Abstract
Cancers of the breast and other tissues arise from aberrant decision-making by cells regarding their survival or death, proliferation or quiescence, damage repair or bypass. These decisions are made by molecular signaling networks that process information from outside and from within the breast cancer cell and initiate responses that determine its survival and reproduction. Because the molecular logic of these circuits is difficult to comprehend by intuitive reasoning alone, we present some preliminary mathematical models of the basic decision circuits in breast cancer cells, with an eye to understanding better their susceptibility or resistance to endocrine therapy.
Cancer is a collection of diseases characterized by misregulation of the biomolecular pathways that control cellular processes of metabolism and growth, DNA replication and repair, mitosis and cell division, autophagy and apoptosis (programmed cell death), de-differentiation, motility and angiogenesis1. Molecular cell biologists have amassed a large body of information about the genes and proteins involved in these pathways and have some good ideas about how they go awry in certain types of cancers. However, most of our understanding of the molecular basis of cancer relies on intuitive reasoning about highly complex networks of biochemical interactions2–4. Intuition is clearly not the most reliable tool for querying the behavior of complex regulatory networks. Wouldn’t it be better if we could frame a reaction network in precise mathematical terms and use computer simulations to work out the implications of how the network functions in normal cells and malfunctions in cancer cells?
Of primary interest to cancer biologists is how cancer cells differ from normal cells in their responses to endogenous signals (such as growth and death factors, cell-cell and cell-matrix contacts) and to exogenous treatments (including cytotoxins, radiation, endocrine therapy). Cell responses—signal transduction, cell-fate decisions, adaptation—are intrinsically dynamic phenomena, so it is essential to understand the temporal evolution of biochemical signaling networks in response to particular stimuli. Ordinary differential equations, based on biochemical reaction kinetics, are an appropriate tool for addressing these questions. In principle, ODE models can provide a comprehensive, unified account of many experimental results, and a reliable tool for predicting novel cell behaviors. ODE models of yeast cell growth and division have lived up to these expectations5–8. But is it possible to build useful models of the considerably more complex regulatory networks in mammalian cells? We intend, in this article, to provide a roadmap for a detailed mathematical model of the estrogen signaling network in breast epithelial cells.
Our roadmap is built on the idea that a cell is an information processing system, receiving signals from its environment and its own internal state, interpreting these signals, and making appropriate cell-fate decisions, such as growth and division, movement, differentiation, self-replication, or cell death9. In plants and animals, these cell-level decisions are crucial to the growth, development, survival and reproduction of the organism. A hallmark of cancer cells is faulty decision-making: they proliferate when they should be quiescent, they survive when they should die, they move around when they should stay put1. To understand the origin, pathology and vulnerabilities of cancer cells, we must understand how normal cells make decisions that promote the survival of the organism as a whole, and how cancer cells make decisions that promote their own survival and reproduction with fatal results for the organism they inhabit10.
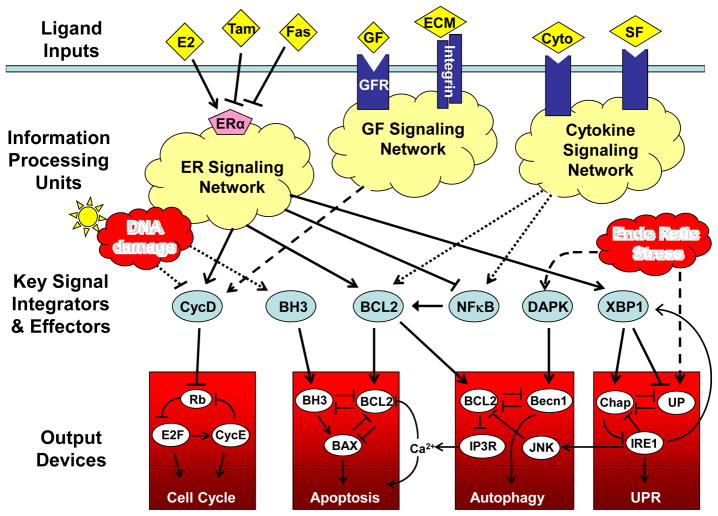
Viewing the living cell as an information processing system, we can (conceptually, at least) distinguish an input level, a processing core, and output devices (FIG. 1). As input, a cell receives information from its surroundings (such as extracellular ligands that bind to cell-surface receptors or to nuclear hormone receptors) and from its internal state (such as DNA damage, misfolded proteins, low energy level and oxidative stress). These signals are processed by chemical reaction networks that integrate information from many sources and compute a response. A response could take the form of the activation or inactivation of key integrator or effector proteins that drive the cell’s functional output devices. Of most interest to cancer biologists are the functional modules that control cell growth and division, motility and invasion, stress responses and apoptosis.
FIGURE 1. The estrogen receptor signaling network in breast epithelial cells.
Extracellular signals, such as estrogen (E2), growth factors (GF), survival factors (SF), cytokines (cyto) and extracellular matrix (ECM), bind to receptor proteins, which initiate a complex series of chemical reactions within the cell, culminating ultimately in the activation of a set of integrator/effector proteins. These proteins sum up the positive and negative signals coming from the information processing units, and then they drive responses in the downstream decision modules and stress modules. The ‘cell cycle module’ coordinates DNA synthesis and mitotic cell division with cell growth and the body’s need for a continuous supply of new cells in the right place at the right time. The ‘apoptosis module’ rids the body of damaged, worn out, or un-needed cells. The ‘unfolded protein module’ is a response to stresses such as starvation and reactive oxygen species. Under conditions of extreme stress, the ‘autophagy module’ can provide the cell with a supply of energy and raw materials. Tamoxifen (Tam) and Faslodex (Fas) are inhibitors of the estrogen receptor (ERα), and they are commonly used to kill estrogen-dependent breast cancer cells.
Although there may be many ways to subdivide the information processing system of a cell, there is clearly a need to divide and conquer the staggering complexity of the system11–13. Fortunately, it is not necessary to model the protein reaction networks in all their complexity, because it is usually possible to identify a set of key ‘integrator’ and ‘decision-making’ proteins that determine the cell’s response to input signals. Unfortunately, living cells are not like human-engineered systems, where modules are designed not to interfere much with one another14. Cellular modules have significant crosstalk and shared components. So although we must divide the system into modules to reduce the initial modeling complexity, we must also put the modules back together into a complete system that properly captures the information processing capabilities of living cells.
A comprehensive model of the information processing system of mammalian cells is not yet available, but we can provide a roadmap of how a modeler might capture, in mathematical form, the molecular events controlling cell growth, proliferation, damage responses and programmed death. Our approach is illustrated by simple mathematical models of the mechanisms involved in the initial susceptibility of breast cancer cells to anti-estrogen therapy and their subsequent development of anti-estrogen resistance. The value of this enterprise will be measured ultimately by new insights provided by the model into the logic and functionality of estrogen-receptor signaling pathways and by the effectiveness of the model as a tool for experimental prediction and design.
The estrogen receptor and breast cancer
The growth and proliferation of breast tissue is normally responsive to estrogen, a steroid hormone that binds to and activates the estrogen receptors (ERα and ERβ), nuclear transcription factors that regulate the expression of genes that orchestrate survival and proliferation. In many neoplastic breast cells, the ER signaling network contributes to controlling the relative rates of cell proliferation and programmed cell death, with pro-survival and proliferation signals overwhelming pro-death and quiescence signals.
Of the 180,000 cases of invasive breast cancer newly diagnosed each year in the USA, more than 70% express ERα (ER+ cells)15. Many of these tumors are initially responsive to endocrine therapy alone, and many also respond to a combination of cytotoxic chemotherapies16,17. Endocrine therapy can consist of anti-estrogens (such as Tamoxifen or Fulvestrant), which bind to and neutralize ER, and/or aromatase inhibitors (such as Letrozole or Exemestane), which block the synthesis of estrogen. Unfortunately, many ER+ tumors recur as incurable, endocrine-resistant cancer cells18.
The advantages and limitations of endocrine therapies have been known for over 30 years. To make significant new advances in the treatment of advanced breast cancer, we need a better understanding of the ER signaling network19. For example, how does ER signaling function in normal breast cells? How does it malfunction in ER+ breast cancer cells that respond to endocrine therapy? How is it further misregulated in antiestrogen-resistant and aromatase inhibitor-resistant cancer cells? And how are cell survival and proliferation maintained in ER cancer cells?
FIGURE 1 provides an overview of the ER signaling network and its major output devices (cell growth and division, apoptosis and autophagy). From a combination of classical molecular biology studies and high-throughput transcriptomic analyses, we identified an initial set of transcription factors that are intimately connected with ER signaling in breast cancer cell lines20. Subsequently, we and others have established the functional relevance of several of these factors, including NFκB, a pro-survival transcription factor that is highly expressed in hormone-resistant cells compared to hormone-sensitive cells21–23; IRF1, a pro-death transcription factor that is down-regulated in endocrine-resistant cells24–27; and XBP1, a transcription factor involved in the unfolded protein response and induction of autophagy, which is highly expressed in its active (spliced) variant in endocrine-resistant cells27,28. Given that FIG. 1 correctly captures some of the key regulatory components and their interactions, interpreting it at a mathematical level should provide novel and useful insights into the decision-making processes in normal and transformed breast cells.
Mathematical modeling perspective
As useful as FIG. 1 is for providing a guide to intuitive reasoning about the probable effects of perturbations to this network, a molecular interaction graph can deliver much more information about the potential dynamic behavior of the control system if it is translated into reasonable mathematical terms suitable for computer simulation. In that case, the computer can keep track of the dynamic consequences of multiple and often conflicting interactions29,30.
In keeping with our roadmap perspective, we will begin by modeling the separate modules in FIG. 1: the ‘decision modules’ (cell cycle and apoptosis), the ‘stress modules’ (autophagy and unfolded protein response), and the ‘signal processing modules’ (ER and growth factor signal transduction networks). As we go along, we will describe how the ‘integrator and effector proteins’ mediate communication between these modules.
Cell cycle module
We start with the module controlling DNA replication and division, events that are triggered by cyclins and cyclin-dependent kinases (CDKs)31,32, the retinoblastoma protein (RB), which regulates members of the E2F-family of transcription factors, and late-G1 and early-S phase cyclins (type A and E cyclins)33–35. RB also down-regulates the expression of ribosomal RNA genes, thereby inhibiting the production of new ribosomes and the cell’s capacity for increased protein synthesis34,36–39. Hence, we can think of RB as a major ‘brake’ on cell growth and division, which must be released before a cell can grow and divide. This release is the job of the cyclin D-dependent kinases (CYCD1–3 in combination with CDK4 or CDK6), which phosphorylate RB and reduce its inhibitory effect on E2Fs33,40. CYCD is an unstable protein, and it is not present in quiescent cells because its transcription regulators, including MYC, AP1 and β-catenin, are inactive. These transcription regulators are activated by proliferative signals, such as growth factors, cytokines, nuclear hormone receptors and integrins, causing the concentration of CYCD to rise. The increasing concentration of CYCD must be converted into a digital decision: shall the cell undergo a new round of DNA replication and division or remain in G1 phase?
This decision is apparently made by a bistable switch, created by the interaction between RB, E2F and cyclin E (CYCE)41,42. The molecular interactions among these three proteins (FIG. 2a) are characterized by a positive feedback loop (E2F up-regulates CYCE, CYCE–CDK2 inactivates RB, RB inactivates E2F) and an auto-activation loop (E2F family members can activate their own transcription). According to mathematical models (Ref. 41 and supplementary information S1 (Box)), these sorts of positive feedback loops create a signal-response curve (FIG. 2b) with alternative stable steady states: an OFF state (RB active, E2F low, CYCE low), and an ON state (RB inactive, E2F high, CYCE high). The OFF state corresponds to quiescent cells (arrested in G1 phase of the cell cycle) and the ON state to proliferating cells (progression through S-G2-M phases)43. Careful measurements of the expression of CYCD and E2F in fibroblast cells responding to changes in serum concentration confirm the predictions of the model (FIG. 2c and d)41. Entry into the mammalian cell cycle in these non-cancerous cells is controlled by a bistable switch that is biased to the OFF state by signals that down-regulate CYCD and E2F (and possibly by signals that up-regulate RB), and that is switched ON by signals that up-regulate CYCD and E2F (see supplementary information S1 (Box) for further information). In cancer cells, this crucial decision point may still be intact44, but it is likely that in many cancers the bistable switch is disrupted by mutations that break the underlying feedback circuits45.
FIGURE 2. Bistable switch controlling the G1-to-S transition in mammalian cells.
a | Wiring diagram. Cyclin E-dependent kinase (CycE) promotes the transition of mammalian cells from G1 phase of the cell cycle into S phase. Quiescent cells are arrested in G1 by the retinoblastoma protein (RB), which binds to and inhibits E2F, a family of transcription factors, some of which can promote CYCE gene expression. Phosphorylation of RB by cyclin-dependent kinases compromises its inhibitory effect on E2Fs. The initial phosphorylation of RB is accomplished by cyclin D-dependent kinase (CYCD). After the G1/S transition is made, RB is maintained in its inactive (phosphorylated) form by CYCE and by cyclin A- and B-dependent kinases that are active in S-G2-M. Quiescent cells, which have very little CYCD, can be induced to proliferate by transcription factors (such as Myc, Fos, Jun) that are upregulated by growth factors in serum. These transcription factors promote the expression of both CYCD and E2F genes, and E2F proteins upregulate their own transcription. b | Signal-response curve. The wiring diagram in panel a is converted into a set of nonlinear differential equations (supplementary information S1 (Box)), and the steady-state levels of CYCD and E2F are plotted as functions of serum concentration in the growth medium. Although CYCD level increases smoothly with serum concentration (‘rheostat’), the E2F distribution exhibits a bimodal dependence on serum concentration (‘toggle switch’)41,43. c and d | Experimental verification in rat embryonic fibroblasts (REF 52 cells); from Yao et al.41, used by permission. Red curves: serum concentration is raised from 0 to final percentage. Green curves: serum concentration is raised to 20% for 5 hours, then lowered to final percentage. GFP fluorescence is driven by an E2F promoter (left) or a CYCD promoter (right). Histograms of CYCD expression shift smoothly up-and-down with final serum percentage. E2F histograms show a bimodal dependence on final serum percentage between ~0.2% and 2%. Notice a distinct hysteresis effect in the E2F response: on shifting serum up (red curves), bistability is not observed until serum level exceeds ~1%, but on shifting serum down (green curves), bistability is maintained to serum levels < 0.2%.
CYCD as a key signal integrator
CYCD is a classic integrator and effector protein: its level integrates the pro- and anti-proliferative signals being received by the cell, and the activity of CYCD-dependent kinases effects the commitment of the cell to a new round of DNA synthesis and cell division. Pro-proliferative signals, such as estrogen acting through ERα, increase CYCD expression by activating its transcription factors. By contrast, cell-cell contacts result in cytoplasmic sequestration of β-catenin and down-regulation of CYCD expression. One of the hallmarks of many cancers is the loss of contact inhibition. A different mode of action is exemplified by the anti-proliferation factor, transforming growth factor β (TGF-β, which up-regulates synthesis of p27Kip1, an inhibitor of CYCD-dependent kinases. In breast cancer cells TGF-β is a key regulator of the anti-proliferative effects of anti-estrogens46,47, and CYCD expression is associated with poor response to Tamoxifen48. In summary, we might think of CYCD level as a rheostat that varies up and down continuously in response to pro- and anti-proliferative signals, respectively41, 43. When CYCD level exceeds a certain threshold, the RB-E2F-CYCE switch converts the CYCD signal into a discrete decision to begin a new round of DNA synthesis and cell division. Triggering this switch is therefore dependent on many factors affecting the level of active CYCD, such as estrogen, β-catenin, p27Kip1 and TGF-β49,50.
Once a cell has committed to the G1/S transition, it will proceed through S, G2 and M phases, even if the pro-proliferative signals are removed and CYCD disappears. However, when this cell divides and the other classes of cyclins (A, B and E) are degraded, RB will return and arrest the cell in a quiescent state.
Apoptosis module
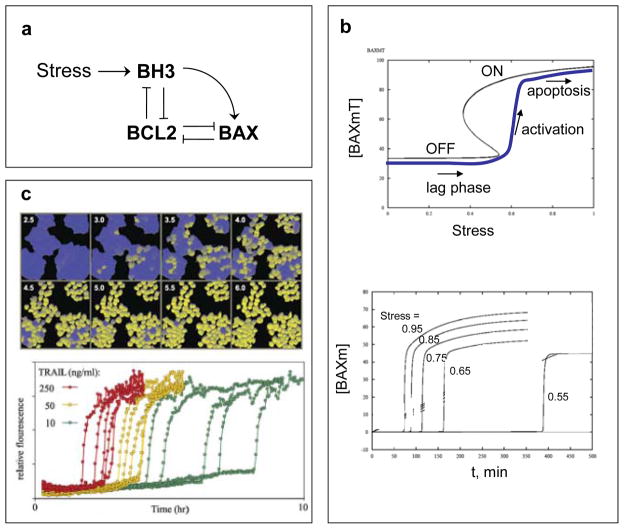
Like the decision to enter a new round of mitotic cell division, the commitment to apoptosis must reach an all-or-none decision point that is biased one way or the other by the summation of pro-death and pro-survival signals. Although the evidence is not conclusive, we believe that the irrevocable commitment to apoptosis is normally made in the activation of BAX and amplified by mitochondrial outer-membrane permeabilization (MOMP)51. In our mathematical models MOMP is governed by a bistable switch involving three families of proteins: BCL2-like, BH3-only and BAX-like proteins (FIG. 3a)52–57. In the OFF state, BAX is inactivated by binding to BCL2. Accumulation of BH3 proteins can displace BCL2 from BAX, leading to the self-amplifying activation of BAX (the ON state). Active BAX proteins create pores in the mitochondrial outer membrane, thereby releasing cytochrome C and SMAC to the cytoplasm, where cytochrome C promotes activation of ‘executioner’ caspases, and SMAC neutralizes the inhibitor of apoptosis (IAP) proteins that inhibit caspases55.
FIGURE 3. Bistable switch controlling apoptosis in mammalian cells.
a | Wiring diagram. Programmed cell death is triggered by activation of BAX proteins in the outer membrane of mitochondria. Active BAX causes the membrane to become permeable to proteins, such as cyctochrome C and SMAC, which induce the activation of proteases (caspases) and other hydrolytic enzymes that disassemble the macromolecules of the cell. BAX is activated by BH3-family proteins and kept inactive by binding to BCL-family proteins. BH3 proteins also bind to BCL proteins55. b | Signal-response curve. A mathematical model of the wiring diagram is presented in supplementary information S2 (Box). Top: steady-state concentration (black curve) of total membrane-bound BAX, [BAXm]T = [BAXm] + [BAXm:BCL2], as a function of [Stress]. For intermediate levels of Stress, the network has two stable steady states: an OFF state with low total level of BAXm, all of it complexed to BCL2 (i.e., [BAXm] ≈ 0); and an ON state with high total level of BAXm, most of it un-complexed to BCL2 (i.e., [BAXm] ≈ [BAXm]T). Bottom: time-course of active BAX. Each simulation is started in the naïve state: no stress, low level of BH3, [BAXm] ≈ 0. At t = 0, [Stress] is raised to a final value that varies (from one simulation to the next) from 0.55 to 0.95. The time-course for final [Stress] = 0.95 is plotted as the blue curve on the upper graph. Notice that, in each simulation, there is a long lag time followed by an abrupt activation of BAX (and subsequently an irreversible activation of caspases). The duration of the lag phase is a decreasing function of [Stress]. c | Experimental verification in HeLa cells (cervical cancer cell line); from Albeck et al.51, used by permission. Top: Cells are treated with 50 ng/ml TRAIL (TNF-related apoptosis-inducing ligand) and assayed for caspase activity by cleavage of an artificial substrate manufactured by the cell. Cells with high caspase activity are pseudo-colored yellow. Notice the long lag time before caspases are activated in any cells, then individual cells activate caspases abruptly, but there is a wide dispersion of activation times among cells. Bottom: traces of caspase activity in single cells activated by different concentrations of TRAIL.
Based on our models (FIG. 3 and supplementary information S2 (Box)), the apoptosis-switch is in the OFF or ON position depending on the balance between BCL2-like proteins (the ‘brakes’) and BH3-only proteins (the ‘accelerators’). When the ratio of accelerators to brakes exceeds a certain critical value (the point in FIG. 3b where the OFF state disappears), then BAX is abruptly activated and MOMP-induced activation of executioner caspases ensues. The ‘snap-action’ kinetics of MOMP are consistent with this view of a bistable switch activating BAX proteins (FIG. 3c).
Whether or not apoptosis is controlled by a bistable ‘decision’ module and (if so) what is its biochemical basis are questions of considerable discussion in the literature51,53–56,58–62. This debate illustrates that fundamentally different mathematical models may be equally consistent with limited experimental data. Fortunately, the different models can be used to design additional experiments that will distinguish between alternative mechanisms. We suppose that apoptosis is governed by a one-way (irreversible) bistable switch, because apoptosis in normal cells is an all-or-nothing affair. We interpret the evidence to suggest that the decision is made upstream of MOMP and that the BH3-BCL-BAX module is the most likely locus for the bistable switch. Although the apoptotic switch may be disabled in some cancer cells, it is likely still functional in most cancers but more difficult to engage. For instance, in breast cancer cell lines, ER-mediated signaling upregulates antiapoptotic proteins, including BCL223,63,64, BCL-W64 and BCL365, making it harder to trigger apoptosis. Endocrine-therapy, by inactivating ER, moves these levels in the opposite direction, making it easier to trigger apoptosis.
Damage-processing modules
Intracellular damage-processing modules have crucial roles in maintaining the viability of cells and organisms. For example, DNA damage activates kinases that phosphorylate and stabilize the transcription factor p53 (Ref.66). p53 up-regulates genes encoding repair enzymes and p21Cip1, which binds to and inhibits the activity of CDKs, thereby preventing the damaged cell from beginning a new round of DNA replication. DNA damage also prevents S- or G2-phase cells from entering mitosis by pathways involving inhibitory phosphorylation of CDKs and the production of stoichiometric CDK inhibitors. If the damage cannot be repaired in a timely fashion, then p53 upregulates production of BH3 proteins in an attempt to activate the apoptosis module. Whether apoptosis occurs or not depends on BH3 level relative to BCL proteins, thereby integrating the influences of pro- and anti-apoptotic agents, including ER-mediated signals. Effective mathematical models of these DNA damage-processing pathways, based on the cell-proliferation and cell-death networks described in FIG. 2 and 3, have been published52,55,66,67.
Other common inducers of stress in normal and cancer cells include hypoxia and oxidative stress68,69. These types of stress cause problems in intermediary metabolism, electron transport in mitochondria and protein folding in the endoplasmic reticulum, and these problems induce characteristic responses by the cell. The first response to low-level stress is a pro-survival mechanism, autophagy, which is thought to provide a steady supply of energy and raw materials by degrading the cell’s own proteins and lipids70. Unremitting stress can lead to cell death, either by excessive autophagy or by activation of apoptosis71.
Autophagy module
The autophagosome is a subcellular organelle containing a selection of cellular proteins and other macromolecules that are destined for destruction. When the autophagosome fuses with a lysosome, its contents are hydrolyzed to amino acids and other small metabolites that can be used by the cell as sources of energy and raw materials for the biosynthesis of essential substances. Autophagy is controlled in large part by Beclin-1 (BECN1), a myosin-like, BCL2-interacting protein. When not bound to BCL2, BECN1 participates in a multi-protein complex that initiates the earliest stages of autophagosome assembly70–72.
In FIG. 4 we propose a simple model for the initiation of autophagy (for details, see supplementary information S3 (Box). In this model, autophagy is regulated not as a toggle switch (as in FIG. 2 and 3) but as a rheostat, ramping up smoothly to higher levels as stress increases. As autophagy ramps up, BCL2 is released from its association with BECN1 and with the inositol trisphosphate receptor (IP3R). The results can be quite variable, including survival (moderate autophagy and inhibition of apoptosis), or apoptotic cell death, or autophagic cell death. Whether the autophagic response is functioning normally or abnormally in breast cancer cell lines is a matter of current investigation.
FIGURE 4. The interplay between autophagy and apoptosis.
a | Wiring diagram. In response to stress, both Beclin-1 and BCL2 are phosphorylated, causing the BCL2:Beclin-1 complex to dissociate103,104. Beclin-1 is phosphorylated by DAPK (death-associated protein kinase), and BCL2 is phosphorylated by JNK, a downstream target of the IRE1 arm of the UPR75,103,104. Detachment from the extracellular matrix provides an additional stress to the cells105. Free Beclin-1 participates (with other components, such as Atg8/LC3) in initiating autophagy71. Autophagy can suppress the stress signal by providing the cell with ATP and raw materials for new protein synthesis. BCL2 phosphorylation also allows the inositol trisphosphate receptor (IP3R) to release calcium from the endoplasmic reticulum to the cytoplasm103,106. If the concentration of calcium in the cytoplasm gets large enough, apoptosis is triggered106. Activated caspases cleave Beclin-1 and turn off autophagy107. Hence, under low stress conditions, autophagy promotes cell survival; at moderate stress, it may lead to autophagic cell death; and for large stress, calcium release may stimulate apoptosis by the intrinsic (mitochondrial) pathway. b | Numerical simulations. The wiring diagram is converted into a set of ordinary differential equations (supplementary information S3 (Box)), and the fraction of cells predicted to stain positive for autophagic vesicles is plotted as a function of time. 1) Detached and high stress; 2) attached and moderate stress; 3) detached and no stress. c, d | Experimental evidence in MCF-7 cells (breast cancer cell line); from Petrovski et al.105, used by permission. Panel c: Percentage of cells that have taken up monodansylcadaverin (MDC) into autophagic vacuoles on days 1—7 after the following treatments: (▽) 10% fetal calf serum (FCS) on poly-HEMA coated plates; non-adherent cells undergo autophagy by day 6 and later die by apoptosis (not shown). (▼) 3% charcoal-stripped FCS on poly-HEMA plates; estrogen-depleted, non-adherent cells undergo autophagy by day 4 and later die by apoptosis. (○) 3% charcoal-stripped FCS + Tamoxifen; estrogen-depleted, ER-inhibited cells undergo autophagy by day 4. (□) 3% charcoal-stripped FCS + Tamoxifen on poly-HEMA plates; estrogen-depleted, ER-inhibited, non-adherent cells undergo autophagy by day 2 and later die by apoptosis. Panel d (top): Transmission electron microscopy of (1) untreated, control cells and of (2) autophagic cells (○) on day 4, showing numerous autophagic vacuoles (white) in the cytoplasm. Panel d (bottom): Western blot analysis of LC3 expression in autophagic cells (○) on days 0—7; c = positive control.
The unfolded protein response (UPR) module
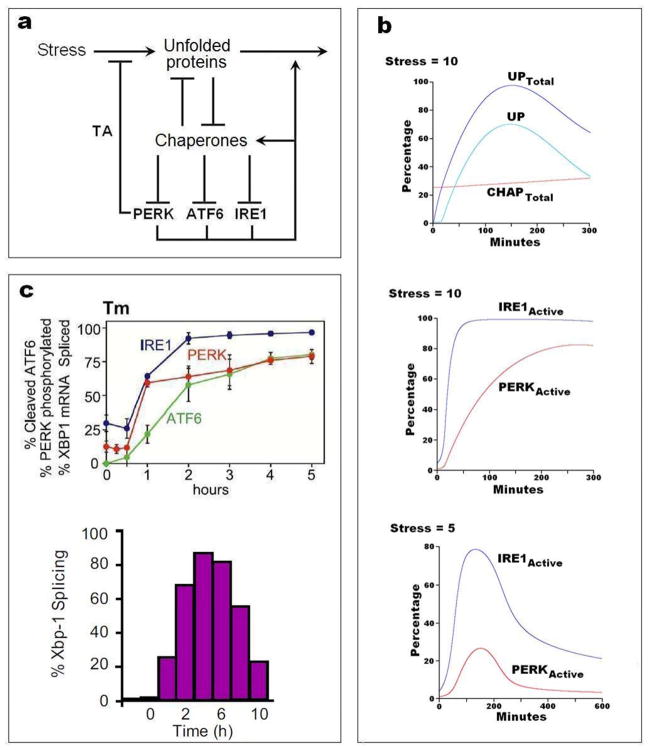
The accumulation of unfolded proteins in the endoplasmic reticulum causes a characteristic response73 that is intended to relieve the immediate problem (by re-folding or degrading the non-functional proteins and reducing the rate of protein synthesis) and to deal with the underlying stress (by inducing autophagy). The molecular basis of the UPR is well understood, and useful mathematical models have been presented in the literature73–76. In FIG. 5 and supplementary information S4 (Box) we present a simplified model of UPR, to illustrate the basic principles of this damage-response module. Both autophagy and the UPR are strongly implicated in the responsiveness of breast cancer cells to anti-estrogens19, 77.
FIGURE 5. The unfolded protein response in mammalian cells.
a | Wiring diagram. The unfolded protein response (UPR) is a coordinated cellular program that is induced by the accumulation of unfolded and misfolded proteins in the lumen of the endoplasmic reticulum73. Elevated levels of unfolded/misfolded proteins are brought down by chaperones, foldases, oxidoreductases, glycosylases and proteases (we refer to all these components simply as chaperones). As unfolded proteins pull chaperones away from the lumenal domains of PERK, ATF6, and IRE1, then these three proteins up-regulate the expression of certain genes that reduce the stress and increase the protein folding capacity of the endoplasmic reticulum. TA = translation attenuation. b | Numerical simulations computed from the mathematical model in supplementary information S4 (Box). Top and middle: at t = 0, with the model at a stable resting state, a stress of 10 (arbitrary units) is added to the differential equations, and the response of the system is plotted in terms of total unfolded protein, total chaperone, and protein species not bound to chaperones (UP, IRE1active and PERKactive). Bottom: response to a stress of 5 a.u. applied at t = 0. c | Experimental verification in non-cancerous cells. Top: Time courses of the three stress sensors after treatment of CHO (chinese hamster ovary) cells with 10 μg/mL of tunicamycin (Tm) to induce protein misfolding; from DuRose et al.108, used by permission. Bottom: Time course of IRE1 activity (assayed as % splicing of XBP1 mRNA) in HEK293 (human embryonic kidney) cells treated with 5 μg/mL of tunicamycin; from Li et al.109, used by permission.
Signaling crosstalk between modules
To impose some order on the tangled web of macromolecular interactions within a living cell it is necessary to think in terms of functional modules. Nonetheless, we must take into account that there is significant crosstalk between modules; for example, the apparent mutual inhibition between autophagy and apoptosis71. Crosstalk between signal transduction pathways is well known; for example, overlapping cell survival pathways are implicated in the notorious plasticity of cells in response to cancer chemotherapeutics, including endocrine therapies49,78–80. Understanding the mechanisms and roles of crosstalk is a crucial concern as we try to assemble modules into more complex networks that can account for the complex responses of cells under realistic conditions, including the development of drug resistance in breast cancer cells.
As an example, consider the epidermal growth factor (EGF) family of signaling pathways. A growing body of evidence demonstrates that endocrine therapy, which is often effective in regression of early-stage ER+ breast cancer, may provoke cellular adaptation processes including activation of a spectrum of estrogen-regulated survival and proliferation genes, such as those involved in EGF signaling81–86. Interestingly, Pratt et al.65 observed that a population of MCF-7 cells could be divided into two sub-groups following withdrawal of estrogen: most cells retain an absolute dependency on estrogen and die as a result of the treatment, but some cells become estrogen-independent by switching to alternative survival and proliferation signals. If endocrine treatment is discontinued within a short period of time, before the resistant cells have established their phenotype by genetic or epigenetic modifications, then the acquired resistance can be reversed. For example, a population of MCF-7 cells that over-express EGFR or HER2 exhibit a bimodal distribution of receptors (FIG. 6c), and this distribution pattern can be reversibly controlled by manipulating the exposure of the cells to estrogen87–89. We take these observations as evidence for a bistable survival-switch working through crosstalk between ER and EGF signaling pathways. Although little is known about how it works, mutual inhibition between the two pathways is a likely source of bistability. In FIG. 6 and supplementary information S5 (Box) we present a simple model that could account for the effects of estrogen withdrawal on MCF-7 cells.
FIGURE 6. Crosstalk between ER and EGF signaling pathways.
a | Wiring diagram. E2 = estrogen, ER = estrogen receptor alpha, GF = growth factors, GFR = growth factor receptors. GFR and its downstream signaling network are suppressed by ligand-dependent ER signaling (‘ER classic’) but promoted by ligand-independent ER signaling (‘ER non-classic’). Increased GF signaling after E2 withdrawal can shift ER from classical to non-classical signaling. GF signaling can up-regulate its own activity. b | Signal-response curve. Differential equations describing the wiring diagram are provided in supplementary information S5 (Box). The steady-state activity of GFR is plotted as a function of [E2] in the growth medium (from 0 to 5 pM). At any given [E2] in this range, a cell may express a low or high level of GFR (upper and lower solid lines; the middle dotted line indicates a branch of unstable steady states). c | Experimental evidence; from Liu et al.88, modified and used by permission. Bimodal distribution of HER2 (ErbB-2) expression in a monoclonal culture of HER2-overexpressing MCF-7 cells. The bimodal distribution pattern is induced by a change in [E2]. Cells grown on charcoal-stripped calf serum (CCS) for 5 weeks or longer are depleted of E2 and constitute a single population of high HER2-expressing cells by flow-cytometric analysis (top plot, abundance of HER2 as detected by fluorescent antibody). Replacing CCS with FCS (fetal calf serum, containing E2) leads to the emergence over time (5 weeks, 3 months, 4 months) of a second population of low HER2-expressing cells. Similar results have also been observed in EGFR (ErbB-1)-overexpressing MCF-7 cells87. d | Model simulation. In supplementary information S5 (Box), noise terms are added to the differential equations to take stochastic effects into account. 2000 cells are simulated with [E2] = 0 (corresponding to CCS) for 3.3 weeks, which results in a single population of high HER2-expressing cells (top plot). Setting [E2] = 5 pM (corresponding to FCS) leads to a time-course of HER2 expression histograms similar to the experimental observations in panel c.
Crosstalk in cell signaling networks generates a large selection of discrete, stable, self-organized states; creating a degree of cell-fate plasticity that is necessary for a cell to switch adaptively and robustly among these different states. Although this plasticity is essential for normal cells to survive in noisy environments and to differentiate properly in response to various developmental cues, it may lead to robust development of resistance to cytotoxic drugs. Hence, understanding how crosstalk controls these phenotypic switches is of first importance for designing more effective cancer treatment strategies.
Present Realities and Future Directions
Mathematical modeling of intracellular molecular regulatory networks is an essential part of a systems-approach to cancer biology90. Intuitive reasoning must be complemented by mathematical models when the molecular regulatory network under consideration is large, complex and interconnected, and when we are dealing with quantitative aspects of signaling and control91. A well crafted mathematical model allows us to integrate crucial information about the genetics, molecular biology and physiology of cancer cells into a quantitative hypothesis, amenable to computer simulation, mathematical analysis, and detailed comparison to experimental data. By computing the behavior of the model under a variety of experimental conditions and comparing these simulations to the observed behavior of cells, we can determine whether our hypothetical molecular mechanism is sufficient to account for the known behavior of cells. If and when our model passes this first test (‘post-diction’), we can use it to predict the behavior of cells under novel experimental conditions, and use these quantitative predictions to test the efficacy of the model. Even when are models are not in full agreement with experiments, we can be confident that the problem is in some part of the model rather than in faulty reasoning about its consequences. Indeed, the model can help us track down the origin of the problem(s) and consider alternative hypotheses.
Mathematical modeling of intercellular control systems related to breast cancer development, though still in its infancy, is beginning to provide some useful insights. For example, a sophisticated model of p53 signaling in MCF-7 cells successfully predicted a novel role for Wip1 in a negative feedback loop from p53 to an upstream kinase in the DNA damage-signaling pathway92. A recent model of the HER2-ER signaling network identified novel drug targets for trastuzumab-resistant cells93. A dynamic model of combinatorial cancer therapy suggested promising treatment strategies that were subsequently verified experimentally94.
In this review, we have presented a roadmap for the mathematical modeling component of an integrative, systems biology of endocrine responsiveness in ER+ breast cancer. The hard work is yet to be done: formulating and verifying models, estimating kinetic parameters, making non-obvious predictions and testing them by quantitative experimental measurements. Is it just a matter of time before an effective, integrated model of regulatory networks in breast cancer cells is informing the next wave of experiments and therapies? Successful ODE models of cell cycle regulation, growth factor signaling, programmed cell death and the unfolded protein response suggest that there are no fundamental barriers to accurate, predictive models of complex control systems in mammalian cells. On the other hand, effective modeling is hampered by many significant differences—genetically and phenotypically—among different types of mammalian cells. Extending models to cancer cells, which are notoriously unstable genetically, will be even more difficult. High-throughput data collection and analysis will be helpful in identifying important differences among cell types and between normal cells and their cancerous derivatives95–97.
Despite the seeming wealth of data on molecular mechanisms controlling mammalian cell proliferation and stress responses, there is often a distinct lack of reliable, quantitative measurements of these mechanisms under conditions that are relevant to model formulation and testing. Another impediment to modeling intracellular control systems stems from the fact that the behavior of populations of cells (e.g., graded response to drug treatment) may not reflect the behavior of single cells (all-or-none decision in response to the drug). At present, modelers are still struggling with how best to cope with all these competing issues.
In addition, there are other relevant theoretical considerations that we have not described in this review. For instance, realistic models of molecular regulation must take into account the compartmentalization of eukaryotic cells. For another, the restricted number of genes, mRNAs and protein molecules in a single cell generate unavoidable stochastic fluctuations in molecular control networks. Intracellular information-processing systems must be robust to these fluctuations in most circumstances, although in some circumstances these fluctuations may be exploited to generate a range of possible outcomes (‘bet-hedging’). Thirdly, our models only bridge the scales from molecular networks to cell physiology. Breast tumors exist in a complex microenvironment that affects the dynamic signaling within and among cancer cells. Modeling these effects adds new layers of complexity. Other kinds of mathematical models are needed to describe how cells are organized into multi-cellular tissues, interacting with extracellular matrix, recruiting vasculature, and eventually metastasizing to distant parts of the body98–100. Models at these higher scales are beginning to be integrated with molecular-level descriptions of intracellular control systems (e.g., the cell cycle) and of intercellular communication (e.g., Wnt signaling)101,102.
We expect that all these modeling challenges will soon be overcome and that a new generation of mathematical models will soon be providing new insights into the molecular foundations of endocrine responsiveness in breast cancer.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants U54-CA149147 (to RC), R01-GM078989 (to JJT and WB), by NSF Grants DMS-0342283 (to JJT and Paul Brazhnik), DBI-0904340 (to AV), and by fellowships to CC and IT provided by the Virginia Tech graduate program in Genetics, Bioinformatics and Computational Biology.
Glossary
- Module
A set of molecular interactions that accomplishes a specific task in a cell, such as committing a cell to a new round of DNA replication
- Crosstalk
Interactions among modules that alter the behavior of the modules in isolation
- Unfolded protein response
Cellular response to the accumulation of stress-induced, misfolded proteins in the endoplasmic reticulum. Cells down-regulate general protein synthesis and up-regulate production of chaperones that help damaged proteins to re-fold properly or to be degraded
- Autophagy
Degradation of a cell’s own components, using its lysosomal machinery, to remove damaged organelles and/or to provide energy and raw materials for adaptation and survival under stressful conditions
- Molecular interaction graph (wiring diagram)
Representation of a set of biochemical reactions involving co-regulated genes and proteins; for example, a signal transduction pathway or a transcription factor network
- Dynamic behavior
The characteristic change over time of a molecular regulatory network in response to a specific pattern of input signals
- Bistable switch
A regulatory network that can persist, under identical external conditions, in either of two stable states (‘on’ or ‘off’) depending on its recent history
- Signal-response curve
The functional dependence of the output of a molecular regulatory network (e.g., the activity of a transcription factor) on changing values of its input (e.g., concentration of a growth factor in the extracellular medium)
- Rheostat
A variable resistor, used to provide continuous control over the current through a circuit (e.g., the dimmer knob on a light fixture)
- Endocrine therapy
Manipulation of endocrine levels or responsiveness to prevent the survival and proliferation of hormone-dependent cancer cells
- Plasticity
The ability of a regulatory network, in the face of interference or damage, to adapt and maintain something akin to its normal function
- Stochastic fluctuations
Random variations in the numbers of molecules of mRNAs and proteins due to the unpredictable nature of chemical reactions at the molecular level
Footnotes
John Tyson’s homepage: http://mpf.biol.vt.edu
Yue Wang’s homepage: http://www.cbil.ece.vt.edu
Robert Clarke’s homepage: http://clarkelabs.georgetown.edu
References
- 1.Hanahan D, Weinberg, Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hornberg JJ, Bruggeman FJ, Westerhoff HV, Lankelma J. Cancer: a Systems Biology disease. Biosystems. 2006;83:81–90. doi: 10.1016/j.biosystems.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Faratian D, Moodie SL, Harrison DJ, Goryanin I. Dynamic computational modeling in the search for better breast cancer drug therapy. Pharmacogenomics. 2007;8:1757–61. doi: 10.2217/14622416.8.12.1757. [DOI] [PubMed] [Google Scholar]
- 4.Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis. 2010;31:2–8. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novak B, Pataki Z, Ciliberto A, Tyson JJ. Mathematical model of the cell division cycle of fission yeast. Chaos. 2001;11:277–286. doi: 10.1063/1.1345725. [DOI] [PubMed] [Google Scholar]
- 6.Chen KC, et al. Integrative analysis of cell cycle control in budding yeast. Mol Biol Cell. 2004;15:3841–62. doi: 10.1091/mbc.E03-11-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberghina L, Coccetti P, Orlandi I. Systems biology of the cell cycle of Saccharomyces cerevisiae: From network mining to system-level properties. Biotechnol Adv. 2009;27:960–78. doi: 10.1016/j.biotechadv.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Barik D, Baumann WT, Paul MR, Novak B, Tyson JJ. A model of yeast cell-cycle regulation based on multisite phosphorylation. Mol Syst Biol. 2010;6:405. doi: 10.1038/msb.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray D. Protein molecules as computational elements in living cells. Nature. 1995;376:307–12. doi: 10.1038/376307a0. [DOI] [PubMed] [Google Scholar]
- 10.Shiraishi T, Matsuyama S, Kitano H. Large-scale analysis of network bistability for human cancers. PLoS Comput Biol. 2010;6:e1000851. doi: 10.1371/journal.pcbi.1000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 12.Rao CV, Arkin AP. Control motifs for intracellular regulatory networks. Annu Rev Biomed Eng. 2001;3:391–419. doi: 10.1146/annurev.bioeng.3.1.391. [DOI] [PubMed] [Google Scholar]
- 13.Wolf DM, Arkin AP. Motifs, modules and games in bacteria. Curr Opin Microbiol. 2003;6:125–34. doi: 10.1016/s1369-5274(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 14.Del Vecchio D, Ninfa AJ, Sontag ED. Modular cell biology: retroactivity and insulation. Mol Syst Biol. 2008;4:161. doi: 10.1038/msb4100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 16.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 17.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–42. [PubMed] [Google Scholar]
- 18.Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- 19.Clarke R, et al. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:8–20. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, et al. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780) Cancer Res. 2002;62:3428–37. [PubMed] [Google Scholar]
- 21.Riggins RB, Zwart A, Nehra R, Clarke R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol Cancer Ther. 2005;4:33–41. [PubMed] [Google Scholar]
- 22.Zhou Y, et al. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nehra R, et al. BCL2 and CASP8 regulation by NF-kappaB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells. FASEB J. 2010;24:2040–55. doi: 10.1096/fj.09-138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouker KB, et al. interferon regulatory factor-1 mediates the proapoptotic but not cell cycle arrest effects of the steroidal antiestrogen ICI 182,780 (faslodex, fulvestrant) Cancer Res. 2004;64:4030–9. doi: 10.1158/0008-5472.CAN-03-3602. [DOI] [PubMed] [Google Scholar]
- 25.Bowie ML, et al. Interferon-regulatory factor-1 is critical for tamoxifen-mediated apoptosis in human mammary epithelial cells. Oncogene. 2004;23:8743–55. doi: 10.1038/sj.onc.1208120. [DOI] [PubMed] [Google Scholar]
- 26.Bouker KB, et al. Interferon regulatory factor-1 (IRF-1) exhibits tumor suppressor activities in breast cancer associated with caspase activation and induction of apoptosis. Carcinogenesis. 2005;26:1527–35. doi: 10.1093/carcin/bgi113. [DOI] [PubMed] [Google Scholar]
- 27.Gomez BP, et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21:4013–27. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 28.Davies MP, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123:85–8. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 29.Tyson JJ, Chen K, Novak B. Network dynamics and cell physiology. Nat Rev Mol Cell Biol. 2001;2:908–16. doi: 10.1038/35103078. [DOI] [PubMed] [Google Scholar]
- 30.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15:221–31. doi: 10.1016/s0955-0674(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 31.Novak B, Tyson JJ. A model for restriction point control of the mammalian cell cycle. J Theor Biol. 2004;230:563–79. doi: 10.1016/j.jtbi.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 32.Gerard C, Goldbeter A. Temporal self-organization of the cyclin/Cdk network driving the mammalian cell cycle. Proc Natl Acad Sci U S A. 2009;106:21643–8. doi: 10.1073/pnas.0903827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol. 1996;8:805–14. doi: 10.1016/s0955-0674(96)80081-0. [DOI] [PubMed] [Google Scholar]
- 34.Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur J Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- 35.Seville LL, Shah N, Westwell AD, Chan WC. Modulation of pRB/E2F functions in the regulation of cell cycle and in cancer. Curr Cancer Drug Targets. 2005;5:159–70. doi: 10.2174/1568009053765816. [DOI] [PubMed] [Google Scholar]
- 36.Cavanaugh AH, et al. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374:177–80. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- 37.White RJ, Trouche D, Martin K, Jackson SP, Kouzarides T. Repression of RNA polymerase III transcription by the retinoblastoma protein. Nature. 1996;382:88–90. doi: 10.1038/382088a0. [DOI] [PubMed] [Google Scholar]
- 38.White RJ. RNA polymerases I and III, growth control and cancer. Nat Rev Mol Cell Biol. 2005;6:69–78. doi: 10.1038/nrm1551. [DOI] [PubMed] [Google Scholar]
- 39.Goodfellow SJ, White RJ. Regulation of RNA polymerase III transcription during mammalian cell growth. Cell Cycle. 2007;6:2323–6. doi: 10.4161/cc.6.19.4767. [DOI] [PubMed] [Google Scholar]
- 40.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–90. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 41.Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol. 2008;10:476–82. doi: 10.1038/ncb1711. [DOI] [PubMed] [Google Scholar]
- 42.Lee TJ, Yao G, Bennett DC, Nevins JR, You L. Stochastic E2F activation and reconciliation of phenomenological cell-cycle models. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novak B, Tyson JJ, Gyorffy B, Csikasz-Nagy A. Irreversible cell-cycle transitions are due to systems-level feedback. Nat Cell Biol. 2007;9:724–8. doi: 10.1038/ncb0707-724. [DOI] [PubMed] [Google Scholar]
- 44.Doisneau-Sixou SF, et al. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer. 2003;10:179–86. doi: 10.1677/erc.0.0100179. [DOI] [PubMed] [Google Scholar]
- 45.Yao G, Tan C, West M, Nevins JR, You L. Origin of bistability underlying mammalian cell cycle entry. Mol Syst Biol. 2011;7:485. doi: 10.1038/msb.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arteaga CL, Koli KM, Dugger TC, Clarke R. Reversal of tamoxifen resistance of human breast carcinomas in vivo by neutralizing antibodies to transforming growth factor-beta. J Natl Cancer Inst. 1999;91:46–53. doi: 10.1093/jnci/91.1.46. [DOI] [PubMed] [Google Scholar]
- 47.Brandt S, Kopp A, Grage B, Knabbe C. Effects of tamoxifen on transcriptional level of transforming growth factor beta (TGF-beta) isoforms 1 and 2 in tumor tissue during primary treatment of patients with breast cancer. Anticancer Res. 2003;23:223–9. [PubMed] [Google Scholar]
- 48.Rudas M, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14:1767–74. doi: 10.1158/1078-0432.CCR-07-4122. [DOI] [PubMed] [Google Scholar]
- 49.Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–66. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- 50.Ren Y, et al. Dual effects of TGF-beta on ERalpha-mediated estrogenic transcriptional activity in breast cancer. Mol Cancer. 2009;8:111. doi: 10.1186/1476-4598-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol. 2008;6:2831–52. doi: 10.1371/journal.pbio.0060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang T, Brazhnik P, Tyson JJ. Exploring mechanisms of the DNA-damage response: p53 pulses and their possible relevance to apoptosis. Cell Cycle. 2007;6:85–94. doi: 10.4161/cc.6.1.3705. [DOI] [PubMed] [Google Scholar]
- 53.Chen C, et al. Modeling of the role of a Bax-activation switch in the mitochondrial apoptosis decision. Biophys J. 2007;92:4304–15. doi: 10.1529/biophysj.106.099606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui J, Chen C, Lu H, Sun T, Shen P. Two independent positive feedbacks and bistability in the Bcl-2 apoptotic switch. PLoS One. 2008;3:e1469. doi: 10.1371/journal.pone.0001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T, Brazhnik P, Tyson JJ. Computational analysis of dynamical responses to the intrinsic pathway of programmed cell death. Biophys J. 2009;97:415–34. doi: 10.1016/j.bpj.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang XP, Liu F, Cheng Z, Wang W. Cell fate decision mediated by p53 pulses. Proc Natl Acad Sci U S A. 2009;106:12245–50. doi: 10.1073/pnas.0813088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang XP, Liu F, Wang W. Coordination between cell cycle progression and cell fate decision by the p53 and E2F1 pathways in response to DNA damage. J Biol Chem. 2010;285:31571–80. doi: 10.1074/jbc.M110.134650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eissing T, et al. Bistability analyses of a caspase activation model for receptor-induced apoptosis. J Biol Chem. 2004;279:36892–7. doi: 10.1074/jbc.M404893200. [DOI] [PubMed] [Google Scholar]
- 59.Legewie S, Bluthgen N, Herzel H. Mathematical modeling identifies inhibitors of apoptosis as mediators of positive feedback and bistability. PLoS Comput Biol. 2006;2:e120. doi: 10.1371/journal.pcbi.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puszynski K, Hat B, Lipniacki T. Oscillations and bistability in the stochastic model of p53 regulation. J Theor Biol. 2008;254:452–65. doi: 10.1016/j.jtbi.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 61.Shoemaker JE, Doyle FJ., 3rd Identifying fragilities in biochemical networks: robust performance analysis of Fas signaling-induced apoptosis. Biophys J. 2008;95:2610–23. doi: 10.1529/biophysj.107.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun T, Lin X, Wei Y, Xu Y, Shen P. Evaluating bistability of Bax activation switch. FEBS Lett. 2010;584:954–60. doi: 10.1016/j.febslet.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 63.Cameron DA, et al. Effective tamoxifen therapy of breast cancer involves both antiproliferative and pro-apoptotic changes. Eur J Cancer. 2000;36:845–51. doi: 10.1016/s0959-8049(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 64.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS One. 2010;5:e8604. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pratt MA, et al. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toettcher JE, et al. Distinct mechanisms act in concert to mediate cell cycle arrest. Proc Natl Acad Sci U S A. 2009;106:785–90. doi: 10.1073/pnas.0806196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer. 2009;9:371–7. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–32. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 72.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 74.Trusina A, Papa FR, Tang C. Rationalizing translation attenuation in the network architecture of the unfolded protein response. Proc Natl Acad Sci U S A. 2008;105:20280–5. doi: 10.1073/pnas.0803476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verfaillie T, Salazar M, Velasco G, Agostinis P. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clarke R, et al. Endoplasmic reticulum stress, the unfolded protein response, and gene network modeling in antiestrogen resistant breast cancer. Hormone Molecular Biology and Clinical Investigation. 2011 doi: 10.1515/hmbci.2010.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin C, et al. Estrogen up-regulation of p53 gene expression in MCF-7 breast cancer cells is mediated by calmodulin kinase IV-dependent activation of a nuclear factor kappaB/CCAAT-binding transcription factor-1 complex. Mol Endocrinol. 2002;16:1793–809. doi: 10.1210/me.2002-0006. [DOI] [PubMed] [Google Scholar]
- 79.Ak P, Levine AJ. p53 and NF-kappaB: different strategies for responding to stress lead to a functional antagonism. FASEB J. 2010;24:3643–52. doi: 10.1096/fj.10-160549. [DOI] [PubMed] [Google Scholar]
- 80.Calzone L, et al. Mathematical modelling of cell-fate decision in response to death receptor engagement. PLoS Comput Biol. 2010;6:e1000702. doi: 10.1371/journal.pcbi.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004;11:623–41. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- 82.Xia W, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gee JM, et al. Deciphering antihormone-induced compensatory mechanisms in breast cancer and their therapeutic implications. Endocr Relat Cancer. 2006;13 (Suppl 1):S77–88. doi: 10.1677/erc.1.01274. [DOI] [PubMed] [Google Scholar]
- 84.Nicholson RI, et al. Growth factor signalling in endocrine and anti-growth factor resistant breast cancer. Rev Endocr Metab Disord. 2007;8:241–53. doi: 10.1007/s11154-007-9033-5. [DOI] [PubMed] [Google Scholar]
- 85.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13:1950–4. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 86.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller DL, et al. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for a role of EGFR in breast cancer growth and progression. Cell Growth Differ. 1994;5:1263–74. [PubMed] [Google Scholar]
- 88.Liu Y, el-Ashry D, Chen D, Ding IY, Kern FG. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 89.Sonne-Hansen K, et al. Breast cancer cells can switch between estrogen receptor alpha and ErbB signaling and combined treatment against both signaling pathways postpones development of resistance. Breast Cancer Res Treat. 2010;121:601–13. doi: 10.1007/s10549-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 90.Aldridge BB, Burke JM, Lauffenburger DA, Sorger PK. Physicochemical modelling of cell signalling pathways. Nat Cell Biol. 2006;8:1195–203. doi: 10.1038/ncb1497. [DOI] [PubMed] [Google Scholar]
- 91.Clarke R, et al. The properties of high-dimensional data spaces: implications for exploring gene and protein expression data. Nat Rev Cancer. 2008;8:37–49. doi: 10.1038/nrc2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell. 2008;30:277–89. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sahin O, et al. Modeling ERBB receptor-regulated G1/S transition to find novel targets for de novo trastuzumab resistance. BMC Syst Biol. 2009;3:1. doi: 10.1186/1752-0509-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bagheri N, Shiina M, Lauffenburger DA, Korn WM. A dynamical systems model for combinatorial cancer therapy enhances oncolytic adenovirus efficacy by MEK-inhibition. PLoS Comput Biol. 2011;7:e1001085. doi: 10.1371/journal.pcbi.1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pujana MA, et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;39:1338–49. doi: 10.1038/ng.2007.2. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, et al. Differential dependency network analysis to identify condition-specific topological changes in biological networks. Bioinformatics. 2009;25:526–32. doi: 10.1093/bioinformatics/btn660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–15. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 99.Anderson AR, Quaranta V. Integrative mathematical oncology. Nat Rev Cancer. 2008;8:227–34. doi: 10.1038/nrc2329. [DOI] [PubMed] [Google Scholar]
- 100.Byrne HM. Dissecting cancer through mathematics: from the cell to the animal model. Nat Rev Cancer. 2010;10:221–30. doi: 10.1038/nrc2808. [DOI] [PubMed] [Google Scholar]
- 101.Owen MR, Alarcon T, Maini PK, Byrne HM. Angiogenesis and vascular remodelling in normal and cancerous tissues. J Math Biol. 2009;58:689–721. doi: 10.1007/s00285-008-0213-z. [DOI] [PubMed] [Google Scholar]
- 102.van Leeuwen IM, et al. An integrative computational model for intestinal tissue renewal. Cell Prolif. 2009;42:617–36. doi: 10.1111/j.1365-2184.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomenius MJ, Distelhorst CW. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J Cell Sci. 2003;116:4493–9. doi: 10.1242/jcs.00829. [DOI] [PubMed] [Google Scholar]
- 104.Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5:720–2. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 105.Petrovski G, et al. Clearance of dying autophagic cells of different origin by professional and non-professional phagocytes. Cell Death Differ. 2007;14:1117–28. doi: 10.1038/sj.cdd.4402112. [DOI] [PubMed] [Google Scholar]
- 106.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 107.Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–9. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 108.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci U S A. 2010;107:16113–8. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.