SUMMARY
How extrinsic stimuli and intrinsic factors interact to regulate continuous neurogenesis in the postnatal mammalian brain is unknown. Here we show that regulation of dendritic development of newborn neurons by Disrupted-in-Schizophrenia 1 (DISC1) during adult hippocampal neurogenesis requires neurotransmitter GABA-induced, NKCC1-dependent depolarization through a convergence onto the AKT-mTOR pathway. In contrast, DISC1 fails to modulate early postnatal hippocampal neurogenesis when conversion of GABA-induced depolarization to hyperpolarization is accelerated. Extending the period of GABA-induced depolarization, or maternal deprivation stress, restores DISC1-dependent dendritic regulation through mTOR pathway during early postnatal hippocampal neurogenesis. Furthermore, DISC1 and NKCC1 interact epistatically to affect risk for schizophrenia in two independent case control studies. Our study uncovers an interplay between intrinsic DISC1 and extrinsic GABA signaling, two schizophrenia susceptibility pathways, in controlling neurogenesis and suggests critical roles of developmental tempo and experience in manifesting the impact of susceptibility genes on neuronal development and risk for mental disorders.
INTRODUCTION
Proper brain function depends on correct formation of neuronal circuits during development, which is well-known to exhibit temporal and spatial precision. During embryonic cortical development, different neuronal subtypes are generated from neural progenitors in a temporally defined and highly predictable manner (Okano and Temple, 2009). Spatially, many neurons migrate over a long distance to their final locations and project axons to specific regions following guidance cues (Tessier-Lavigne and Goodman, 1996). While the importance of spatial precision in neuronal development is well appreciated, the significance of strict temporal regulation is less understood. In the hippocampus, dentate granule neurons are generated continuously from neural stem cells throughout life and appear to exhibit similar morphological, histological, and physiological properties upon maturation, regardless of whether they are born during embryonic, early postnatal, or adult neurogenesis (Ming and Song, 2011). One major difference is that neurons born in the adult brain take a significantly longer time to develop (Overstreet-Wadiche et al., 2006; Zhao et al., 2006). Interestingly, neuronal activity, such as seizures, accelerates development of adult-born neurons (Ma et al., 2009; Overstreet-Wadiche et al., 2006) and prolonged seizures lead to inappropriate integration of these new neurons (Jessberger et al., 2007). What are fundamental mechanisms that govern the tempo of neurogenesis? What intrinsic properties and extrinsic factors regulate this tempo? These are critical questions not only for developmental neurobiology, but also for the goal of realizing therapeutic cell replacement in the adult nervous system.
The process of adult neurogenesis, ranging from proliferation of neural stem cells to development of their neuronal progeny, is governed by both extrinsic niche signals and intrinsic cellular properties (Ihrie and Alvarez-Buylla, 2011; Ming and Song, 2011). Among extrinsic factors, neurotransmitter γ-aminobutyric acid (GABA) regulates the proliferation of neural progenitors and new neuron development in the adult brain (Ge et al., 2007; Platel et al., 2010). As a classic inhibitory neurotransmitter, GABA hyperpolarizes mature neurons, which maintain low intracellular chloride content ([Cl−]i) through high level expression of a neuronal K+-Cl− co-transporter (KCC2, a Cl− exporter) (Owens and Kriegstein, 2002). In contrast, immature neurons are depolarized by GABA due to their high [Cl−]i as a result of high level expression of a Na+-K+-2Cl− co-transporter (NKCC1, a Cl− importer). Down-regulating NKCC1 or up-regulating KCC2 in immature neurons abolishes GABA-induced depolarization, resulting in defects in dendritic growth and synapse formation during both embryonic and adult neurogenesis (Ge et al., 2007; Platel et al., 2010). Among intrinsic regulators of adult neurogenesis, Disrupted-in-Schizophrenia 1 (DISC1) controls multiple aspects of neuronal development (Ming and Song, 2009). DISC1 knockdown (KD) by short-hairpin RNA (shRNA) accelerates the tempo of adult hippocampal neurogenesis, resulting in premature cell cycle exit of neural progenitors (Mao et al., 2009) and precocious dendritic development of newborn neurons (Duan et al., 2007). Adult-born neurons with DISC1 KD also exhibit soma hypertrophy, ectopic primary dendrites, and aberrant positioning (Duan et al., 2007). It is unclear whether the role of DISC1 is conserved in early postnatal hippocampal neurogenesis.
Aberrant neuronal development is believed to contribute to the pathogenesis of mental disorders such as schizophrenia and autism (Geschwind and Levitt, 2007; Lewis and Levitt, 2002; Weinberger, 1987). GABA signaling and DISC1 have both been implicated in schizophrenia and other major mental disorders (Balu and Coyle, 2011). There is strong evidence implicating deficits of GABA signaling in the pathophysiology of schizophrenia (Hyde et al., 2011; Lewis et al., 2005; Perry et al., 1979). Several GABAAR subunits and GAD67 have been linked to increased risk for schizophrenia and related disorders in genetic association studies and many of them exhibit abnormal expression in postmortem patient tissues (Charych et al., 2009; Straub et al., 2007). DISC1 was initially identified at the break-point of a balanced chromosomal translocation (1;11)(q42; q14) that co-segregates with schizophrenia and other major mental illness in a large Scottish family (Millar et al., 2000). Further genetic association studies support an expanded role of DISC1 in influencing risks for schizophrenia, bipolar disorders, major depression, and autism (Chubb et al., 2008). To understand how DISC1 dysfunction contributes to a broad spectrum of mental disorders, it is important to clarify biological function and signaling mechanisms of DISC1 in the normal brain. While recent studies have begun to delineate DISC1 intracellular signaling mechanisms (Chubb et al., 2008), very little is known about how DISC1 interacts with specific extracellular signaling to control different aspects of neuronal development in vivo.
A central question in stem cell biology is how dynamic interactions between extrinsic niche signaling and intrinsic factors influence stem cell behavior and their development in vivo. Despite recent progress in identifying individual molecular players (Duan et al., 2008), little is known about the relationship between intrinsic and extrinsic signaling in regulating adult neurogenesis. Recent findings that reveal common processes of adult neurogenesis affected by DISC1 and GABA raise a tantalizing possibility that intrinsic factor DISC1 may regulate extrinsic GABA signaling. Here we employed a “single-cell” genetic approach to investigate the interaction between DISC1 and GABA signaling in regulating development of newborn dentate granule neurons during adult and early postnatal neurogenesis in vivo. We further explored whether these molecular interactions might contribute to mental illness by testing genetic interactions between single-nucleotide polymorphisms (SNPs) in DISC1 and SLC12A2 (which encodes human NKCC1) on risk for schizophrenia in three independent case control samples. Our study reveals a surprising role of developmental temporal dynamics and animal experience in determining the impact of genetic factors associated with schizophrenia and has important implications for understanding the pathogenesis of mental disorders.
RESULTS
Depolarizing GABA-signaling is Required for DISC1 KD-induced Acceleration of Dendritic Development during Adult Neurogenesis
To examine whether DISC1 and GABA signaling interacts during adult neurogenesis, we first characterized the time-course of neuronal development regulated by these two pathways. To knockdown DISC1 specifically in adult-born new neurons, engineered retroviruses co-expressing GFP and a previously characterized shRNA against mouse disc1 (shRNA-DISC1#1; shRNA-D1) were stereotaxically injected into the dentate gyrus at postnatal day 42 (P42; See Experimental Procedures) (Duan et al., 2007). shRNA-D1/GFP+ neurons exhibited accelerated dendritic growth compared with those expressing a control shRNA (shRNA-C1) at 14 days post viral injection (dpi; Figure 1A), as well as soma hypertrophy, ectopic primary dendrites and aberrant positioning as previously reported (Figures S1A to S1C) (Duan et al., 2007). Interestingly, the effect of DISC1 KD on newborn neurons, including increased dendritic length and complexity, manifested only after 7 dpi (Figures 1B and S1D). We previously showed that expression of a specific shRNA against mouse nkcc1 (shRNA-NK1) abolishes GABA-induced depolarization of newborn neurons in the adult hippocampus (Ge et al., 2006). Consistent with a critical role of depolarizing GABA signaling in adult neurogenesis, shRNA-NK1+/GFP+ new neurons exhibited significant decreases in total dendritic length and complexity at 14 dpi (Figures 1A, 1B and S1D). There was no apparent effect of shRNA-NK1 expression on soma size, number of primary dendrites and positioning of new neurons at all time points examined (Figures S1A to S1C). Notably, the major effect of depolarizing GABA signaling and DISC1 KD on dendritic growth of new neurons exhibited a similar time-course, but in the opposite direction (Figure 1B).
Figure 1. DISC1 KD-induced precocious dendritic growth of newborn neurons in the adult dentate gyrus requires GABA-induced depolarization.
(A) A schematic diagram of the experimental design and sample projected confocal images of newborn neurons with retrovirus-mediated co-expression of GFP and a control shRNA (C1), shRNA-DISC1 (D1), or shRNA-NKCC1 (NK1) in the adult mouse dentate gyrus at 14 dpi. Scale bar: 50 µm.
(B) Summary of total dendritic length of GFP+ neurons under different conditions. Values represent mean ± SEM (n = 4 animals with a total of 29–36 neurons for each condition; *: P < 0.05; ANOVA).
(C–F) Effect of retrovirus-mediated double NKCC1 and DISC1 KD on dendritic development and AKT/mTOR signaling in newborn neurons in the adult dentate gyrus. Shown in (C) are schematic diagrams of retroviral vectors and experimental design, and sample projected confocal images of a newborn neuron with double KD at 14 dpi. Shown are summaries of total dendritic length (D) and quantifications of pAKT (E) and pS6 (F) levels in the cytosol of new neurons at 14 dpi. Numbers associated with the bar graph represent total numbers of neurons examined under each condition. Values represent mean ± SEM (n = 4 animals for each condition; *: P < 0.05; ANOVA).
To directly examine whether intrinsic DISC1 interacts with extrinsic GABA signaling in regulating new neuron development, we used a double knockdown strategy with two retroviruses: one co-expressing shRNA-NK1 and GFP and the other co-expressing shRNA-D1 and DsRed (Figure 1C). GFP+DsRed+ new neurons exhibited similar dendritic growth as those expressing shRNA-C1 at 14 dpi (Figures 1D and S1D). In contrast, other defects from DISC1 KD were not rescued by shRNA-NK1 expression (Figures S1A to S1C). To ensure that GABA, but not Cl− signaling, is required for DISC1-dependent regulation of newborn neurons, we developed specific shRNA against the γ2 subunit of GABAARs (shRNA-γ2; Figures S1E to S1F), a critical subunit involved in GABA signaling. Expression of shRNA-γ2 in newborn neurons rescued the DISC1 KD-induced acceleration of dendritic growth (Figures 1D and S1D), but not other defects (Figures S1A to S1C). To identify the mechanistic link between GABA and DISC1 signaling, we examined AKT, a DISC1 target, and subsequent mTOR activation in newborn neurons (Kang et al., 2011; Kim et al., 2009). Interestingly, DISC1 KD-induced increases of pAKT and pS6 levels were significantly attenuated by shRNA-NK1 or shRNA-γ2 expression (Figures 1E to 1F). Together, these results suggest a specific requirement of depolarizing GABA signaling in DISC1-dependent regulation of AKT/mTOR signaling and dendritic growth during adult neurogenesis.
Increasing GABA Signaling Enhances DISC1 KD-induced Acceleration of Dendritic Development during Adult Neurogenesis
To further assess the functional interaction between DISC1 and GABA signaling during adult neurogenesis, we examined the effect of enhancing GABA signaling on DISC1-dependent regulation of dendritic development in vivo. Our previous time course analysis of different modes of GABA signaling showed that new neurons are initially activated tonically by ambient GABA followed by both tonic and phasic/synaptic GABA activation starting from 7 dpi in the adult brain (Ge et al., 2006). Importantly, both tonic and synaptic activation by GABA remain depolarizing until at least 14 dpi (Ge et al., 2006). In the first experiment, we injected the GABA transaminase inhibitor vigabatrin (25 µg/g body weight per day from 3 to 6 dpi, i.p.) (Wu et al., 2003) to increase tonic GABA signaling in newborn neurons (Figure 2A). shRNA-D1/GFP+ neurons with vigabatrin treatment exhibited a significant increase in total dendritic length at 7 dpi, whereas vigabatrin by itself or expression of shRNA-D1 alone had no effect (Figures 2A to 2B). Co-manipulation also significantly increased pAKT and pS6 levels in newborn neurons more than either manipulation alone (Figures 2C to 2D and Table S1). Thus, there is a synergistic interaction between DISC1 and GABA signaling in regulating AKT/mTOR signaling and dendritic growth of newborn neurons in the adult brain. Other aspects of new neuron development appeared to be normal (Figures S2A to S2C), supporting the specificity of the synergistic interaction.
Figure 2. DISC1 KD-induced precocious dendritic development of newborn neurons in the adult dentate gyrus is enhanced by activation of GABA signaling.
(A–D) Effect of vigabatrin (VGA) on dendritic development and AKT/mTOR signaling in newborn neurons in the adult hippocampus with or without DISC1 KD. Shown in (A) are the schematic diagram of the experimental design and sample projected confocal images of new neurons with retrovirus-mediated co-expression of GFP and a control shRNA (C1) or shRNA-DISC1 (D1), with or without VGA injection (25 µg/g body weight per day from 3 to 6 dpi, i.p.) and examined at 7 dpi. Scale bar: 50 µm. Shown are summaries of total dendritic length (B) and quantifications of pAKT (C) and pS6 (D) levels in the cytosol of newborn neurons at 7 dpi. Values represent mean ± SEM (n = 4 animals; *: P < 0.05; n.s.: P > 0.1; ANOVA).
(E–H) Same as in (A–D), except that pentobarbital (25 µg/g per day from 6 to 10 dpi; i.p.) was injected and analysis was performed at 11 dpi.
(H–K) Same as in (B–D), except that newborn neurons expressing shRNA-control (C1), KIAA1212 (K1), shRNA-NK1 (NK1), or both K1 and NK1 were analyzed at 14 dpi.
(L) A model of interaction between depolarizing GABA and DISC1 signaling in regulating dendritic growth of newborn neurons in the adult dentate gyrus.
In the second experiment, we injected the GABAAR agonist pentobarbital (25 µg/g per day from 6 to 10 dpi, i.p.) to enhance synaptic GABA signaling in new neurons (Figure 2E). While there was little effect on shRNA-C1/GFP+ neurons, pentobarbital treatment further enhanced DISC1 knockdown-induced dendritic growth and pAKT and pS6 levels at 11 dpi (Figures 2E to 2H), again suggesting a synergistic interaction between DISC1 and GABA signaling. The effect of pentobarbital treatment is also specific for dendritic growth because other defects from DISC1 knockdown were not affected (Figures S2D to S2F).
To further establish a mechanistic link between depolarizing GABA and DISC1 signaling, we co-manipulated NKCC1 and KIAA1212/Girdin, which binds directly to both DISC1 and AKT (Enomoto et al., 2009; Kim et al., 2009). KIAA1212 promotes AKT activation and is phosphorylated by AKT. shRNA-NK1 expression abolished KIAA1212-induced acceleration of dendritic growth and significantly attenuated pAKT and pS6 level increases in new neurons at 14 dpi (Figures 2I to 2K), whereas other defects were not significantly affected (Figures S2G to S2I). Taken together, these results demonstrate that DISC1 KD-induced acceleration of dendritic development of adult-born neurons not only requires, but also synergistically acts with, depolarizing GABA signaling. These results support a model that intrinsic factor DISC1 functionally gates extrinsic GABA signaling through the AKT/mTOR pathway during adult hippocampal neurogenesis (Figure 2L).
DISC1 KD Fails to Affect New Neuron Development during Early Postnatal Hippocampal Neurogenesis
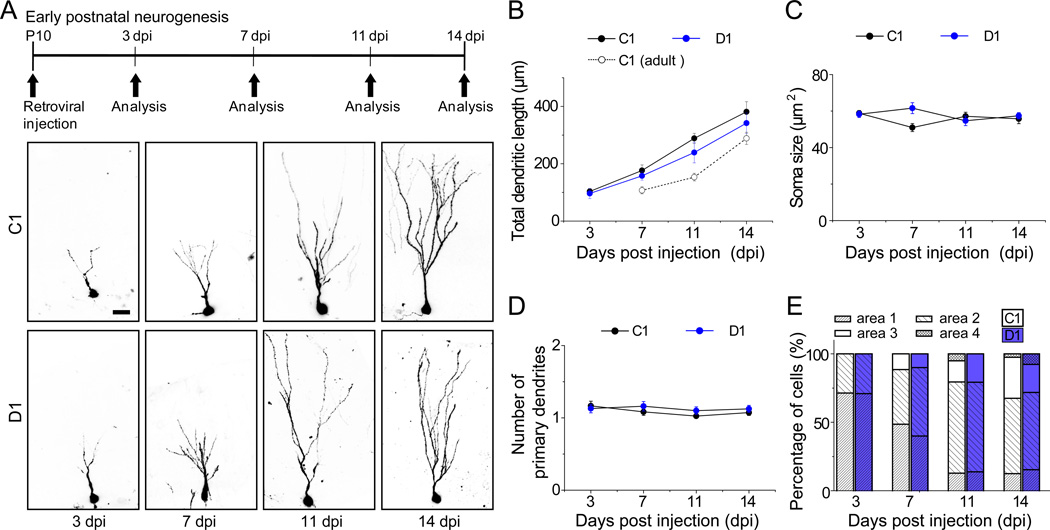
Generation of dentate granule neurons in the hippocampus peaks during the early postnatal period and continues throughout life (Angevine, 1965). DISC1 expression in the dentate gyrus starts from embryonic stages and is maintained in adulthood (Austin et al., 2004). To assess whether DISC1 also regulates early postnatal hippocampal neurogenesis, we stereotaxically injected retroviruses expressing shRNA-D1 or shRNA-C1 into the dentate gyrus at postnatal day 10 (P10; Figure 3A). Consistent with previous results (Zhao et al., 2006), time course analysis showed that shRNA-C1/GFP+ newborn neurons in the early postnatal hippocampus exhibited significantly accelerated dendritic development compared to those in the adult brain (Figure 3B). Notably, shRNA-D1/GFP+ neurons in the early postnatal hippocampus did not exhibit any accelerated dendritic growth when compared to those expressing shRNA-C1 (Figures 3A, 3B and S3A). Furthermore, there was no significant difference in the soma size, primary dendrite number, or neuronal positioning at time points examined (Figures 3C to 3E). Expression of shRNA-C1 or shRNA-D1 also did not affect neuronal subtype differentiation of neural progenitors into Prox1+ dentate granule neurons (Figure S3B). Taken together, these results show that development of the same neuronal subtype exhibits differential DISC1-dependency during early postnatal and adult hippocampal neurogenesis.
Figure 3. DISC1 KD fails to affect development of newborn dentate granule neurons during early postnatal hippocampal neurogenesis.
(A) A schematic diagram of the experimental design and sample projected confocal images of newborn neurons with retrovirus-mediated co-expression of GFP and a control shRNA (C1), or shRNA-DISC1 (D1), in the dentate gyrus of neonatal mouse hippocampus. Scale bar: 50 µm.
(B–D) Summary of the mean total dendritic length (B), soma size (C), primary dendrite number (D) of GFP+ neurons under different conditions. The same data on the time course of dendritic development of shRNA-C1/GFP+ neurons in the adult hippocampus (P42) as in Figure 1B are replotted in (B) for direct comparison. Values represent mean ± SEM (n = 4–6 animals with a total of 36–45 neurons for each condition; P > 0.05; ANOVA).
(E) Positioning of newborn neurons in the dentate gyrus under different conditions. Shown is the summary of the distribution of soma of GFP+ neurons within the four domains in the dentate gyrus (as defined in Figure S1C). The same group of neurons as in (B–D) was used.
Early Postnatal and Adult Hippocampal Neurogenesis Exhibit Differential Tempo in Shifting the Polarity of GABA Responses
Could properties of GABA signaling in newborn neurons explain the differential dependence on DISC1 between early postnatal and adult hippocampal neurogenesis? A much prolonged time course of neuronal maturation in the adult brain represents the major difference between early postnatal and adult neurogenesis (Zhao et al., 2006). One physiological hallmark of neuronal maturation is the polarity switch of GABAergic responses from depolarization to hyperpolarization due to NKCC1 down-regulation and KCC2 up-regulation (Owens and Kriegstein, 2002). Our previous electrophysiological analysis demonstrated a complete polarity switch of GABAergic responses in newborn neurons after 14 dpi during adult hippocampal neurogenesis (Ge et al., 2006). Consistent with this result, Ca2+ live-imaging analysis of newborn neurons in slices acutely prepared from animals with retroviral injection at P42 showed a significant Ca2+ rise in response to the GABAAR agonist muscimol (10 µM) from 3 to 14 dpi (Figure 4A). To control for Ca2+ dye loading efficacy, we normalized all responses to those of Ca2+ ionophore ionomycin (10 µM) for each cell. No significant decrease in the peak amplitude of muscimol-induced Ca2+ responses was observed up to 11 dpi (Figure 4B). Furthermore, muscimol-induced Ca2+ rise was absent in shRNA-NK1+ neurons at 7 dpi (Figures 4A to 4B). These results provide additional evidence that GABA depolarizes newborn neurons up to 14 dpi in an NKCC1-dependent fashion during adult hippocampal neurogenesis.
Figure 4. Newborn neurons during adult and early postnatal neurogenesis exhibit differential tempo in shifting the polarity of GABA responses.
(A) A schematic diagram of the experimental design and sample Ca2+ imaging analysis of new neurons expressing RFP and shRNA-C1 in P42 animals. Sample confocal images of RFP and Ca2+ dye Fluo4-AM, as well as Ca2+ responses were shown at the basal level, followed by application of GABAAR agonist muscimol (musc.; 10 µM), and then Ca2+ ionophore ionomycin (iono.; 10 µM). White arrowheads point to RFP+Fluo4+ newborn cells. Scale bar: 20 µm.
(B) Summary of mean peak Ca2+ responses to muscimol for newborn neurons expressing shRNA-C1 (C1), shRNA-NKCC1 (NK1), or shRNA-D1 (D1) in the adult dentate gyrus. The values of Ca2+ responses are normalized to the mean fluorescence intensity measured at the baseline condition (set as 0%) and after the ionomycin treatment (set as 100%) in the same cell. Values represent mean ± SEM (n = 6–7 cells; *: P < 0.01; ANOVA).
(C and D) Same as (A and B), except that retroviruses co-expressing RFP and shRNA-C1 (C1), shRNA-KCC2 (K2), or shRNA-D1 (D1) were injected into P10 animals.
We then performed Ca2+ imaging analysis of new neurons during early postnatal neurogenesis after retroviral labeling at P10 (Figure 4C). While muscimol induced a large Ca2+ rise in new neurons at 3 dpi, the peak Ca2+ responses were significantly decreased by 7 dpi and largely diminished by 11 dpi (Figure 4D). Thus, there is an accelerated tempo in the polarity shift of GABAergic responses by new neurons during early postnatal neurogenesis when compared to adult neurogenesis. Consistent with our model that DISC1 functions downstream of Ca2+ signaling (Figure 2L), DISC1 KD did not significantly affect muscimol-induced Ca2+ responses in new neurons during early postnatal or adult neurogenesis (Figures 4B and 4D). These results raise the possibility that newborn neurons during early postnatal neurogenesis escape the DISC1 control of GABAergic responses due to a reduced time-window of depolarizing GABA action.
Extending Period of Depolarizing GABA Signaling Restores DISC1-dependent Regulation of Early Postnatal Hippocampal Neurogenesis
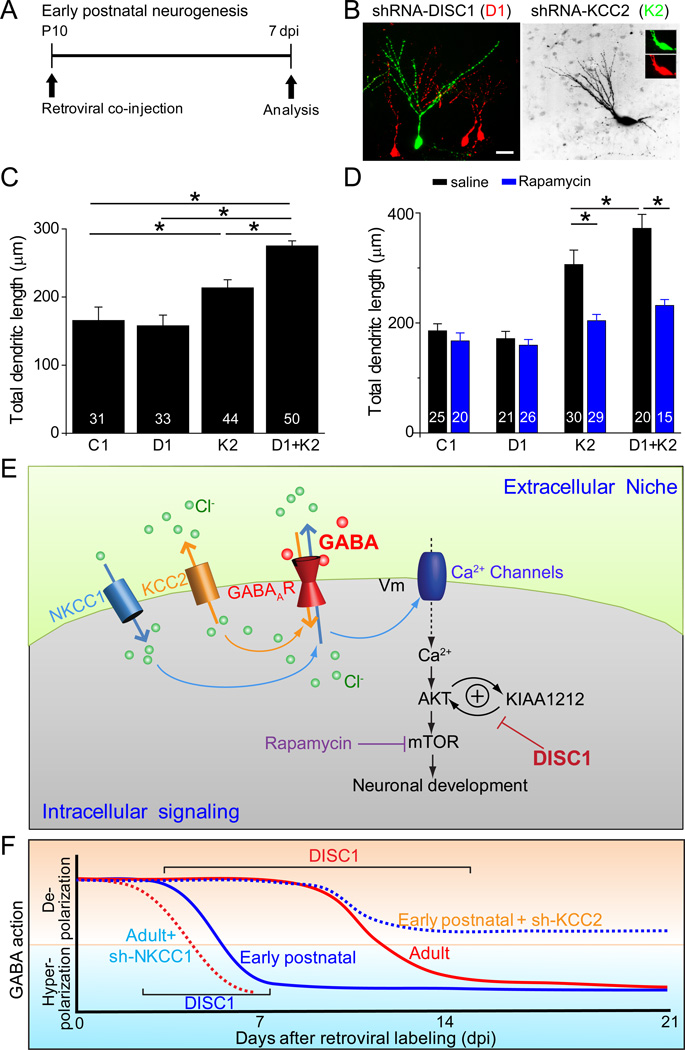
To directly test our hypothesis, we developed a genetic means to extend the period of GABA-induced depolarization specifically in newborn neurons during early postnatal neurogenesis. Previous studies have shown that KCC2 up-regulation is a primary factor underlying the functional switch of GABAergic responses from depolarization to hyperpolarization during neuronal maturation (Rivera et al., 1999). We designed several shRNAs against mouse kcc2 (shRNA-K2) and identified effective ones with in vitro analysis (Figure S4). To test the shRNA efficacy in vivo, we injected retroviruses into the dentate gyrus of P10 animals. Ca2+ imaging analysis showed that shRNA-K2+ new neurons at 7 dpi exhibited a significantly larger Ca2+ rise compared to those expressing shRNA-C1 (Figures 4C to 4D). Functionally, expression of shRNA-K2 itself led to increased total dendritic length and complexity of new neurons at 7 dpi in the early postnatal hippocampus (Figures 5A to 5C), supporting a conserved role of depolarizing GABA signaling in promoting dendritic growth of immature neurons during neuronal development.
Figure 5. DISC1 KD induces precocious dendritic development during early postnatal neurogenesis in the presence of extended period of GABA-induced depolarization.
(A) A schematic diagram of the experimental design.
(B) Sample confocal images of newborn neurons after stereotaxic injection of a mixture of retroviruses co-expressing shRNA-DISC1 (D1)/DsRed and those co-expressing shRNA-KCC2 (K2)/GFP into the dentate gyrus of the P10 animal and analyzed at 7 dpi. Scale bar: 20 µm.
(C–D) Summary of the mean total dendritic length of newborn neurons at 7 dpi under different conditions. Rapamycin (20 mg/kg body weight) or saline was i.p. injected daily after viral injection in (D). Values represent mean ± SEM (n = 6–8 animals with a total of 31–50 neurons for each condition; *: P < 0.05; ANOVA).
(E– F) Summary models of interaction between DISC1 and depolarizing GABA in regulating dendritic development in a context-dependent fashion. Shown in (E) is a schematic diagram of interaction between depolarizing GABA and DISC1 signaling in regulating dendritic development. Shown in (F) is a model of DISC1 signaling in regulating neuronal development during early postnatal and adult hippocampal neurogenesis and its temporal constraints.
We next examined the effect of DISC1 KD on newborn neurons with an extended period of GABA-induced depolarization during early postnatal neurogenesis. Interestingly, co-expression of shRNA-D1/DsRed and shRNA-K2/GFP led to a significant further increase of dendritic growth of new neurons than shRNA-K2 expression alone at 7 dpi (Figures 5A to 5C and S5A). Importantly, the increased dendritic growth from shRNA-K2 expression with or without shRNA-D1 was abolished by mTOR inhibition with rapamycin (Figures 5D and S5E), similar to what occurs during adult neurogenesis with DISC1 KD (Kim et al., 2009), suggesting a shared molecular mechanism. On the other hand, there were no apparent effects on soma size, number of primary dendrites, or positioning of newborn neurons under any conditions (Figure S5).
Taken together, these results demonstrate that extending the duration of GABA-induced depolarization specifically elicits DISC1-dependent regulation of dendritic development of new neurons through the mTOR pathway during early postnatal neurogenesis and provide further evidence on the requirement of concomitant depolarizing GABA signaling over an extended period for DISC1-dependent regulation of dendritic growth (Figures 5E to 5F and Table S1).
Maternal Deprivation Stress Synergizes with DISC1 in Regulating Early Postnatal Hippocampal Neurogenesis
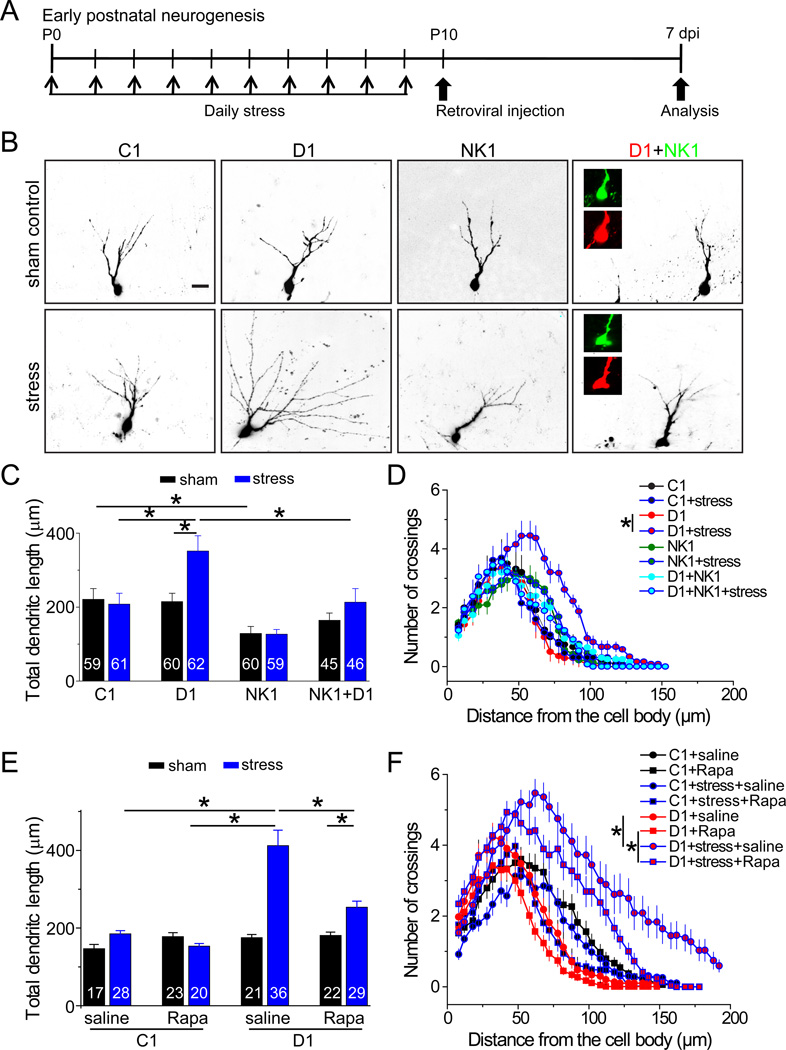
Recent studies have shown that stress affects neuronal maturation (Tamura et al., 2011), as well as KCC2 expression and function in neurons (Hewitt et al., 2009; Wake et al., 2007). Therefore, we explored whether behavioral manipulations also modulate the impact of DISC1 function during early postnatal neurogenesis using a well-established maternal deprivation stress paradigm (Figure 6A) (Meaney et al., 1996). Ca2+ imaging analysis showed a significant muscimol-induced Ca2+ rise of new neurons at 7 dpi after stress (Figure S6A), suggesting a delayed polarity shifting of GABA responses. Importantly, shRNA-D1/GFP+ neurons at 7 dpi exhibited accelerated dendritic growth after maternal deprivation stress, but not after the sham treatment (Figures 6B to 6D). Interestingly, after maternal deprivation stress, shRNA-D1/GFP+ neurons, but not shRNA-C1/GFP+ neurons, also exhibited soma hypertrophy, ectopic primary dendrites, and aberrant neuronal positioning at 7 dpi (Figures S6B to S6D), resembling the full array of neurodevelopment defects observed during adult neurogenesis. Given the lack of effects from either maternal deprivation stress or DISC1 KD alone, our result provides a striking example of a synergistic interaction between environmental contributions and genetic susceptibility in regulating neuronal development.
Figure 6. Maternal deprivation stress synergizes with DISC1 KD in regulating early postnatal hippocampal neurogenesis.
(A) A diagram of the experimental design.
(B) Sample confocal images of new neurons at 7 dpi after retrovirus-mediated expression of control shRNA (C1), shRNA-DISC1 (D1), shRNA-NKCC1 (NK1), or co-expression of D1 and NK1, in P10 dentate gyrus with or without maternal deprivation stress. Scale bar: 50 µm.
(C–F) Summaries of mean total dendritic length (C and E) and Sholl analysis (D and F) of new neurons at 7 dpi in the early postnatal dentate gyrus. Rapamycin (20 mg/kg body weight) was i.p. injected daily after viral injection for some animals. Values represent mean ± SEM (n = 4–8 animals; P < 0.01; ANOVA).
We next examined whether neurodevelopmental defects from a synergistic interaction between stress and DISC1 KD also requires depolarizing GABA signaling during early postnatal neurogenesis. Expression of shRNA-NK1 completely suppressed DISC1 KD-induced dendritic growth following maternal deprivation stress (Figures 6C to 6D). Interestingly, co-expression of shRNA-NK1 also largely suppressed soma hypertrophy and ectopic primary dendrite formation from shRNA-D1 expression at 7 dpi (Figures S6B to S6C), whereas aberrant neuronal positioning was not rescued (Figure S6D). Furthermore, treatment of rapamycin rescued DISC1 KD-induced defects of newborn neurons during early postnatal neurogenesis after maternal deprivation (Figures 6E to 6F, S6E to S6H and Table S1), suggesting a conserved mechanism underlying DISC1-dependent regulation of neuronal development between early postnatal neurogenesis after stress and during normal adult neurogenesis.
DISC1 and NKCC1 Interact Epistatically to Affect Risk for Schizophrenia
The molecular interactions of DISC1 and NKCC1-dependent GABA depolarization have potential implications for understanding the mechanism of DISC1-associated genetic risk for mental illnesses. We tested this possibility directly in a clinical genetic study of variations in DISC1 and in SLC12A2 (the gene encoding human NKCC1) and risk for schizophrenia. Three independent case control datasets of patients with schizophrenia and healthy controls, all of European ancestry, were studied (n = 2961 individuals). Common haplotype tagging SNPs in DISC1 and in SLC12A2 were identified and genotyped (Figures 7A to 7B); allele and genotype frequencies between cases and controls were compared independently and in a combined dataset (Table S2). A SNP (rs1000731) in DISC1 and a SNP (rs10089) in SLC12A2 showed significant epistatic effects in the two larger datasets (Scottish: P = 0.032; German: P = 0.0294; Figure 7C; Table S2). Importantly, these same SNPs interacted significantly on risk for schizophrenia in the combined, three-sample dataset (P = 0.0017). Individuals who were minor allele carriers at both rs10089 in SLC12A2 and rs1000731 in DISC1 were positively associated with risk for schizophrenia compared with all other genotypes (OR = 1.42, P = 0.001, LRT P = 0.0037; Table S2). None of these SNPs showed main association effects with schizophrenia on their own (Table S2). These results suggest that DISC1 and NKCC1 may interact to affect clinical risk for the development of schizophrenia.
Figure 7. DISC1 and NKCC1 interact epistatically to affect risk for schizophrenia.
(A) Haploview LD plot (R-Squared; based on the Scottish sample) of SNPs on the Illumina chip in DISC1 showing interactions with SNPs in NKCC1.
(B) Linkage disequilibrium (R-Squared measure) of SNPs in NKCC1 (HapMAP CEU data) showing two SNPs on the Illumina chip that were used for the interaction analysis with DISC1.
(C) Interaction between rs10089 (NKCC1) and rs1000731 (DISC1) and risk for schizophrenia in three independent case-control samples of European ancestry.
The DISC1 SNP maps close to the translocation breakpoint in the Scottish family (Millar et al., 2000) and may affect or be in linkage disequilibrium with SNPs that affect splicing of short DISC1 transcripts that are upregulated in postmortem brains from patients with schizophrenia (Nakata et al., 2009). The SNP in NKCC1 is in the 3’UTR where microRNA regulation of gene function occurs and it is predicted in silico to affect microRNA mechanisms (Friedman et al., 2009). In an effort to uncover other evidence that these SNPs proxy variants impact on the function of these two genes, we interrogated a public database for genetic regulation of transcript expression in human brains, which includes SNP genotype statistical associations with the expression of specific exons (Heinzen et al., 2008). Interestingly, rs1000731 predicts the expression of a probe of exon 3 in the DISC1 gene (P < 0.006), and rs10089 predicts the expression of an expressed sequence in the 5’ region of the NKCC1 gene (P < 0.02). Thus, each of these SNPs, which interact to affect risk for schizophrenia, is associated with variable expression of their respective transcripts in the human brain. These data add to the evidence that these SNPs are marking functional domains within the gene that impact gene function.
DISCUSSION
Neurogenesis in the developing and adult nervous system is dynamically regulated by both intrinsic factors and extrinsic niche cues. While a number of molecular pathways, in isolation, have been shown to impact specific processes of adult neurogenesis (Duan et al., 2008), we are just beginning to unravel how some of these signaling complexes may interact to orchestrate the dynamic interaction of neurogenesis-related events. Our study reveals a critical interaction between extrinsic GABA and intrinsic DISC1 signaling in regulating dendritic growth of newborn neurons and how the functional impact of this interaction is dictated by developmental tempo and experience. Interestingly, DISC1 and depolarizing GABA signaling also interact synergistically to affect risk for schizophrenia. There are a number of important implications from our results.
First, our study represents one of the first attempts to dissect the molecular interaction between extrinsic niche signaling and intrinsic regulators of neurogenesis in the mammalian brain. Since its initial discovery as a prominent susceptibility gene for schizophrenia and other major mental illnesses, DISC1 has been shown to regulate multiple neurodevelopmental processes in animal models (Chubb et al., 2008). During adult neurogenesis, DISC1 is thought to function as an intrinsic modulator to constrain stimulating effects of unknown extrinsic mechanisms in maintaining proper neurogenesis. Here we showed that DISC1 gates signaling from GABA-induced depolarization in regulating dendritic growth during adult neurogenesis. During early postnatal neurogenesis, extending the period of GABAergic depolarization through KCC2 KD leads to further enhancement of dendritic growth with concurrent DISC1 KD, whereas DISC1 KD alone has minimal effect. Therefore, DISC1 appears to be specifically recruited during periods of neuronal development driven by prolonged GABA-mediated depolarization. Multiple neurotransmitters, including GABA, have been shown to activate the AKT pathway through Ca2+ signaling (Yano et al., 1998). Using a series of genetic, pharmacological and immunohistological approaches, we provided evidence supporting the model that AKT serves as a point of convergence and DISC1 gates depolarizing GABA-induced AKT/mTOR signaling to regulate dendritic growth of newborn neurons in a context-dependent fashion (Figures 5E to 5F). Given DISC1 interacts with many partners, it is possible that DISC1 also gates other signaling pathways in regulating different aspects of neuronal development and function.
Second, our study demonstrates that a single molecular player has a dramatically different effect on development during early postnatal and adult neurogenesis. Previous studies have largely found conserved roles of extrinsic morphogens, growth factors and neurotransmitters, and intrinsic transcriptional factors and cytoplasmic signaling molecules (Ming and Song, 2011). Here we show that DISC1 keeps the tempo of neuronal maturation in check during adult neurogenesis to prevent runaway signaling from a positive feed-back loop that functions to promote dendritic growth of new neurons: increased depolarizing GABAergic signaling leads to increased dendritic growth and potentially more GABAergic inputs, which in turn drive more dendritic growth. This gating role of DISC1 is diminished during early postnatal neurogenesis when GABA-induced depolarization is transient (Figure 5F). These results support the notion that DISC1 serves as an important determinant, instead of direct mediator, of extrinsic stimulation in regulating neuronal development. Our findings provide novel molecular insights into the differential regulation of early postnatal and adult neurogenesis and indicate that adult neurogenesis is not simply a continuation of ongoing neuronal development into adulthood.
Third, our study supports the importance of tempo regulation as a key contextual element in neuronal development and gene-gene interactions. Previous studies have revealed a defined temporal sequence in generating different neuronal subtypes and glia cells during embryonic cortical neurogenesis (Okano and Temple, 2009). Neuronal maturation is also temporally controlled during both early development and adult neurogenesis. We show that this temporal control is essential for the proper neuronal development and dictates DISC1 function in vivo.
Fourth, our study links two important susceptibility factors for schizophrenia, DISC1 and GABA signaling, within a common pathway that regulates neuronal development. These results therefore support the emerging theme that many risk genes converge to regulate common neurotransmitter systems and signaling pathways (Balu and Coyle, 2011). Previous studies have implicated GABA and GABAARs in risk for schizophrenia and emphasized the inhibitory GABAergic action in the pathophysiology of schizophrenia (Lewis et al., 2005; Perry et al., 1979). Our results specifically point to a critical role for depolarizing GABA action, involving two Cl− transporters NKCC1 and KCC2, in DISC1-dependent regulation of neuronal development. The NKCC1 (SLC12A2) locus has been linked to schizophrenia in a meta-analysis (Lewis et al., 2003) and resides within the chromosome 5 region that has been implicated repeatedly in schizophrenia (Almasy et al., 2008). A genome-wide association study also identified NKCC1 as a potential susceptibility gene for schizophrenia (Potkin et al., 2009). Furthermore, NKCC1 expression in the dorsolateral prefrontal cortex of some schizophrenia patients was over seven times higher (Dean et al., 2007) and the ratio of NKCC1/KCC2 is increased in the hippocampus of patients with schizophrenia and related to genetic variation in GAD1 (Hyde et al., 2011). Our hypothesis-driven genetic association study of schizophrenia showed genetic epistasis between DISC1 and NKCC1, which, though relatively weak on a statistical level in individual samples, was replicated in two larger datasets with the same SNPs and combinations of genotypes. Importantly, the epistatic interaction remained significant in the combined analysis of three clinical case control datasets from three different countries. These typed SNPs tag common haplotypes that may contain functional variants and are proxies for untyped functional alleles. Indeed, these two specific SNPs appear to mark functional domains within the gene that impact gene function and show association with expression of specific exons within their respective genes in a brain mRNA expression dataset. In the context of the molecular data and given the consistency of the same genotypes showing predicted interactions across diverse clinical datasets, the convergent results support a role for interaction between DISC1 and NKCC1 in affecting risk for mental illness.
Fifth, our study may provide new insight into the relationship between developmental stress and schizophrenia. Both genetic predisposition and environmental factors, as well as interactions and correlations between them, are thought to contribute to the etiology of psychiatric disorders (Caspi and Moffitt, 2006; Tsuang et al., 2004). We present an example of how a genetic risk factor and an environmental stimulus, each relatively innocuous by itself, can have profound effects on neuronal development when combined. These results may provide one mechanistic explanation for how genetic risk alone can remain causally insufficient for disease manifestation, even in the Scottish family with the chromosomal translocation that disrupted DISC1 (Millar et al., 2000). Analogous gene-environment interactions on risk for schizophrenia have recently been reported in the context of obstetric complications (Nicodemus et al., 2008). Importantly, interaction between DISC1 and depolarizing GABA signaling operates both during normal adult neurogenesis and early postnatal neurogenesis after stress and converge on the downstream mTOR pathway. Our results suggest that adult hippocampal neurogenesis may be more sensitive to dysfunction of susceptibility genes for mental disorders and support the possibility that defects in adult neurogenesis may contribute to the adult onset manifestations of schizophrenia (Ming and Song, 2009).
In summary, we have identified the extrinsic neurotransmitter GABA-induced depolarizing signaling as a key target of the intrinsic factor DISC1 in regulating distinct aspects of neuronal development during adult neurogenesis and early postnatal neurogenesis after stress. A synergistic interaction between DISC1 and depolarizing GABA signaling also appears to affect risk for schizophrenia. Our results suggest a context-dependent role for a prominent schizophrenia susceptibility gene in neuronal development. Thus, elucidating specific contextual conditions that can trigger the deleterious effects of susceptibility genes in neuronal development may be critical for understanding the pathogenesis of mental disorders.
EXPERIMENTAL PROCEDURES
In Vivo Birth-dating, Genetic Manipulation of Neural Progenitors with Engineered Retroviruses
Engineered self-inactivating murine oncoretroviruses were used to co-express shRNA and a fluorescent marker in proliferating cells and their progeny as previously described (Ge et al., 2006). Specific shRNAs against mouse disc1 (shRNA-DISC1#1) (Duan et al., 2007; Faulkner et al., 2008; Kang et al., 2011; Kim et al., 2009) and mouse nkcc1 (shRNA-NKCC1) (Ge et al., 2006) have been characterized previously. Three shRNAs against mouse kcc2, two shRNAs against mouse γ2 subunit of GABAARs, one control shRNA were also examined.
Adult female C57BL/6 mice (P42) housed under standard conditions were anaesthetized and concentrated retroviruses were stereotaxically injected into the dentate gyrus as previously described (Duan et al., 2007). P10 pups (C57BL/6 or CD1) were anaesthetized and retroviruses were stereotaxically injected at 2 sites (from Bregma in mm): anterioposterior = − 1.8, lateral = ± 1.8, ventral = 1.5. All animal procedures were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee.
Pharmacological Manipulations and Maternal Deprivation Stress
For pharmacological manipulations, vigabatrin (Sigma, 25 µg/g body weight) (Wu et al., 2003), pentobarbital (25 µg/g body weight), rapamycin (LC Laboratories; 20 mg/kg body weight) (Kim et al., 2009) or corresponding vehicle control was intraperitoneally (i.p.) delivered once daily for a defined period of time as indicated. Maternal deprivation stress was carried out in a similar manner as previously described (Meaney et al., 1996). Different groups of pups were stereotaxically injected with retroviruses expressing shRNA-C1 or shRNA-D1 at P10 after the maternal separation procedures were finished. Tissue samples were processed and the same set of cellular phenotypes was analyzed in a double-blind manner.
Immunohistology, Confocal Imaging and Analysis
Coronal brain sections (40-µm thick) were prepared from injected mice and processed for immunostaining as described (Ge et al., 2006). Images were acquired on a META multiphoton confocal system (Zeiss LSM 510) using a multi-track configuration. Analysis of neuronal development and pAKT/pS6 were performed as previously described (Duan et al., 2007; Kim et al., 2009). All experiments were carried out in a blind fashion to experimental conditions. Statistic significance was determined by ANOVA or student t-test as indicated.
Ca2+ Imaging Analysis
Brain slices (275 µm) were acutely prepared from retroviral injected animals and focally loaded with 5 µl of 1 mM Fluo4-AM (Invitrogen). Ca2+ imaging was performed as previously described (Shim et al., 2009). Different groups of cells were treated with muscimol (10 µM), followed by ionomycin (10 µM). Cells were excited at 488 nm, and Fluo-4 signal was collected at 505–550 nm. Images were acquired every 5 sec and analyzed using NIH Image J software. The Ca2+ signal change was determined by ΔF/F [ΔF/F = [(F1-B1)-(F0-B0)]/(F0-B0)], which was normalized to the mean fluorescence intensity measured at the baseline condition (set as 0%) and after the ionomycin treatment (set as 100%).
Human Genetic and Gene Expression Analysis
Genetic interaction analysis of NKCC1 and DISC1 included three independent sample cohorts of cases with schizophrenia and healthy controls collected from Scotland, Germany and the United States (Table S2). The Scottish and German samples were genotyped using the Illumina 330K platforms. All SNPs in these two genes contained on this SNP platform were used in our genetic analyses. Pair-wise interaction analyses were carried out between SNPs in NKCC1 (rs10067555 and rs10089) and the SNPs in DISC1 (Table S2B) first in the Scottish sample, and the other two sample cohorts were used as replication samples to control for false positive findings. Logistic regression based on an additive model was used to assess the two-SNP interactions in each sample separately (Table S2C). The combined sample of all three cohorts together was also analyzed while controlling for sex and sample cohorts (Table S2A). To gain maximal power for interaction analysis, we combined genotypes at both SNPs into binary variables. Odds ratios (OR) were calculated for interaction at minor allele carriers of the two SNPs verse all other genotypes, and a likelihood ratio test was performed for assessing the significance of the interaction. We also interrogated genotype associations with brain transcript expression in a public database about genetic regulation of transcript expression in the human brain for rs1000731 in DISC1 and rs10089 in SLC12A2 (Heinzen et al., 2008).
Supplementary Material
ACKNOWLEDGEMENT
We thank members of Ming and Song Laboratories for help and critical comments, L. Liu, Y. Cai, N. Powanpangkul, and Q. Hussaini for technical support, A. Chiang, J. Wang for help with tissue processing. This work was supported by NIH (NS048271, HD069184), MSCRF and NARSAD to G-l.M., by NIH (NS047344, MH087874), and IMHRO to H.S., by the NIMH intramural program to D.W., and by postdoctoral fellowships from MSCRF to J.Y.K., Z.W., and K.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Almasy L, Gur RC, Haack K, Cole SA, Calkins ME, Peralta JM, Hare E, Prasad K, Pogue-Geile MF, Nimgaonkar V, Gur RE. A genome screen for quantitative trait loci influencing schizophrenia and neurocognitive phenotypes. Am J Psychiatry. 2008;165:1185–1192. doi: 10.1176/appi.ajp.2008.07121869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB., Jr. Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol. 1965 Suppl 2:1–70. [PubMed] [Google Scholar]
- Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev. 2011;35:848–870. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Charych EI, Liu F, Moss SJ, Brandon NJ. GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Dean B, Keriakous D, Scarr E, Thomas EA. Gene expression profiling in Brodmann's area 46 from subjects with schizophrenia. Aust N Z J Psychiatry. 2007;41:308–320. doi: 10.1080/00048670701213245. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Asai N, Namba T, Wang Y, Kato T, Tanaka M, Tatsumi H, Taya S, Tsuboi D, Kuroda K, et al. Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron. 2009;63:774–787. doi: 10.1016/j.neuron.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci U S A. 2009;106:7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Heinzen EL, Ge D, Cronin KD, Maia JM, Shianna KV, Gabriel WN, Welsh-Bohmer KA, Hulette CM, Denny TN, Goldstein DB. Tissue-specific genetic control of splicing: implications for the study of complex traits. PLoS Biol. 2008;6:e1. doi: 10.1371/journal.pbio.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, et al. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr., Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Burdick KE, Kim JY, Duan X, Guo JU, Sailor KA, Jung DE, Ganesan S, Choi S, Pradhan D, et al. Interaction between FEZ1 and DISC1 in regulation of neuronal development and risk for schizophrenia. Neuron. 2011;72:559–571. doi: 10.1016/j.neuron.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. DISC1 partners with GSK3beta in neurogenesis. Cell. 2009;136:990–992. doi: 10.1016/j.cell.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, Vakkalanka R, Barenboim M, Sei Y, Weinberger DR, Kleinman JE. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci U S A. 2009;106:15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, Weinberger DR. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- Okano H, Temple S. Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol. 2009;19:112–119. doi: 10.1016/j.conb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Perry TL, Kish SJ, Buchanan J, Hansen S. Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet. 1979;1:237–239. doi: 10.1016/s0140-6736(79)90767-0. [DOI] [PubMed] [Google Scholar]
- Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res Rev. 2010;63:60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Guffanti G, Lakatos A, Fallon JH, Nguyen DD, Mathalon D, Ford J, Lauriello J, Macciardi F. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull. 2009;35:96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Shim S, Yuan JP, Kim JY, Zeng W, Huang G, Milshteyn A, Kern D, Muallem S, Ming GL, Worley PF. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron. 2009;64:471–483. doi: 10.1016/j.neuron.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Tamura M, Sajo M, Kakita A, Matsuki N, Koyama R. Prenatal Stress Inhibits Neuronal Maturation through Downregulation of Mineralocorticoid Receptors. J Neurosci. 2011;31:11505–11514. doi: 10.1523/JNEUROSCI.3447-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.