Abstract
Neurotransmission requires anterograde axonal transport of dense-core vesicles (DCVs) containing neuropeptides and active zone components from the soma to nerve terminals. However, it is puzzling how one-way traffic could uniformly supply sequential release sites called en passant boutons. Here Drosophila neuropeptide-containing DCVs are tracked in vivo for minutes with a new method called simultaneous photobleaching and imaging (SPAIM). Surprisingly, anterograde DCVs typically bypass proximal boutons to accumulate initially in the most distal bouton. Then excess distal DCVs undergo dynactin-dependent retrograde transport back through proximal boutons into the axon. Just before reentering the soma, DCVs again reverse for another round of anterograde axonal transport. While circulating over long distances, both anterograde and retrograde DCVs are captured sporadically in en passant boutons. Therefore, vesicle circulation, which includes long range retrograde transport and inefficient bidirectional capture, overcomes the limitations of one-way anterograde transport to uniformly supply release sites with DCVs.
Introduction
Neurotransmission relies on axonal transport of neuropeptides and active zone components packaged in dense-core vesicles (DCVs) to the nerve terminal (Zupanc, 1996; Ahmari et al., 2000). Many terminals feature sequential varicose sites called en passant boutons, which release neuropeptides to regulate neuronal circuits or large postsynaptic targets, such as muscle cells. In en passant boutons, neuropeptide stores are typically uniform reflecting equivalent accumulation of DCVs that reside in each bouton for many hours (Shakiryanova et al., 2006). The kinesin responsible for anterograde axonal transport of DCVs has been identified (Jacob and Kaplan, 2003; Barkus et al., 2008). However, it is not known how anterograde transport equivalently supplies DCVs to en passant boutons to ensure that neurotransmission is robust.
The difficulty in distributing DCVs generated in the soma among en passant boutons is evident when possible models based on one-way anterograde transport are considered. For example, if boutons were filled in order, then the most proximal bouton would be supplied first and distal boutons might be starved for resources. Even with stochastic delivery, this problem persists: with a fixed probability of delivering anterograde DCVs to each bouton, delivery would still be highest for the most proximal bouton and decrement for each subsequent bouton. In principle, this issue could be overcome by routing cargoes specifically to each en passant bouton, but there is no known address system for directing delivery of DCVs from the soma to a potentially large and dynamically changing number of boutons. Therefore, although en passant boutons are common throughout the nervous system, the mechanism for uniformly maintaining their DCV pools by anterograde transport is unknown.
Here the “rules of the road” for neuronal DCVs are determined by combining Drosophila genetic methods with a technique that enables tracking of neuropeptide-containing DCVs in native nerve terminals for minutes. Anterograde axonal transport, which had been thought to fully account for delivery to the terminal, is shown to be just the first step in a surprising, but elegant, routing strategy that produces uniform presynaptic neuropeptide stores.
Results
Preferential anterograde transport of DCVs to the most distal bouton
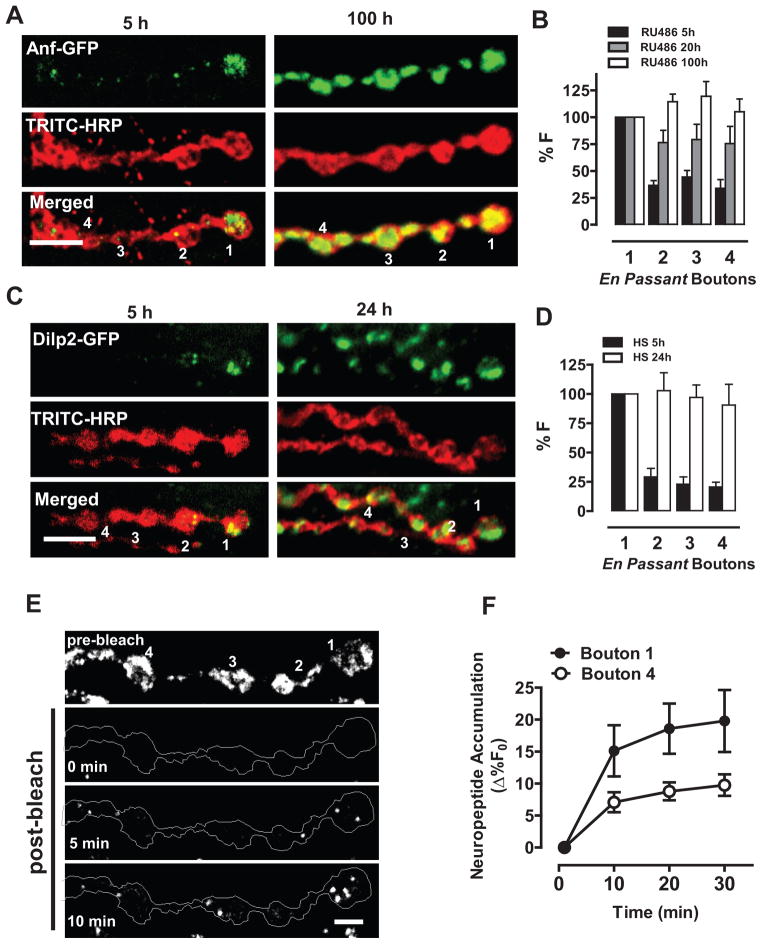
To determine how the uniform neuropeptide stores in Drosophila motoneuron type Ib boutons (Anderson et al., 1988) are supplied, the Geneswitch (GS) system (Nicholson et al. 2008) was used to induce expression of Emerald GFP-tagged Atrial Natriuretic Factor (Anf-GFP), a reporter of native neuropeptide packaging and release in Drosophila larvae (Rao et al., 2001; Husain and Ewer, 2004; Heifetz and Wolfner, 2004; Kula et al., 2006; Shakiryanova et al., 2006; Loveall and Deitcher, 2010). Independent of Anf-GFP labeling, boutons were detected with a TRITC-conjugated anti-horseradish peroxidase antibody (TRITC-HRP) and numbered from distal to proximal (Fig. 1A). Surprisingly, neuropeptide accumulated initially in the most distal bouton (#1) and only later was distributed uniformly among en passant boutons #1–4 (Fig. 1A,B). Similar results were obtained with heat shock induction of GFP-tagged Drosophila proinsulin-like peptide 2 (Dilp2-GFP, Fig. 1C,D). Therefore, the initial distal accumulation cannot be attributed to a particular neuropeptide or induction mechanism. Finally, when neuropeptide release was inhibited by removing extracellular Ca2+, fluorescence recovery after photobleaching (FRAP) of constitutively expressed Anf-GFP (i.e., driven by elav-GAL4) also revealed preferential neuropeptide accumulation in the most distal bouton (Fig. 1E,F, Movie S1). This finding independently verifies the polarized neuropeptide accumulation detected with pulse labeling and furthermore proves that this pattern cannot be explained by differential release by en passant boutons.
Figure 1. Neuropeptide accumulates initially in the most distal bouton.
A. Nerve terminal Anf-GFP fluorescence images after RU486 induction for 5 and 100 hours. TRITC-conjugated HRP labeling localized the neuronal membrane. En passant boutons are numbered from the most distal (#1) to proximal (Scale bar, 5 μm). B. Quantification of fluorescence relative to the most distal bouton (%F) after RU486 (5 h, n=11; 20 h, n=10; 100 h, n=12). Note that data are displayed from the most distal bouton #1 to proximal bouton #4. C. Nerve terminal Dilp2-GFP images after heat shock induction for 5 or 24 hours. Scale bar, 5 μm. D. Quantification of Dilp2-GFP fluorescence after heat shock induction for 5 h (n=8) and 24 h, n=5). Note that data are displayed from the most distal bouton #1 to proximal bouton #4. E. Time-lapse images before and after photobleaching (Scale bar, 2.5 μm). Contrast was adjusted to visualize DCV puncta. F. FRAP quantified in the most distal bouton (#1) and a proximal bouton (#4) (n=12). In all figures, error bars are standard error of the mean. See also Movie S1.
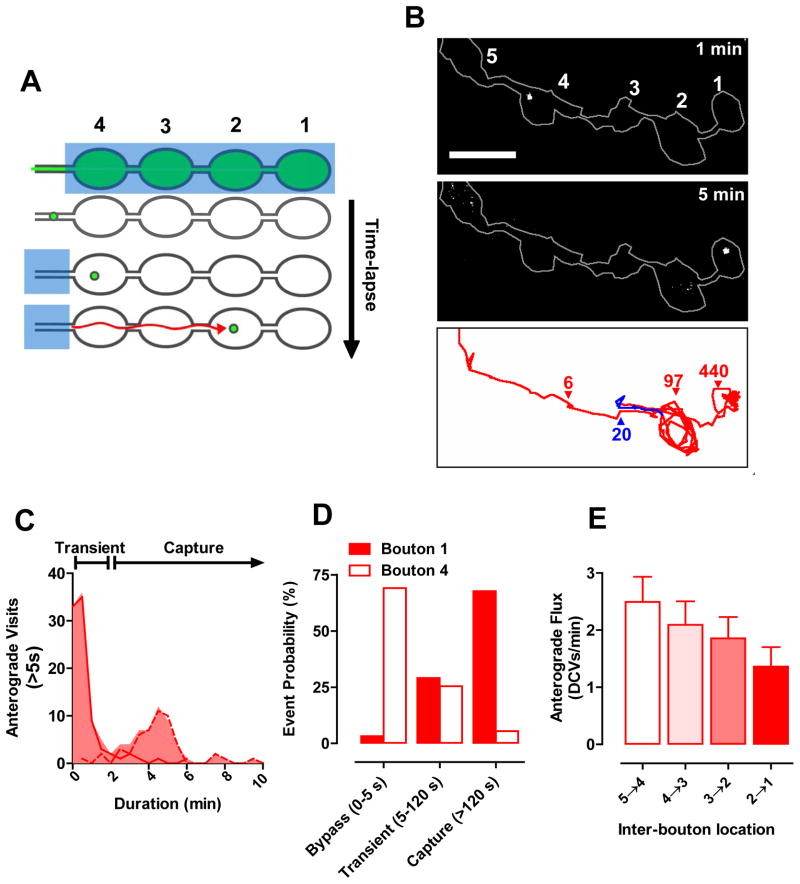
The above experiments exclude many potential models for maintaining neuropeptide stores in en passant boutons including delivery in order, stochastic delivery and parallel address-based delivery. Instead, there must be a routing mechanism that preferentially produces DCV accumulation in the most distal bouton at first, but eventually results in evenly distributed DCVs. To gain insight into this mechanism, DCVs were tracked as they populated the nerve terminal with a new approach called simultaneous photobleaching and imaging (SPAIM). In initial SPAIM experiments the distal region of the nerve terminal was photobleached. Then after a punctum likely corresponding to a single DCV (Shakiryanova et al., 2006) entered the photobleached region, a laser beam was positioned to photobleach subsequent newcomers. Finally, while continually photobleaching newcomers, a second independent scanning beam was used to simultaneously image the unbleached DCV for minutes (Fig. 2A). This allowed the initial unbleached bright DCV to be distinguished from the nearly completely photobleached newcomers (Movie S2).
Figure 2. Complex anterograde DCV traffic in the terminal.
A. SPAIM experimental design. Blue boxes, photobleaching regions; Red arrow, possible trajectory of the newcomer DCV. At least 4 boutons were examined in each experiment. B. Anterograde DCV images (upper and middle) and trajectory (bottom). Red, anterograde; Blue, retrograde. Colored numbers indicate pause durations in seconds. Scale bar, 5 μm. C. Frequency distribution of visits >5 s (n= 138 from 62 tracked DCVs in 17 animals, 30 s binning) at en passant boutons, which comprise 55% of total events. Dashed line indicates events that were interrupted by the end of time-lapse data acquisition. D. Probability of DCVs to bypass (duration 0–5 s), transiently visit (duration 5–120 s) or be captured (duration >120 s) at boutons #1 and #4 (n=86 events from 14 DCVs in 14 animals). E. Anterograde DCV flux between boutons in 5 minutes after photobleaching (n=15). See also Movie S2.
SPAIM revealed complex anterograde DCV routing within the nerve terminal. For example, an individual trajectory from a proximal bouton to the most distal bouton contained multiple pauses, a transient change in direction and looping exploration of boutons before coming to rest (Fig. 2B, Movie S2). Bouton visits fell into 3 kinetic classes: (1) DCVs moving 0.75 ± 0.03 μm/s (n=62) bypassed a bouton within 5 s in 45% of cases, (2) visited boutons transiently (5–120 s) or (3) stayed longer than 5–10 minute time-lapse experiments (Fig. 2C,D), which were classified as capture. Because proximal capture was infrequent (Fig. 2D, open bars), anterograde flux dropped at each proximal bouton by ~10% (Fig. 2E). This result has two implications: first, there is nearly uniform accumulation of anterograde DCVs in each proximal bouton because there is modest depletion of DCV flux at each bouton, and second, capture in proximal boutons is inefficient so that excess DCVs remain to accumulate in the most distal bouton (Fig. 2D filled bars, E). These experiments show that DCVs do not move directly to their ultimate destinations, but instead can pause and make detours along the way. Furthermore, the initially polarized accumulation of neuropeptide is caused by the sporadic proximal capture of anterograde DCVs as they make their complex journey through the terminal.
Retrograde DCV transport delivers neuropeptide to proximal boutons
Given that anterograde transport is biased to the most distal bouton, how is uniform neuropeptide storage eventually accomplished? The above SPAIM experiments showed that changes in transport direction were rare and transient in proximal boutons. However, in 10 of 62 initially anterograde trajectories acquired, DCVs converted to retrograde transport in the most distal bouton. Analysis of these events showed that their number was limited because conversion to retrograde transport was slow (i.e., comparable to the time scale of these experiments). Thus, retrograde flux had not yet reached values found at steady state. Nevertheless, this result raised two new questions. First, if newcomer anterograde DCVs can leave the distal bouton, might retrograde transport dissipate the initial distal gradient in neuropeptide accumulation? Second, what is the fate of DCVs that leave the distal bouton by retrograde transport?
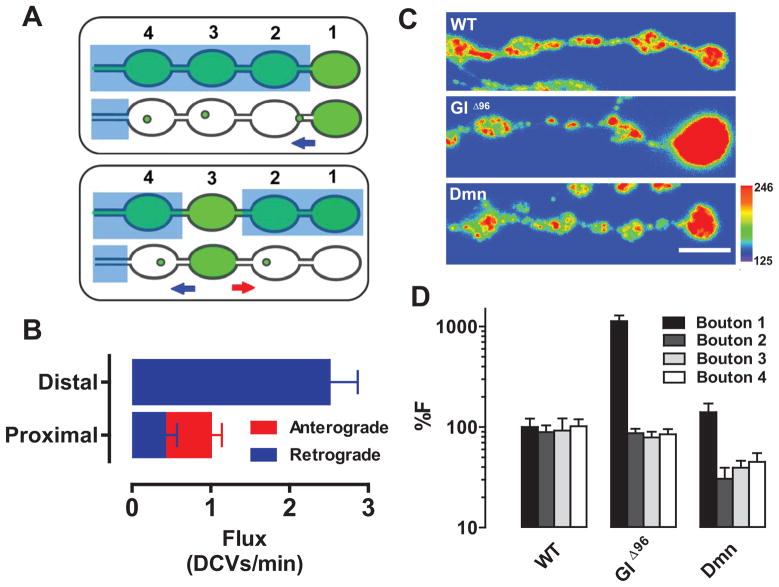
To address these questions, SPAIM experiments were designed to detect retrograde traffic of DCVs that had already accumulated in the most distal bouton. In this case, proximal boutons were photobleached, but the most distal bouton was spared. Then anterograde newcomers were continually photobleached by a laser beam located upstream of the region of interest while simultaneously imaging DCVs leaving the most distal bouton with the second scanner (Fig. 3A top). For comparison, SPAIM was also used to detect traffic out of a proximal bouton (Fig. 3A bottom). These experiments showed that retrograde DCV flux from the distal bouton was far greater than DCV flux from a proximal bouton (Fig. 3B, Movies S3 and S4), suggesting that retrograde DCV transport out of the most distal bouton removes excess DCVs supplied by anterograde transport.
Figure 3. Retrograde transport of excess DCVs out of the distal bouton.
A. SPAIM strategies for detecting DCV efflux from distal (Top) and proximal (bottom) boutons in 5 minutes after photobleaching large blue rectangles. The green boutons were spared from photobleaching and then anterograde newcomers were photobleached by the beam positioned at the location of the small blue squares. Flux was measured at the position of the arrows (Red, anterograde; Blue, retrograde). B. Quantification of DCV flux from proximal (n=10) and distal (n=13) boutons. C. Pseudo-color images showing distal Anf-GFP accumulation after inhibiting dynactin with GlΔ96 or Dmn. Scale bar, 5 μm. Warmer colors represent higher fluorescence. D. Anf-GFP intensity relative to the wild type control (WT) distal bouton (%F) (WT, n=18; GlΔ96, n=20; Dmn, n=7).
Consistent with this conclusion, inhibition of retrograde transport resulted in excess neuropeptide accumulation in the most distal bouton. Because retrograde transport depends on activation of the motor dynein by the dynactin multisubunit complex, it can be inhibited by genetically affecting dynactin subunits (e.g. by driving expression of dominant negative p150 Glued (GlΔ96) (Allen et al., 1999) or dynamitin (Dmn) overexpression (Burkhardt et al., 1997)). Transgenic expression of GlΔ96 or Dmn resulted in excess Anf-GFP accumulation in the most distal bouton (Fig. 3C,D). The effect of inhibiting retrograde transport was not limited to Anf-GFP because, in the absence of any GFP construct expression, GlΔ96 also promoted distal accumulation of the native neuropeptide proproctolin (Fig. S1). Therefore, dynactin-dependent retrograde DCV transport is required to dissipate the neuropeptide gradient produced by anterograde transport.
Interestingly, SPAIM showed that retrograde transport in the terminal shares many features found with anterograde transport. First, retrograde DCVs moving 0.73 ± 0.03 μm/s (n=63) often (i.e., 56% of events) bypassed proximal boutons (Fig. 4A). Second, trajectories featured both transient visits and capture in proximal boutons, as well as rare transient reversals in direction (Fig. 4A,B). Third, capture was inefficient and uniform among proximal boutons so that flux dropped by ~10% per bouton (Fig. 4C). This similarity to anterograde capture ensures that any subtle proximal-distal gradient produced on the way toward the distal bouton is counterbalanced by a symmetric gradient in capture on way back from the distal bouton. Most importantly, inefficient sporadic capture of retrograde DCVs contributes to neuropeptide accumulation in proximal boutons.
Figure 4. Retrograde DCV traffic and capture in the terminal.
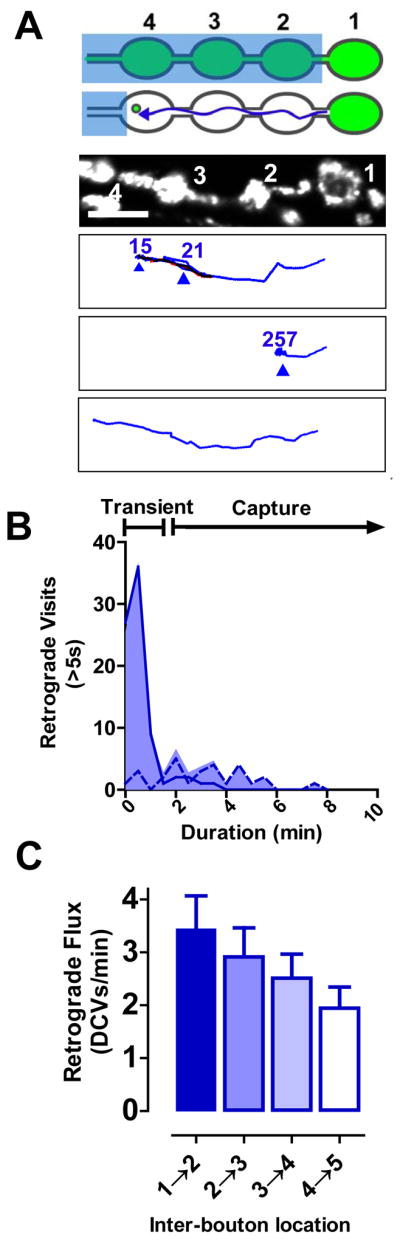
A. Top two panels, SPAIM strategy for detecting retrograde DCVs leaving the most distal bouton. Third panel, image of boutons prior to photobleaching. Bottom three panels, sample retrograde trajectories. The first shows transient visits and an anterograde reversal. The second shows capture. The third shows bypasses to exit region of interest. Blue, retrograde; Red, anterograde; Overlapping anterograde and retrograde, black; Scale bar, 5 μm. B. Frequency distribution of retrograde visits >5 s (n=106 events in 63 tracked DCVs in 20 animals), which comprise 57% of total events. Dashed line indicates events that were interrupted by the end of time-lapse data acquisition. C. Retrograde flux of DCVs from bouton #1 in 5 minutes after photobleaching (n=7).
Uncaptured retrograde DCVs stop just before reentering the soma
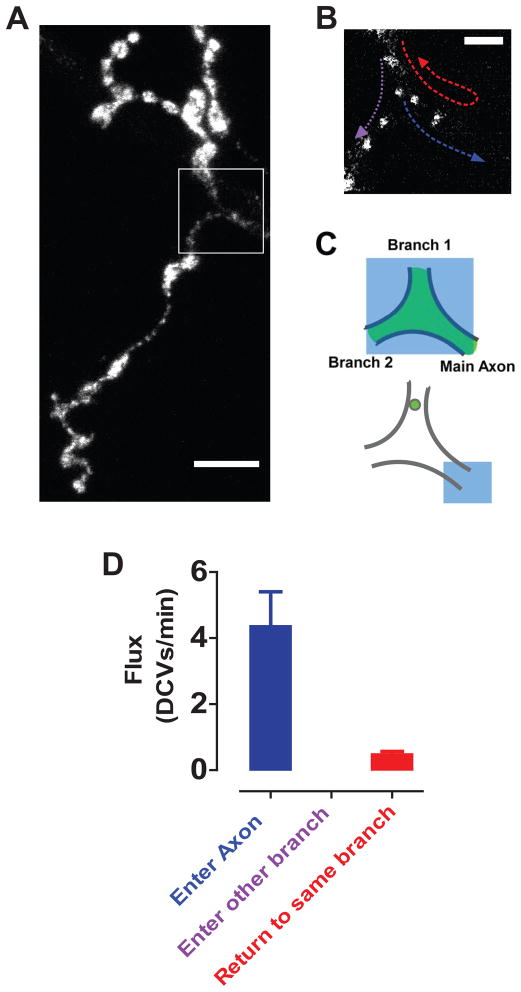
Another consequence of inefficient capture was that some retrograde DCVs were not captured in any of the proximal boutons. Because Drosophila motoneuron terminals are branched structures (Fig. 5A), retrograde DCVs must therefore either change direction to reenter the same or a neighboring terminal branch or continue on into the axon (Fig. 5B). SPAIM was used to distinguish between these possibilities: after photobleaching a branch point, retrograde traffic was imaged while simultaneously photobleaching anterograde newcomers (Fig. 5C). Time-lapse imaging showed that retrograde DCVs never turned to enter another terminal branch, only rarely reversed direction to return to the same branch and usually continued on into the axon (Fig. 5D, Movie S5). Therefore, uncaptured retrograde DCVs are not typically retained in the terminal, but instead proceed toward the soma.
Figure 5. Uncaptured retrograde DCVs leave the terminal to enter the axon.
A. Projection stack showing Anf-GFP in a muscle 4 terminal with photobleached branch point indicated with a box. Scale bar, 10 μm. B. Single image of the photobleached region showing DCVs and possible retrograde trajectories (colored arrows). Scale bar, 2.5 μm. C. SPAIM strategy for viewing retrograde DCVs entering branch point. Following photobleaching of the branch point (large blue box), anterograde newcomers are photobleached (small blue box) while simultaneously imaging retrograde DCVs. D. Quantification of retrograde DCV flux at the branch point (n=7). Note that the preferential traffic into the axon was also seen at muscle 6/7 terminals. See also Movie S5.
There is extensive retrograde transport of neuropeptidergic DCVs in the axon (Alonso and Assenmacher, 1983). A conventional hypothesis would be that retrograde DCVs return to the soma to be degraded. However, SPAIM demonstrated that many DCVs contribute to retrograde transport simply because they were not captured on the way to and from the most distal bouton. Therefore, transport back through the proximal axon into the soma was imaged to determine the fate of retrograde DCVs. Unfortunately, resolving this traffic in motoneurons was not feasible for three reasons. First, the soma and axon are often not in a single plane of focus, which is needed for continual imaging of transport. Second, available motoneuron drivers label multiple clustered somata, which makes it difficult to discriminate individual axon-soma pairs. Finally, because motoneuron somata are located in the central nervous system (i.e., the ventral ganglion), light scattering by the surrounding tissue degrades the confocal signal from individual DCVs and interferes with photobleaching. Because of these limitations, retrograde DCV traffic was studied in an optically accessible neuron that is amenable to imaging traffic back through the proximal axon to the soma.
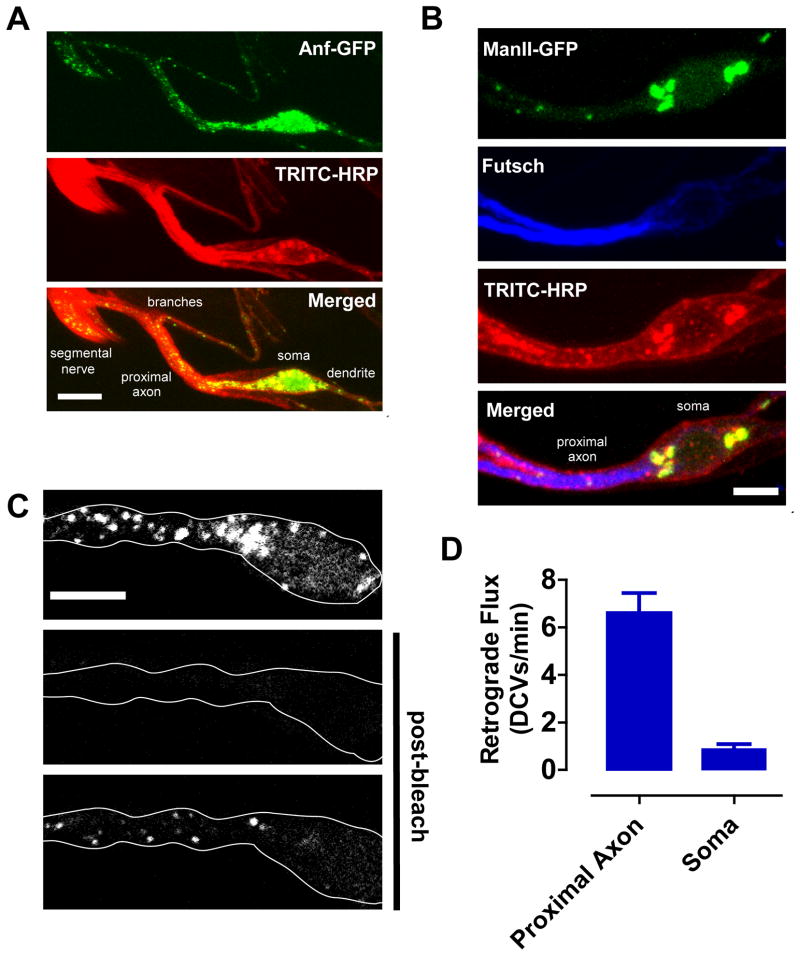
Specifically, we focused on the lateral td neuron, which has its soma located on the surface of the body wall near muscle 8 and produces an anterior projecting dendrite and a posterior projecting axon (Bodmer and Jan, 1987). In the larva, the lateral td neuron expresses Anf-GFP in response to the peptidergic cell driver 386Y-GAL4 (Fig. 6A). Importantly, the soma, proximal processes and the first axonal branch of lateral td neurons can be imaged in a single plane of focus (Fig. 6A). Little DCV traffic entered the proximal dendrite, as expected for a presynaptic cargo (Rolls, 2011). Therefore, we determined the fate of retrograde DCVs moving from the segmental nerve and peripheral axons toward the td neuron soma, which is distinguished from the proximal axon by morphology and the reciprocal distributions of the microtubule associated protein futsch and the Golgi marker mannosidase II-egfp (ManII-GFP; Ye et al., 2007) (Fig. 6B). Surprisingly, in 5 minute FRAP experiments, retrograde DCVs accumulated in the proximal axon (i.e., the region between the soma and the first axonal branch) far more than in the soma (Fig. 6C, Movie S6). Time-lapse experiments showed that this occurred because flux into the soma was ~7.5-fold less than into the proximal axon (Fig. 6D). Thus, only a small fraction of retrograde DCVs traverse the proximal axon to enter the soma.
Figure 6. Accumulation of retrograde DCVs in the proximal axon.
A. Anf-GFP (Top) expressed in a lateral td neuron. Neuronal membrane was labeled with TRITC-HRP antibody. Scale bar, 10 μm. B. Localization of the Golgi marker (Man II-GFP, green) and the microtubule associated protein futsch detected by immunofluorescence (blue). Scale bar, 5 μm. C. Representative images of the lateral td neuron soma and proximal axon before and after photobleaching. Scale bar, 5 μm. D. Quantification of retrograde DCV flux into the proximal axon and soma (n=10). See also Movie S6.
Retrograde DCVs circulate back into the axon
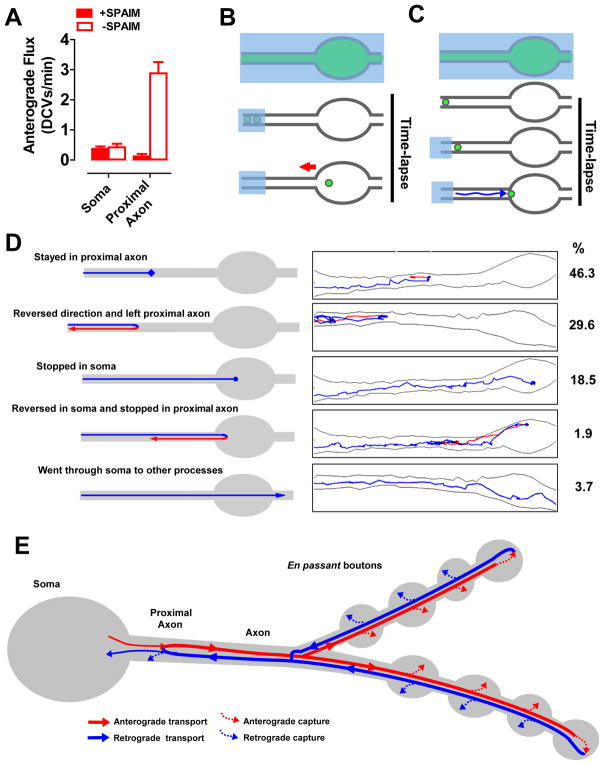
Three independent lines of experimentation demonstrated that DCVs switch from retrograde to anterograde transport in the proximal axon. First, the soma and proximal axon were photobleached. SPAIM was then used to detect nascent anterograde DCVs in the absence of retrograde newcomers. As expected, the flux of nascent DCVs, which originated only from the soma, was very low in the 5 minutes after photobleaching (Fig. 7A, Soma, B). Yet, anterograde flux out of the proximal axon measured in this time period was easily detected when retrograde DCVs were not photobleached (Fig. 7A, proximal axon open bar, Movie S6). Therefore, anterograde DCVs exiting the proximal axon must have been former retrograde newcomers. Second, the effect of photobleaching retrograde newcomers from the axon on anterograde flux out of the proximal axon was determined. When the supply of retrograde DCVs from the axon was cut off, anterograde exit of DCVs from the proximal axon was nearly eliminated (Fig. 7A, proximal axon open and filled bars). Again, this result is consistent with reversal of retrograde DCVs in the proximal axon. Finally, in 5 minute trajectories detected by SPAIM (Fig. 7C), ~30% of retrograde DCVs reversed and left the proximal axon by anterograde transport (Fig. 7D, Movie S7). In contrast, reversals in the soma only occurred in ~2% of trajectories, showing that the proximal axon is the major turnaround site for retrograde DCVs. Interestingly, after converting to anterograde transport, DCVs left either by the branch they used to approach the soma or the other possible axonal branch. Therefore, retrograde axonal transport provides DCVs that circulate back from the proximal axon to generate anterograde transport toward all release sites, where DCVs can be captured while traveling to and from the most distal boutons.
Figure 7. Retrograde DCVs reverse in the proximal axon to reenter the axon.
A. Anterograde flux from the proximal axon and soma in 5 minutes after photobleaching. +SPAIM indicates that retrograde newcomers were photobleached. B. SPAIM experimental design to prevent retrograde newcomers from the axon entering the large photobleached region so flux from nascent DCVs moving out of the soma (red arrow) can be quantified. C. SPAIM experimental design to determine trajectories of retrograde DCVs entering the proximal axon. Note that photobleaching of retrograde newcomers began only after a one DCV entered the proximal axon from the axon. D. 5 minute trajectories of retrograde DCVs after returning to the proximal axon. Left images describe trajectory classes. Right images show representative data. Percentage of tracked DCVs (n=54) for each class is presented on the right. Blue lines, retrograde; Red lines, anterograde. E. Vesicle circulation model for synaptic neuropeptide delivery. Anterograde DCVs are routed from the soma toward the most distal bouton in each branch. After reversing, dynactin-mediated retrograde transport routes DCVs past branch points into the axon. Once arriving in the proximal axon, many retrograde DCVs reverse to journey toward release sites again, thus avoiding degradation in the soma. While undergoing long-distance circulation, there is a low probability of being captured at each proximal bouton. This produces initial accumulation in the most distal bouton. However, because capture is slow compared to vesicle flux, neuropeptide stores in en passant boutons eventually fill equally despite differences in distance from the soma. See also Movies S6 and S7.
Discussion
Supplying en passant boutons by vesicle circulation
One-way anterograde transport of DCVs was believed to fully explain neuropeptide delivery to nerve terminals. However, SPAIM in different neuronal compartments (i.e., en passant boutons, axonal branches, the proximal axon, and the soma) showed that neuropeptide accumulation in nerve terminal release sites is mediated by sporadic anterograde and retrograde capture of DCVs circulating between the proximal axon and distal boutons (Fig. 7E). These findings cannot be attributed to phototoxicity as they are supported by SPAIM-independent induction and mutant experiments, the match between flux measured without photobleaching (Shakiryanova et al., 2006), the consistency of DCV behavior after multiple bouts of photobleaching, and the detection of release from single DCVs (Wong and Levitan, unpublished results), which demonstrates viability and function.
It was surprising that DCVs travel such far distances and that their presynaptic capture is inefficient, but this elegantly overcomes the limitations of relying solely on one-way transport (see Introduction) to produce the uniform neuropeptide accumulation that characterizes en passant boutons. Indeed, the conundrum of how to ensure that all potential release sites can function effectively regardless of distance from the soma or presence on different branches, which is critical for neurons, is resolved by vesicle circulation. Vesicle circulation also accounts for the long known, but mysterious, abundance of retrograde DCVs in axons (Alonso and Assenmacher, 1983) and ensures that DCVs do not readily return to the soma to be degraded. Furthermore, vesicle circulation provides a novel explanation for the finding that perturbing retrograde DCV transport in neurons affects anterograde transport (Kwinter et al., 2009): interrupting retrograde transport prevents circulating retrograde DCVs from contributing to anterograde flux and so would reduce total anterograde transport. Finally, because the same kinesin motor transports neuropeptides and many other presynaptic proteins (Hall and Hedgecock, 1991; Okada et al., 1995; Jacob and Kaplan, 2003; Pack-Chung et al., 2007; Barkus et al., 2008), vesicle circulation could be a general strategy for maintaining the synaptic function of en passant boutons. In this light, it is interesting that retrograde transport is compromised in neurodegenerative diseases (Ström et al., 2008; Perlson et al., 2010). A previously unconsidered contributing factor to the early onset loss of synaptic function could be that diseases that affect dynactin-dependent retrograde transport perturb vesicle circulation that normally maintains the terminal. Therefore, vesicle circulation may be significant under physiological and pathological conditions.
SPAIM: a new tool for studying traffic
SPAIM detects DCVs in a native synapse for many minutes. In contrast, we found that photoactivation-based approaches using PA-GFP, tdEosFP and mOrange did not work well with Drosophila DCVs. This may be due to suboptimal targeting, difficulty in inducing photoactivation in the mildly acidic (Sturman et al., 2006) and oxidizing lumen of Drosophila DCVs, and the lower brightness and poorer folding of many photoactivatable proteins compared to emerald GFP. Given the availability of bright, well characterized GFP constructs, SPAIM represents a tenable method for tracking DCVs in native Drosophila neurons. In addition to detecting vesicle circulation, SPAIM could be used to study how presynaptic DCV mobilization is induced by activity in a native synapse and how DCV traffic changes with synaptic development and plasticity. Furthermore, as noted above, SPAIM can detect release from single DCVs induced by nerve stimulation, which opens the possibility of studying peptidergic transmission at the level of individual vesicles in a native synapse. Therefore, SPAIM could answer many questions concerning neuronal DCVs.
In principle, SPAIM could be applied to any fluorescent organelle. However, a dual scanner confocal microscope, which was used here, might not be ideal for detecting very dim signals. When optical sectioning is not required, this limitation could be overcome by using a single scanner system for initially photobleaching a region of interest and then for continually photobleaching newcomers while simultaneously imaging individual organelles with a sensitive camera. In fact, performing SPAIM experiments by coupling a camera with a common single scanner confocal microscope may be an attractive option to overcome both the insensitivity and the expense of dual scanner systems. With the appropriate setup, SPAIM could be used to study traffic in terminals, dendrites, cilia, and filopodia.
Experimental Procedures
Drosophila
Emerald GFP was inserted at the EcoRV cut site in the preproDilp2 C-peptide sequence (kindly provided by E. Rulifson) and the resultant Dilp2-GFP fragment was cloned into the pUAST vector at the EcoRI and NotI sites for generation of transgenic UAS-Dilp2-GFP/CyO flies.
The following lines were maintained as double homozygotes for both transgenes, with female larvae selected for imaging experiments: elav>Anf-GFP (elav-Gal4C155 UAS-preproAnf-EMD), 386Y>Anf-GFP (w, UAS-preproAnf-EMD;386Y-Gal4), HS>Dilp2-GFP (w; UAS-Dilp2-GFP/CyO; Hsp70-Gal4) and GS>Anf-GFP (GS 3550-2, UAS-preproAnf-EMD). To study the role of dynactin, male wild type (Canton S), UAS-GlΔ96 and UAS-Dmn flies were crossed with female GS>Anf-GFP flies and F1 male larvae were selected for imaging. To localize the Golgi in lateral td neurons, UAS-ManII-GFP (Ye et al., 2007; kindly provided by Y.N. Jan) males were crossed with female 386Y-Gal4 flies and F1 larvae were used for imaging.
All animals were raised at 25°C, except in heat shock induction experiments in which larvae grown at 18°C were incubated at 37°C for 2 h and then maintained at 29°C. For RU486 induction, GS>Anf-GFP larvae were transferred to food containing 100 μg/ml RU486, which was made from a stock ethanol solution containing 100 mg/ml RU486 (Mifepristone; Sigma) mixed into freshly cooked Jazzmix food (Fisher Scientific) that had been cooled down to 60°C. In induction experiments, 3rd instar larvae, selected at specific time points after heat shock or RU486 exposure, were filleted and incubated with TRITC-conjugated anti-HRP (1:100, Jackson Immunoresearch) for 20–30 minutes on ice to stain neuronal membranes prior to GFP imaging.
Immunocytochemistry and Antibodies
Filleted larvae were fixed in 4% parfomaldehyde with 7% picric acid for 1 hour followed by permeabilization with 0.3% Triton X-100. For futsch labeling, the following antibodies were used: anti-Futsch/22C10 (1:100, Developmental Studies Hybridoma Bank, University of Iowa) and Alexa 405-conjugated goat anti-mouse IgG (1:1000, Invitrogen). For proctolin precursor labeling, preparations were incubated in polyclonal anti-proctolin precursor serum (1:1000; Taylor et al., 2004; kindly provided by D.R. Nässel) at 4°C for 2 days followed by Dylight 488-conjugated goat anti-rabbit IgG (1:1000, Jackson Immunoresearch).
Imaging
Transport was quantified in muscle 6/7 type Ib boutons, muscle 4 motoneuron branches (although consistent results were obtained in muscle 6/7 branches) and lateral td neurons. In all cases, wandering 3rd instar larvae were filleted and imaged (Levitan et al., 2007) in Ca2+-free HL3 solution (in mM: 70 NaCl, 5 KCl, 0.5 Na3EGTA, 20 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, and 5 HEPES, pH 7.2) with an upright Olympus Fluoview FV1000 confocal laser scanning microscope equipped with an 80/20 beamsplitter in the laser combiner, a second “SIM” scanner, a dichroic mirror to recombine the SIM and main scanner beams, and a Olympus LUMFL 60X (NA 1.1) water immersion objective. GFP and TRITC were imaged using 488 nm and 559 nm excitation lasers, respectively. All prebleached images and images taken for quantification purpose were acquired at a minimum resolution 17 pixels/μm at laser power and detection settings that did not generate saturated signals or produce significant photobleaching. When time-lapse was not required, fluorescence intensity for each en passant bouton was measured in Z stacks (29 images at 0.35 μm steps) taken of type Ib terminals on muscle 6/7. For td neurons, single images were acquired, with the trachea left intact. Photobleaching was performed using 100% 488 nm laser power from the SIM scanner in the line scanning mode. Time-lapse images were taken at 1 frame/s at a single plane of focus. To enable detection of single DCVs in flux and tracking experiments, time-lapse images were taken with a 5-fold increase in 488 nm main scanning laser power compared to prebleach images.
In SPAIM experiments, initial photobleaching was performed using 488 nm SIM scanning at speed of 40–100 μm/pixel and 100% SIM laser power. Then the SIM scanner setting was reduced to 20%–30% power (i.e., 110 μW below the objective) for simultaneous photobleaching while acquiring images with the main scanner using ~4 μW of power below the objective scanning at 4 μm/pixel. All SPAIM experiments lasted at least 5 minutes or until the DCV reversed direction and left the region of interest in tracking experiments.
Image Analysis
Images were analyzed using ImageJ (NIH) software. For intensity measurements, stacks were first transformed into averaged Z projections. A region of interest was drawn around each bouton and background subtracted GFP fluorescence was measured as the mean grey value unless otherwise specified. In pulse-labeling experiments, boutons were identified with HRP labeling. FRAP (Δ%Fo) of each bouton was calculated as relative fluorescence to the corresponding prebleached bouton subtracted by the residual fluorescence immediately after photobleaching. In dynactin perturbation experiments, fluorescence was measured as total grey value from the averaged Z projection. Flux was determined by manually counting DCVs moving through a ROI over a period of minutes. Single DCVs were tracked and trajectories were plotted using the Manual Tracking plugin in ImageJ.
Supplementary Material
Acknowledgments
We thank Daniel Altschuler (University of Pittsburgh) for comments, Haig Keshishian (Yale University) for the Geneswitch flies, Eric Rulifson (UCSF) for the preproDilp2 clone, Dick Nässel (Stockholm University) for anti-proctolin precursor serum, and Yuh-Nung Jan (UCSF) for the UAS-ManII-EGFP flies. This work was supported by a grant from the National Institutes of Health (NIH R01 NS32385 to E.S.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Allen MJ, et al. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J Neurosci. 1999;19:9374–9384. doi: 10.1523/JNEUROSCI.19-21-09374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Assenmacher I. Retrograde axoplasmic transport of neurosecretory material. An immunocytochemical and electron-microscopic study of transected axons in normal and colchicine-treated rats. Cell Tissue Res. 1983;233:183–196. doi: 10.1007/BF00222242. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: characterization of muscle fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R, Jan YN. Morphological differentiation of the embryonic peripheral neurons in Drosophila. Roux’s Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero G, Reiff DF, Agarwal G, Ball RW, Borst A, Goodman CS, Isacoff EY. Heterogeneity in synaptic transmission along a Drosophila larval motor axon. Nat Neurosci. 2005;8:1188–1196. doi: 10.1038/nn1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haifetz Y, Wolfner MF. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc Natl Acad Sci (USA) 2004;101:6261–6266. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Husain QM, Ewer J. Use of targetable gfp-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J Neurobiol. 2004;59:181–91. doi: 10.1002/neu.10309. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23:2122–3210. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula E, Levitan ES, Pyza E, Rosbash M. PDF cycling in the dorsal protocerebrum of the Drosophila brain is not necessary for circadian clock function. J Biol Rhythms. 2006;21:104–117. doi: 10.1177/0748730405285715. [DOI] [PubMed] [Google Scholar]
- Kwinter DM, Lo K, Mafi P, Silverman MA. Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience. 2009;162:1001–1010. doi: 10.1016/j.neuroscience.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat Protoc. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]
- Loveall BJ, Deitcher DL. The essential role of bursicon during Drosophila development. BMC Dev Biol. 2010;10:92. doi: 10.1186/1471-213X-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L, et al. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics. 2008;178:215–234. doi: 10.1534/genetics.107.081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–989. doi: 10.1038/nn1936. [DOI] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Rolls MM. Neuronal polarity in Drosophila: Sorting out axons and dendrites. Dev Neurobiol. 2011;71:419–429. doi: 10.1002/dneu.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Levitan ES. Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci. 2006;9:896–900. doi: 10.1038/nn1719. [DOI] [PubMed] [Google Scholar]
- Ström AL, et al. Retrograde axonal transport and motor neuron disease. J Neurochem. 2008;106:495–505. doi: 10.1111/j.1471-4159.2008.05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Shakiryanova D, Hewes RS, Deitcher DL, Levitan ES. Nearly neutral secretory vesicles in Drosophila nerve terminals. Biophys J. 2006;90:L45–47. doi: 10.1529/biophysj.106.080978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Winther AM, Siviter RJ, Shirras AD, Isaac RE, Nässel DR. Identification of a proctolin preprohormone gene (Proct) of Drosophila melanogaster: expression and predicted prohormone processing. J Neurobiol. 2004;58:379–391. doi: 10.1002/neu.10301. [DOI] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GK. Peptidergic transmission: from morphological correlates to functional implications. Micron. 1996;27:35–91. doi: 10.1016/0968-4328(95)00028-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.