Abstract
OBJECTIVE
Evaluations of stents by MDCT from studies performed at single centers have yielded variable results with a high proportion of unassessable stents. The purpose of this study was to evaluate the accuracy of 64-MDCT angiography (MDCTA) in identifying in-stent restenosis in a multicenter trial.
MATERIALS AND METHODS
The Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography Using 64 Detectors (CORE-64) Multicenter Trial and Registry evaluated the accuracy of 64-MDCTA in assessing 405 patients referred for coronary angiography. A total of 75 stents in 52 patients were assessed: 48 of 75 stents (64%) in 36 of 52 patients (69%) could be evaluated. The prevalence of in-stent restenosis by quantitative coronary angiography (QCA) in this subgroup was 23% (17/75). Eighty percent of the stents were ≤ 3.0 mm in diameter.
RESULTS
The overall sensitivity, specificity, positive predictive value, and negative predictive value to detect 50% in-stent stenosis visually using MDCT compared with QCA was 33.3%, 91.7%, 57.1%, and 80.5%, respectively, with an overall accuracy of 77.1% for the 48 assessable stents. The ability to evaluate stents on MDCTA varied by stent type: Thick-strut stents such as Bx Velocity were assessable in 50% of the cases; Cypher, 62.5% of the cases; and thinner-strut stents such as Taxus, 75% of the cases. We performed quantitative assessment of in-stent contrast attenuation in Hounsfield units and correlated that value with the quantitative percentage of stenosis by QCA. The correlation coefficient between the average attenuation decrease and ≥ 50% stenosis by QCA was 0.25 (p = 0.073). Quantitative assessment failed to improve the accuracy of MDCT over qualitative assessment.
CONCLUSION
The results of our study showed that 64-MDCT has poor ability to detect in-stent restenosis in small-diameter stents. Evaluability and negative predictive value were better in large-diameter stents. Thus, 64-MDCT may be appropriate for stent assessment in only selected patients.
Keywords: angiography, cardiac imaging, CORE-64, MDCT, restenosis, stents
On average, 900,000 angioplasty and stenting procedures are performed yearly in the United States and 350,000 in Europe [1]. Although vessel patency has improved tremendously with stents in comparison with balloon angioplasty [2, 3], the rate of in-stent restenosis due to smooth-muscle cell proliferation remains 10–30% for bare metal stents, especially in higher-risk populations such as diabetic patients [4]. The rates of in-stent restenosis have decreased with the use of drug-eluting stents (sirolimus- and paclitaxel-coated stents); however, recent reports of increased stent thrombosis risk as a late complication [5, 6] are changing the practice of the interventional cardiologist toward the more frequent use of bare metal stents.
In-stent restenosis is typically heralded by angina symptoms, but in up to 19% of patients, it may present as myocardial infarction [7–10]. Thus, screening for in-stent restenosis with a noninvasive technique such as MDCT angiography (MDCTA) may be clinically useful. MDCTA has a good negative predictive value (NPV) for ruling out coronary artery stenosis in studies with low disease prevalence [11–13]; however, the assessment of stents has been limited by blooming artifacts, which are more significant in stents < 3.0 mm in diameter [14–16]. In this first multicenter international single-blinded study and registry using 64-MDCT, we performed a post-hoc analysis to assess the accuracy of MDCTA in detecting in-stent restenosis.
Materials and Methods
The institutional review boards at all nine participating hospitals and the coordinating center approved this HIPAA-compliant study, and written informed consent was obtained from all participants. The trial is also registered at Clinical-Trials.gov as NCT 00738218.
Patient Selection
Between December 2005 and January 2007, the Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography Using 64 Detectors (CORE-64) Multicenter Trial and Registry enrolled 405 patients with symptoms compatible with coronary artery disease who were scheduled to undergo coronary angiography, met the inclusion criteria, and gave written informed consent. Of those patients, 52 (12.8%) had previously placed stents, making the rate of previous percutaneous coronary intervention (PCI) in this population 8.6%. The detailed study protocol and methodology have been described in previous publications [17, 18]. Strict inclusion and exclusion criteria were observed, and details about prior coronary interventions were obtained [18].
Patient Characteristics
Baseline information and history were collected including demographic information (age, race, sex, height, and weight), all past and current illnesses, allergies, past surgical and medical procedures, and concomitant medications as well as allergies and adverse drug reactions, cardiovascular risk factors and habits, and smoking and alcohol use history. All patients had a brief physical examination including vital signs and body mass index (BMI) measurement before MDCT and ECG.
In addition, patient history was reviewed for type (bare metal vs drug eluting), manufacturer, and number of stents placed; vessel or vessels that were stented (left main, left anterior descending, left circumflex, or right coronary) and vessel segment or segments, according to the modified 19-segment American Heart Association classification system [19]; and time elapsed since the prior stenting procedures.
MDCT Protocol and Image Acquisition
Beta-blockers were administered if a patient’s resting heart rate was ≥ 70 beats per minute (bpm). A patient whose heart rate could not be reduced to less than 80 bpm was excluded from the study (two of 405 patients) [18]. Nitroglycerine, 0.4 mg, was given before contrast injection in 90% of the patients. MDCTA was performed on an MDCT system (Aquilion 64, Toshiba America Medical Systems).
Unenhanced scanning was performed to establish the origin of the coronary arteries at the aortic root including the base of the heart and was used to calculate the Agatston calcium score. Unenhanced images were acquired with prospective ECG gating, a slice thickness of 3 mm with no overlap, temporal resolution of 200 milliseconds, field of view (FOV) of 320 mm, and pixel size of 0.39 mm2 with spatial resolution of 0.4 mm. The peak tube voltage was 120 kVp and the tube current was 300 mA, with an effective dose of 2.0 mSv. Between 35 and 40 images were acquired in the z-direction to ensure coverage of the entire heart, 12.5 cm, to set the scanning area for CT angiography (CTA). Axial images were reviewed to determine whether a stent was present before calculating the Agatston calcium score.
The MDCTA parameters were based on each subject’s BMI. Images were obtained with continuous helical acquisition; a gantry rotation of 400 milliseconds (temporal resolution = 200 milliseconds); retrospective ECG gating; a slice thickness of 0.5 mm with a weight-adjusted overlap pitch factor of 0.225 autoselected to heart rate; 120 kVp; and 270–400 mA depending on the patient’s weight, which varied from 59 to 125 kg. The FOV was 320 mm with a pixel size of 0.39 mm2. Multisegmental reconstruction was used to achieve an average temporal resolution of 106 milliseconds to minimize artifacts due to cardiac motion. The effective dose was 12.3–16.9 mSv. Because of institutional review board and safety monitoring board restrictions, the maximum total radiation dose allowed was < 20 mSv.
IV contrast material (iopamidol, 370 mg I/mL) was administered through an automated power injector at 4 mL/s for a total of 80–100 mL depending on body mass (59–125 kg) and was injected at 3.5–5 mL/s followed by a 40-mL saline chase bolus at 4 mL/s. Images were triggered to 180 HU in the descending aorta and were acquired in 12–14 seconds. The images were analyzed on a workstation (Vitrea 2 workstation, Vital Images; or Advantage workstation, GE Healthcare). The scanning parameters were recorded for each subject.
MDCTA Data Analysis
A copy of the blinded raw data coded without patient identifiers for each subject was forwarded to the MDCTA Core Laboratory for analysis. Data sets were evaluated on original axial, multiplanar, and curved multiplanar reformations. To assess for significant restenosis (≥ 50%), we evaluated the stent and the artery segments within 5 mm of the stent distally and proximally. The stents were visually judged as assessable or not by two experienced readers based on the ability to assess contrast opacification within the stent. Image quality was graded as 1, good, without motion or other artifacts; 2, adequate, assessable with limitations (i.e., presence of minor motion or other artifacts); 3, poor, assessable with substantial limitations (i.e., presence of major motion and other artifacts); or 4, unassessable [12, 20, 21]. In assessable stents, the stent lumen was visually assessed as no disease, < 50% stenosis, ≥ 50% stenosis, or total occlusion (Appendix 1). Discrepancies between the readers’ qualitative assessments were resolved through consensus by a third reader. All three readers had at least level 3 training in MDCTA interpretation. A dedicated stent convolution kernel (FC05) was used to reduce metal-associated artifacts [15].
APPENDIX 1.
Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) to Detect Nondiseased Stents, < 50% stenosis, ≥ 50% In-Stent Restenosis, and Totally Occluded Stents by Qualitative MDCT Angiography
| Performance Measure | No Diseasea | < 50% Stenosisb | ≥ 50% Stenosisc | 100% Stenosisd |
|---|---|---|---|---|
| Prevalence (%) | 4 | 71 | 21 | 4 |
| Sensitivity (%) | 0 (0–84) | 88 (73–97) | 20 (3–56) | 50 (1–99) |
| Specificity (%) | 98 (89–100) | 29 (8–58) | 90 (75–97) | 100 (92–100) |
| PPV (%) | 0 (0–98) | 75 (59–87) | 33 (4–78) | 100 (3–100) |
| NPV (%) | 96 (86–100) | 50 (16–84) | 81 (66–91) | 98 (89–100) |
Note—Data in parentheses are 95% CIs.
Quantitative coronary angiography values of 0–9% diameter stenosis.
Quantitative coronary angiography values of 10–49% diameter stenosis.
Quantitative coronary angiography values of 50–99% diameter stenosis.
Quantitative conventional coronary angiography value of 100% diameter stenosis.
Quantitative assessments of attenuation in the stent and in the segment were performed to improve the accuracy of MDCT to detect in-stent restenosis by two independent experienced readers different from the readers who performed the qualitative assessments. For this purpose, attenuations within the lumen center were recorded at 2-mm intervals throughout the stent as well as within 10 mm proximal and distal to the stent. A reduction in density values of 50% or 100 HU lower than the immediate proximal segment was considered significant for in-stent restenosis [22]. Attenuation values obtained by the two readers were averaged for the final analysis.
Coronary Angiography Procedure and Quantitative Analysis
Coronary angiography was performed within 30 days of MDCT via a 5- or 6-French sheath and using 5- or 6-French catheters. Intracoronary nitroglycerin was administered (150–200 mcg) before the first images of the left coronary artery system and right coronary artery were obtained to standardize the vasomotor state of the coronary artery and eliminate any potential for catheter-induced spasm. Coronary angiographic images were saved in the universal DICOM format and forwarded to the Angiographic Core Laboratory for analysis of quantitative coronary angiography (QCA). QCA was performed using standard, validated analysis software (CAAS II QCA software, research version 2.0.1, PIE Medical Imaging). Reference vessel diameter (RVD) and minimal luminal diameter (MLD) were computed automatically in the vessel proximal to the stent, in the stent, and in the vessel distal to the stent. The diameter stenosis (DS) was calculated as a percentage using the following formula:
where RVD was averaged between the proximal and distal segments unless a major branch was present. A stenosis with a diameter of 50% or more was considered significant. No disease was defined as less than 10% diameter stenosis by QCA.
Statistical Analysis
Statistical analysis was performed at Johns Hopkins Bloomberg School of Public Health using statistics software (SAS, version 9.0, SAS Institute). The primary hypothesis of this study was that MDCTA is able to detect in-stent restenosis of ≥ 50% within stents of ≥ 2.5 mm diameter with at least 90% sensitivity and at least 90% specificity (both sensitivity and specificity expressed as a 95% CI with at most ± 5% precision).
Continuous variables are reported as means ± 1 SD. Categoric variables are presented as percentages and counts. All continuous variables that were normally distributed, including differences between MLD, RVD, and DS between stented proximal and distal segments, were evaluated using the unpaired Student’s t test. All tests were two-tailed, and a p value of < 0.05 was considered significant. The diagnostic accuracy (sensitivity, specificity, NPV, and positive predictive value [PPV]) of MDCTA for the detection of in-stent restenosis of ≥ 50% was compared with coronary angiography. Correlations and regression analysis were performed for attenuation analysis.
Results
Patient Population and Characteristics
Fifty-two patients from the CORE-64 Trial and Registry underwent cardiac catheterization to evaluate the patency of previously implanted stents. This group represents 12.8% of the 405 patients enrolled in the trial. The baseline clinical characteristics of these 52 patients are summarized in Table 1 and are similar to the overall characteristics of patients in the CORE-64 study [18]. The average number of stents per patient was 1.44 ± 0.1 (SD), with 37 of 52 patients having one stent. The mean interval between stent implantation (i.e., the last PCI) and MDCT was 3.88 ± 0.47 (SD) years. Thirty-two of the 52 patients had undergone one PCI, and 20 patients had undergone at least two PCI procedures.
TABLE 1.
Patient Baseline Characteristics
| Characteristic | Value |
|---|---|
| Age (y) | |
| Median (IQR) | 61 (56–67) |
| Male sex | |
| % (no. of patients) | 89 (46) |
| BMI | |
| Median (IQR) | 28 (25–32) |
| % (no.) of patients with | |
| Diabetes | 37 (19) |
| Hypertension | 73 (38) |
| Hypercholesterolemia | 87 (45) |
| Smoking history | |
| Current | 17 (9) |
| Former | 56 (29) |
| Never | 27 (14) |
| Family history of coronary artery disease | 48 (25) |
| Prior myocardial infarction | 69 (36) |
| Stroke history | 4 (2) |
| Peripheral vascular disease | 10 (5) |
| Positive stress test | 10 (5) |
| Heart rate (bpm) | |
| Median (IQR) | 59 (52–64) |
| Agatston calcium score | |
| Median (IQR) | 431 (141–853) |
Note—IQR = interquartile range, BMI = body mass index, bpm = beats per minute.
Characteristics of Previously Implanted Stents
We evaluated 75 stents in 52 patients. The locations of the stents evaluated are summarized in Table 2. Eighty percent of the stents evaluated were ≤ 3.0 mm in diameter. For only 26 of these 75 stents was the manufacturer known. In the cases in which the stent manufacturer and stent size were unknown, the length and diameter of the stent were measured by MDCT. Sixteen were drug-eluting stents: eight Cypher (Cordis) and eight Taxus (Boston Scientific) stents. The remaining 10 were bare metal non-drug-eluting stents: Multi-Link (Abbott Vascular), Driver (Medtronic), S660 or S670 (Medtronic), or Bx Velocity (Cordis).
TABLE 2.
Stent Distribution by Native Vessel
| Vessel | No. of Stents (n = 75) | No. of Assessable Stents
|
||
|---|---|---|---|---|
| Reader 1 (n = 47) | Reader 2 (n = 41) | Consensus (n = 48) | ||
| LADa | ||||
| Proximal | 25 | 18 | 17 | 15 |
| Distal | 4 | 2 | 1 | 4 |
| LCXb | ||||
| Proximal | 9 | 7 | 7 | 6 |
| Distal | 3 | 0 | 0 | 1 |
| RCAc | ||||
| Proximal | 15 | 12 | 9 | 8 |
| Distal | 10 | 3 | 3 | 6 |
| LM | 1 | 0 | 0 | 1 |
| Ramus | 2 | 0 | 0 | 2 |
| First obtuse marginal | 6 | 5 | 4 | 5 |
Note—LAD = left anterior descending coronary artery, LCX = left circumflex coronary artery, RCA = right coronary artery, LM = left main coronary artery.
Difference between stent evaluability in proximal versus distal vessels: p = 0.118 (chi-square test).
Difference between stent evaluability in proximal versus distal vessels: p = 0.310 (chi-square test).
Difference between stent evaluability in proximal versus distal vessels: p = 0.742 (chi-square test).
The ability of the readers to evaluate stent patency appeared to be lower in the thicker-strut stainless-steel stents, such as the Bx Velocity and Cypher stents (50% and 62.5% evaluability, respectively), and to be better in stents with thinner struts, such as the Driver (100%) and Taxus (75%) stents; however, given the low number of stents per stent type and the many stents of unknown type, the effect of strut thickness on stent evaluability was difficult to determine (Appendix 2). We did not observe a statistically significant difference in stent assessment between stents placed in proximal versus distal vessels (Table 2).
APPENDIX 2.
Number and Characteristics of Stents Assessed on MDCT Angiography (MDCTA)
| Stent (Name of Manufacturer) | No. of Stents (n = 75) | Diameter (mean ± SD) | Length (mean ± SD) | % Assessable on MDCTA |
|---|---|---|---|---|
| Cypher (Cordis) | 8 | 2.93 ± 0.07 | 24.70 ± 2.79 | 62.5 |
| Taxus (Boston Scientific) | 8 | 2.88 ± 0.16 | 14.57 ± 2.42 | 75.0 |
| Bx Velocity (Cordis) | 4 | 3.00 ± 0.00 | 23.00 ± 0.00 | 50.0 |
| Multi-Link Penta (Abbott Vascular) | 1 | 3.00 ± 0.00 | 8.00 ± 0.00 | 100.0 |
| Driver (Medtronic) | 1 | 3.00 ± 0.00 | 15.00 ± 0.00 | 100.0 |
| S660 or S670 (Medtronic) | 4 | 2.88 ± 0.13 | 17.25 ± 2.56 | 50.0 |
| Other stents (unknown) | 49 | 3.91 ± 0.09 | 15.73 ± 0.82 | 63.3 |
MDCTA Results
Qualitative assessment
The prevalence of in-stent restenosis was 23% using qualitative coronary angiography as the gold standard. Forty-eight of 75 stents (64%) could be adequately evaluated by qualitative visual assessment on MDCTA. The inability of MDCTA to detect or evaluate stents was due to severe calcification (one stent), motion artifacts (seven stents), beam hardening (two stents), and poor contrast-to-noise ratio (one stent); 16 could not be visualized by MDCT because of severe calcification, beam hardening, or motion. The overall sensitivity, specificity, PPV, and NPV to detect ≥ 50% in-stent restenosis by qualitative MDCTA were 33.3%, 91.7%, 57.1%, and 80.5%, respectively, with an overall accuracy of 77.1% for the 48 assessable stents. The overall sensitivity, specificity, PPV, and NPV to detect nondiseased stents, < 50% stenosis, ≥ 50% in-stent restenosis, and totally occluded stents by qualitative MDCT are listed in Appendix 1.
The ability of MDCT to detect in-stent restenosis varied between stents of different diameters but did not reach statistical significance because of the low number of stents per group. In the group of patients with stents with a diameter of less than 2.5 mm, 70% were assessable. In the group with stents with a diameter of 2.5–3.0 mm, 58% were considered assessable. In the group with stents that were greater than 3.0 mm in diameter, 67% were considered assessable by the two readers, respectively. Sensitivity to identify in-stent restenosis was 20% for stents less than 2.5 mm in diameter, 25% for stents between 2.5 and 3.0 mm, and 67% for stents > 3.0 mm (Table 3).
TABLE 3.
Accuracy of MDCT in Assessing In-Stent Restenosis by Stent Diameter
| Reader | Stent Size
|
||
|---|---|---|---|
| < 2.5 mm (n = 20) | 2.5–3.0 mm (n = 40) | > 3.0 mm (n = 15) | |
| Reader 1 | |||
| % assessable segmentsa | 45 | 58 | 62 |
| Sensitivity (%) | 50 (12–88) | 50 (12–88) | 100 |
| Specificity (%) | 80 (31–97) | 74 (52–88) | 57 (20–87) |
| Reader 2 | |||
| % assessable segmentsa | 45 | 53 | 46 |
| Sensitivity (%) | 25 (3–76) | 25 (3–76) | 100 |
| Specificity (%) | 80 (31–97) | 88 (65–97) | 40 (10–80) |
Note—Data in parentheses are 95% CI.
A chi-square test with 2 degrees of freedom was performed to evaluate the difference between assessable and nonassessable segments across stent diameter categories. A statistical difference in evaluability was not observed in either reader 1 (p = 0.590) or reader 2 (p = 0.838).
Quantitative association of attenuation with in-stent restenosis
Given the relatively low sensitivity and specificity of visual qualitative MDCT assessment of in-stent restenosis, a detailed attenuation analysis of in-stent and in-segment regions was performed every 2 mm (Fig. 1). Correlation of the minimum attenuation value in the stent lumen center with QCA was weak, with a correlation coefficient of 0.24 (regression line: Y = minimum HU × (−0.06) + 52.47). Based on our regression analysis, we found that a minimum attenuation value of 38.78 HU is moderately associated with a QCA diameter stenosis value of 50% or more (p = 0.091).
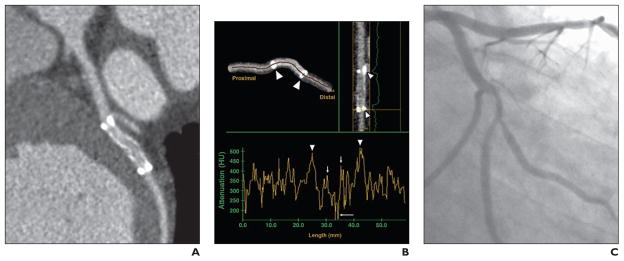
Fig. 1. 42-year-old symptomatic man with known coronary artery disease who had bare metal 3.0 × 20.0 mm stent (Lekton, Biotronik, GmbH) placed 8 months before MDCT angiography and quantitative coronary angiography (QCA). Image quality is good.
A, MDCT angiography image used for qualitative assessment shows 50% restenosis,
B, Quantitative assessment of centerline plot at 2-mm intervals throughout segment and stent shows metal markers at stent edges (arrowheads) with ballooning artifacts preventing evaluation of inflow and outflow. Attenuation in stent shows drop from 350 HU (short arrows) to 225 HU (long arrow), indicating in-stent restenosis of 50%.
C, Stenosis was assessed on QCA image to be 61%.
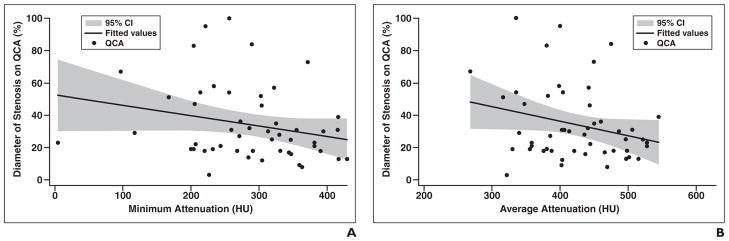
Similarly, the average attenuation value in the stent gave a correlation coefficient of 0.255 with QCA; in regression analysis, an attenuation value of 247.17 HU was weakly associated with a QCA diameter stenosis value of 50% (p = 0.073) (regression line: Y = average HU × (−0.089731) + 72.1787) (Fig. 2). This detailed quantitative analysis did not improve our ability to detect ≥ 50% in-stent restenosis using MDCTA.
Fig. 2. Correlation of attenuation within stents with invasive quantitative coronary angiography (QCA).
A, Correlation of QCA and minimum CT attenuation value in Hounsfield units within stents (p = 0.091). Correlation coefficient is −0.2419. Regression line: Y = minimum attenuation in Hounsfield units × (−0.0637445) + 52.47177.
B, Correlation of QCA and average CT attenuation value in Hounsfield units within stents (p = 0.073). Correlation coefficient is −0.2555. Regression line: Y = average attenuation in Hounsfield units × (−0.089731) + 72.1787.
Overall Image Quality and Ability to Assess Stents
To elucidate the reason for the lower-than-expected accuracy of MDCT for stent assessment, we investigated the relationship between overall image quality of MDCT in all vessels versus the ability to assess stented segments (Table 4). In patients with images characterized as showing good overall image quality, 13 of 24 stents (54%) were correctly diagnosed by visual qualitative consensus. In patients with images considered of adequate image quality, 23 of 44 stents (52%) were correctly evaluated (Fig. 3). Seven patients had images with poor image quality, and only one of seven stents (14%) could be diagnosed correctly with respect to patency in those patients. The differences did not reach statistical significance (p = 0.568). The overall image quality was good in 32% of patients and adequate in 62% of patients. Image quality and the ability to assess stents using MDCT was good in only 6% of patients and was adequate in 33%. The remaining stented segments (61%) either had poor image quality (42%) or were not assessable (19%).
TABLE 4.
Overall Image Quality and Stent Evaluation Accuracy Using MDCT Angiography (MDCTA)
| Image Qualitya | Correct Diagnosis | Misdiagnosis | Nonassessable | Not Seen | Total No. of Stents |
|---|---|---|---|---|---|
| Good | 13 | 3 | 3 | 5 | 24 |
| Adequate | 23 | 7 | 6 | 8 | 44 |
| Poor | 1 | 1 | 2 | 3 | 7 |
|
| |||||
| Total | 37 | 11 | 11 | 16 | 75 |
A chi-square test with 6 degrees of freedom was performed to evaluate the difference in MDCTA evaluability across overall image quality strata. A statistical difference in evaluability across image quality strata was not observed (p = 0.568).
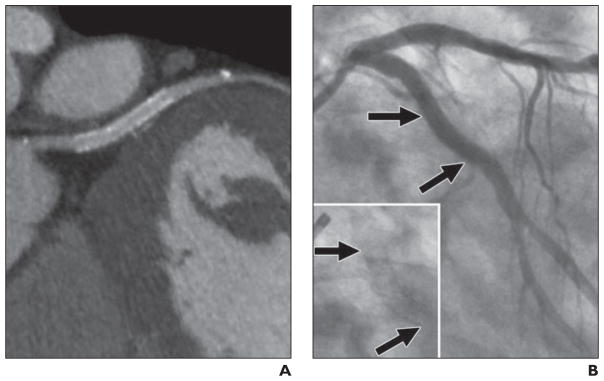
Fig. 3. 62-year-old symptomatic man with coronary artery disease who had bare metal 3 × 15 mm stent placed in left circumflex coronary artery 6 months 23 days before studies.
A, MDCT angiography image quality was good. Qualitative and quantitative assessments show restenosis of 50% and 27%, respectively.
B, Quantitative coronary angiography assessment shows 73% in-stent restenosis (arrows).
Discussion
This study is the first multicenter single-blinded study of patients with suspected or known coronary artery disease, to our knowledge, and includes 52 of 405 patients with prior stent implantation and is considered an “intention-to-diagnose” study. Our study shows that despite advancements in MDCT technology, MDCT remains limited in stent assessment in “real world” patients, many of whom have stents that are less than 3 mm in diameter [16]. We report the overall sensitivity, specificity, PPV, and NPV to detect 50% in-stent restenosis by visual MDCT as 33.3%, 91.7%, 57.1%, and 80.5%, respectively, with an overall accuracy of 77.1% for 48 assessable stents. This accuracy is considerably lower than the accuracies in other recently reported studies [26–32] because of blooming artifacts resulting from stents with thick struts, high metal content, small diameter in 80% of vessels, motion artifacts, calcification, and perhaps a lower tube current setting.
As noted by others [26–32], we found that MDCT evaluation of the stents in our study was limited because of beam-hardening and stent-strut artifacts—that is, the blooming effect. It is particularly problematic with early stent designs where the strut thickness is greater and metals such as tantalum and gold were used. Indeed, a high proportion of the stents in our study had a thick-strut design and a high metal content. Newer stents have much thinner struts and are made predominantly of stainless steel, cobalt chromium, and nitinol. In addition, recent advances in MDCT technology, such as improvement in the z-resolution, faster tube rotation, and the development of special dedicated kernels for stent evaluation [23], have the potential to improve the ability of MDCT to accurately detect in-stent restenosis. In phantom studies with 3-mm stents, Beohar et al. [24] showed that both 16- and 64-MDCT scanners underestimate lumen size by as much as 1.2 mm in newer-generation stents of different strut thicknesses ranging from 80 to 180 μm.
Data from 16-MDCT have been promising, showing that more than 80% of stents with diameters greater than 3.0 mm could be evaluated for in-stent restenosis [14]. In other smaller studies, investigators have reported a sensitivity of 75–83% and specificity of 98–100% [25, 26]. The evaluation of stents in the left main coronary artery, which are usually 3.5 mm or larger, with 16- and 64-MDCT also gave an accuracy of 98% with the exception of complex bifurcation stenting (with more than three layers of stents), where accuracy was only 83% because of stent-strut artifact [27]. A key finding from prior studies [28] is that MDCT’s accuracy to detect in-stent restenosis is favorable in large stents (≥ 3.5 mm) and is less favorable in smaller stents.
Our results confirm the poor performance of MDCT in the evaluation of small stents, which represented most of our cases. Because only 15 of 75 stents (20%) in the study group were 3.0 mm or greater, we cannot make a definitive assessment regarding the accuracy of MDCT in the evaluation of larger stents in our study. Our study took advantage of the technologic advances in 64-MDCT and its improved reliability, and similar to Mühlenbruch et al. [23], we developed a dedicated convolution kernel for stent assessment. However, despite improvements in technology, we found that in the real world situation—that is, when most of the stents are < 3.0 mm [16] with concomitant calcifications and motion artifacts—MDCT assessment of in-stent restenosis remains problematic and the evaluability and accuracy is still too low to make this technique applicable to most patients with stents [26–30] (Fig. 4).
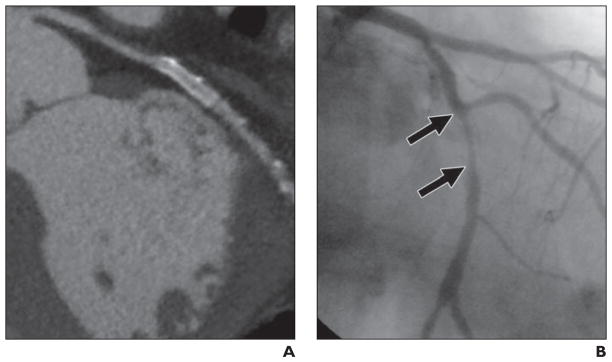
Fig. 4. 73-year-old symptomatic man with coronary artery disease who had drug-eluting 2.5 × 24 mm stent (Taxus, Boston Scientific) placed in left circumflex coronary artery 6 months and 10 days before studies. Image quality was good.
A, Qualitative and quantitative assessments by MDCT angiography did not show in-stent restenosis.
B, Quantitative coronary angiography shows no in-stent restenosis (arrows). Inset shows area of interest magnified.
The peak kilovoltage (kVp) that we used was similar to those used in other studies; however, the tube current varied from 270 to 400 mA depending on patient weight, which ranged from 59 to 125 kg. This tube current is significantly lower than those of others who used tube currents of 600–800 mA [26, 29–31]. Our results might have been better in terms of a greater number of assessable stents if we had used a higher tube current (mA). Better image quality and stent assessment must be balanced against the risks of increased radiation doses when multiple examinations are expected during a patient’s lifetime.
Our findings are in agreement with those of Hecht et al. [32] in that a quantitative approach, such as using attenuation measurements, does not improve the accuracy of MDCT over subjective qualitative visual assessment by an experienced reader. However, our results differ from that study with regard to the accuracy of qualitative assessment of in-stent restenosis, possibly because of different stent types and sizes encountered and different study designs. Our results differ considerably from those of Cademartiri et al. [26]; most of the patients in their study group had Xience V stents (Abbott), which have thinner struts (81 μm) than Cypher stents (140 μm), the stent predominantly used in the United States and many other countries. In addition, the interval from stent implantation to MDCT in that study [26] was 6 months; thus, that patient group had a lower prevalence of in-stent restenosis than our cohort, with a mean time interval from PCI of 3.88 years. The accuracy of MDCT in our study is also lower than that reported recently by Pugliese et al. [29] using a 64-MDCT dual-source scanner. The predominant stent type in their study was Taxus (130-μm struts), and most stents were > 3.0 mm. Small stents in their study were also often unassessable [29]. In addition, only a minority of patients had a high BMI, which can also influence the evaluability of stents. Stent evaluability in our study is also lower than that reported by Schepis et al. [33].
Further improvements in spatial resolution using novel detector designs as well as better reconstruction algorithms may increase the utility of CT for the assessment of stents in the near future. Until then, patients must be carefully selected before undergoing MDCT evaluation for in-stent restenosis. Patients with a low BMI and the ability to breath-hold and achieve low heart rates as well as patients with large (> 3.0 mm) thin-strut, recently implanted stents and low calcium scores should be considered for noninvasive assessment of in-stent restenosis rather than being sent directly to cardiac catheterization.
In conclusion, in this multicenter intention-to-diagnose trial, 64-MDCTA showed poor ability to detect in-stent restenosis. This finding may be at least partly related to the small stent sizes evaluated, which reflects the real world patient population. We believe that the use of this technology cannot be recommended for routine stent assessment at present but rather should be reserved for selected cases.
Acknowledgments
This study was supported by grants from Toshiba Medical Systems; the Davis Duke Charitable Foundation; the National Heart, Lung, and Blood Institute (grants RO1-HL66075-01 and HO1-HC95162-01); the National Institute on Aging (RO1-AG021570-01); and the Donald W. Reynolds Foundation.
A. Arbab-Zadeh received grant support from CT Core Laboratory. J. M. Miller, N. Paul, and J. Lima received grant support from Toshiba Medical Systems. N. Paul, J. Hoe, and J. Lima received speakers’ fees from Toshiba Medical Systems and N. Paul, advisory fees from Vital Images. J. Hoe served as director of the Cardiac CT Training Course sponsored by Toshiba Medical Systems, Asia, and received speaker’s fees from GE Biosciences. J. Lima received grant support from GE Healthcare.
References
- 1.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 3.Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 4.Serruys PW, Foley DP, Suttorp MJ, et al. A randomized comparison of the value of additional stenting after optimal balloon angioplasty for long coronary lesions: final results of the additional value of NIR stents for treatment of long coronary lesions (ADVANCE) study. J Am Coll Cardiol. 2002;39:393–399. doi: 10.1016/s0735-1097(01)01760-0. [DOI] [PubMed] [Google Scholar]
- 5.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Ellis SG, Colombo A, et al. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007;115:2842–2847. doi: 10.1161/CIRCULATIONAHA.106.687186. [DOI] [PubMed] [Google Scholar]
- 7.Nayak AK, Kawamura A, Nesto RW, et al. Myocardial infarction as a presentation of clinical in-stent restenosis. Circ J. 2006;70:1026–1029. doi: 10.1253/circj.70.1026. [DOI] [PubMed] [Google Scholar]
- 8.Walters DL, Harding SA, Walsh CR. Acute coronary syndrome is a common clinical presentation of in-stent restenosis. Am J Cardiol. 2002;89:491–494. doi: 10.1016/s0002-9149(01)02285-8. [DOI] [PubMed] [Google Scholar]
- 9.Bossi I, Klersy C, Black AJ, et al. In-stent restenosis: long-term outcome and predictors of subsequent target lesion revascularization after repeat balloon angioplasty. J Am Coll Cardiol. 2000;35:1569–1576. doi: 10.1016/s0735-1097(00)00584-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen MS, John JM, Chew DP. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Abdulla J, Abildstrom SZ, Gotzsche O. 64-mul-tislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 12.Garcia MJ, Lessick J, Hoffmann MH. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Gilard M, Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart. 2006;92:58–61. doi: 10.1136/hrt.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahnken AH, Buecker A, Wildberger JE, et al. Coronary artery stents in multislice computed tomography: in vitro artifact evaluation. Invest Radiol. 2004;39:27–33. doi: 10.1097/01.rli.0000095471.91575.18. [DOI] [PubMed] [Google Scholar]
- 16.Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163–1173. doi: 10.1016/S0140-6736(08)61244-1. [DOI] [PubMed] [Google Scholar]
- 17.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 19.Ringqvist I, Fisher LD, Mock M, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Invest. 1983;71:1854–1866. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–666. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 21.Kuettner A, Beck T, Drosch T, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005;45:123–127. doi: 10.1016/j.jacc.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 22.Ohnuki K, Yoshida S, Ohata M, et al. New diagnostic technique in multi-slice computed tomography for in-stent restenosis: pixel count method. Int J Cardiol. 2006;108:251–258. doi: 10.1016/j.ijcard.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Mühlenbruch G, Mahnken AH, Das M, et al. Evaluation of aortocoronary bypass stents with cardiac MDCT compared with conventional catheter angiography. AJR. 2007;188:361–369. doi: 10.2214/AJR.06.0120. [DOI] [PubMed] [Google Scholar]
- 24.Beohar N, Robbins JD, Cavanaugh BJ, et al. Quantitative assessment of in-stent dimensions: a comparison of 64 and 16 detector multislice computed tomography to intravascular ultrasound. Catheter Cardiovasc Interv. 2006;68:8–10. doi: 10.1002/ccd.20786. [DOI] [PubMed] [Google Scholar]
- 25.Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94:427–430. doi: 10.1016/j.amjcard.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Cademartiri F, Schuijf JD, Pugliese F, et al. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J Am Coll Cardiol. 2007;49:2204–2210. doi: 10.1016/j.jacc.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Van Mieghem CA, Cademartiri F, Mollet NR, et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation. 2006;114:645–653. doi: 10.1161/CIRCULATIONAHA.105.608950. [DOI] [PubMed] [Google Scholar]
- 28.Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006;27:2567–2572. doi: 10.1093/eurheartj/ehl303. [DOI] [PubMed] [Google Scholar]
- 29.Pugliese F, Weustink AC, Van Mieghem C, et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart. 2008;94:848–854. doi: 10.1136/hrt.2007.126474. [DOI] [PubMed] [Google Scholar]
- 30.Ehara M, Kawai M, Surmely JF, et al. Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography: comparison with invasive coronary angiography. J Am Coll Cardiol. 2007;49:951–959. doi: 10.1016/j.jacc.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 31.Gaspar T, Dvir D, Peled N. The role of 16-slice computed tomography angiography in the diagnosis of coronary artery disease: large sample analysis. Isr Med Assoc J. 2005;7:424–427. [PubMed] [Google Scholar]
- 32.Hecht HS, Zaric M, Jelnin V, et al. Usefulness of 64-detector computed tomographic angiography for diagnosing in-stent restenosis in native coronary arteries. Am J Cardiol. 2008;101:820–824. doi: 10.1016/j.amjcard.2007.09.117. [DOI] [PubMed] [Google Scholar]
- 33.Schepis T, Koepfli P, Leschka S, et al. Coronary artery stent geometry and in-stent contrast attenuation with 64-slice computed tomography. Eur Radiol. 2007;17:1464–1473. doi: 10.1007/s00330-006-0502-0. [DOI] [PubMed] [Google Scholar]