Abstract
Background
Hyponatremia is common in patients with conditions such as congestive heart failure, and is associated with increased mortality in hospitalized patients. Congestive heart failure is common in patients with chronic kidney disease (CKD), but the association of serum sodium concentration with mortality in such patients is not well characterized.
Methods and Results
We examined the association of serum sodium concentration (SeNa) with all-cause mortality in a nationally representative cohort of 655,493 US veterans with non-dialysis dependent CKD (95,961 (15%) of them with congestive heart failure). Associations were examined in time-dependent Cox models with adjustment for potential confounders. During a median follow-up of 5.5 years a total of 193,956 patients died (mortality rate, 95% confidence interval [CI]: 62.5/1000 patient-years, 62.2-62.8). The association of serum sodium level with mortality was U-shaped, with the lowest mortality seen in patients with sodium level of 140 mEq/l, and with both lower and higher levels showing significant associations with increased mortality. Patients with serum sodium levels of <130, 130-135.9, 145.1-150 and ≥150 compared to 136-145 mEq/l had multivariable adjusted mortality hazard ratios (95%CI) of 1.93 (1.83-2.03), 1.28 (1.26-1.30), 1.33 (1.28-1.38) and 1.56 (1.33-1.83), p<0.001 for all. The associations remained consistent in subgroups of patients with and without congestive heart failure.
Conclusions
Both lower and higher serum sodium levels are independently associated with higher mortality in patients with non-dialysis dependent CKD, irrespective of the presence of absence of congestive heart failure.
Keywords: hyponatremia, hypernatremia, kidney, heart failure, epidemiology
Introduction
Hyponatremia is one of the most common electrolyte abnormalities that has been described primarily in hospitalized patients; the prevalence of hyponatremia in hospitalized patients has been reported to be as high as 42% in some studies.1, 2 Hyponatremia has been associated with various adverse clinical outcomes such as increased mortality,3-20 length of inpatient stay,20, 21 gait imbalance and falls,22 rhabdomyolysis23 and bone fractures.24-26 Additionally, hyponatremia has also been linked to significantly increased healthcare costs.27-29 Most of the studies that examined outcomes associated with hyponatremia studied hospitalized patients at single medical centers and many restricted their analyses to patients with various pre-existing pathologic conditions known to cause hyponatremia, such as CHF and liver cirrhosis. While most studies that examined outcomes associated with abnormal serum sodium levels have focused on low serum sodium (hyponatremia), elevated serum sodium (hypernatremia) has also been associated with an increase in mortality in hospitalized patients.20
Patients with chronic kidney disease (CKD) may be more susceptible to the development of dysnatremias by virtue of their diminished ability to maintain water homeostasis in the face of decreasing kidney function. In spite of this, short of a single study in hemodialysis patients19 to our knowledge there have been no attempts to explore the association of abnormal serum sodium levels in patients with CKD. We examined the association of serum sodium levels measured repeatedly over time with all-cause mortality in a large, nationally representative cohort of US veterans with non-dialysis dependent CKD. We examined associations both with mild and moderate-to-severe hyponatremia and hypernatremia.
Methods
Cohort definition
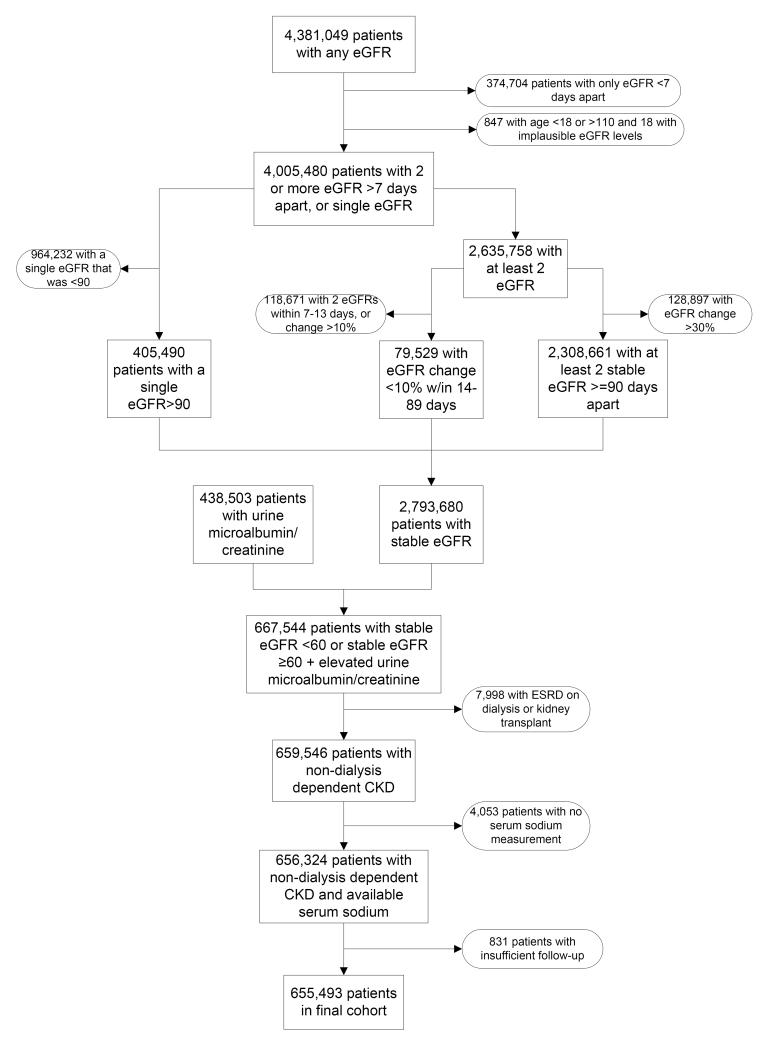
We used laboratory data on serum creatinine from the VA Decision Support System (DSS) National Data Extracts Laboratory Results file (a VA-wide database containing select laboratory results obtained in the clinical setting)30 to identify patients with CKD based on a stable estimated GFR (eGFR) and the presence of an elevated spot urine microalbumin/creatinine ratio (for those with eGFR ≥60).31 GFR was estimated from serum creatinine measurements and demographic characteristics by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.32 The algorithm for cohort definition is shown in Figure 1. Of a total of 4,381,049 patients with any available eGFR between October 1, 2004 and September 30, 2006 we identified 655,493 patients with non-dialysis dependent CKD and available serum sodium measurements.
Figure 1.
Algorithm used to define the study cohort. eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease.
Socio-demographic characteristics and comorbidities
Data on patient age, gender, race, geographic location (Veteran Integrated Service Network (VISN) number) and blood pressure was obtained through the VA Corporate Data Warehouse (CDW). Information on race was complemented with data obtained from Medicare through the VA-Medicare data merge project.33 All blood pressure values available from the October 1, 2004-October 1, 2009 time period were recorded and grouped by calendar quarters, and their quarterly-averaged values were used for analyses. Data on comorbidities (including the presence of CHF, liver disease, malignancies and depression) was collected from the VA Inpatient and Outpatient Medical SAS Datasets34, 35 using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic and procedure codes and Current Procedural Terminology codes recorded during the October 1, 2004 to September 30, 2006 time period. These databases contain up to 12 diagnostic and/or procedure codes for every inpatient, long term care and outpatient VA encounter, as well as non-VA encounters. Prevalent cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina or myocardial infarction, or procedure codes for percutaneous coronary interventions or coronary artery bypass grafting. We calculated the Charlson comorbidity index using the Deyo-modification for administrative datasets, without including kidney disease.36
Laboratory characteristics
Data on laboratory variables was collected from the October 1, 2004-September 30, 2009 time period by using the DSS National Data Extracts Laboratory Results file.30 To minimize random variability all available laboratory values (including all serum sodium levels) were grouped by calendar quarters, and their quarterly-averaged values were used in analyses.
Statistical analyses
Descriptive analyses were performed and skewed variables were log-transformed. Due to the large sample size traditional statistical testing of differences in baseline characteristics was statistically significant for all variables; hence the significance of differences was established based on what we deemed to be biologically meaningful differences. Data points were missing for race (1.4%), blood pressure (17.5%), serum albumin (13.0%), hemoglobin (13.2%), white blood cell count (WBC, 16.1%), aspartate aminotransferase (AST, 7.4%), alanine aminotransferase (ALT, 5.3%), total bilirubin (9.4%), alkaline phosphatase (10.3%) and blood glucose (0.5%). There were a total of 509,906 patients (78% of the total study population) with complete data available for the fully adjusted multivariable analyses. Compared to patients with missing data, patients with complete data were of similar age (73.8±9.6 vs. 74.0±10.1 years), gender (2.7% vs. 3.1% females), race (87% vs. 91% white, 10% vs. 7% black), and had similar prevalence of diabetes mellitus (44% vs. 41%), cardiovascular disease (44% vs. 39%) and congestive heart failure (15% vs. 12%). Missing values were not imputed in primary analyses, and were substituted by using multiple imputation procedures in sensitivity analyses. Missing values were replaced by multiple imputations with a multivariate normal regression method using data augmentation with an iterative Markov chain Monte Carlo procedure37, 38 in Stata’s “mi” command suite. Ten imputed datasets were generated, primary analyses were performed on each imputed dataset and Rubin’s combination rules were used to form one set of results.39 The start of the follow-up period was the date of the first available serum sodium measurement after October 1, 2004. Patients were followed until death or were censored at the date of the last health care or administrative VA encounter, as documented in the VA Vital Status Files (VSF). The VA VSF is a registry containing dates of death or last medical/administrative encounter from all available sources in the VA system (the Beneficiary Identification Records Locator Subsystem (BIRLS), the Patient Treatment File (PTF), Medicare and Social Security Administration (SSA)). The sensitivity and specificity of the Vital Status Files using the National Death index as gold standard were shown to be very high (98.3% and 99.8% respectively).40 For 2,956 patients (0.005%) with missing VSF data the date of the last available laboratory measurement was used as the censoring date. The association of serum sodium level with all-cause mortality was examined in time-dependent Cox models, with adjustment for potential confounders. Variables were included in multivariable models if they could be considered confounders41 based on theoretical considerations and after examination of baseline associations with serum sodium. Associations were examined sequentially in models with incremental multivariable adjustments: unadjusted (Model 1), age, gender, race and geographic location (Model 2), model 2 plus comorbid conditions (diabetes, ASCVD, CHF, liver disease, malignancy, depression and Charlson comorbidity index) (Model 3) and model 3 plus systolic blood pressure, eGFR, serum albumin, alkaline phosphatase, AST, ALT, total bilirubin, blood hemoglobin, glucose and white blood cell count (Model 4). Variables which were measured repeatedly during follow-up (serum sodium, blood pressure and all other laboratory covariates) were handled as time-dependent variables in Cox models. We hypothesized that the association of serum sodium with mortality will be non-linear; hence we examined sodium by using restricted cubic splines. Due to the small number of patients with serum sodium levels <115 mEq/liter (N=76) and >160 mEq/liter (N=61) the spline models were limited to patients with serum sodium levels of 115-160 mEq/liter. We also compared patients with mild-moderate and severe hyponatremia (serum sodium <130 and 130-135 mEq/l) and hypernatremia (serum sodium 146-150 and >150 mEq/l) to those with normal serum sodium (serum sodium 136-145 mEq/l). Due to their smaller numbers patients with hypernatremia were analyzed as a single category (serum sodium >145 mEq/l) in subgroup analyses. In order to better assess the short term vs. the long term effects of serum sodium levels on mortality time-stratified Cox models were constructed to examine 1-year mortality hazard ratios associated with baseline hypo- and hypernatremia (defined by serum sodium levels at the cohort entry and maintained constant throughout the examined time periods) and with time-varying hypo-and hypernatremia (defined based on repetitive quarterly serum sodium measurements in each examined year) in the first, second, third, fourth and fifth years of follow-up, conditional on surviving to the beginning of the examined (first, second, etc.) year.42
The association of serum sodium with mortality was examined separately in subgroups of patients categorized by CKD stage, by key sociodemographic characteristics, presence or absence of key comorbid conditions and by their levels of relevant laboratory values. Sensitivity analyses were performed by using imputed values of independent variables and by using serum sodium levels corrected for serum glucose level. Statistical analyses were performed using STATA MP version 11 (STATA Corporation, College Station, TX). The study protocol was approved by the Research and Development Committee at the Salem VAMC.
Role of the Funding Source
This study was supported by grant 1R01DK078106-01 from NIDDK to CPK and KKZ, and by resources from the Department of Veterans Affairs. The funding sources had no role in the design of the study, the data analysis or the writing of the manuscript.
Results
The mean±SD age of the cohort at baseline was 73.9±9.8 years, 87% and 9% of patients were white and black, respectively, and the mean estimated GFR (eGFR) was 50.2±14.1 ml/min/1.73m2. The mean±SD baseline serum sodium was 140±3 mEq/l. At baseline 85,855 patients (13.5%) had hyponatremia (serum sodium <136 mEq/l) and 13,289 (2%) had hypernatremia (serum sodium of >145 mEq/l); during the entire duration of follow-up 169,158 patients (26%) had at least one episode of hyponatremia and 45,666 (7%) had at least one episode of hypernatremia. Baseline characteristics in patients categorized by their baseline serum sodium levels are shown in Table 1. Patients with hyponatremia were younger, more likely to be diabetic and to have CHF, liver disease and depression, had a higher eGFR, blood glucose and white blood cell count, and a lower serum albumin and blood hemoglobin. Patients with hypernatremia on the other hand were older, and had a lower eGFR, serum total bilirubin and blood glucose.
Table 1.
Baseline characteristics of individuals stratified by baseline serum sodium level
| Serum sodium (mEq/l) | |||||
|---|---|---|---|---|---|
| <130 (N=2,729) |
130-135.9 (N=83,126) |
136-144.9 (N=556,349) |
145-149.9 (N=12,807) |
≥150 (N=482) |
|
| Age (years) | 71.5 ± 11.3 | 71.7 ± 10.7 | 74.1 ± 9.6 | 75.5 ± 8.9 | 76.1 ± 8.5 |
| Race white | 2,346 (87) | 70,905 (87) | 482,718 (88) | 11,245 (89) | 423 (89) |
| black | 217 (8) | 8,206 (10) | 51,704 (9) | 1,163 (9) | 40 (8) |
| Hispanic | 49 (2) | 998 (1) | 7,107 (1) | 146 (1) | 9 (2) |
| other | 76(3) | 1566(2) | 7694(1) | 136(1) | 5 (1) |
| Gender (male) | 2,631 (96) | 80,637 (97) | 541,195 (97) | 12,430 (97) | 474 (98) |
| DM | 1,302 (48) | 45,761 (55) | 230,989 (42) | 4,873 (38) | 185 (38) |
| ASCVD | 1,112 (41) | 34,868 (42) | 237,429 (43) | 5,778 (45) | 221 (46) |
| CHF | 556 (20) | 14,076 (17) | 79,374 (14) | 1,880 (15) | 75 (16) |
| Liver disease | 101 (4) | 1,257 (2) | 3,390 (0.6) | 51 (0.4) | 1 (0.2) |
| Malignancy | 454 (17) | 13,949 (17) | 98,068 (18) | 2,445 (19) | 82 (17) |
| Depression | 205 (8) | 5,740 (7) | 30,295 (5) | 692 (5) | 24 (5) |
| Comorbidity index | 3 (2-5) | 3 (2-5) | 3 (2-5) | 3 (2-5) | 3 (2-4) |
| SBP (mmHg) | 137 ± 20 | 137 ± 18 | 137 ± 18 | 138 ± 18 | 135 ± 19 |
| DBP (mmHg) | 72 ± 11 | 72 ± 11 | 72 ± 11 | 72 ± 11 | 70 ± 12 |
| eGFR (ml/min/1.73m2) |
55.2 ± 19.3 | 53.1 ± 17.3 | 49.8 ± 13.5 | 47.1 ± 12.6 | 47.0 ± 13.1 |
| Serum Albumin (g/dl) |
3.8 ± 0.5 | 3.9 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.5 |
| Total cholesterol (mg/dl) |
173 ± 67 | 178 ± 46 | 173 ± 39 | 171 ± 38 | 170 ± 37 |
| Serum calcium (mg/dl) |
9.1 ± 0.5 | 9.2 ± 0.5 | 9.3 ± 0.5 | 9.5 ± 0.5 | 9.5 ± 0.6 |
| Serum AST (U/L) | 24 (19-31) | 22 (18-28) | 22 (19-27) | 24 (20-29) | 25 (21-30) |
| Serum ALT (U/L) | 22 (16-32) | 22 (16-31) | 22 (16-30) | 25 (18-32) | 26 (19-33) |
| Serum total bilirubin (mg/dl) |
0.7 (0.5-0.9) | 0.7 (0.5-0.9) | 0.7 (0.5-0.9) | 0.6 (0.4-0.7) | 0.5 (0.4-0.7) |
| Serum ALP (U/l) | 91 ± 46 | 81 ± 38 | 79 ± 32 | 85 ± 30 | 87 ± 31 |
| Serum bicarbonate (mEq/l) |
26.0 ± 3.0 | 26.6 ± 2.8 | 27.4 ± 2.9 | 27.9 ± 3.3 | 28.0 ± 4.2 |
| Blood Hgb (g/dl) | 13.1 ± 1.9 | 13.7 ± 1.8 | 13.9 ± 1.7 | 13.9 ± 1.7 | 14.0 ± 1.7 |
| Blood WBC (1000/mm3) |
7.9 ± 3.0 | 7.8 ± 3.6 | 7.3 ± 4.1 | 7.3 ± 3.4 | 7.4 ± 2.2 |
| Blood glucose (mg/dl) |
166 ± 126 | 151 ± 48 | 119 ± 40 | 109 ± 28 | 110 ± 32 |
Data is presented as means ± SD, number (% of total) or median (Q1-Q3). DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; CHF, congestive heart failure; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; Hgb, hemoglobin; WBC, white blood cell count.
All p values for comparing differences between categories were statistically significant.
Mortality
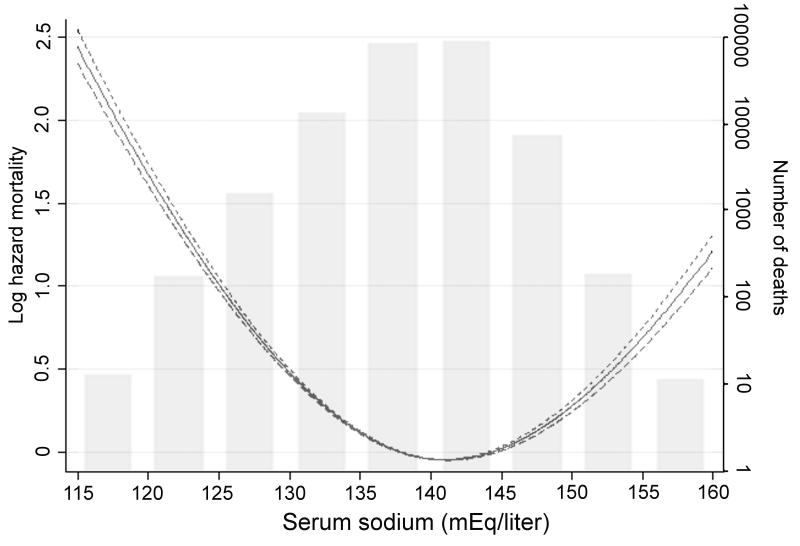
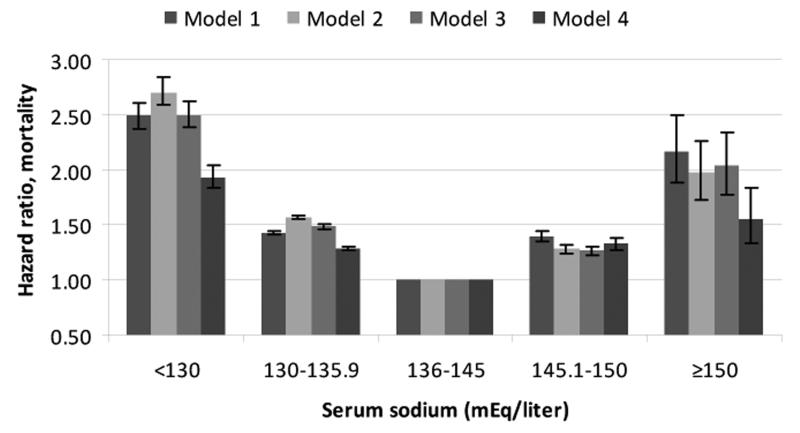
A total of 193,956 patients died (mortality rate: 62.5/1000 patient-years, 95% confidence interval [CI]: 62.2-62.8) during a median follow-up of 5.5 years. The number of deaths in patients with different serum sodium levels were 1,773 (<130); 30,199 (130-135.9); 158,103 (136-144.9); 3,680 (145-149.9) and 201 (≥150 mEq/liter). The association of serum sodium with mortality was U-shaped, with both lower and higher serum sodium showing a significant association with higher mortality even after multivariable adjustment (Figure 2). Patients with serum sodium levels of <130, 130-135.9, 145.1-150 and ≥150 compared to 136-145 mEq/l had unadjusted mortality hazard ratios (95%CI) of 2.49 (2.38-2.61), 1.43 (1.41-1.44), 1.40 (1.34-1.35) and 2.17 (1.89-2.49), p<0.001 for all; after full multivariable adjustment the hazard ratios (95%CI) for the same groups were 1.93 (1.83-2.03), 1.28 (1.26-1.30), 1.33 (1.28-1.38) and 1.56 (1.33-1.83), p<0.001 for all (Figure 3). In time-stratified Cox models concomitantly assessing 1-year mortality rates associated with both baseline and time-varying serum sodium categories, time-varying hypo-and hypernatremia both showed stronger associations with mortality compared to baseline hypo- and hypernatremia, which displayed weak or non-significant associations (Table 2).
Figure 2.
Multivariable adjusted log hazard ratios (95% confidence intervals) of all-cause mortality associated with serum sodium levels in a time-dependent Cox model using restricted cubic splines, adjusted for age, gender, race, geographic location, diabetes mellitus, ASCVD, CHF, liver disease, malignancy, depression, the Charlson comorbidity index, systolic blood pressure, eGFR, serum albumin, alkaline phosphatase, aspartate and alanine aminotransferase, total bilirubin, blood hemoglobin, glucose and white blood cell count. The bars represent the number of deaths in patients with serum sodium levels grouped in increments of 5 mEq/liter from 115 to 160 mEq/liter, on a logarithmic scale.
Figure 3.
Unadjusted and multivariable adjusted hazard ratios (95% confidence intervals) of all-cause mortality associated with various levels of serum sodium in time-dependent Cox models. The groups with serum sodium 136-145 served as referent. Models represent unadjusted association (Model 1) and associations after adjustment for age, gender, race and geographic location (Model 2), Model 2 variables + diabetes mellitus, ASCVD, CHF, liver disease, malignancy, depression and the Charlson comorbidity index (Model 3) and Model 3 variables + systolic blood pressure, eGFR, serum albumin, alkaline phosphatase, aspartate and alanine aminotransferase, total bilirubin, blood hemoglobin, glucose and white blood cell count (Model 4). All comparisons were significant at p<0.001 level.
Table 2.
One-year unadjusted mortality hazard ratios (95% confidence intervals) associated with baseline and with time-dependent serum sodium levels of <130 , 130-135.9 and >145 mEq/L, compared to 136-145 mEq/L in the first, second, third, fourth and fifth year of follow-up, conditional on surviving to the beginning of the examined year.
| Year of follow-up | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Serum sodium (mEq/L) | Baseline | <130 | 1.14 (0.93-1.40) | 0.99 (0.85-1.17) | 1.04 (0.90-1.21) | 1.30 (1.11-1.51) | 1.13 (0.95-1.33) |
| Time-dependent | <130 | 2.00 (1.67-2.38) | 2.04 (1.81-2.30) | 2.05 (1.82-2.30) | 1.63 (1.43-1.86) | 1.32 (1.15-1.51) | |
| Baseline | 130-135.9 | 1.03 (0.98-1.09) | 1.05 (1.01-1.09) | 1.06 (1.03-1.10) | 1.04 (1.01-1.08) | 1.06 (1.02-1.09) | |
| Time-dependent | 130-135.9 | 1.34 (1.27-1.41) | 1.28 (1.23-1.32) | 1.30 (1.26-1.34) | 1.27 (1.23-1.32) | 1.17 (1.13-1.21) | |
| Baseline | >145 | 1.09 (0.94-1.27) | 1.18 (1.08-1.29) | 1.17 (1.08-1.27) | 1.12 (1.02-1.22) | 1.02 (0.93-1.11) | |
| Time-dependent | >145 | 1.40 (1.20-1.64) | 1.40 (1.28-1.53) | 1.37 (1.27-1.49) | 1.30 (1.19-1.42) | 1.20 (1.10-1.31) | |
Hazard ratios are estimated from Cox models that included both baseline and time-dependent serum sodium categories, and were adjusted for age, gender, race, geographic location, diabetes mellitus, ASCVD, CHF, liver disease, malignancy, depression, the Charlson comorbidity index, systolic blood pressure, eGFR, serum albumin, alkaline phosphatase, aspartate and alanine aminotransferase, total bilirubin, blood hemoglobin, glucose and white blood cell count. Baseline serum sodium categories were established based on measurements in the first three months of cohort participation for all models and kept constant throughout follow-up. Time-dependent serum sodium categories were established using repetitive quarterly serum sodium levels measured throughout follow-up.
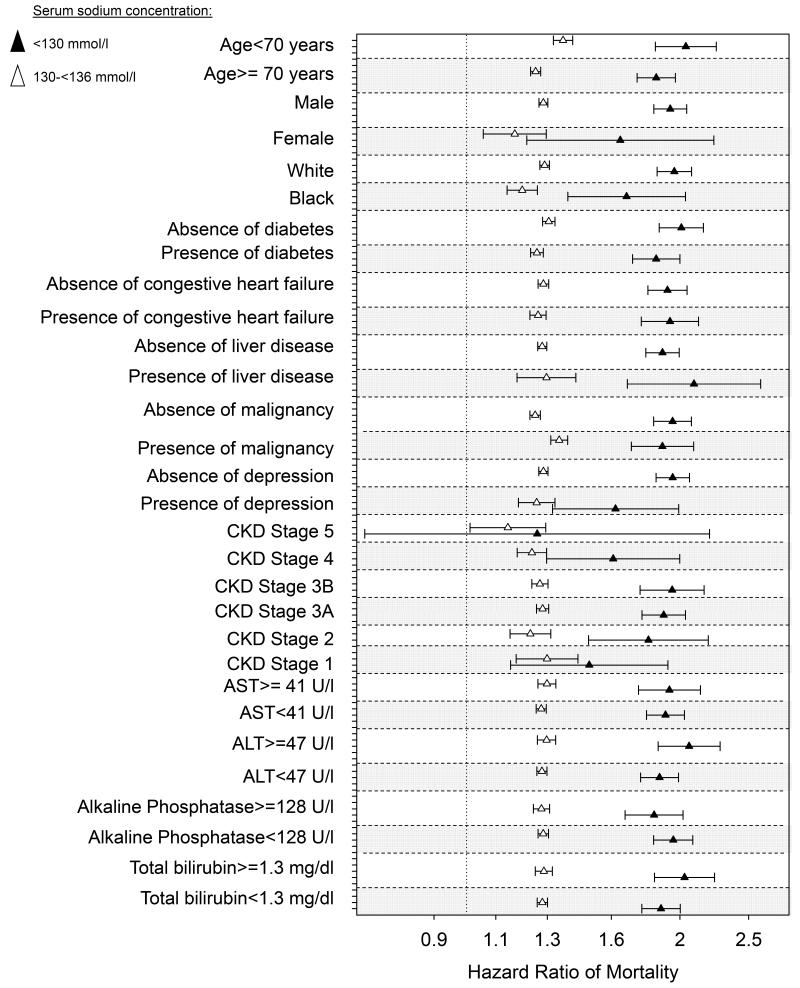
The association of lower and higher serum sodium with mortality was present in all examined subgroups, including patients with and without CHF, liver disease, malignancy and depression, in patients with high and with low levels of the composite Charlson comorbidity index, and in patients with normal or elevated serum levels of hepatic enzymes (Figure 4). There was no linear trend in the mortality hazard ratios associated with hyponatremia in patients with different stages of CKD (Figure 4, panel A); however, mortality associated with hypernatremia appeared to be relatively lower in patients with more advanced stages of CKD (Figure 4, panel B).
Figure 4.
Forest plot of the multivariable adjusted natural log-transformed mortality hazard ratios (95%CI) associated with mild (130-135.9 mEq/l) and moderate-to-severe (<130 mEq/l) hyponatremia (Panel A), and with hypernatremia (serum sodium >145 mEq/l, Panel B) in various pre-specified subgroups of patients. Groups with normal (136-145 mEq/l) serum sodium levels served as referent. Estimates are from time-dependent Cox models adjusted for age, gender, race, geographic location, diabetes mellitus, ASCVD, CHF, liver disease, malignancy, depression, the Charlson comorbidity index, systolic blood pressure, eGFR, serum albumin, alkaline phosphatase, aspartate and alanine aminotransferase, total bilirubin, blood hemoglobin, glucose and white blood cell count.
Results of analyses using imputed values for missing variables yielded similar results: the multivariable adjusted hazard ratios (95%CI) in patients with serum sodium levels of <130, 130-135.9, 145.1-150 and ≥150 compared to 136-145 mEq/l were 1.94 (1.85-2.03), 1.28 (1.27-1.30), 1.29 (1.25-1.34) and 1.58 (1.38-1.82), p<0.001 for all. The results of analyses examining serum sodium levels that were adjusted for blood glucose levels were also not different from the results detailed above (data not shown).
Discussion
We describe an association between abnormally decreased and elevated levels of serum sodium and higher all-cause mortality in a large, nationally representative group of US veterans with non-dialysis dependent CKD. These associations were linearly proportional with the severity of the underlying hyponatremia and hypernatremia, and were independent from comorbid conditions such as CHF or liver disease. Both lower and higher serum sodium were associated more strongly with mortality when modeling them in a time-dependent manner, with weak or no effects associated with baseline levels of the same factors, suggesting that both hypo- and hypernatremia represent acute (short term), rather than chronic (long term) risk factors for mortality. Severity of kidney disease did not appear to affect the mortality associated with hyponatremia, but patients with more advanced CKD displayed a relatively lower mortality associated with hypernatremia compared to patients with less severe stages of CKD.
Studies examining the predictive value of serum sodium level have concentrated largely on hyponatremia, have examined mostly patients who were hospitalized, and were usually derived from data obtained from single medical centers. Such studies have described associations of hyponatremia with a variety of adverse outcomes including all-cause mortality,3-20 length of inpatient stay,20, 21 gait imbalance and falls,22 rhabdomyolysis,23 bone fractures24-26 and higher hospitalization costs.27-29 Some of the studies examined unselected groups of patients,18, 20 but others focused on groups with some underlying comorbid condition such as CHF,7, 11, 14 cardiovascular9, 10 or liver disease.4, 13 Irrespective of the setting or the patients included, all studies have found that hyponatremia is associated with an increased risk of the studied end points. Similar associations were reported for hypernatremia too,20 although this abnormality has been in general under-emphasized.
Ours is the first study that examined patients with non-dialysis dependent CKD, and the first to provide data that is nationally representative for the US. The uniqueness of the CKD population is that kidney disease affects the organ responsible for maintaining water homeostasis, and as such it is possible that both the prevalence of dysnatremias and their clinical consequences could be magnified. While we did report a relatively high prevalence and incidence of hyponatremia (13% of the patients in our study had hyponatremia at baseline, and twice as many had at least one episode of hyponatremia during follow-up), the lack of information about the general population prevents us from determining whether CKD results in an increased incidence or prevalence of dysnatremias. Regarding outcomes, we did not find differences in the association of hyponatremia with mortality in patients with different severities of CKD, but hypernatremia appeared to predict less severe outcomes in patients with more advanced stages of CKD. This latter observation could be the result of end-organ adaptation to a state of increased extracellular osmolality in patients with advanced CKD who experience a gradual accumulation of various uremic solutes with advancing severity of kidney disease. One implication of our results is that it establishes hypo- and hypernatremia as robust outcome predictors in patients with all stages of CKD. On the other hand, it is unclear yet if these abnormalities should be considered treatment targets in these patients. Both hypo- and hypernatremia can have direct adverse effects on various organs’ function, most notably on the central nervous system.43-46 This underlying pathophysiology could serve as one potential explanation of why abnormal serum sodium level is associated with increased mortality; our results indicating a more marked association with short term, rather than long term mortality also supports this hypothesis. Since abnormal water homeostasis usually develops as a result of another underlying pathology it is also possible that hyponatremia and hypernatremia are merely surrogate markers of more severe disease states. Similar to other studies, we did not detect any effect modification by known disease states that affect serum sodium level, which makes it more likely that abnormal serum sodium level has an independent effect on survival. Furthermore, a study of maintenance hemodialysis patients enrolled in the Hemodialysis (HEMO) study also reported a significant association of hyponatremia with mortality, even though in anuric dialysis patients the development of low serum sodium is unrelated to the stimulation of arginine-vasopressin by underlying comorbidities.19 Arguing against a causal effect of hyponatremia was a recent study in hospitalized patients in whom the authors could link fatalities associated with severe hyponatremia (<120 mEq/l) to more severe underlying disease processes rather than the hyponatremia itself.47 Nevertheless, observational studies cannot completely overcome the problem of residual confounding, which can be better addressed by randomized controlled trials. One such large trial in patients that examined the effects of the vasopressin V2 receptor antagonist tolvaptan on mortality in patients with CHF did not detect a significant benefit on mortality,48 but more interventional studies are needed to determine if other patient populations, other treatment regimens, or patients with different severities of hyponatremia might show different outcomes.
Our study is notable for its very large sample size and event numbers, and for it being representative of the entire geographic United States. Our study also has a number of limitations that need to be considered when interpreting its results. Our study population consisted mostly of male patients; hence the results may not apply to females. We used data obtained during the course of clinical practice, hence selection bias is possible. However, the key laboratory variables used for cohort definition (serum creatinine and sodium) are part of routine panels that are measured in most patients receiving healthcare, hence it is unlikely that a significant proportion of actively enrolled veterans would have been excluded. We defined CKD using the CKD-EPI equation as it is more accurate than other estimating equations (such as the Modification of Diet in renal Disease (MDRD) equation) in patients with normal and mildly decreased GFR. The CKD-EPI equation was, however, meant to be used with serum creatinine measured by the IDMS-traceable method, which was not ubiquitous at the time when our cohort was defined (2005-2006), and hence it is unclear how accurate the staging of CKD in our cohort was. The associations between serum sodium and mortality did not change, however, if we used the MDRD equation to estimate GFR in our cohort participants (data not shown). We did not collect information about hospitalizations, hence we cannot determine if the mortality associated with hypo- or hypernatremia occurred in context of hospitalizations. We adjusted our analyses for a significant number of potential confounders, but we cannot rule out the presence of residual confounding. We used diagnostic codes to define comorbid conditions that could act as confounders in the association of serum sodium with mortality, which may have resulted in underestimation of their prevalence. By including laboratory variables reflecting abnormal liver function and/or structure we were able to alleviate this concern as far as the confounding imparted by liver disease, but we did not have similar data to assess the presence and/or severity of the other relevant comorbid conditions. We had relatively few patients with extremely high or extremely low serum sodium levels, hence our ability to characterize outcomes associated with very severe degrees of hypo- and hypernatremia may be limited.
Conclusions
Both hyponatremia and hypernatremia are associated with increased all-cause mortality in patients with non-dialysis dependent CKD. This association is independent of comorbid conditions and severity of kidney disease. Abnormal serum sodium levels can be used as predictors of outcomes in this patient population, and could be considered as treatment targets that need to be tested in clinical trials.
Acknowledgements
Parts of this material were accepted for presentation at the American Society of Nephrology Renal Week 2011, November 8-13, 2011, Philadelphia, PA.
Funding Sources: This study is supported by grant 1R01DK078106-01 to CPK and KKZ and by the Department of Veterans Affairs. CPK, EHL and SMM are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs.
Footnotes
Conflict of Interest Disclosures: Dr. Kalantar-Zadeh has received honoraria from Otsuka Pharmaceuticals. The other authors declared no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30–S35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol. 2009;29:227–238. doi: 10.1016/j.semnephrol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985;102:164–168. doi: 10.7326/0003-4819-102-2-164. [DOI] [PubMed] [Google Scholar]
- 4.Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605–610. doi: 10.1016/s1590-8658(00)80844-0. [DOI] [PubMed] [Google Scholar]
- 5.Chung HM, Kluge R, Schrier RW, Anderson RJ. Postoperative hyponatremia. A prospective study. Arch Intern Med. 1986;146:333–336. [PubMed] [Google Scholar]
- 6.Clayton JA, Jeune IR Le, Hall IP. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. QJM. 2006;99:505–511. doi: 10.1093/qjmed/hcl071. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Abraham WT, Albert NM, Gattis SW, Greenberg BH, O’Connor CM, She L, Yancy CW, Young J, Fonarow GC. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 8.Gill G, Huda B, Boyd A, Skagen K, Wile D, Watson I, van HC. Characteristics and mortality of severe hyponatraemia--a hospital-based study. Clin Endocrinol (Oxf) 2006;65:246–249. doi: 10.1111/j.1365-2265.2006.02583.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D. Prognostic importance of hyponatremia in acute ST-elevation myocardial infarction. Am J Med. 2004;117:242–248. doi: 10.1016/j.amjmed.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg A, Hammerman H, Petcherski S, Nassar M, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Beyar R, Markiewicz W, Aronson D. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med. 2006;166:781–786. doi: 10.1001/archinte.166.7.781. [DOI] [PubMed] [Google Scholar]
- 11.Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr., Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111:2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 12.Lee CT, Guo HR, Chen JB. Hyponatremia in the emergency department. Am J Emerg Med. 2000;18:264–268. doi: 10.1016/s0735-6757(00)90118-9. [DOI] [PubMed] [Google Scholar]
- 13.Londono MC, Guevara M, Rimola A, Navasa M, Taura P, Mas A, Garcia-Valdecasas JC, Arroyo V, Gines P. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130:1135–1143. doi: 10.1053/j.gastro.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed AA, van Kimmenade RR, Richards M, Bayes-Genis A, Pinto Y, Moore SA, Januzzi JL., Jr. Hyponatremia, natriuretic peptides, and outcomes in acutely decompensated heart failure: results from the International Collaborative of NT-proBNP Study. Circ Heart Fail. 2010;3:354–361. doi: 10.1161/CIRCHEARTFAILURE.109.915280. [DOI] [PubMed] [Google Scholar]
- 15.Sterns RH. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107:656–664. doi: 10.7326/0003-4819-107-5-656. [DOI] [PubMed] [Google Scholar]
- 16.Terzian C, Frye EB, Piotrowski ZH. Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med. 1994;9:89–91. doi: 10.1007/BF02600208. [DOI] [PubMed] [Google Scholar]
- 17.Tierney WM, Martin DK, Greenlee MC, Zerbe RL, McDonald CJ. The prognosis of hyponatremia at hospital admission. J Gen Intern Med. 1986;1:380–385. doi: 10.1007/BF02596422. [DOI] [PubMed] [Google Scholar]
- 18.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med. 2011;124:77–84. doi: 10.1016/j.amjmed.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170:294–302. doi: 10.1001/archinternmed.2009.513. [DOI] [PubMed] [Google Scholar]
- 21.Sherlock M, O’Sullivan E, Agha A, Behan LA, Rawluk D, Brennan P, Tormey W, Thompson CJ. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2006;64:250–254. doi: 10.1111/j.1365-2265.2006.02432.x. [DOI] [PubMed] [Google Scholar]
- 22.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71–78. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Morita S, Inokuchi S, Yamamoto R, Inoue S, Tamura K, Ohama S, Nakagawa Y, Yamamoto I. Risk factors for rhabdomyolysis in self-induced water intoxication (SIWI) patients. J Emerg Med. 2010;38:293–296. doi: 10.1016/j.jemermed.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5:275–80. doi: 10.2215/CJN.06120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gankam KF, Andres C, Sattar L, Melot C, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101:583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- 26.Ayus JC, Moritz ML. Bone disease as a new complication of hyponatremia: moving beyond brain injury. Clin J Am Soc Nephrol. 2010;5:167–168. doi: 10.2215/CJN.09281209. [DOI] [PubMed] [Google Scholar]
- 27.Callahan MA, Do HT, Caplan DW, Yoon-Flannery K. Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study. Postgrad Med. 2009;121:186–191. doi: 10.3810/pgm.2009.03.1991. [DOI] [PubMed] [Google Scholar]
- 28.Hirth RA, Messana JM. What does a serum sodium cost? J Am Soc Nephrol. 2008;19:654–655. doi: 10.1681/ASN.2008020143. [DOI] [PubMed] [Google Scholar]
- 29.Shea AM, Hammill BG, Curtis LH, Szczech LA, Schulman KA. Medical costs of abnormal serum sodium levels. J Am Soc Nephrol. 2008;19:764–770. doi: 10.1681/ASN.2007070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VIReC Research User Guide: Veterans Health Administration Decision Support System Clinical National Data Extracts. 2nd Edition 2009.
- 31.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Department of Veterans Affairs. VA Information Resource Center Data Quality Update: Race. 2009.
- 34.VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006. U.S. Department of Veterans Affairs. VA Information Resource Center; Hines, IL: 2007. [Google Scholar]
- 35.VIReC Research User Guide; VHA Medical SAS Outpatient Datasets FY2006. U.S. Department of Veterans Affairs. VA Information Resource Center; Hines, IL: 2007. [Google Scholar]
- 36.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 37.Li KH. Imputation using Markov chains. J Stat Comput Simul. 1988;30:57–79. [Google Scholar]
- 38.Tanner MA, Wong WH. The calculation of posterior distributions by data augmentation. J Am Stat Assoc. 1987;82:528–550. [Google Scholar]
- 39.Rubin DB. Multiple imputation for nonresponse in surveys. J.Wiley & Sons; New York: 1987. [Google Scholar]
- 40.Arnold N, Sohn M, Maynard C, Hynes DM. VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. 2006.
- 41.Thadhani R, Tonelli M. Cohort studies: marching forward. Clin J Am Soc Nephrol. 2006;1:1117–1123. doi: 10.2215/CJN.00080106. [DOI] [PubMed] [Google Scholar]
- 42.Dekker FW, de MR, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74:994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 43.Arieff AI, Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976;10:104–116. doi: 10.1038/ki.1976.82. [DOI] [PubMed] [Google Scholar]
- 44.Mount DB. The brain in hyponatremia: both culprit and victim. Semin Nephrol. 2009;29:196–215. doi: 10.1016/j.semnephrol.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Sterns RH, Silver SM. Brain volume regulation in response to hypo-osmolality and its correction. Am J Med. 2006;119:S12–S16. doi: 10.1016/j.amjmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Nguyen MK, Chang R, Kurtz I. Fatal hyponatremia in a young woman after ecstasy ingestion. Nat Clin Pract Nephrol. 2006;2:283–288. doi: 10.1038/ncpneph0167. quiz. [DOI] [PubMed] [Google Scholar]
- 47.Chawla A, Sterns RH, Nigwekar SU. Cappuccio JD. Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6:960–965. doi: 10.2215/CJN.10101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]