Abstract
Alzheimer’s disease (AD) is characterized by the deposition of β-amyloid (Aβ) peptides in the brain, inducing neuronal cell death and microglial activation. Endoplasmic reticulum (ER) stress has been proposed to be a mediator of Aβ neurotoxicity. In this study, we test whether salubrinal, an ER stress inhibitor, can protect against Aβ-mediated neurotoxicity. We show in rat primary cortical neurons and mouse microglial BV-2 cells that short-term treatment with salubrinal attenuates Aβ-induced neuronal death and microglial activation. Remarkably, our results show that salubrinal’s neuroprotective effects are not due to inhibition of ER stress. Rather, we demonstrate that salubrinal exerts its effects through the inhibition of IκB kinase (IKK) activation, IκB degradation and the subsequent nuclear factor-kappa B (NF-κB) activation. These results elucidate inhibition of the NF-κB pathway as a new mechanism responsible for the protective effects of salubrinal against Aβ neurotoxicity. This study also suggests that modulation of Aβ-induced NF-κB activation could be a potential therapeutic strategy for AD.
Keywords: Alzheimer’s disease, Salubrinal, β-amyloid, NF-κB
1. Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that is characterized by memory and cognitive impairment. Senile plaques, neurofibrillary tangles, neuronal cell death and microglial activation are important pathological characteristics in AD brains (Selkoe, 2004; Binder et al., 2005). It is widely accepted that β-amyloid (Aβ) peptides, the main constituent of senile plaques, play a central role in AD pathogenesis. Aβ is derived from proteolytic cleavages of the amyloid precursor protein (APP) by β- and γ-secretase (Cole and Vassar, 2007; Sisodia and St George-Hyslop, 2002; Zhang and Xu, 2007). There is compelling evidence that the excessive generation and accumulation of Aβ initiates the pathological cascade in AD, leading to neuronal cell dysfunction and death (Ballard et al., 2011). The underlying mechanism of Aβ-induced neurotoxicity is not yet fully understood but appears to involve several pathways associated with apoptosis (Loo et al., 1993; Yao et al., 2005). Aβ deposits also trigger microglia-mediated neuroinflammation, postulated to contribute to the pathogenesis and progression of AD (Giulian et al., 1995; Minghetti, 2005; Floden et al., 2005; Luo et al., 2010). Activated microglia surrounding the senile plaques release proinflammatory cytokines and free radicals, causing neuronal damage (Bamberger and Landreth, 2002; Gao and Hong, 2008; Frank-Cannon et al., 2009). Epidemiological studies reveal that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) reduces the risk of developing AD (in t’ Veld et al., 2001; Etminan et al., 2003; Vlad et al., 2008), suggesting that anti-inflammatory treatment can be beneficial to AD patients.
The nuclear factor-kappa B (NF-κB) pathway plays an important role in regulating a variety of key biological processes, including inflammatory responses and the induction of apoptosis (Li and Verma, 2002; Kucharczak et al., 2003). The mammalian NF-κB family is comprised of five structurally related proteins: c-Rel, RelA/p65, RelB, p50, and p52 (Baldwin, 1996; Chen et al., 1999). These proteins can form either homo- or heterodimers which remain inactive in the cytoplasm in unstimulated cells. NF-κB can be activated by diverse stimuli via distinct signal transduction pathways. These signals phosphorylate and activate the enzyme IκB kinase (IKK) complex which in turn phosphorylates IκB, the inhibitory protein of NF-κB, inducing IκB degradation and thereby activating NF-κB. The activated NF-κB then translocates from the cytoplasm to the nucleus where it initiates the transcription of specific genes (Karin, 1999). It has also been reported that there is a constitutively low basal level of NF- κB in the nuclei of unstimulated cells, indicating that NF-κB may regulate basal gene expression (Carlotti et al., 2000; Ashburner et al., 2001). Activation of the NF-κB pathway has been linked to Aβ neurotoxicity. NF-κB can be activated by Aβ treatment in both neuronal cells and microglial cells (Longpré et al., 2006; Casal et al., 2004). NF-κB activation has also been detected in the brains of AD patients (Kaltschmidt et al., 1997; Boissière et al., 1997). Therefore, modulation of Aβ-induced activation of NF-κB pathway could be a potential therapeutic strategy for the treatment of AD.
Salubrinal is a phosphatase inhibitor that selectively inhibits dephosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α). Long-term incubation (> 36 h) with salubrinal can protect cells against endoplasmic reticulum (ER) stress-induced apoptosis (Boyce et al., 2005). Since ER stress has been proposed to be involved in Aβ-induced cell apoptosis (Nakagawa et al., 2000; Ferreiro et al., 2006; Nishitsuji et al., 2009), herein we test whether salubrinal can protect against Aβ-mediated neurotoxicity and show that short-term treatment with salubrinal attenuates Aβ-induced neuronal cell death and microglial activation. Remarkably, we demonstrate that salubrinal exerts its effects through inhibition of the NF-κB pathway, rather than through inhibition of ER stress. Therefore, our study provides evidence of a novel mechanism by which salubrinal exerts its neuroprotective effects.
2. Methods
2.1. Chemicals and antibodies
Salubrinal was obtained from Tocris Bioscience. Synthetic Aβ1-42 peptide was obtained from American Peptide Company. To induce fibril formation, the peptides were dissolved in distilled water and incubated for one week at 37°C before use. Antibodies used in this study were: anti-cleaved caspase-3, anti-Bip, anti-PDI, anti-NF-κB p65, anti-IL-1β, anti- eIF2α, IKKα/β, IκBα and their phospho-antibodies from Cell Signaling Technology; anti-Lamin B1 from Abcam; anti-β-actin from Sigma.
2.2. Cell cultures and treatment
Primary cortical neuronal cells from embryonic day 17 (E17) rat embryos were maintained in neurobasal medium supplemented with B27 and 0.8 mM L-Glutamine. Mouse microglial BV-2 cells were maintained in DMEM supplemented with 10% FBS. For drug treatment, primary neurons and BV-2 cells were treated with 25 μM Aβ1-42, 50 or 100 μM salubrinal, or Aβ plus salubrinal for different time periods.
2.3. TUNEL assay
To identify apoptotic neurons, TUNEL assays using an in situ cell death detection kit (Roche Diagnostics) were performed according to the manufacturer’s instructions, followed by counterstaining with 0.1μg/ml DAPI. The number of TUNEL-positive cells was counted in 10 randomized fields under a fluorescent microscope.
2.4. Cell viability assay
The viability of the neurons and BV-2 cells after treatment was evaluated by the WST-8 assay using a cell counting kit (Dojindo Molecular Technologies). Briefly, cells grown in 96-well plates were incubated for 2 h in culture medium containing 10% WST-8 reagent and the absorbance was measured at 450 nm by a microplate reader. Decreased absorbance indicates a reduction in cell viability.
2.5. Nuclear extraction
Nuclear extracts from primary neurons and BV-2 cells were prepared using a nuclear extract kit (Active Motif) according to the manufacturer’s instructions. The Nuclear extracts were snap frozen immediately and stored at −80°C until used. The protein concentration of the nuclear extract was determined using the Bradford assay.
2.6. ELISA assay
For the ELISA assay of interleukin-1β (IL-1β) levels, the culture supernatants of BV-2 cells were collected after treatment and processed using the IL-1β ELISA Kit (BD Biosciences) according to the manufacturer’s instructions.
2.7. Western blot analysis
Western blot analysis was performed as described previously (Chen et al., 2009). Briefly, protein extracts (20 µg) were separated by electrophoresis on 4-20% SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were sequentially incubated with primary antibodies, horseradish peroxidase (HRP)-conjugated secondary antibodies, and enhanced chemiluminescence (ECL) solution and followed by autoradiography. The intensity of the blots was analyzed using ImageJ software.
2.8. Immunocytochemistry analysis
Primary neurons and BV-2 cells were fixed, permeabilized and immunostained with p65 antibody, incubated with an Alexa Fluor 488-conjugated secondary antibody, counterstained with 0.1 μg/ml DAPI, and visualized under a fluorescence microscope.
2.9. Statistical analysis
All data are presented as mean ± SEM. The Student’s t test in Microsoft Excel was used for statistical comparison. P value < 0.05 was considered to be statistically significant.
3. Results
3.1. Salubrinal attenuates Aβ-induced neuronal death
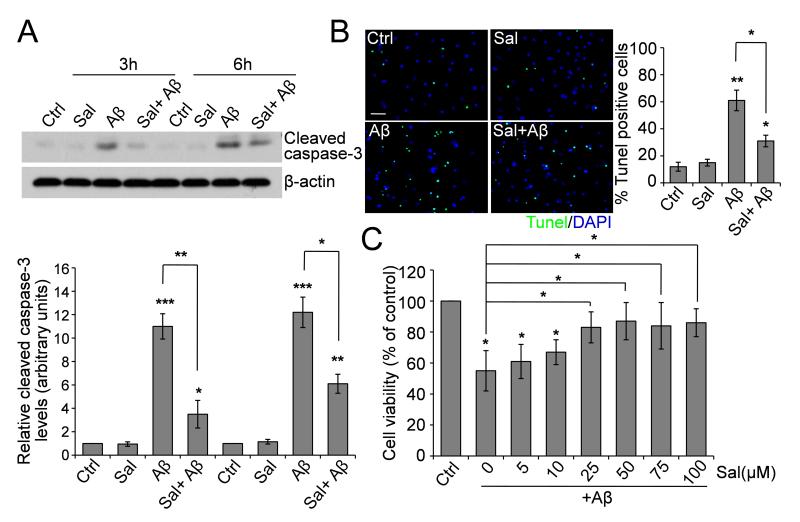
Long-term incubation with salubrinal protects rat pheochromocytoma PC12 cells against ER stress-induced apoptosis through inhibition of eIF2α dephosphorylation (Boyce et al., 2005). Here we asked whether incubation with salubrinal can protect against neuronal death. To answer this question, we treated cultured primary cortical neurons (14 DIV) with Aβ1-42 peptide (25 μM), salubrinal (50 μM) or Aβ plus salubrinal and found that upon 3- and 6 h treatments, Aβ1-42 already induced dramatic activation of caspase-3, a well-known apoptotic marker, while salubrinal suppressed the activation of caspase-3 induced by Aβ (Fig. 1A).
Fig. 1.
Effect of Salubrinal on Aβ-induced neuronal death. (A) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for the indicated time points. Cell lysates were immunoblotted for cleaved caspase-3. Representative blots and densitometric analysis are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, n = 3. (B) Representative immunostaining and quantitative assessment of apoptotic cell death by TUNEL assay. Fluorescent images (10× magnification): green, TUNEL; blue, DAPI. Scale bar, 100 μm. * p < 0.05, ** p < 0.01, n = 3. (C) Dose-dependent protection by salubrinal of primary neurons treated with 25 μM Aβ1-42 for 6 h. Quantitative analysis of cell viability using the WST-8 assay is shown. * p < 0.05, n = 3.
We then carried out TUNEL assay to confirm the neuronal apoptosis. Primary neurons were treated with 25 μM Aβ, 50 μM salubrinal or Aβ plus salubrinal for 6 h and TUNEL assay was performed. The number of neurons undergoing apoptosis, induced by Aβ, was significantly reduced by salubrinal (Fig. 1B), consistent with the results of caspase-3 activation. We also examined the cell viability using a WST-8 assay. As shown in Fig. 1C, while cell viability of neurons was decreased after Aβ treatment for 6 h, salubrinal significantly inhibited Aβ-induced neuronal cell death in a dose-dependent manner.
3.2. Salubrinal attenuates Aβ-induced microglial activation and cell death
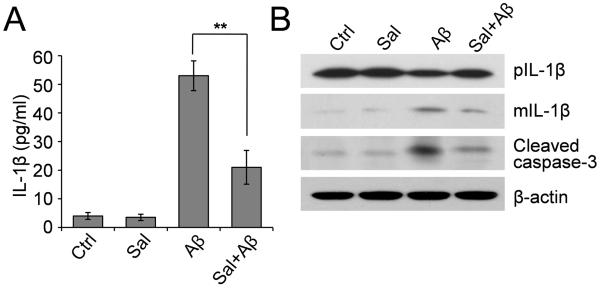
Microglial activation is an important pathological change associated with AD. To investigate whether salubrinal can inhibit microglial activation, we treated mouse microglial BV-2 cells with Aβ1-42 (25 μM), salubrinal (100 μM) or Aβ plus salubrinal for 3 and 6 h. The amount of pro-inflammatory cytokine interleukin-1β (IL-1β) secreted into the culture medium from BV-2 cells was examined by ELISA. Similar results were seen when BV-2 cells were treated for 3 and 6 h, so we only present the results at the 6- h time point. Exposure of BV-2 cells to Aβ increased the secreted IL-1β levels by about 10-fold while salubrinal significantly attenuated Aβ-induced IL-1β secretion (Fig. 2A). We then examined intracellular IL-1β production. Western blot analysis of BV-2 cell lysates showed that Aβ increased cleavage of the precursor of IL-1β to generate the secretory mature IL-1β and salubrinal significantly inhibited the mature IL-1β production induced by Aβ (Fig. 2B). We also examined the levels of cleaved caspase-3 in BV-2 cells treated with Aβ, salubrinal or Aβ plus salubrinal for 6 h, and found that similar to the results from rat primary cortical neurons, caspase-3 was activated by Aβ treatment and such an activation was reversed by salubrinal (Fig. 2B), suggesting that salubrinal can also inhibit Aβ-induced microglial cell death.
Fig. 2.
Effect of Salubrinal on Aβ-induced microglial activation and cell death. (A) Mouse microglial BV-2 cells were treated with 25 μM Aβ1-42, 100 μM salubrinal or Aβ plus salubrinal for 6 h. The IL-1β level in the culture supernatants was examined by ELISA. ** p < 0.01, n = 3. (B) Western blot analysis of precursor and mature IL-1β and cleaved caspase-3 in BV-2 cell lysates.
3.3 The neuroprotective effects of short-term incubation with salubrinal are not due to inhibition of ER stress
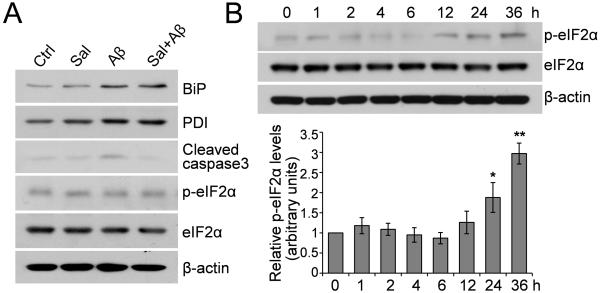
Since Aβ is known to induce ER stress (Ferreiro et al., 2004; Resende et al., 2008) and salubrinal is known to protect against ER stress (Boyce et al., 2005), we asked whether salubrinal exerts its neuroprotective effects against Aβ through the inhibition of ER stress. When primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for 6 h, we found that Aβ treatment induced the accumulation of two ER stress markers BiP/Grp78 and protein disulfide isomerase (PDI). However, salubrinal did not attenuate the Aβ-induced BiP and PDI increases (Fig. 3A). We also examined the phosphorylation status of eIF2α upon salubrinal treatment and found no alterations in either total or phosphorylated eIF2α levels during such a short-term incubation (Fig. 3A). We further conducted a time-course study to investigate the changes in phosphorylated eIF2α levels at different time points after salubrinal treatment. The results revealed that eIF2α phosphorylation was unaltered during short-term incubation with salubrinal and only increased at the 24- and 36-h time points after salubrinal treatment (Fig. 3B). Taken together, these results indicate that the neuroprotective effects of short-term incubation with salubrinal do not occur through the inhibition of ER stress.
Fig. 3.
Salubrinal’s neuroprotective effects are not due to inhibition of ER stress. (A) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for 6 h. Cell lysates were immunoblotted for Bip, PDI, cleaved caspase-3, phospho- and total eIF2α. Representative blots are shown. (B) Primary neurons were treated with 50 μM salubrinal for the indicated time points. Cell lysates were immunoblotted for phospho- and total eIF2α. The representative blots and densitometric analysis are shown. * p < 0.05, ** p < 0.01, n = 3.
3.4. Salubrinal inhibits Aβ-induced NF-κB nuclear translocation
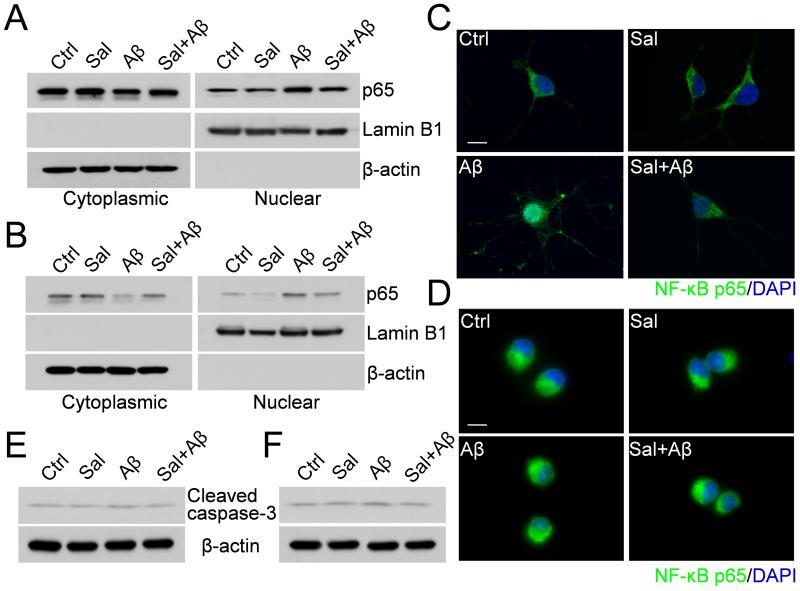
Aβ has been shown to stimulate NF-κB activation, which is associated with neuronal cell death and microglial activation. Therefore, we asked whether salubrinal exerts its effects through the inhibition of NF-κB activation. We treated the primary neurons (Fig. 4A) and BV-2 cells (Fig. 4B) with 25 μM Aβ1-42, 50 or 100 μM salubrinal or Aβ plus salubrinal for 2 h. Cytoplasmic and nuclear extracts from these cells were then subjected to Western blot analysis to detect NF-κB p65. The results showed that there was a low basal level of p65 in the nuclei of untreated cells and Aβ treatment induced a further translocation of p65 from the cytoplasm to the nucleus, while salubrinal significantly attenuated the p65 translocation induced by Aβ (Fig. 4A and 4B). These results were confirmed by immunostaining of p65 in both primary neurons (Fig. 4C) and BV-2 cells (Fig. 4D), indicating that salubrinal can attenuate Aβ-induced NF-κB nuclear translocation. We also found that at the 2- h Aβ treatment time point, caspase-3 was only marginally activated in both primary neurons (Fig. 4E) and BV-2 cells (Fig. 4F), suggesting that NF-κB nuclear translocation precedes caspase-3 activation upon Aβ treatment.
Fig. 4.
Salubrinal inhibits Aβ-induced NF-κB nuclear translocation in primary neurons and BV-2 cells. (A) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for 2 h. Cytoplasmic and nuclear extracts were immunoblotted for NF-κB p65, Lamin B1 (nuclear marker) and β-actin (cytoplasmic marker). (B) BV-2 cells were treated with 25 μM Aβ1-42, 100 μM salubrinal or Aβ plus salubrinal for 2 h. Cytoplasmic and nuclear extracts were immunoblotted for NF-κB p65, Lamin B1 and β-actin. (C) Immunocytochemistry showing the effect of salubrinal on Aβ-induced NF-κB nuclear translocation in primary neurons. Fluorescent images (40× magnification): green, p65; blue, DAPI. Scale bar, 25 μm. (D) Immunocytochemistry showing the effect of salubrinal on Aβ-induced NF-κB nuclear translocation in BV-2 cells. Fluorescent images (40× magnification): green, p65; blue, DAPI. Scale bar, 25 μm. (E) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for 2 h. Cell lysates were immunoblotted for cleaved caspase-3. (F) BV-2 cells were treated with 25 μM Aβ1-42, 100 μM salubrinal or Aβ plus salubrinal for 2 h. Cell lysates were immunoblotted for cleaved caspase-3.
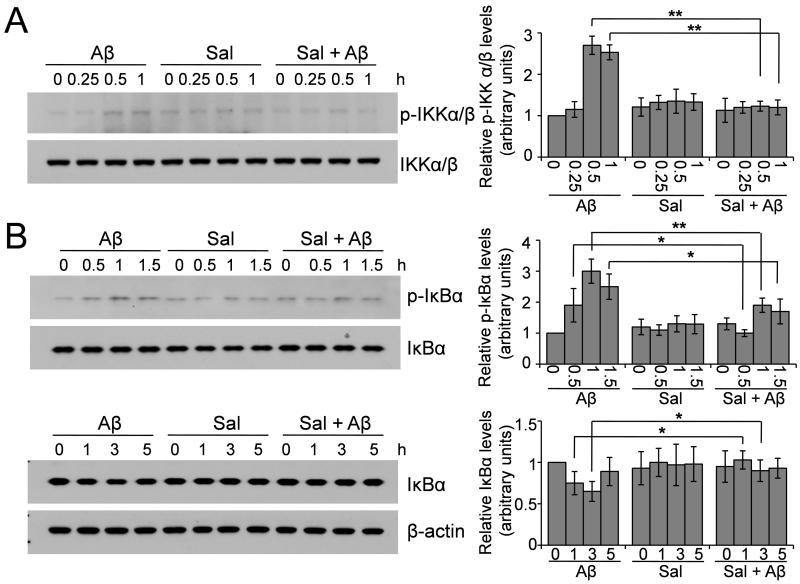
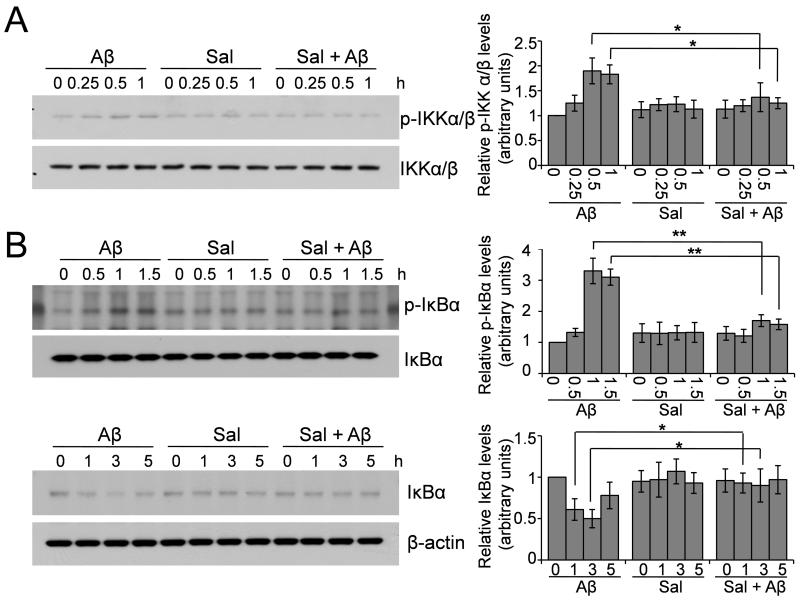
3.5. Salubrinal inhibits Aβ-induced IKK activation and IκB degradation
The activation of IKK and degradation of IκB are required for NF-κB nuclear translocation; we therefore examined whether salubrinal could affect these upstream signaling cascades involved in the activation of NF-κB. Primary neurons (Fig. 5) and BV-2 cells (Fig. 6) were treated with 25 μM Aβ1-42, 50 or 100 μM salubrinal or Aβ plus salubrinal for 15 min to 1 h. Whole cell lysates were then subjected to Western blot analysis to detect the levels of phosphorylated and total IKK. We found that Aβ treatment induced the phosphorylation of IKK at 0.5- and 1- h time points and salubrinal significantly suppressed Aβ’s effect (Fig. 5A and Fig. 6A). We then treated these cells for up to 5 h and examined the levels of phosphorylated and total IκB. The results showed that Aβ induced phosphorylation of IκB at the 0.5- and 1.5- h time points, causing the subsequent degradation of IκB at the 1- and 3- h time points, and salubrinal suppressed the phosphorylation and degradation of IκB induced by Aβ (Fig. 5B and Fig. 6B). Taken together, these data suggest that salubrinal can inhibit Aβ-induced IKK activation and IκB degradation, the upstream signaling cascades that lead to NF-κB activation.
Fig. 5.
Salubrinal inhibits Aβ-induced IKK activation and IκB degradation in primary neurons. (A) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for the indicated time points. Cell lysates were immunoblotted for phospho- and total IKKα/β. The representative blots and densitometric analysis are shown. ** p < 0.01, n = 3. (B) Primary neurons were treated with 25 μM Aβ1-42, 50 μM salubrinal or Aβ plus salubrinal for the indicated time points. Cell lysates were immunoblotted for phospho- and total IκBα. The representative blots and densitometric analysis are shown. * p < 0.05, ** p < 0.01, n = 3.
Fig. 6.
Salubrinal inhibits Aβ-induced IKK activation and IκB degradation in BV-2 cells. (A) BV-2 cells were treated with 25 μM Aβ1-42, 100 μM salubrinal or Aβ plus salubrinal for the indicated time points. Cell lysates were immunoblotted for phospho- and total IKKα/β. The representative blots and densitometric analysis are shown. * p < 0.05, n = 3. (B) BV-2 cells were treated with 25 μM Aβ1-42, 100 μM salubrinal or Aβ plus salubrinal for the indicated time points. Cell lysates were immunoblotted for phospho- and total IκBα. The representative blots and densitometric analysis are shown. * p < 0.05, ** p < 0.01, n = 3.
4. Discussion
In the present report, we provide data showing that short-term treatment with salubrinal attenuates Aβ-induced neuronal death and microglial activation. We also elucidate the underlying mechanism, i.e., salubrinal inhibits IKK activation, IκB degradation and the subsequent NF-κB activation. These results reveal that salubrinal protects against Aβ neurotoxicity through a new mechanism of inhibition of the NF-κB pathway.
Apoptotic neuronal death is the central feature of AD. Although the role of NF-κB in inflammatory responses has been well documented, whether NF-kB promotes or inhibits apoptosis is still controversial. The activation of NF-κB might provide protection from apoptosis in non-neuronal cells (Guo et al., 1998) but potentiate apoptosis in neuronal cells (Schneider et al., 1999; Yang et al., 2002). Thus, the precise role of NF-κB in apoptosis may depend on the specific cell type. Herein, we show that Aβ-induced NF-κB translocation precedes caspase-3 activation. Moreover, when NF-κB translocation was inhibited by salubrinal, Aβ-induced caspase-3 activation was also suppressed. These results strongly indicate that NF-κB plays a role in pro-apoptotic signaling in neurons. Remarkably, inhibition of the NF-κB pathway by salubrinal suppresses both neuronal death and microglial activation, two major features of AD, suggesting that potential therapeutic strategies that target Aβ-induced NF-κB activation may be beneficial for AD patients.
Salubrinal is an inhibitor of protein Ser/Thr phosphatase 1(PP1) complex which acts on eIF2α and has been demonstrated to enhance the phosphorylation of eIF2α and to protect cells against ER stress-induced apoptosis (Boyce et al., 2005). Enhanced eIF2α phosphorylation attenuates translation initiation of most mRNAs and reduces protein synthesis, which allows the cells to restore protein folding capacity and recover from ER stress (Harding et al., 1999). A recent study has demonstrated that phosphorylation of eIF2α increases the translation of β-site APP cleaving enzyme 1 (BACE1) and long-term incubation (48 h) with salubrinal directly increases BACE1 levels and Aβ production in primary neurons (O’Connor et al., 2008), indicating that salubrinal may promote amyloidogenesis through eIF2a phosphorylation-mediated translational control of BACE1.
In this study we found that, while salubrinal had no effect on eIF2α phosphorylation during short-term (within 6 h) treatment, it did suppress the phosphorylation of IKK complex and the subsequent NF-κB activation after Aβ exposure, suggesting that salubrinal negatively regulates the NF-κB pathway through a different mechanism. One possibility is that salubrinal may regulate IKK kinases (IKKKs) that phosphorylate and activate the IKK complex, such as MAP kinase kinase kinase-1 (MEKK1) (Lee et al., 1997; Lee et al., 1998) and NF-κB-inducing kinase (NIK) (Ling et al., 1998). Alternatively, salubrinal may affect IKK phosphorylation indirectly through inhibition of IKK phosphatases. More extensive efforts are needed therefore to identify the direct target of salubrinal that is involved in the suppression of the NF-κB pathway.
Acknowledgements
This work was supported by National Institutes of Health grants (R01AG021173, R01NS046673, R01AG030197 and R03AG034366 to H.X.), and grants from the Alzheimer’s Association (to H.X. and Y.-w.Z.), the American Health Assistance Foundation (to H.X.), National Natural Science Foundation of China (30973150 to Y.-w.Z.), the 973 Prophase Project (2010CB535004 to Y.-w.Z.), and Natural Science Foundation of Fujian Province of China (2009J06022 to Y.-w.Z.). Y.-w.Z. is supported by the Program for New Century Excellent Talents in Universities (NCET), the Fundamental Research Funds for the Central Universities, and Fok Ying Tung Education Foundation. Y.C. was the recipient of the 2010 Young Scholar Award from the Alzheimer’s association San Diego/Imperial Chapter. We thank Robert C. Thompson and Traci Fang Newmeyer for technical assistance.
Footnotes
Disclosure statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner BP, Westerheide SD, Baldwin AS., Jr. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Landreth GE. Inflammation, apoptosis, and Alzheimer’s disease. Neuroscientist. 2002;8:276–283. doi: 10.1177/1073858402008003013. [DOI] [PubMed] [Google Scholar]
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Boissière F, Hunot S, Faucheux B, Duyckaerts C, Hauw JJ, Agid Y, Hirsch EC. Nuclear translocation of NF-κB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport. 1997;8:2849–2852. doi: 10.1097/00001756-199709080-00009. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Carlotti F, Dower SK, Qwarnstrom EE. Dynamic shuttling of nuclear factor kappa B between the nucleus and cytoplasm as a consequence of inhibitor dissociation. J. Biol. Chem. 2000;275:41028–41034. doi: 10.1074/jbc.M006179200. [DOI] [PubMed] [Google Scholar]
- Casal C, Serratosa J, Tusell JM. Effects of β-AP peptides on activation of the transcription factor NF-κB and in cell proliferation in glial cell cultures. Neurosci. Res. 2004;48:315–323. doi: 10.1016/j.neures.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. Br. Med. J. 2003;327:128–132. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-β peptide. J. Neurosci. Res. 2004;76:872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM. An endoplasmicreticulum-specific apoptotic pathway is involved in prion and amyloid-β peptides neurotoxicity. Neurobiol. Dis. 2006;23:669–678. doi: 10.1016/j.nbd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Floden AM, Li S, Combs CK. β-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. J. Neurosci. 2005;25:2566–2575. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP. Secreted β-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF-κB and stabilization of calcium homeostasis. J. Biol. Chem. 1998;273:12341–12351. doi: 10.1074/jbc.273.20.12341. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-κB is activated in primary neurons by amyloid β peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The beginning of the end: IκB kinase (IKK) and NF-κB activation. J. Biol. Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gélinas C. To be, or not to be: NF-κB is the answer-role of Rel/NF-κB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Lee FS, Peters RT, Dang LC, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Ling L, Cao Z, Goeddel DV. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longpré F, Garneau P, Christen Y, Ramassamy C. Protection by EGb 761 against β-amyloid-induced neurotoxicity: involvement of NF-κB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol. Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K, Tomiyama T, Ishibashi K, Ito K, Teraoka R, Lambert MP, Klein WL, Mori H. The E693Δ mutation in amyloid precursor protein increases intracellular accumulation of amyloid β oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am. J. Pathol. 2009;174:957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hébert SS, De Strooper B, Haass C, Bennett DA, Vassar R. Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-β peptide 1-42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann. Intern. Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. γ-Secretase, Notch, Aβ and Alzheimer’s disease: where do the presenilins fit in? Nat. Rev. Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J. Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Pike CJ. β-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J. Neurosci. 2005;25:1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Xu H. Molecular and cellular mechanisms for Alzheimer’s disease: understanding APP metabolism. Curr. Mol. Med. 2007;7:687–696. doi: 10.2174/156652407782564462. [DOI] [PubMed] [Google Scholar]