Abstract
OBJECTIVE
The purpose of the study was to investigate patient characteristics associated with image quality and their impact on the diagnostic accuracy of MDCT for the detection of coronary artery stenosis.
MATERIALS AND METHODS
Two hundred ninety-one patients with a coronary artery calcification (CAC) score of ≤ 600 Agatston units (214 men and 77 women; mean age, 59.3 ± 10.0 years [SD]) were analyzed. An overall image quality score was derived using an ordinal scale. The accuracy of quantitative MDCT to detect significant (≥ 50%) stenoses was assessed using quantitative coronary angiography (QCA) per patient and per vessel using a modified 19-segment model. The effect of CAC, obesity, heart rate, and heart rate variability on image quality and accuracy were evaluated by multiple logistic regression. Image quality and accuracy were further analyzed in subgroups of significant predictor variables. Diagnostic analysis was determined for image quality strata using receiver operating characteristic (ROC) curves.
RESULTS
Increasing body mass index (BMI) (odds ratio [OR] = 0.89, p < 0.001), increasing heart rate (OR = 0.90, p < 0.001), and the presence of breathing artifact (OR = 4.97, p ≤ 0.001) were associated with poorer image quality whereas sex, CAC score, and heart rate variability were not. Compared with examinations of white patients, studies of black patients had significantly poorer image quality (OR = 0.58, p = 0.04). At a vessel level, CAC score (10 Agatston units) (OR = 1.03, p = 0.012) and patient age (OR = 1.02, p = 0.04) were significantly associated with the diagnostic accuracy of quantitative MDCT compared with QCA. A trend was observed in differences in the areas under the ROC curves across image quality strata at the vessel level (p = 0.08).
CONCLUSION
Image quality is significantly associated with patient ethnicity, BMI, mean scan heart rate, and the presence of breathing artifact but not with CAC score at a patient level. At a vessel level, CAC score and age were associated with reduced diagnostic accuracy.
Keywords: angiography, body mass index, CORE-64, coronary artery calcium, heart rate, hemodynamics, image quality, MDCT
MDCT for the noninvasive detection of coronary artery disease has been implemented in a variety of patients with suspected coronary artery disease because of its diagnostic accuracy and reliability, as shown in previous studies using 16-MDCT [1-6], 32-MDCT [7], and 64-MDCT [8-14] technology. Despite advances in scanners to improve both spatial and temporal resolution [15-17], physiologic factors such as high heart rate [12, 18-24], arrhythmia [19, 25, 26], obesity [12, 27-29], and high coronary calcium burden [10, 12, 20, 24] continue to limit the diagnostic accuracy of MDCT compared with conventional coronary angiography. The diagnostic ability of any imaging method is directly dependent on image quality. In a recent study, Brodoefel et al. [30] on a per-segment basis assessed the effect of heart rate, heart rate variability, and coronary artery calcification (CAC) on the image quality and diagnostic accuracy of dual-source CT. The aim of the current study was to investigate among patients enrolled in the Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography Using 64 Detectors (CORE-64) Multicenter Trial the influence of body mass index (BMI), ethnicity, age, sex, heart rate, heart rate variability, obesity, and CAC on image quality and their impact on the per-patient and per-vessel diagnostic accuracy of 64-MDCT to detect significant coronary artery disease as determined by conventional coronary angiography.
Materials and Methods
Study Population
The CORE-64 Trial prospectively enrolled 316 patients with a CAC score of ≤ 600 Agatston units. Twenty-five patients were excluded: four had major protocol deviations, 10 had incomplete CT angiography scans, and 11 had incomplete conventional angiograms [31]. Two hundred ninety-one patients (214 men, 77 women; mean age, 59.3 ± 10 years [SD]) were included in this single-blinded cohort study conducted at nine centers in seven countries. One hundred ninety-six patients were white; 66, Asian; 18, black; and 11, other. The median BMI was 27 kg/m2 (range, 16-40 kg/m2); median heart rate, 60 beats per minute (bpm) (range, 39-79 bpm); and median CAC score, 80 Agatston units (range, 0-579 Agatston units). Sixty-six percent of patients had a history of hypertension; 60%, dyslipidemia; 23%, diabetes; 19%, current smokers; 41%, former smokers; 40%, never smokers; and 25%, a family history of coronary artery disease. One hundred thirty-four subjects (46%) received β-blockers and 263 (90%) received nitroglycerin before CT data acquisition. Demographic data are listed in Table 1. All MDCT studies were performed ≤ 30 days before conventional coronary angiography.
TABLE 1. Predictors of Image Quality: Patient Analysis.
| Image Quality |
|||||
|---|---|---|---|---|---|
| Predictors | Optimal (n = 128) | Adequate (n = 134) | Poor (n = 29) | Odds Ratio | p |
| Age (y) | 0.98 | 0 .10 | |||
| Mean | 59.4 | 5 9.1 | 59.8 | ||
| SD | 10.0 | 9.7 | 12.2 | ||
| Sex (%) (no. / total no. in image quality category) | |||||
| Male (n = 214) | 78 (100/128) | 72 (96/134) | 62 (18/29) | 1.00 | Reference |
| Female (n = 77) | 22 (28/128) | 28 (38/134) | 38 (11/29) | 1.49 | 0.49 |
| Ethnicity (%) (no. / total no. in image quality category) |
|||||
| White (n = 196) | 58 (74/128) | 75 (100/134) | 76 (22/29) | 1.00 | Reference |
| Asian (n = 66) | 35 (45/128) | 14 (19/134) | 7 (2/29) | 2.67 | 0.06 |
| Black (n = 18) | 3 (4/128) | 7 (9/134) | 17 (5/29) | 0.58 | 0.04 |
| Other (n = 11) | 4 (5/128) | 4 (6/134) | 0 (0/29) | 2.83 | 0 .19 |
| Body mass index (kg/m2) | |||||
| Mean | 26.2 | 28.3 | 29.8 | 0.89 | < 0.001 |
| SD | 4 .1 | 4.3 | 4.6 | ||
| CAC score (Agatston units) | |||||
| Mean | 143.4 | 140.4 | 128.6 | 1.00 | 0.77 |
| SD | 159.6 | 15 9.1 | 15 6.1 | ||
| Heart rate (bpm) | |||||
| Mean | 55.8 | 59.8 | 66.5 | 0.90 | < 0.001 |
| SD | 10.7 | 12 .1 | 7. 8 | ||
| Heart rate variability (beats per scan) | 1.00 | 0.75 | |||
| Mean | 4.8 | 6.3 | 10.8 | ||
| SD | 16 .1 | 12.7 | 1 7.7 | ||
| Breath artifact present (no.)a | 0 | 15 | 7 | 4.97 | < 0.001 |
| Ectopy present (no.)a | 2 | 7 | 3 | 2.69 | 0 .16 |
Note—CAC = coronary artery calcifcation, bpm = beats per minute.
These results are also on the per-patient level; for example, in two patients with optimal image quality at least one ectopic beat was present.
Inclusion criteria were symptomatic patients > 40 years old scheduled for coronary angiography who were willing to provide written informed consent [31, 32]. Exclusion criteria were a history of allergic reaction to iodinated contrast media, renal failure, multiple myeloma, previous organ transplantation, elevated serum creatinine level (> 1.5 mg/dL or calculated creatinine clearance of < 60 mL/), atrial fibrillation, tachyarrhythmia, advanced atrioventricular block, evidence of severe symptomatic heart failure (New York Heart Association class III or IV), severe aortic stenosis, previous coronary artery bypass or other cardiac surgery, coronary artery intervention within the past 6 months, contraindication to β-blockers, BMI > 40 kg/m2, and presence or history of any condition that the site principal investigator considered sufficient for exclusion. The study protocol and informed consent form were approved by the institutional review board of each center as well as a centralized institutional review board at the Johns Hopkins Hospital. The details of the methods of the CORE-64 Trial have been published previously [32]. Here, we present a brief summary of all methodologic aspects relevant to this analysis of predictors of image quality and diagnostic accuracy of 64-MDCT angiography (MDCTA).
Calcium Scoring and CT Coronary Angiography
Calcium scanning was performed using prospective ECG gating at 120 kV and 300 mA with a gantry rotation of 0.4 second and detector collimation of 4.0 × 3.0 mm. For the MDCT coronary angiography, retrospective ECG gating was implemented using gantry rotation times of 350-400 milliseconds autoselected to avoid synchrony with the heartbeat to enable efficient adaptive multisegment image reconstruction [33]. On the basis of each patient’s BMI, an acquisition tube current of 240-400 mAs was used with the tube voltage remaining unchanged at 120, as described in detail elsewhere [32], to meet a predetermined sex-specific effective radiation dose of approximately 12-15 mSv not to exceed 20 mSv according to the German Federal Department for Radiation Protection. Iopamidol (Isovue 370, Bracco Diagnostics) was administered using an 18- or 20-gauge IV line (preferably in the right brachial veins) with image acquisition triggered automatically at 180 HU in the descending aorta (bolus-tracking method). The flow of the contrast agent was adjusted according to each patient’s weight [32]. The volume in milliliters of contrast agent administered for the helical CT acquisition was also calculated individually for each patient using the following formula: volume (in mL) = [(CT data acquisition time in seconds + 10) × flow in mL/s] [32].
Images were acquired during one breath-hold. All images were acquired using a 64 × 0.5 mm MDCT scanner (Aquilion 64, Toshiba Medical Systems). Beta-blockers were given when the resting heart rate was > 70 bpm to reduce it to < 65 bpm. If the mean heart rate could not be reduced to < 80 bpm before or during image acquisition, the patient was excluded from the primary analysis. A dose of short-acting nitrates was routinely given if systolic blood pressure was above 110 mm Hg.
MDCT Image Analysis
Raw image data sets were transferred to the CT Core Laboratory for analysis and reconstructed at a 0.5-mm slice thickness with an overlap of 0.3 mm to optimize image quality by an adaptive multisegment reconstruction algorithm [33]. Software (ImageXact, Toshiba Medical Systems) was used to select the systolic and diastolic phases with the least cardiac motion using both a standard (FC43) and a hard (FC05) convolution kernel. A temporal window of ± 20 milliseconds was used to optimize reconstruction on per-patient and per-vessel bases in both systolic and diastolic reconstructions. ECG editing was used in 26 of the 291 patients to exclude arrhythmias and decrease cardiac motion [34].
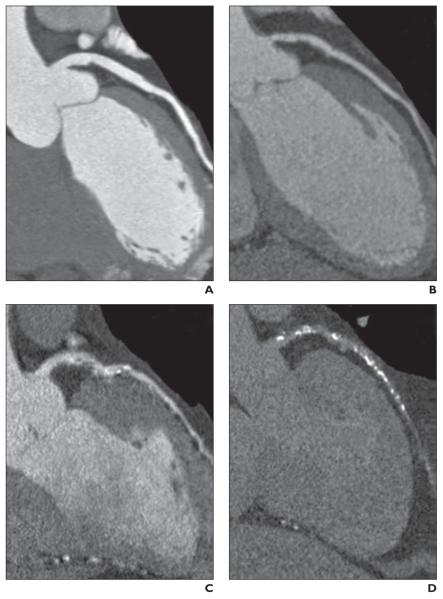
An overall assessment of patient- and segment-level image quality was performed in the CT Core Laboratory. To determine the vessel-level image quality score, the average score of all segments comprising the vessel was used. The following image quality ordinal scale was implemented: 1, optimal quality (absence of motion artifact and optimal contrast opacification); 2, adequate quality (minor imaging artifacts); 3, poor (significant motion artifact, calcification artifact, or poor contrast opacification); or 4, nonassessable (absence of contrast opacification or incomplete scan), which were considered nondiagnostic for the evaluation of diagnostic accuracy [5, 35] (Fig. 1).
Fig. 1.
Assessment of image quality as shown on four representative curved multiplanar reformations along left anterior descending coronary artery.
A, Optimal image quality was defined as absence of motion artifact and optimal contrast opacification, as shown in this MDCT image of 63-year-old man.
B, Adequate image quality was defined as minor imaging artifacts and noisier image, as shown in this MDCT image of 54-year-old woman.
C, Poor image quality was defined as significant motion artifact, calcification artifact, or poor contrast opacification, as shown in this MDCT image of 58-year-old man.
D, Nonassessable was defined as absence of contrast opacification or incomplete scan, as shown in this MDCT image of 66-year-old man.
Total coronary calcium burden and regional coronary calcium burden were measured while carefully avoiding stents and calcium of the mitral valve using the Agatston method [36] and standardized software (Vitrea 2, version 3.9.0.1, Vital Images). MDCT data were reconstructed, probed, and segmented by the CT Core Laboratory technician. Reconstructed images in multiple cardiac phases were sent to the Core Imaging Laboratory for image interpretation by two investigators who were blinded to any patient information; they used independent workstations with dedicated cardiac MDCT analysis software (Vitrea 2, version 3.9.0.1, Vital Images). Quantitative assessment of the degree of diameter stenosis was performed in cross-sectional and longitudinal projections after visual identification of ≥ 30% stenosis using a semiautomatic contour-detection algorithm, electronic calipers, and rulers in cross-sectional and longitudinal projections. Final determinations of reference and lumen diameters were made only after manual contour editing [32]. Segments with significant discrepancies underwent a consensus process that incorporated a third experienced observer. In segments in which the difference between the two principal readers was not significant, a consensus score was derived through averaging. A significant difference was defined as the maximum percentage diameter stenosis crossing the 50% or 70% thresholds. Additionally, if any one reader judged a segment nonassessable, the segment underwent a consensus interpretation. For the quantitative analysis, segments were deemed nonassessable only if there were no quantitative measurements made by any of the readers.
Conventional Coronary Angiography
Conventional coronary angiography was performed using standard angiographic techniques within 30 days after MDCT. Intracoronary nitroglycerine was administered (150-200 mcg) before acquisition of the first image of the left and right coronary arteries to standardize coronary vasomotor tone and reduce the potential for catheter-induced spasm. Coronary angiographic images were saved in DICOM format and forwarded to the Angiographic Core Laboratory for analysis.
Quantitative coronary angiography (QCA) was performed using an edge-detection algorithm (CAAS II QCA software, research version 2.0.1, PIE Medical Imaging). The most significant stenosis within each coronary segment was analyzed with quantitative assessment of the degree of stenosis (QCA). Segments that could not be accurately visualized because of reduced image quality were excluded. Segmental disease was analyzed in each vessel using a 19-segment model used by MDCT. Lesions causing ≥ 50% reduction of the lumen were considered significant. After analysis completion, all measurements were locked, and an adjudication process was performed to ensure MDCT and QCA measurements were obtained from the same segments. Scores were reassigned to the appropriate segments in cases of misalignment.
Coronary Artery Segmentation
Although prior MDCT studies have used a 15-segment model [37], which is a variation of the standard American Heart Association (AHA) model for conventional coronary angiography (AHA/American College of Cardiology, 29 segments) [38, 39], we developed a modified 19-segment model to accommodate a more comprehensive coronary tree description without placing undue emphasis on very small and very distant territories. We excluded the acute marginal, first septal, and third diagonal. Additionally, we grouped together the right posterolaterals and the distal circumflex with the left posterolaterals. We reported the tightest lesion in the right posterior lateral group and the tightest lesion in the distal circumflex group [31, 32].
Statistical Analysis
Statistical analysis was performed with standard statistical analysis software (Intercooled Stata, version 10, Stata Press). Proportional odds logistic regression was used to investigate patient characteristics (sex, age, ethnicity, BMI, CAC score, heart rate, heart rate variability, breathing artifact, ectopy) associated with optimal image quality. Multivariate logistic regression was performed between patient predictor characteristics and concordance between MDCTA and conventional coronary angiography on a per-patient level and a per-vessel level to test the association between patient characteristics and overall accuracy. Age, BMI, heart rate, heart rate variability, and CAC score were considered continuous variables, ethnicity was considered a categoric variable, and sex, ectopy, and breathing artifact were considered binary.
To take into account the effect of vessels related to individual patients, the effect of clustering was incorporated. To assess the diagnostic accuracy of MDCT compared with invasive angiography across image quality, age, sex, coronary calcium, BMI, acquisition heart rate, and heart rate variability strata, we considered the output from MDCT as a continuous measure and used the area under the receiver operating characteristic (ROC) curve as the measure of diagnostic accuracy. The reference standard for all ROC analysis was ≥ 50% diameter stenosis by QCA. All tests were two-tailed, and differences were considered significant when the p value was equal or less than 0.05.
Results
Image Quality Parameters
The patient characteristics used to evaluate the influence of optimal image quality included the following: age, sex, ethnicity, BMI, CAC score, heart rate, heart rate variability, breathing artifact, and ectopy. A significant relationship for predicting image quality was determined for BMI (odds ratio [OR] = 0.89, p < 0.001), mean scan heart rate (OR = 0.90, p < 0.001), the presence of a breathing artifact (OR = 4.97, p < 0.001), and black ethnicity (OR = 0.58, p = 0.04; Table 1).
In the patient-based analysis, optimal image quality was achieved in 128 patients (44%), adequate quality in 134 (46%), and poor quality in 29 (10%) (Table 2). In the vessel-based analysis, optimal image quality was achieved in 384 vessels (44%), adequate quality in 397 (46%), and poor quality in 85 (10%) (Table 3).
TABLE 2. The Effect of Sex, Age, Image Quality, Coronary Calcification, Body Mass Index, Heart Rate, and Variability of Heart Rate on Diagnostic Accuracy of Quantitative MDCT Versus Quantitative Coronary Angiography: Patient Analysis.
| Patient Characteristic | AUC (95% CI) | Sensitivity (%) |
Specifcity (%) |
PPV (%) | NPV (%) | Accuracy (%) | PLR | NLR | DOR |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male (n = 214) | 0.94 (0.90–0.97) | 86 (114/133) | 91 (74/ 81) | 9 4 (114 / 12 1) | 80 (74/93) | 88 (188/214) | 9.92 | 0 .16 | 62.00 |
| Female (n = 77) | 0.89 (0.81–0.98) | 83 (25/30) | 87 (41/47) | 81 (2 5 /31) | 89 (41/46) | 86 (66/77) | 6.53 | 0 .19 | 34.37 |
| Age | |||||||||
| 40–49 y (n = 53) | 0.95 (0.89–1.00) | 9 4 (29/ 31) | 91 (20/22) | 9 4 (2 9 / 31) | 91 (20/22) | 92 (49/53) | 10.29 | 0.07 | 144.93 |
| 50–59 y (n = 97) | 0.96 (0.92–0.99) | 86 (42/49) | 92 (44/48) | 91 (42/46) | 86 (44/51) | 89 (86/97) | 10.29 | 0 .16 | 65.96 |
| 60–69 y (n = 93) | 0.90 (0.84–0.96) | 87 (48/55) | 82 (31/38) | 87 (48/55) | 82 (31/38) | 85 (79/93) | 4.74 | 0 .16 | 29.63 |
| ≥ 70 y (n = 48) | 0.91 (0.82–0.99) | 71 (20/28) | 100 (20/20) | 100 (20/20) | 71 (13/18) | 83 (40/48) | 29.70 | 0.30 | 98.90 |
| Image quality | |||||||||
| Optimal (n = 128) | 0.95 (0.91–0.99) | 91 (67/74) | 87 (47/54) | 91 (67/74) | 87 (47/54) | 8 9 (114 /128) | 7.0 0 | 0 .10 | 67.67 |
| Adequate (n = 134) | 0.92 (0.87–0.96) | 84 (61/73) | 90 (55/61) | 91 (61/67) | 82 (55/67) | 8 7 (116 /13 4) | 8.40 | 0 .18 | 46.67 |
| Poor (n = 29) | 0.91 (0.80–1.00) | 6 9 (11/ 16) | 100 (13/13) | 100 (13/13) | 72 (13/18) | 83 (24/29) | 69.00 | 0.31 | 222.58 |
| Calcium score (Agatston units) | |||||||||
| 0–100 (n = 151) | 0.89 (0.83–0.95) | 75 (40/53) | 92 (90/98) | 83 (40/48) | 87 (90/103) | 86 (130/151) | 9.25 | 0.27 | 34.26 |
| 101–300 (n = 81) | 0.95 (0.90–1.00) | 90 (55/61) | 85 (17/20) | 95 (55/58) | 74 (17/23) | 89 (7 2 / 81) | 6.01 | 0 .12 | 50.08 |
| 301–600 (n = 59) | 0.93 (0.87–1.00) | 90 (44/49) | 80 (8/10) | 96 (44/46) | 62 (8/13) | 88 (52/59) | 4.49 | 0 .13 | 34.54 |
| Body mass index | |||||||||
| Normal weighta (n = 66) | 0.93 (0.86–1.00) | 84 (32/38) | 93 (26/28) | 94 (32/34) | 81 (26/32) | 88 (58/66) | 12.00 | 0 .17 | 69.75 |
| Overweightb (n = 141) | 0.92 (0.87–0.96) | 86 (72/84) | 88 (50/57) | 91 (72/79) | 81 (50/62) | 8 7 (12 2 / 141) | 7.17 | 0 .16 | 45.05 |
| Obesec (n = 84) | 0.96 (0.92–1.00) | 8 5 (3 5 / 41) | 91 (39/43) | 90 (35/39) | 87 (39/45) | 88 (74/84) | 9.44 | 0 .16 | 5 7. 3 0 |
| Heart rate | |||||||||
| < 60 bpm (n = 168) | 0.93 (0.90–0.97) | 85 (80/94) | 92 (68/74) | 93 (80/86) | 83 (68/82) | 88 (148/168) | 10.50 | 0 .16 | 64.76 |
| 60–69 bpm (n = 95) | 0.93 (0.87–0.98) | 86 (44/51) | 89 (39/44) | 90 (44/49) | 85 (39/46) | 87 (83/95) | 7. 5 9 | 0 .15 | 49.03 |
| ≥ 70 bpm (n = 28) | 0.94 (0.85–1.00) | 83 (15/18) | 80 (8/10) | 88 (15/17) | 7 3 (8 /11) | 82 (23/28) | 4 .17 | 0.21 | 20.00 |
| Heart rate variability | |||||||||
| ≤ 6 beats per scan (n = 241) | 0.93 (0.90–0.96) | 8 6 (117/ 13 6) | 89 (93/105) | 91 (117/ 12 9) | 83 (93/112) | 87 (210/241) | 7. 82 | 0 .16 | 49.70 |
| > 6 beats per scan (n = 50) | 0.93 (0.85–1.00) | 81 (22/27) | 96 (22/23) | 96 (22/23) | 81 (22/27) | 88 (44/50) | 20.25 | 0.20 | 102.31 |
Note—AUC = area under receiver operating characteristic curve, PPV = positive predictive value, NPV = negative predictive value, PLR = positive likelihood ratio (sensitivity / 1 – specifcity), NLR = negative likelihood ratio (1 – sensitivity / specifcity), DOR = diagnostic odds ratio (PLR / NLR), bpm = beats per minute.
< 25 kg/m2.
25–29.9 kg/m2.
≥ 30 kg/m2.
TABLE 3. The Effect of Sex, Age, Image Quality, Coronary Calcification, Body Mass Index, Heart Rate, and Variability of Heart Rate on Diagnostic Accuracy of Quantitative MDCT Versus Quantitative Coronary Angiography: Vessel Analysis.
| Patient Characteristic | AUC (95% CI) | Sensitivity (%) |
Specifcity (%) |
PPV (%) | NPV (%) | Accuracy (%) | PLR | NLR | DOR |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male (n = 636) | 0.91 (0.88–0.93) | 76 (168/222) | 92 (382/414) | 84 (168/200) | 88 (382/436) | 86 (550/636) | 9.79 | 0.26 | 3 7.6 5 |
| Female (n = 230) | 0.89 (0.83–0.95) | 70 (33/47) | 93 (171/183) | 73 (33/45) | 92 (171/185) | 89 (204/230) | 10.71 | 0.32 | 33.47 |
| Age | |||||||||
| 40–49 y (n = 158) | 0.93 (0.89–0.98) | 80 (40/50) | 94 (102/108) | 87 (40.46) | 91 (102 /112) | 90 (142/158) | 14.40 | 0.21 | 68.57 |
| 50–59 y (n = 289) | 0.93 (0.90–0.97) | 7 5 ( 6 1/ 81) | 95 (198/208) | 8 6 (61/ 71) | 91 (198/218) | 90 (259/289) | 15.66 | 0.26 | 60.46 |
| 60–69 y (n = 276) | 0.88 (0.84–0.93) | 77 (75/97) | 89 (160/179) | 80 (75/94) | 88 (160/182) | 85 (235/276) | 7. 2 8 | 0.25 | 2 9 .12 |
| ≥ 70 y (n = 143) | 0.87 (0.80–0.94) | 61 (2 5 /41) | 91 (93/102) | 74 (25/34) | 85 (3/109) | 83 (118/143) | 6.91 | 0.43 | 16 .14 |
| Image quality | |||||||||
| Optimal (n = 384) | 0.93 (0.90–0.96) | 83 (96/115) | 91 (244/269) | 79 (9 6/121) | 93 (244/263) | 89 (340/384) | 9.22 | 0 .19 | 49.35 |
| Adequate (n = 397) | 0.91 (0.87–0.94) | 73 (93/127) | 94 (254/270) | 85 (93/109) | 88 (254/288) | 87 (347/397) | 12 .17 | 0.29 | 41.97 |
| Poor (n = 85) | 0.81 (0.72–0.92) | 44 (12/27) | 95 (55/58) | 80 (12/15) | 79 (55/70) | 79 (67/85) | 8.80 | 0.59 | 14.94 |
| Calcium score (Agatston units) | |||||||||
| 0 (n = 382) | 0.89 (0.82–0.96) | 61 ( 2 5 /41) | 96 (329/ 3 41) | 68 (25/37) | 95 (329/345) | 93 (354/382) | 17. 3 3 | 0.41 | 42.27 |
| 1–100 (n = 335) | 0.86 (0.81–0.90) | 7 6 (10 7/ 141) | 89 (172/194) | 83 (107/129) | 83 (172/206) | 83 (279/335) | 6.91 | 0.27 | 25.62 |
| 101–600 (n = 149) | 0.89 (0.84–0.94) | 79 (69/87) | 84 (52/62) | 87 (69/79) | 74 (52/70) | 81 (121/149) | 4.91 | 0.25 | 19.64 |
| Body mass index | |||||||||
| Normal weighta (n = 197) | 0.91 (0.86–0.95) | 72 (46/64) | 8 9 (119 / 13 3) | 77 (46/60) | 87 (119/137) | 84 (165/197) | 6.83 | 0.31 | 25.30 |
| vOerweightb (n = 417) | 0.90 (0.86–0.93) | 76 (106/139) | 92 (255/278) | 82 (106/129) | 89 (255/288) | 87 (361/417) | 9.22 | 0.26 | 35.46 |
| Obesec (n = 252) | 0.92 (0.88–0.96) | 74 (49/66) | 96 (179/186) | 88 (49/56) | 91 (179/196) | 90 (228/252) | 19.73 | 0.27 | 73.07 |
| Heart rate | |||||||||
| < 60 bpm (n = 502) | 0.91 (0.88–0.94) | 76 (112 /147) | 92 (328/355) | 81 (112 /139) | 90 (328/363) | 88 (440/502) | 10.02 | 0.26 | 38.87 |
| 60–69 bpm (n = 264) | 0.90 (0.86–0.94) | 74 (64/87) | 93 (165/177) | 84 (64/76) | 88 (165/188) | 87 (229/264) | 10.85 | 0.28 | 38.26 |
| ≥ 70 bpm (n = 100) | 0.90 (0.84–0.97) | 71 (25/35) | 92 (60/65) | 83 (25/30) | 86 (60/70) | 85 (85/100) | 9.29 | 0.31 | 30.00 |
| Heart rate variability | |||||||||
| ≤ 6 beats per scan (n = 699) | 0.91 (0.88–0.93) | 74 (160/215) | 91 (442/484) | 79 (160/202) | 89 (442/497) | 86 (602/699) | 8.58 | 0.28 | 30.64 |
| > 6 beats per scan (n = 167) | 0.92 (0.86–0.97) | 76 (41/54) | 9 8 (111/ 113 ) | 95 (41/43) | 9 0 (111 / 12 4 ) | 91 (152/167) | 42.90 | 0.25 | 171.6 |
Note—AUC = area under receiver operating characteristic curve, PPV = positive predictive value, NPV = negative predictive value, PLR = positive likelihood ratio (sensitivity / 1 – specifcity), NLR = negative likelihood ratio (1 – sensitivity / specifcity), DOR = diagnostic odds ratio (PLR / NLR), bpm = beats per minute.
< 25 kg/m2.
25–29.9 kg/m2.
≥ 30 kg/m2.
Predictors of Diagnostic Accuracy
Influence of image quality
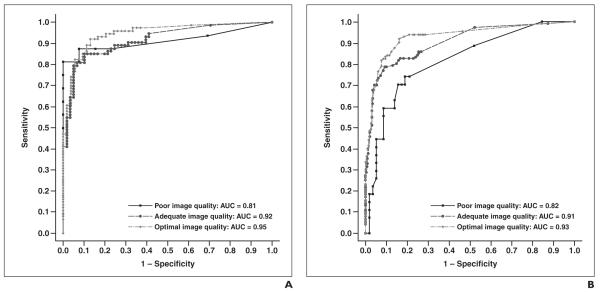
In the patient-based analysis, the area under the ROC curve (AUC) for optimal image quality was 0.95 (95% CI, 0.91-0.99); for adequate quality, 0.92 (0.87-0.96); and for poor quality, 0.91 (0.80-1.00) A significant difference was not observed in AUCs between image quality strata (p = 0.56; Fig. 2A).
Fig. 2.
To assess diagnostic accuracy of MDCT compared with invasive angiography stratified by image quality, we considered output from MDCT as continuous measure and used the area under receiver operating characteristic (ROC) curve as a measure of diagnostic accuracy. ROC curve analysis is a method of describing the intrinsic accuracy of a diagnostic test apart from decision thresholds. An ROC curve is a plot of a diagnostic test’s sensitivity (plotted on y-axis) versus its false-positive rate (1 – specificity) (plotted on x-axis). An ROC analysis plots the relationship between sensitivity and specificity across all cut points of a test and calculates the area under the ROC curve (AUC) and its standard error. A diagnostic test with an AUC of 1 is perfectly accurate, whereas one with an AUC of 0.5 is performing no better than chance. Use of ROC curve as a measure of accuracy allows the following interpretations [50]: first, the average value of sensitivity for all possible values of specificity; second, the average value of specificity for all possible values of sensitivity; and, third, the probability that randomly selected patient with the condition of interest has diagnostic test result indicating greater suspicion than that of randomly chosen patient without the condition of interest.
A, ROC curve shows diagnostic ability of coronary MDCT angiography to distinguish patient with—as opposed to without—at least one ≥ 50% coronary stenosis as defined by quantitative coronary angiography (QCA). Curve has been stratified by image quality. Difference was not observed in AUCs between image quality strata (p = 0.562).
B, ROC curve shows diagnostic ability of coronary MDCT angiography to distinguish vessel with—as opposed to without—at least one ≥ 50% coronary stenosis as defined by QCA. Curve has been stratified by image quality. Statistical trend was observed in differences in AUCs between image quality strata (p = 0.079).
In a vessel-based analysis, the AUC for optimal quality was 0.93 (95% CI, 0.90-0.96); for adequate quality, 0.91 (0.87-0.94); and for poor quality, 0.81 (0.72-0.92). A trend was observed in differences in AUCs across vessel image quality strata (p = 0.08) (Fig. 2B). The effect of image quality on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
Influence of age and sex
The influences of age and sex on the diagnostic accuracy of MDCT were assessed. Age was categorized into the following groups: 40-49 years, 50-59 years, 60-69 years, and 70 years or greater. In a patient-based analysis, the AUC for individuals 40-49 years old was 0.95 (95% CI, 0.89-1.00); for individuals 50-59 years old, 0.96 (0.92-0.99); for individuals 60-69 years old, 0.90 (0.84-0.96); and for individuals 70 years old or older, 0.91 (0.82-0.99). In a vessel-based analysis, the AUC for individuals 40-49 years old was 0.93 (95% CI, 0.89-0.98); for individuals 50-59 years old, 0.93 (0.90-0.97); for individuals 60-69 years old, 0.88 (0.84-0.93); and for individuals 70 years old or older, 0.87 (0.80-0.94). A significant difference was not observed in the AUCs between age strata in the patient-based analysis (p = 0.43) or the vessel-based analysis (p = 0.13). The effect of age on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
In a patient-based analysis, the AUC for women was 0.89 (95% CI, 0.81-0.98) and for men was 0.94 (0.90-0.97), whereas in a vessel-based analysis the AUC for women was 0.89 (0.83-0.95) and for men was 0.91 (0.88-0.93). A significant difference in the AUC was not observed between men and women in either the patient-based analysis (p = 0.38) or the vessel-based analysis (p = 0.57). The effect of sex on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
Influence of coronary calcium
Coronary calcification was categorized into the following groups: low calcium (0-100 Agatston units), moderate calcium (101-300 Agatston units), and elevated calcium (301-600 Agatston units) for a patient-based analysis and low calcium (0 Agatston units), moderate calcium (1-100 Agatston units), and elevated calcium (≥ 101 Agatston units) for vessel-based analysis [40]. In a patient-based analysis the AUC for individuals in the low calcium category was 0.89 (95% CI, 0.83-0.95); for those with moderate calcium, 0.95 (0.90-1.00); and for individuals with elevated calcium, 0.93 (0.87-1.00). In a vessel-based analysis, the AUC for individuals in the low calcium category was 0.89 (95% CI, 0.82-0.96); for those with moderate calcium, 0.86 (0.81-0.90); and for individuals with elevated calcium, 0.89 (0.84-0.94). A significant difference was not observed in AUCs between individuals with low, moderate, or elevated calcium scores in a patient-based analysis (p = 0.33) or a vessel-based analysis (p = 0.55). The effect of coronary calcification on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
The effect of lesion calcification on the event rate of false-negative and false-positive findings was assessed. Lesion calcification was assessed in a cross-sectional projection using an ordinal scale: no calcification, mild calcification (focal, arc < 90°), moderate calcification (arc = 90-179°), and severe calcification (arc > 179°) [41]. In the patients with false-positive findings, there was a total of 11 lesions: 45.5% (5/11) had no calcium, 45.5% (5/11) had mild calcium, and 9% (1/11) had moderate calcification. In the patients with false-negative findings, there was a total of 49 lesions: 65% (32/49) had no calcification, 20% (10/49) had mild calcification, 12% (6/49) had moderate calcification, and 2% (1/49) had severe calcification.
Influence of obesity
The influence of obesity on the diagnostic accuracy of MDCT was examined. BMI was categorized into three groups: normal weight (< 25 kg/m2), overweight (25-29.9 kg/m2), and obese (≥ 30 kg/m2). In a patient-based analysis, the AUC for normal-weight individuals was 0.93 (95% CI, 0.86-1.00); for overweight individuals, 0.92 (0.87-0.96); and for individuals who were obese, 0.96 (0.92-1.00). In a per-vessel analysis the AUC for normal-weight individuals was 0.91 (95% CI, 0.86-0.95); for overweight individuals, 0.90 (0.86-0.93); and for obese individuals, 0.92 (0.88-0.96). A significant difference was not observed in AUCs between BMI strata in either a patient-based analysis (p = 0.39) or a vessel-based analysis (p = 0.86). The effect of obesity on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
Influence of heart rate and heart rate variability
The influence of categoric heart rates on the diagnostic accuracy of MDCTA was assessed. Heart rate was categorized into three groups: < 60 bpm, 60-69 bpm, and ≥ 70 bpm. In a patient-based analysis, the AUC for heart rates < 60 bpm was 0.93 (95% CI, 0.90-0.97); for heart rates 60-69 bpm, 0.93 (0.87-0.98); and for heart rates ≥ 70 bpm, 0.94 (0.85-1.00). In a vessel-based analysis the AUC for heart rates < 60 bpm was 0.91 (95% CI 0.88-0.94); for heart rates 60-69 bpm, 0.90 (0.86-0.94); and for heart rates ≥ 70 bpm, 0.90 (0.84-0.97). A significant difference was not observed in the AUCs between heart rate strata in either a patient-based analysis (p = 0.97) or a vessel-based analysis (p = 0.88). The effect of acquisition heart rate on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
The influence of heart rate variability on the diagnostic accuracy of MDCTA was evaluated. Heart rate variability was defined as the maximum difference in heart rate during scanning based on the acquisition ECG. Heart rate variability was categorized into two groups: ≤ 6 beats per scan and > 6 beats per scan. In a patient-based analysis, the AUC for heart rate variability of ≤ 6 beats per scan was 0.93 (95% CI, 0.90-0.96), and for heart rate variability of > 6 beats per scan the AUC was 0.93 (0.85-1.00). In a vessel-based analysis, the AUC for heart rate variability of ≤ 6 beats per scan was 0.91 (95% CI, 0.88-0.93) and for heart rate variability of > 6 beats per scan, 0.92 (0.86-0.97). A significant difference was not observed in the AUCs between heart rate variability strata in either a patient-based analysis (p = 0.93) or a vessel-based analysis (p = 0.73). The effect of heart rate variability on the diagnostic accuracy of quantitative MDCTA versus QCA in a patient-based analysis is presented in Table 2 and in a vessel-based analysis in Table 3.
Multivariate Analyses
Univariate and multivariate logistic regressions were performed between patient characteristics (age, sex, ethnicity, BMI, CAC score in increments of 10 Agatston units, heart rate, heart rate variability, breathing artifact, and ectopy) and diagnostic accuracy on a per-patient level and a per-vessel level to test the association between patient characteristics and diagnostic accuracy. Diagnostic accuracy was defined as agreement between MDCT and conventional coronary angiography. In the per-patient univariate analysis, no statistically significant relationship was found between patient characteristics and diagnostic accuracy (p value range, 0.15-0.98); similarly, in the multivariate per-patient analysis, no statistically significant relationship was determined (p value range, 0.23-0.99).
In the per-vessel univariate analysis, a statistically significant relationship was determined between age and diagnostic accuracy (OR = 1.03, p = 0.02) and CAC score and diagnostic accuracy (OR = 1.03, p = 0.002). In the multivariate analysis, a statistically significant relationship was found between age and diagnostic accuracy (OR = 1.02, p = 0.04) and CAC score and diagnostic accuracy (OR = 1.0019, p = 0.003) when controlling for all other patient characteristics (age, sex, ethnicity, BMI, CAC, heart rate, heart rate variability, breathing artifact, and ectopy).
Discussion
This study is the first, to our knowledge, to report on the influence of different determinates of image quality of MDCT using 64 simultaneous detector rows and their influence on diagnostic accuracy on a per-patient level and a per-vessel level as compared with conventional coronary angiography in a large international multicenter study. We found a relevant influence of BMI, heart rate, and breathing artifact on the degradation of image quality. A large effect of ethnicity on image quality was found, but was not highly statistically significant because the analysis was not based on a large number of patients. We also examined the influence of subgroups of sex, age, CAC burden, BMI, heart rate, and heart rate variability on the diagnostic accuracy of 64-MDCT and could show an association with reduced accuracy only for increasing calcium burden in a vessel, even when only patients with a CAC score ≤ 600 were analyzed. The adaptation of tube current to each patient’s BMI [32] might explain the independence of the diagnostic accuracy results from obesity. Finally, our study shows that the patient characteristic predictor variables, although still important as determinates of image quality in 64-MDCT 0.5-mm-slice-thickness CT scanners, are less influential than in previous studies performed with fewer detector rows and a wider section thickness.
The 64-MDCT scanners have a faster gantry rotation time and faster volume coverage compared with previous-generation scanners, thus enabling a more robust examination of the coronary arteries that is less susceptible to respiratory artifact and patient movement. The improved temporal and spatial resolution of the 64-MDCT scanners has been translated into clinical application, and several studies have shown higher image quality and diagnostic accuracy on 64-MDCT scanners compared with previous-generation scanners [9-12, 16, 17, 42]. Despite the advancement of scanners from single-slice to 4 slices to 16-64 slices, motion artifacts have negatively affected image quality and remain a limitation of the technology [18, 19, 43]. With each generation of scanners, motion artifacts reappear as a major cause of image quality degradation during CT coronary angiography [4, 5, 18, 44]. Residual cardiac motion artifacts have been reported to be the main cause of image quality degradation with 4- and 16-MDCT [5, 18, 19]. The small diameter of the coronary [9-12, 16, 17, 42] segments, complex 3D geometry, and rapid movement through the cardiac cycle represent major challenges for artifact-free CT angiography [19].
In a recent study, Brodoefel et al. [30] analyzed the influence of heart rate and heart rate variability on both the image quality and diagnostic accuracy of 64-MDCT. They reported that elevated heart rate and heart rate variability have a negative effect on image quality; however, this effect did not deteriorate diagnostic accuracy. These findings contrast with the findings of Raff et al. [12] and Dewey et al. [16] who reported a significant decline in diagnostic accuracy at heart rates above 70 and 65 bpm using standard halfscan reconstruction [12, 21]. Our data support the finding of Brodoefel et al. [30] in which higher heart rate was a predictor of reduced image quality; however, in both patient and vessel analyses, heart rate was not associated with significantly reduced diagnostic accuracy. Of note, in our trial the mean heart rate was 60 bpm (range, 39-86 bpm); 46% of the patients received β-blockades before scanning. Similarly, heart rate variability was not associated with a degradation of image quality or reduced diagnostic accuracy in both the patient and vessel analyses. This finding is likely attributable to ECG editing, which allows the elimination of variable R-R intervals [34], multisegment reconstruction [33], and optimized reconstruction of each artery per patient.
In recent studies, investigators examining the influence of CAC as related to MDCTA diagnostic accuracy have concluded that despite a high overall accuracy, patients with calcium scores over 400 Agatston units remain a challenge to diagnose [12, 30]. In the current study, CAC was not associated with reduced image quality or reduced diagnostic accuracy in a patient-level analysis. However, in a vessel-level analysis, CAC was associated with reduced diagnostic accuracy (p = 0.012) using multivariable logistic regression. For each increase of 10 Agatston units in CAC score in a vessel, the risk of a misdiagnosis is increased by 3.0% (p = 0.002). The influence of CAC in our data suggests that if a predefined upper limit of CAC is to be determined it will likely occur at a vessel level rather than a patient level.
The results of our study confirm that certain patient characteristics, such as patient size, must be considered when adjusting scanner settings such as kV and mA to scan individuals undergoing coronary CT angiography. Moreover, training patients with breath-hold testing (e.g., “mock breathing instructions”) [45] before scanning is important to avoid breathing artifacts that limit image quality.
Previous studies have investigated sex differences in the capability of MDCT to detect significant coronary artery disease as compared with conventional coronary angiography. Dewey et al. [46] and Meijboom et al. [47] have shown reduced capability of MDCT to detect significant coronary artery disease in women compared with men. They have attributed these differences to women having smaller coronary arteries and milder stenosis severity. In a patient-based analysis, Meijboom et al. showed a significant difference in specificity, positive predictive value, and overall diagnostic accuracy between men and women; however, the sensitivity was nearly equal because of few false-negative studies. They noted that the lower disease prevalence of coronary artery disease in women likely contributed to the differences in specificity [47].
Similarly, Dewey et al. [46] reported that overall diagnostic accuracy, sensitivity, and positive predictive value were all significantly lower for women than men. They acknowledge that the difference in disease prevalence in women and men may have contributed to the differences in diagnostic performance. Additionally, Halon et al. [48] showed in multivariable analysis that women have a higher incidence of nonassessable segments (OR = 3.1, p < 0.001) than men. This finding may be because women have smaller coronary arteries; however, the authors acknowledge that they did not routinely measure coronary artery size in conventional coronary angiography arteries > 2 mm [48]. On the other hand, Pundziute et al. [49] have shown in both patient- and vessel-based analyses high diagnostic capability of MDCT compared with conventional coronary angiography for both men and women. In the current study using 64 × 0.5 mm detector collimation, no significant differences in the diagnostic capability of MDCT were shown between men and women in either a patient-based or a vessel-based analysis.
Our study has some limitations. Individuals with heart rates greater than 80 bpm or a BMI of > 40 kg/m2 were excluded from the study. Additionally, segments with a vessel diameter of less than 1.5 mm and individuals with a CAC score of ≤ 600 Agatston units were excluded from the analysis. These exclusions limit, to a great extent, the applicability of the current study results to a real-world setting in which a relevant portion of patients present with obesity, an elevated heart rate, increased CAC score, or a combination of these characteristics. Thus, additional analyses (ideally multicenter) including those patients would be of distinct clinical and practical value. We also acknowledge that the image quality scoring system could have been influenced by subjectivity bias. The 64-MDCT scanner with 0.5-mm detector collimation represents state-of-the-art at the time of study conduct. More recent scanner configurations, such as dual-source CT (improvements in temporal resolution) [30] and 320-MDCT (single-heartbeat acquisitions) [50, 51], have the potential to improve image quality of coronary CT angiography, further making the test even more independent of patient characteristics.
In summary, observations from this multicenter international study show that 64-MDCT scanner technology provides a high-quality noninvasive coronary arteriogram that accurately delineates the presence or absence of significant lesions in a broad spectrum of patients, including patients who are obese, have high heart rates or heart rate variability, and have CAC. Age and CAC in a vessel-based analysis were the only factors associated with reduced diagnostic accuracy of MDCT for the detection of significant CAD compared with conventional coronary angiography.
Acknowledgments
M. Dewey has research grants from GE Healthcare (Amersham), Bracco Diagnostics, Guerbet, and Toshiba Medical Systems and is a member of the speaker’s bureau for Toshiba Medical Systems and Bayer-Schering. A. Arbab-Zadeh received grant support from CT Core Laboratory. J. M. Miller, N. Paul, and J. A. Lima received grant support from Toshiba Medical Systems. N. Paul, J. Hoe, and J. Lima received speakers’ fees from Toshiba Medical Systems and N. Paul, advisory fees from Vital Images. J. Hoe served as director of the Cardiac CT Training Course sponsored by Toshiba Medical Systems, Asia, and received speaker’s fees from GE Biosciences. J. A. Lima received grant support from GE Healthcare.
Footnotes
M. Dewey and A. L. Vavere contributed equally to this work.
References
- 1.Garcia MJ, Lessick J, Hoffmann MH, CATSCAN Study Investigators Accuracy of 16-row multide-tector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann MH, Shi H, Schmitz BL, et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005;293:2471–2478. doi: 10.1001/jama.293.20.2471. [DOI] [PubMed] [Google Scholar]
- 3.Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–2270. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106:2051–2054. doi: 10.1161/01.cir.0000037222.58317.3d. [DOI] [PubMed] [Google Scholar]
- 5.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–666. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 6.Dewey M, Teige F, Schnapauff D, et al. Noninvasive detection of coronary artery stenoses with multislice computed tomography or magnetic resonance imaging. Ann Intern Med. 2006;145:407–415. doi: 10.7326/0003-4819-145-6-200609190-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro MA, Miller JM, Schmidt A, et al. Noninvasive half millimetre 32 detector row computed tomography angiography accurately excludes significant stenoses in patients with advanced coronary artery disease and high calcium scores. Heart. 2006;92:589–597. doi: 10.1136/hrt.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48:1896–1910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Hamon M, Morello R, Riddell JW, Hamon M. Coronary arteries: diagnostic performance of 16-versus 64-section spiral CT compared with invasive coronary angiography—meta-analysis. Radiology. 2007;245:720–731. doi: 10.1148/radiol.2453061899. [DOI] [PubMed] [Google Scholar]
- 10.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–1487. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaou K, Knez A, Rist C, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR. 2006;187:111–117. doi: 10.2214/AJR.05.1697. [DOI] [PubMed] [Google Scholar]
- 12.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–582. doi: 10.1007/s00330-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 15.Pugliese F, Mollet NR, Hunink MG, et al. Diagnostic performance of coronary CT angiography by using different generations of multisection scanners: single-center experience. Radiology. 2008;246:384–393. doi: 10.1148/radiol.2462070113. [DOI] [PubMed] [Google Scholar]
- 16.Dewey M, Hoffmann H, Hamm B. CT coronary angiography using 16 and 64 simultaneous detector rows: intraindividual comparison. Rofo. 2007;179:581–586. doi: 10.1055/s-2007-963112. [DOI] [PubMed] [Google Scholar]
- 17.Hausleiter J, Meyer T, Hadamitzky M, et al. Noninvasive coronary computed tomographic angiography for patients with suspected coronary artery disease: the Coronary Angiography by Computed Tomography with the Use of a Submillimeter Resolution (CACTUS) trial. Eur Heart J. 2007;28:3034–3041. doi: 10.1093/eurheartj/ehm150. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann MH, Shi H, Manzke R, et al. Noninvasive coronary angiography with 16-detector row CT: effect of heart rate. Radiology. 2005;234:86–97. doi: 10.1148/radiol.2341031408. [DOI] [PubMed] [Google Scholar]
- 19.Leschka S, Wildermuth S, Boehm T, et al. Noninvasive coronary angiography with 64-section CT: effect of average heart rate and heart rate variability on image quality. Radiology. 2006;241:378–385. doi: 10.1148/radiol.2412051384. [DOI] [PubMed] [Google Scholar]
- 20.Hamoir XL, Flohr T, Hamoir V, et al. Coronary arteries: assessment of image quality and optimal reconstruction window in retrospective ECG-gated multislice CT at 375-ms gantry rotation time. Eur Radiol. 2005;15:296–304. doi: 10.1007/s00330-004-2541-8. [DOI] [PubMed] [Google Scholar]
- 21.Dewey M, Teige F, Laule M, Hamm B. Influence of heart rate on diagnostic accuracy and image quality of 16-slice CT coronary angiography: comparison of multisegment and halfscan reconstruction approaches. Eur Radiol. 2007;17:2829–2837. doi: 10.1007/s00330-007-0685-z. [DOI] [PubMed] [Google Scholar]
- 22.Husmann L, Leschka S, Desbiolles L, et al. Coronary artery motion and cardiac phases: dependency on heart rate—implications for CT image reconstruction. Radiology. 2007;245:567–576. doi: 10.1148/radiol.2451061791. [DOI] [PubMed] [Google Scholar]
- 23.Giesler T, Baum U, Ropers D, et al. Noninvasive visualization of coronary arteries using contrast-enhanced multidetector CT: influence of heart rate on image quality and stenosis detection. AJR. 2002;179:911–916. doi: 10.2214/ajr.179.4.1790911. [DOI] [PubMed] [Google Scholar]
- 24.Cademartiri F, Mollet NR, Runza G, et al. Diagnostic accuracy of multislice computed tomography coronary angiography is improved at low heart rates. Int J Cardiovasc Imaging. 2006;22:101–105. doi: 10.1007/s10554-005-9010-6. discussion, 107-109. [DOI] [PubMed] [Google Scholar]
- 25.Nieman K, Rensing BJ, van Geuns RJ, et al. Noninvasive coronary angiography with multislice spiral computed tomography: impact of heart rate. Heart. 2002;88:470–474. doi: 10.1136/heart.88.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oncel D, Oncel G, Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology. 2007;245:703–711. doi: 10.1148/radiol.2453070094. [DOI] [PubMed] [Google Scholar]
- 27.Stanford W, Burns TL, Thompson BH, Witt JD, Lauer RM, Mahoney LT. Influence of body size and section level on calcium phantom measurements at coronary artery calcium CT scanning. Radiology. 2004;230:198–205. doi: 10.1148/radiol.2301020807. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura N, Sabir A, Kubo T, Lin PJ, Clouse ME, Hatabu H. Correlation between image noise and body weight in coronary CTA with 16-row MDCT. Acad Radiol. 2006;13:324–328. doi: 10.1016/j.acra.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Husmann L, Leschka S, Boehm T, et al. Influence of body mass index on coronary artery opacification in 64-slice CT angiography [in German] Rofo. 2006;178:1007–1013. doi: 10.1055/s-2006-926871. [DOI] [PubMed] [Google Scholar]
- 30.Brodoefel H, Burgstahler C, Tsiflikas I, et al. Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology. 2008;247:346–355. doi: 10.1148/radiol.2472070906. [DOI] [PubMed] [Google Scholar]
- 31.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 32.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewey M, Laule M, Krug L, et al. Multisegment and halfscan reconstruction of 16-slice computed tomography for detection of coronary artery stenoses. Invest Radiol. 2004;39:223–229. doi: 10.1097/01.rli.0000115201.27096.6e. [DOI] [PubMed] [Google Scholar]
- 34.Cademartiri F, Mollet NR, Runza G, et al. Improving diagnostic accuracy of MDCT coronary angiography in patients with mild heart rhythm irregularities using ECG editing. AJR. 2006;186:634–638. doi: 10.2214/AJR.04.1797. [DOI] [PubMed] [Google Scholar]
- 35.Kuettner A, Beck T, Drosch T, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005;45:123–127. doi: 10.1016/j.jacc.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 36.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 37.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(suppl 4):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 38.Alderman EL, Stadius M. The angiographic definitions of the Bypass Angioplasty Revascularization Investigation. Cor Art Dis. 1992;3:1189–1207. [Google Scholar]
- 39.Ringqvist I, Fisher LD, Mock M, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Invest. 1983;71:1854–1866. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 41.Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease: a statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–1965. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- 42.Dewey M, Dubel HP, Schink T, Baumann G, Hamm B. Head-to-head comparison of multislice computed tomography and exercise electrocardiography for diagnosis of coronary artery disease. Eur Heart J. 2007;28:2485–2490. doi: 10.1093/eurheartj/ehl148. [DOI] [PubMed] [Google Scholar]
- 43.Matt D, Scheffel H, Leschka S, et al. Dual-source CT coronary angiography: image quality, mean heart rate, and heart rate variability. AJR. 2007;189:567–573. doi: 10.2214/AJR.07.2078. [DOI] [PubMed] [Google Scholar]
- 44.Wintersperger BJ, Nikolaou K, von Ziegler F, et al. Image quality, motion artifacts, and reconstruction timing of 64-slice coronary computed tomography angiography with 0.33-second rotation speed. Invest Radiol. 2006;41:436–442. doi: 10.1097/01.rli.0000202639.99949.c6. [DOI] [PubMed] [Google Scholar]
- 45.Engelken F, Lembcke A, Hamm B, Dewey M. Determining optimal acquisition parameters for computed tomography coronary angiography: evaluation of a software-assisted, breathhold exam simulation. Acad Radiol. 200;16:239–243. doi: 10.1016/j.acra.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Dewey M, Rutsch W, Hamm B. Is there a gender difference in noninvasive coronary imaging? Multislice computed tomography for noninvasive detection of coronary stenoses. BMC Cardiovasc Disord. 2008;8:2. doi: 10.1186/1471-2261-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meijboom WB, Weustink AC, Pugliese F, et al. Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in women versus men with angina pectoris. Am J Cardiol. 2007;100:1532–1537. doi: 10.1016/j.amjcard.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 48.Halon DA, Gaspar T, Adawi S, et al. Uses and limitations of 40 slice multi-detector row spiral computed tomography for diagnosing coronary lesions in unselected patients referred for routine invasive coronary angiography. Cardiology. 2007;108:200–209. doi: 10.1159/000096778. [DOI] [PubMed] [Google Scholar]
- 49.Pundziute G, Schuijf JD, Jukema JW, et al. Gender influence on the diagnostic accuracy of 64-slice multislice computed tomography coronary angiography for detection of obstructive coronary artery disease. Heart. 2008;94:48–52. doi: 10.1136/hrt.2007.116715. [DOI] [PubMed] [Google Scholar]
- 49.Dewey M, Zimmermann E, Laule M, Rutsch W, Hamm B. Three-vessel coronary artery disease examined with 320-slice computed tomography coronary angiography. Eur Heart J. 2008;29:1669. doi: 10.1093/eurheartj/ehm626. [DOI] [PubMed] [Google Scholar]
- 50.Dewey M, Zimmermann E, Deissenrieder F, et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120:867–875. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 51.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]