored as previous research has shown sex differences in brain and behavior relationships in FHP and FHN youth (Silveri et al., 2008). Second, while a previous study in adolescents found a relationship between FHD scores and brain response during spatial working memory vs. vigilance (Spadoni et al., 2008), the relatively low FHD scores in this sample may have precluded finding an association between FHD and behavior or brain response in the brain regions of group difference. A wider range of FHD scores with a larger sample size might allow for better detection of these relationships. Finally, the relatively easy nature of the current task may have precluded finding group differences on task accuracy. Thus, future work should investigate how working memory capacity is related to family history risk for alcoholism by increasing working memory load.
This study was the first to report weaker PFC brain response during working memory in FHP compared to FHN youth. Weaker brain response in regions important for executive functioning during working memory could lead to poor decision-making and may be a marker of risk for alcoholism (Finn, 2002), but longitudinal studies are needed to understand how working memory brain response may be predictive of future alcohol abuse. Further research should explore whether slower processing speed in FHP youth is present across other neuropsychological measures and how it relates to brain activity and performance across other measures of executive functioning in order to identify common neurobiological and behavioral phenotypes that may be associated with developing alcohol dependence.
Acknowledgments
Nate Spofford, Madison Stroup, and Emily Maxwell are thanked for their assistance with participant scheduling and data collection.
Role of Funding Source: This research was supported by the National Institute on Alcohol Abuse and Alcoholism (T32 AA007468, Cservenka; F31 AA 019866, Herting; R01 AA017664, Nagel), pilot funds from the Portland Alcohol Research Center (P60 AA010760, Nagel), and the National Institute of Neurological Disorders and Stroke (K08 NS052147, Nagel). The NIAAA, Portland Alcohol Research Center, and the NINDS had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributers: Authors A.C. and M.H. completed statistical analyses, author A.C. wrote the first draft of the manuscript, and author B.J. designed the study and protocol and read and revised the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: None.
References
- Ahveninen J, Jaaskelainen IP, Pekkonen E, Hallberg A, Hietanen M, Naatanen R, Schroger E, Sillanaukee P. Increased distractibility by task-irrelevant sound changes in abstinent alcoholics. Alcohol. Clin. Exp. Res. 2000;24:1850–1854. [PubMed] [Google Scholar]
- Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol. Clin. Exp. Res. 2001;25:185–191. [PubMed] [Google Scholar]
- Baddeley A, Hitch G. Working memory. In: Bower GA, editor. The Psychology of Learning and Motivation. San Diego: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Appl. Prev. Psychol. 1994;3:61–73. [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb. Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J. Stud. Alcohol. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Corral MM, Holguin SR, Cadaveira F. Neuropsychological characteristics in children of alcoholics: familial density. J. Stud. Alcohol. 1999;60:509–513. doi: 10.15288/jsa.1999.60.509. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res. Cogn. Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcohol. Clin. Exp. Res. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcoholism. Behav. Cogn. Neurosci. Rev. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J. Abnorm. Psychol. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol. Clin. Exp. Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock V, Bauer LO, Hesselbrock MN, Gillen R. Neuropsychological factors in individuals at high risk for alcoholism. Recent Dev. Alcohol. 1991;9:21–40. [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol. Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, Cohen M, Balaban V, Woodruff BA, Bin F, Musa GJ, Mei L, Cantor PA, Aber JL, Cohen P, Susser E. Psychopathology among New York city public school children 6 months after September 11. Arch. Gen. Psychiatry. 2005;62:545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl.) 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Tinklenberg JR, Roth WT, Kopell BS. Slowing of short-term memory scanning in alcoholics. J. Stud. Alcohol. 1978;39:1908–1915. doi: 10.15288/jsa.1978.39.1908. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Ohannessian A, Cummins K. Performance dissociation during verbal and spatial working memory tasks. Percept. Mot. Skills. 2007;105:243–250. doi: 10.2466/pms.105.1.243-250. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J. Abnorm. Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Noel X, Paternot J, Van der Linden M, Sferrazza R, Verhas M, Hanak C, Kornreich C, Martin P, De Mol J, Pelc I, Verbanck P. Correlation between inhibition, working memory and delimited frontal area blood flow measure by 99mTc-Bicisate SPECT in alcohol-dependent patients. Alcohol Alcohol. 2001;36:556–563. doi: 10.1093/alcalc/36.6.556. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Sohn S, Park JE, Kim SH, Yu IK, Sohn JH. Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scand. J. Psychol. 2010;52:1–7. doi: 10.1111/j.1467-9450.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Tobin-Richard M, Boxer A. Measuring Pubertal Status: Reliability and Validity of a Self-Report Measure. University Park: Pennsylvania State University; 1985. [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J. Stud. Alcohol. 1992;53:154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimenez R, Avila C, Garcia-Navarro C, Bagney A, Aragon AM, Ventura-Campos N, Martinez-Gras I, Forn C, Ponce G, Rubio G, Jimenez-Arriero MA, Palomo T. Differential dorsolateral prefrontal cortex activation during a verbal n-back task according to sensory modality. Behav. Brain Res. 2009;205:299–302. doi: 10.1016/j.bbr.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann. N.Y. Acad. Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcohol. Clin. Exp. Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: effects of age, sex and risk for drug use. Addiction. 2008;103:1509–1520. doi: 10.1111/j.1360-0443.2008.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol. Clin. Exp. Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Suhr J, Hammers D. Who fails the Iowa Gambling Test (IGT)? Personality, neuropsychological, and near-infrared spectroscopy findings in healthy young controls. Arch. Clin. Neuropsychol. 2010;25:293–302. doi: 10.1093/arclin/acq017. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional Coplanar Stereotaxic Atlas of the Human Brain Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol. Clin. Exp. Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect Behav. Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]