Abstract
Objectives
This study was designed to evaluate whether the absence of coronary calcium could rule out ≥50% coronary stenosis or the need for revascularization.
Background
The latest American Heart Association guidelines suggest that a calcium score (CS) of zero might exclude the need for coronary angiography among symptomatic patients.
Methods
A substudy was made of the CORE64 (Coronary Evaluation Using Multi-Detector Spiral Computed Tomography Angiography Using 64 Detectors) multicenter trial comparing the diagnostic performance of 64-detector computed tomography to conventional angiography. Patients clinically referred for conventional angiography were asked to undergo a CS scan up to 30 days before.
Resutls
In all, 291 patients were included, of whom 214 (73%) were male, and the mean age was 59.3 ± 10.0 years. A total of 14 (5%) patients had low, 218 (75%) had intermediate, and 59 (20%) had high pre-test probability of obstructive coronary artery disease. The overall prevalence of ≥50% stenosis was 56%. A total of 72 patients had CS = 0, among whom 14 (19%) had at least 1 ≥50% stenosis. The overall sensitivity for CS = 0 to predict the absence of ≥50% stenosis was 45%, specificity was 91%, negative predictive value was 68%, and positive predictive value was 81%. Additionally, revascularization was performed in 9 (12.5%) CS = 0 patients within 30 days of the CS. From a total of 383 vessels without any coronary calcification, 47 (12%) presented with ≥50% stenosis; and from a total of 64 totally occluded vessels, 13 (20%) had no calcium.
Conclusions
The absence of coronary calcification does not exclude obstructive stenosis or the need for revascularization among patients with high enough suspicion of coronary artery disease to be referred for coronary angiography, in contrast with the published recommendations. Total coronary occlusion frequently occurs in the absence of any detectable calcification.
Keywords: coronary artery disease, calcium score, computed tomography, coronary angiography, coronary stenosis
Coronary artery calcification is highly specific for atherosclerosis (1,2) and is thought to be originated as part of the healing mechanism of usually subclinical plaque rupture events (1,3). Nonetheless, calcification is only 1 of the components of atherosclerotic plaques and develops late in the atherosclerotic process. Because ruptured culprit plaques are not necessarily calcified (1,4), it is widely assumed that coronary calcification predicts events based on the overall atherosclerosis burden rather than the detection of vulnerable plaques per se (5). Thus, it is intuitive to hypothesize that patients without any coronary calcification might present with symptoms caused by significant stenosis.
Coronary calcium scans used to determine calcium score (CS) entail relatively low risks; the radiation exposure is limited to 1.0 to 2.0 mSv, and the test does not utilize iodinated contrast. The main intrinsic limitations of both conventional coronary angiography (CCA) and multidetector computed tomography (MDCT) angiography in terms of patient safety include the use of roentgenograms (6) as well as the need for iodinated contrast agents with their associated risk of adverse reactions (7). The greater the radiation dose, the greater the risk of deterministic and stochastic injury (6). Additionally, mechanical complications of CCA, although infrequent, can be serious. The definition of a CS threshold below which significant obstructive CAD could be virtually excluded would potentially serve as a gatekeeper to coronary angiography. Importantly, the American College of Cardiology (ACC) together with the American Heart Association (AHA) have issued an Expert Consensus Document that states: “For the symptomatic patient, exclusion of measurable coronary calcium may be an effective filter before undertaking invasive diagnostic procedures or hospital admission. Scores <100 are typically associated with a low probability (<2%) of abnormal perfusion on nuclear stress tests, and <3% probability of significant obstruction (>50% stenosis) on cardiac catheterization” (5).
Our aims were to evaluate the prevalence of obstructive and nonobstructive CAD in a multicenter international trial involving patients with suspected CAD and CS = 0 clinically referred for CCA, as well as to evaluate the rate of clinically driven revascularization in those patients. Additionally, we sought to further investigate the in vivo relationship between the absence of coronary calcification and vessel total occlusion.
Methods
The CORE64 (Coronary Evaluation Using Multi-Detector Spiral Computed Tomography Angiography Using 64 Detectors) trial (8) was designed to prospectively include patients ≥40 years of age with suspected CAD and planned clinically-indicated CCA within 30 days to compare the CCA with new-generation 64-detector MDCT angiography. The complete protocol and methods of the CORE64 trial are described elsewhere (9). Briefly, patients were recruited from 9 centers around the world (3 in the U.S., and 1 each in Brazil, Germany, Japan, Singapore, Canada, and the Netherlands). Institutional review boards from all of the participating centers approved the study protocol, and all patients signed the same informed consent form. All local protocols were approved by a central institutional review board at Johns Hopkins Hospital.
Exclusion criteria included known allergy to iodinated contrast media or history of contrast-induced nephropathy, history of multiple myeloma or previous organ transplantation, calculated creatinine clearance of <60 ml/min, atrial fibrillation or uncontrolled tachyarrhythmia, second- or third-degree atrioventricular block, evidence of New York Heart Association functional class III or IV heart failure, known or suspected moderate or severe aortic stenosis, previous coronary cardiac surgery, coronary artery intervention within the last 6 months, known or suspected intolerance to beta-blockers, body mass index >40 kg/m2, and pregnancy. While there were no restrictions to enrollment based on coronary CS to avoid selection bias, as a pre-specified criterion, only patients with Agatston CS ≤600 were included in the primary analysis; patients with CS > 600 were scanned and included in a separate registry. The MDCT raw image as well as CCA digital datasets were transferred for reconstruction and interpretation at distinct core laboratories, where analysis was performed in completely blinded fashion relative to clinical data and cross-modality results.
MDCT acquisition protocol and data analysis
All patients were scanned by similar commercially-available 64-detector MDCT scanners (Aquilion, Toshiba Medical Systems, Tochigi, Japan). The CS scans were obtained using standard techniques with slice collimation 4 × 3.0 mm, 300 mA, 120 kV, and gantry rotation time 0.4 s (5). Offline analyses in remote workstations with dedicated cardiac analysis software (Vitrea2 version 3.0.9.1, Vital Images, Minnetonka, Minnesota) were used to calculate Agatston CS.
CCA data acquisition and analysis
Coronary angiography was performed using standard angiographic techniques within 30 days from the CS study and was clinically driven. Coronary angiographic images were saved in DICOM format and forwarded to the CCA core laboratory.
The coronary tree was divided into 19 segments on the basis of a combination of the CASS (Coronary Artery Surgery Study) and AHA segmentation standards (5). All coronary segments >1.5 mm in diameter were visually analyzed, and in every ≥30% stenosis, quantitative coronary angiography (QCA) was performed using edge-detection techniques (CAAS II QCA Research Version 2.0.1, PIE Medical Imaging, Maastricht, the Netherlands). Segments that could not be accurately visualized because of reduced image quality or inadequate contrast filling were deemed unevaluable. The most significant stenosis was noted for each vessel and patient. Significant stenosis by CCA was defined by luminal diameter narrowing of ≥50% as measured by QCA.
Clinical data collection and interpretation
Research coordinators from each site entered clinical data into a Web-based data management system. All patients referred for CCA from the emergency department (ED) were defined as ED patients; electrocardiogram (ECG), troponin levels, and symptoms were obtained after admission and before CCA. Chest pain at rest was defined as the presence of acute chest, epigastric, neck, jaw, or arm pain or discomfort or pressure without apparent noncardiac cause, ≥20 min in duration within 48 h of hospital admission. Troponin results were adjusted for each specific laboratory standard. The presence of ischemic changes on the ECG (i.e., ≥0.5 mV ST-segment deviation on multiple contiguous leads) denoted a “positive ECG.” The pre-test probability of CAD was derived using the method validated by Morise et al. (10) that uses a scoring system to define low, intermediate, and high pre-test probability of CAD.
Statistical analysis
Continuous data are expressed as mean and SD or median and interquartile ranges when appropriate. Proportion comparisons were performed by chi-square tests, and differences in mean values were compared using the Student t test. Logistic univariable and multivariable analyses were used for determining associations with the presence of coronary obstructive lesions, and clustering by patient was employed for vessel analyses. The accuracy of continuous variables for the detection of obstructive CAD was measured as the area under the receiver-operating characteristic curve (AUC), and the association between the CS and the maximum degree of coronary stenosis in a patient was determined by linear regression analysis. All p values <0.05 were considered to indicate statistical significance. All p values are 2-sided.
Results
Of 316 eligible patients, 4 were excluded because of major protocol deviations, 11 were excluded because CCA was cancelled or performed with small catheters that were inappropriate for QCA analysis (protocol deviation), and 10 were excluded because of MDCT technical failure or ineligibility at the time of scanning, resulting in a total of 291 patients included in the study (mean age 59.3 ± 10.0 years; 78 [26.8%] women) from November 2005 through January 2007. Clinical demographics are summarized in Table 1.
Table 1.
Baseline Characteristics
| Calcium Score
|
||||
|---|---|---|---|---|
| 0 (n = 72) | 1–10 (n = 24) | >10 (n = 195) | p Value | |
| Age, yrs | 55.9 ± 9.9 | 60.1 ± 9.5 | 60.5 ± 9.9 | 0.003 |
|
| ||||
| Men | 43 (60) | 16 (67) | 154 (79) | 0.005 |
|
| ||||
| Race | 0.014 | |||
| White | 41 (57) | 18 (75) | 137 (70) | |
| Black | 11 (15) | 1 (4) | 6 (3) | |
| Asian | 16 (22) | 5 (21) | 45 (23) | |
| Other | 4 (6) | 0 | 7 (4) | |
|
| ||||
| BMI, kg/m2* | 27 ± 4.8 | 28 ± 4.7 | 28 ± 4.3 | 0.55 |
|
| ||||
| Hypertension | 43 (60) | 16 (67) | 133 (68) | 0.43 |
|
| ||||
| Diabetes mellitus | 12 (17) | 3 (13) | 53 (27) | 0.083 |
|
| ||||
| Dyslipidemia | 35 (49) | 14 (58) | 126 (65) | 0.059 |
|
| ||||
| Smoking | 0.048 | |||
| Current | 15 (21) | 3 (13) | 38 (19) | |
| Past | 22 (31) | 7 (29) | 90 (46) | |
| Never | 35 (49) | 14 (58) | 67 (34) | |
|
| ||||
| Family history of CAD | 16 (22) | 4 (17) | 74 (25) | 0.39 |
|
| ||||
| Chest pain (within 30 days) | 38 (53) | 11 (48) | 120 (62) | 0.25 |
|
| ||||
| ED presentation† | 16 (22) | 12 (50) | 61 (31) | 0.035 |
|
| ||||
| Presence of any ≥50% stenosis‡ | 14 (19) | 11 (46) | 138 (71) | <0.001 |
|
| ||||
| Disease distribution by CCA‡ | <0.001 | |||
| No disease | 56 (78) | 11 (46) | 53 (27) | |
| Single-vessel disease | 14 (19) | 10 (42) | 49 (25) | |
| 2-vessel disease | 2 (3) | 3 (13) | 61 (31) | |
| 3-vessel disease | 0 | 0 | 32 (16) | |
|
| ||||
| Revascularization in 30 days | 9 (13) | 6 (25) | 85 (44) | <0.001 |
Values are mean ± SD or n (%).
Calculated as weight in kilograms divided by height in meters squared.
Patients referred for angiography from the emergency department (ED).
Defined by quantitative conventional coronary angiography (CCA).
BMI = body mass index; CAD = coronary artery disease.
Patient-based analysis
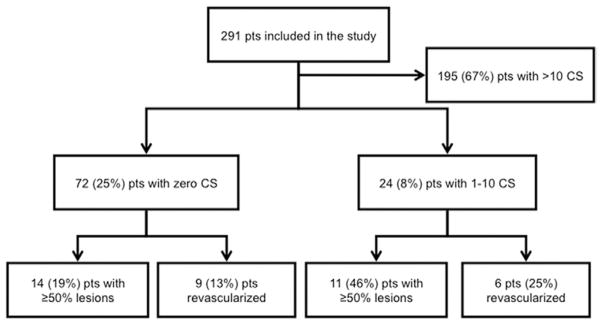
Figure 1 provides a summary of the patient-based results for the prevalence of coronary stenosis and revascularization according to CS. Overall prevalence of ≥50% coronary stenosis by CCA was 56%, and for ≥70% stenosis it was 45%. Patients with 0 CS were younger than patients with >0 CS (age 55.9 ± 9.9 years vs. 60.5 ± 9.8 years, p = 0.001) and the proportion of women was significantly higher (40.3% vs. 22.4%, p = 0.003).
Figure 1. Distribution of Patients on the Basis of CS and Coronary Stenosis.
CS = calcium score; pts = patients.
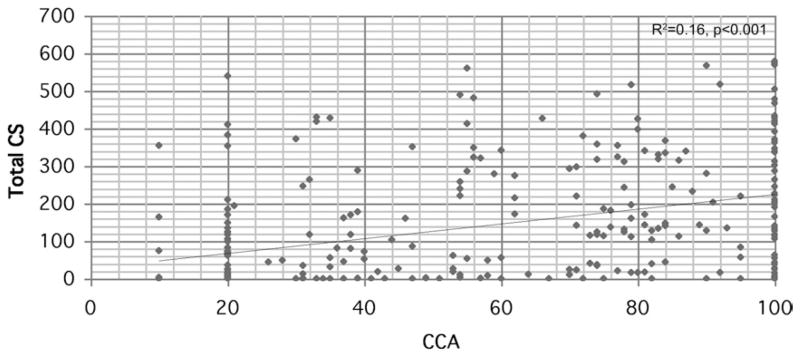
Calcium score weakly correlated with the highest degree of coronary stenosis among individual patients (R2 = 0.16, p < 0.001), as can be seen in Figure 2, and the ability of CS to predict the presence of significant lesions was moderate, with an AUC of 0.77 (95% confidence interval [CI]: 0.72 to 0.83, p < 0.001). The presence of any coronary calcium (CS > 0) significantly increased the chance of a patient having ≥50% coronary stenosis (odds ratio [OR]: 8.1, p < 0.001 after adjusting for age, sex, hypertension, dyslipidemia, family history of premature CAD, diabetes, race, and hospitalization). Conversely, the absence of detectable coronary calcium (CS = 0) significantly increased the likelihood of a patient being free of ≥50% coronary stenosis (OR: 8.8, p < 0.001 after adjusting for the same variables).
Figure 2. Individual Values of CS and Maximum Coronary Stenosis in Subjects.
Linear regression plot showing the poor correlation between the maximum degree of coronary stenosis by conventional coronary angiogram (CCA) and the calcium score (CS) in a patient.
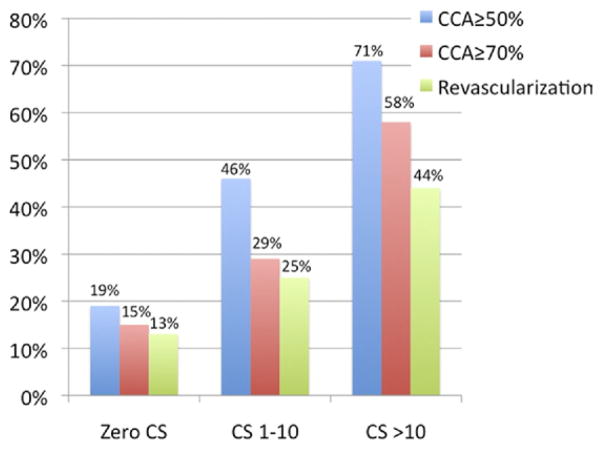
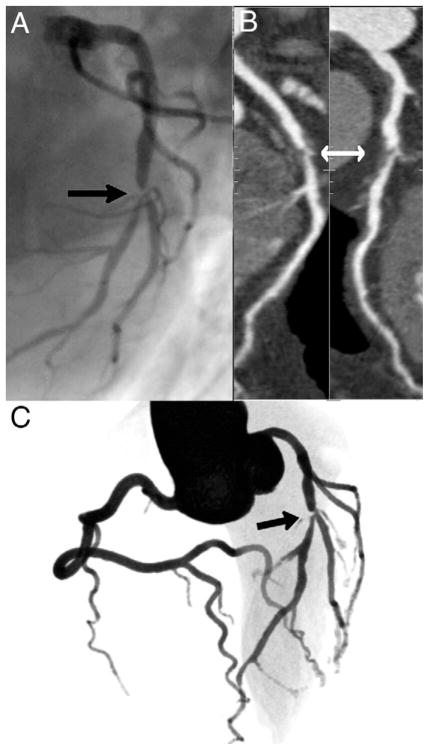
Nonetheless, among 72 patients with 0 CS, 14 (19%, 95% CI: 10% to 28%) had at least 1 ≥50% (mean age 53.8 ± 9.5 years; age range 41 to 76 years) and 11 (15%, 95% CI: 7% to 23%) had at least 1 ≥70% stenosis by CCA, as shown in Figure 3. The overall sensitivity for CS = 0 to predict the absence of ≥50% lesions by CCA on individual participants was 45%, specificity was 91%, negative predictive value was 68%, and positive predictive value was 81%. Figure 4 demonstrates an example of a CS = 0 outpatient who presented with a severe (>70%) stenosis in the left descending coronary artery. The only variable significantly associated with the presence of obstructive CAD in CS = 0 patients was ED presentation, found in both univariable and multivariable analyses after accounting for the risk factors listed in Table 2.
Figure 3. Prevalence of Obstructive CAD and Need for Clinically Indicated Revascularization Among Patients in Different CS Categories.
As the CS increases, the prevalence of obstructive coronary artery disease (CAD) and revascularization also increase (p < 0.001 for all trends). Note that the group of 0 CS patients has high prevalence of obstructive CAD and revascularization. The blue bars indicate CCA ≥50%; the red bars indicate CCA ≥70%; and the green bars indicate revascularization. Abbreviations as in Figure 2.
Figure 4. A Patient From Our Study.
Example of an outpatient 53-year-old man with 0 calcium score and severe stenosis on the midportion of the left descending coronary (arrows), as can be seen on the conventional angiogram (A), on the multiplanar reformatted image (B), and on the 3-dimensional volume rendered image (C) from coronary computed tomography angiogram. The stenosis also extends to a diagonal branch, as can be seen in A and C.
Table 2.
Univariable (Crude) and Multivariable (Adjusted) Logistic Regression Analysis of Factors That May Be Associated With Presence of ≥50% Coronary Lesions in Patients With 0 Coronary Calcium Score (n = 72)
| Crude
|
Adjusted
|
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 0.97 | 0.91–1.03 | 0.375 | 0.99 | 0.92–1.06 | 1.06 |
|
| ||||||
| Male | 1.27 | 0.38–4.26 | 0.698 | 1.18 | 0.26–5.25 | 0.83 |
|
| ||||||
| Race | 0.85 | 0.45–1.59 | 0.291 | 0.85 | 0.43–1.68 | 0.64 |
|
| ||||||
| Diabetes mellitus | 1.48 | 0.34–6.40 | 0.596 | 1.91 | 0.37–10.01 | 0.44 |
|
| ||||||
| Family history of CAD | 2.37 | 0.66–8.50 | 0.184 | 2.62 | 0.66–10.50 | 0.17 |
|
| ||||||
| Dyslipidemia | 1.07 | 0.33–3.44 | 0.908 | 1.5 | 0.37–6.12 | 0.56 |
|
| ||||||
| Hypertension | 1.27 | 0.38–4.27 | 0.698 | 1.31 | 0.36–4.89 | 0.68 |
|
| ||||||
| ED presentation | 3.60 | 1.02–12.68 | 0.046 | 4.7 | 1.13–19.75 | 0.03 |
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 1.
In all, 100 patients were revascularized within 30 days of the CCA procedure, 88 percutaneously and 12 surgically. Revascularization was performed in 9 (13%, 95% CI: 4% to 20%) of the 72 patients with CS = 0, 6 (25%, 95% CI: 8% to 42%) of the 24 patients with CS = 1 to 10, and 85 (44%, 95% CI: 36% to 50%) of the 195 patients with CS > 10 (p < 0.001 for the trend), as shown in Figure 3. Among the 9 patients with CS = 0 who were revascularized, 4 were referred from outpatient clinics and 5 were referred from the ED.
Of the 42 patients who presented to the ED with negative ECG and troponin tests, 8 had CS = 0, of whom 3 (38%) presented with obstructive CAD on CCA. This is contrasted with a lower prevalence (8 [14%] patients of 56 with CS = 0, p = 0.117 for the proportion between ED and ambulatory patients) of obstructive CAD among those who presented as outpatients with 0 CS.
A total of 14 (5%, 95% CI: 2% to 7%) patients had low (median CS = 0; range 0 to 540), 218 (75%, 95% CI: 69% to 79%) had intermediate (median CS = 63; range 0 to 579), and 59 (20%, 95% CI: 15% to 25%) had high (median CS = 118; range 0 to 567) pre-test probability of obstructive CAD (9). For each point increment in the Morise score, a 15% increase in the probability of a significant stenosis by CCA was observed (p = 0.003, 95% CI: 1.05 to 1.26). Among patients with 0 CS, none of the 8 patients with low CAD probability, 12 (21%) of 57 patients with intermediate probability, and 2 (29%) of the 7 patients with high probability had at least 1 significant stenosis on CCA (p < 0.001 for the trend).
Importantly, low CAD probability alone did not rule out significant stenosis, as 4 (29%, 95% CI: 5% to 52%) of 14 patients had obstructive CAD, as compared with 122 (56%, 95% CI: 48% to 62%) of 218 patients with intermediate probability and 37 (63%, 95% CI: 50% to 74%) of 59 patients with high probability. In fact, although the Morise score was associated with obstructive CAD in univariable analysis (OR: 1.15, 95% CI: 1.05 to 1.26, p = 0.003), it did not reach statistical significance when adjusted to CS (OR: 1.09, 95% CI: 0.99 to 1.20), the latter of which was significantly associated in both univariable analysis (OR: 1.006, 95% CI: 1.004 to 1.009, p < 0.001) and after adjusting to the Morise score (OR: 1.006, 95% CI: 1.004 to 1.008, p < 0.001).
Vessel-based analysis
Of the 873 vessels analyzed, 271 (31%) and 189 (22%) had at least 1 ≥50% or ≥70% coronary stenosis by CCA, respectively. The absence of coronary calcification did not exclude the presence of obstructive CAD, because 47 (12%, 95% CI: 9% to 15%) of the 383 coronaries without any calcification had at least 1 ≥50% lesion (28 of them presented as outpatients). Accordingly, the ability of vessel CS to predict ≥50% lesions was only moderate, with an AUC curve of 0.75 (95% CI: 0.72 to 0.78, p < 0.001). Importantly, in this regard, of 64 vessels with complete obstructions, 13 (20%, 95% CI: −10% to 30%) had no calcium.
Discussion
CS as a gatekeeper to angiography
Because CS scans are fast, offer low radiation dose, and predict cardiovascular events over and above conventional risk factors for CAD (5,11,12), they are frequently performed before MDCT angiography in the clinical setting. The main objective of this study was to evaluate the ability of a negative CS scan to rule out significant (≥50%) coronary stenosis, thus assessing the value of a calcium scan as a gatekeeper for angiographic studies in symptomatic patients with suspected CAD. This would prevent iodinated contrast and increased ionizing radiation exposures, prevent the risk of invasive angiography, and reduce cost and patient anxiety. A significant strength of our study was the prospective nature and the thorough analysis of the CCAs and CS scans in blinded, centralized core laboratories. Additionally, the use of QCA as the method for stenosis assessment made the CCA results more robust, given the known overestimation of stenosis severity by visual assessment (13,14).
As expected, we found a positive association between coronary calcium and stenosis, as demonstrated by the linear correlation of the maximum degree of stenosis and the total CS per patient, as well as the strong and independent association between any coronary calcium and the presence of ≥50% stenoses. This is in agreement with perfusion scintigraphic studies that demonstrated that as the CS increases, so does the prevalence and severity of myocardial perfusion abnormalities (15). Nonetheless, our results demonstrate that in patients with clinical indication for CCA, a CS = 0 cannot be used as a gatekeeper, because 19% of these patients had obstructive CAD. The sensitivity and negative predictive value for a CS = 0 to predict the absence of ≥50% lesions on CCA were low (45% and 68%, respectively). Importantly, that 12.5% of patients with CS = 0 were revascularized attests to the clinical significance of such lesions. Although coronary calcification is in general associated with obstructive stenosis and the absence of calcification is correlated with the absence of stenosis, our results indicate that CS cannot be used to safely exclude the presence of obstructive CAD. Emergency department admission was the only variable associated with CS = 0 and obstructive CAD, highlighting that calcium scans are especially not reliable in this setting.
These results are in resonance with previous single-center studies documenting a 24% prevalence of obstructive CAD in patients with 0 CS referred to CCA (16). In another study, 7% of chest pain patients with CS = 0 had obstructive CAD (17), whereas in a third study, the authors found a 16% incidence of coronary plaque in CS = 0 patients (18). Similarly, another, more recent single-center study found that 20% of the patients with CS = 0 presenting to the ED had at least 1 obstructive coronary lesion (19). Conversely, a previous single-center study reported discordant results. In that study, the authors found that CS = 0 patients had <1% prevalence of obstructive CAD by CCA (20). Interestingly, in addition to differences in clinical presentation, the above discrepancies may reflect known racial or ethnic differences in calcification patterns (12), which may also play an important role in the limited ability of CS to exclude significant stenosis.
The present data are particularly important in light of the current Expert Consensus Document endorsed by the ACC and the AHA (5). Based on our findings, this strategy would lead to a high percentage of patients with a missed diagnosis of obstructive CAD in a group of patients with high enough clinical suspicion for CAD to assure an indication for invasive coronary angiography. This strategy could potentially be applicable to patients with a calculated low pre-test probability of CAD and no coronary calcium, because none of these patients had obstructive CAD in our study, although the limited number of such patients (only 8 had no calcium and low pre-test probability of CAD) warrants further investigation before definitive conclusions can be drawn.
Biological considerations
Although highly specific for atherosclerosis, coronary calcification has been shown to develop late in the atherosclerotic process. One of the most accepted classifications of the progression of atherosclerosis endorsed by the AHA and published by Stary et al. (1) proposes an usual pattern of disease progression, in which coronary calcification appears on type V lesions (fibroatheroma) downstream from type IV lesions (formed atheroma). Importantly, the authors recognize that type IV lesions can already rupture and become unstable, leading to acute coronary events (1). This finding is in agreement with results from ex vivo studies documenting that atherosclerotic lesions develop in 5 steps, namely, phase 1, early; phase 2, advanced; phases 3 and 4, acute complicated thrombosed plaques (symptomatic or not, stenotic or not); and phase 5, in which the lesion calcifies, as part of the rupture-healing process (21). In this regard, we demonstrated that significant atherosclerosis can occur in the absence of CT-detectable calcification. Moreover, 20% of the totally occluded vessels had no calcium, emphasizing that calcification is not required for coronary occlusion, often associated with plaque rupture and acute coronary events.
Presence of atherosclerosis and risk for coronary events
It should be emphasized, however, that CS is usually measured in asymptomatic patients with intermediate risk for events, and it has been consistently shown that a CS = 0 in this patient group confers a low risk of coronary events in 5 to 10 years (5,12). Our patient group of those with suspected CAD is at much higher risk, because they represent patients referred for CCA. In this setting, our results indicate that the absence of coronary calcification might not be as protective, because obstructive CAD also has prognostic value. The extent, location, and severity of coronary obstructive lesions are important predictors of outcome and identify subgroups of patients likely to benefit from revascularization (22–24). Importantly, more recent studies have shown that stenosis severity as assessed by MDCT angiography may also independently predict events and total mortality (25–28).
Study limitations
It should be noted that there is an important limitation of the present study: we did not evaluate the prognostic importance of obstructive CAD in patients with CS = 0. This evaluation would be important, although indirect evidence supports the location and severity of coronary stenosis as a prognostic marker.
Conclusions
The present study demonstrates that although coronary calcification is associated with significant stenosis, the absence of coronary calcification does not exclude obstructive CAD or the need for clinically indicated coronary revascularization both in the outpatient department as well as in the ED among patients with a high enough suspicion of CAD prompting an indication for CCA. The absence of coronary calcification should not be used as a gatekeeper and should not prevent a symptomatic patient from undergoing angiography. Furthermore, a large percentage of totally occluded vessels had no evidence of calcium by CT, emphasizing that calcification is not indispensable for plaque rupture and acute coronary events.
Acknowledgments
This study is supported by grants from Toshiba Medical Systems, the Doris Duke Charitable Foundation, the National Heart, Lung, and Blood Institute (RO1-HL66075-01 and HO1-HC95162-01), the National Institute on Aging (RO1-AG021570-01), and the Donald W. Reynolds Foundation. Dr. Miller receives grant support from Toshiba Medical Systems. Dr. Dewey receives speaker’s fees from Bayer, Schering, and Toshiba Medical Systems and grant support from GE Healthcare, Bracco, and Toshiba Medical Systems. Dr. Bush receives speaker’s fees from Bristol-Myers Squibb, Sanofi-Aventis, and Toshiba Medical Systems. Dr. Paul receives advisory fees from Vital Images and grant support and speakers’ fees from Toshiba Medical Systems. Dr. Lima receives grant support from GE Medical Systems and grant support and speakers’ fees from Toshiba Medical Systems. Rita Redberg, MD, served as Guest Editor for this article.
Abbreviations and Acronyms
- AHA
American Heart Association
- AUC
area under the receiver-operating characteristic curve
- CAD
coronary artery disease
- CCA
conventional coronary angiography
- CI
confidence interval
- CS
calcium score
- ECG
electrocardiogram
- ED
emergency department
- MDCT
multidetector computed tomography
- OR
odds ratio
- QCA
quantitative coronary angiography
References
- 1.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 2.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Briguori C, Colombo A, Airoldi F, et al. Nephrotoxicity of low-osmolality versus iso-osmolality contrast agents: impact of N-acetylcysteine. Kidney Int. 2005;68:2250–5. doi: 10.1111/j.1523-1755.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 9.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;19:816–28. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med. 1997;102:350–6. doi: 10.1016/s0002-9343(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 12.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:133–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 13.Desmet W, Willems J, Van Lierde J, Piessens J. Discrepancy between visual estimation and computer-assisted measurement of lesion severity before and after coronary angioplasty. Cathet Cardiovasc Diagn. 1994;31:192–8. doi: 10.1002/ccd.1810310306. [DOI] [PubMed] [Google Scholar]
- 14.Fleming RM, Kirkeeide RL, Smalling RW, Gould KL. Patterns in visual interpretation of coronary arteriograms as detected by quantitative coronary arteriography. J Am Coll Cardiol. 1991;18:945–51. doi: 10.1016/0735-1097(91)90752-u. [DOI] [PubMed] [Google Scholar]
- 15.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Haberl R, Tittus J, Bohme E, et al. Multislice spiral computed tomographic angiography of coronary arteries in patients with suspected coronary artery disease: an effective filter before catheter angiography? Am Heart J. 2005;149:1112–9. doi: 10.1016/j.ahj.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 17.Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99:472–5. doi: 10.1016/j.amjcard.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Hausleiter J, Meyer T, Hadamitzky M, Kastrati A, Martinoff S, Schomig A. Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48:312–8. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 19.Henneman MM, Schuijf JD, Pundziute G, et al. Noninvasive evaluation with multislice computed tomography in suspected acute coronary syndrome: plaque morphology on multislice computed tomography versus coronary calcium score. J Am Coll Cardiol. 2008;52:216–22. doi: 10.1016/j.jacc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Knez A, Becker A, Leber A, et al. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol. 2004;93:1150–2. doi: 10.1016/j.amjcard.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque. Part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Zucker D, Peduzzi P, et al. for the CASS Participating Investigators and Staff. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 23.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69:729–32. doi: 10.1016/0002-9149(92)90495-k. [DOI] [PubMed] [Google Scholar]
- 24.Alderman EL, Corley SD, Fisher LD, et al. for the CASS Participating Investigators and Staff. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS) J Am Coll Cardiol. 1993;22:1141–54. doi: 10.1016/0735-1097(93)90429-5. [DOI] [PubMed] [Google Scholar]
- 25.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto N, Sato Y, Yoda S, et al. Prognostic value of non-obstructive CT low-dense coronary artery plaques detected by multi-slice computed tomography. Circ J. 2007;71:1898–903. doi: 10.1253/circj.71.1898. [DOI] [PubMed] [Google Scholar]
- 27.Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 28.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–43. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]