Abstract
Blood flow is a key factor in the efficacy of radiofrequency (RF) ablation treatment of solid tumors. We hypothesized that vasoactive drugs can modulate tumor blood flow and thereby improve the outcome of this thermal ablation approach. To verify this hypothesis, we measured the tumor perfusion changes in response to phenylephrine (PE) and hydralazine (HYZ) using a CT perfusion method in a rat subcutaneous tumor model. The coagulation sizes induced by RF ablation alone, RF ablation with PE and RF ablation with HYZ were compared. Results demonstrated that HYZ produced a marked decrease in entire tumor and tumor rim blood flow of 31.1 and 29.1%; while PE insignificantly change tumor blood flow (5.1% decrease in whole tumor and 6.0% decrease in tumor rim). A markedly greater coagulation area (0.59 cm2 ± 0.24) was observed when HYZ was administered before RF ablation. No difference was noted in the coagulation area induced by RF ablation alone or the combination of PE injection followed by RF ablation (0.29 cm2 ± 0.13 vs. 0.30 cm2 ± 0.18). Results suggest that HYZ decreases subcutaneous tumor blood flow and enhances the coagulation size induced by RF ablation. PE has little influence on tumor blood flow and does not improve ablation.
Keywords: Radiofrequency ablation, CT perfusion, Blood flow, Vasomodulation, Phenylephrine, Hydralazine
INTRODUCTION
Image guided percutaneous radiofrequency (RF) ablation has become a widely accepted minimally invasive treatment for solid tumors since its introduction in 1995.37 This technique provides efficacious, cost-effective management of localized cancer sites.13 Although many technical innovations, such as perfusion electrode,15 internally-cooled10 or multi-tined expandable electrodes,19 have been utilized to increase the volume of ablation, incomplete tumor coagulation and recurrence still occur as a result of large tumor size, irregular contours and elevated blood flow. Solbiati et al.42 reported that the recurrence rate of liver metastasis after RF ablation correlated with tumor size, with a recurrence rate of 16.5% for lesions < 3 cm and 56.1% for lesions > 3 cm in a 6–52 months follow-up period. Hori et al.24 reported that in 104 patients after RF ablation the 3 year recurrence rate was 14.8% for tumors < 2.5 cm and > 50% for tumors > 2.5 cm. Further research is needed to enhance the ablation size and decrease the recurrence rate.
One strategy for increasing RF ablation efficacy is decreasing tumor blood flow to reduce the “heat sink” effect. Mechanical methods such as intravascular embolization45 and surgical or intravascular occlusion 46 of tumor or organ supply vessels have been used to decrease tumor blood flow prior to ablation. However, these methods are invasive, interrupt the blood flow of both tumor and surrounding normal organ tissue, and have the potential to increase morbidity. In contrast, systemic administration of vasoactive drugs is less invasive and more convenient to perform. Several groups explored the correlation between the change of organ blood flow induced by vasoactive drugs, such as vasopressin,17 epinephrine,26 arsenic trioxide,23 and the size of coagulation induced by RF ablation. The results suggest that decreasing organ blood flow enhances the effect of RF ablation and increasing organ blood flow decreases the effect of RF ablation. While somewhat successful, these efforts to modulate the blood flow had a systemic effect and were non-specific to the tumor-bearing organ, or affected the cardiac output. A more tumor-specific strategy would be of significant benefit.
Previous research has shown that tumor vessels are immature, lack normal smooth muscle32 and pericyte structure,32,41 or keep maximal vasodilation due to low pH produced by hypermetabolism,31 and thus do not react to vasoactive drugs. The response of normal vessels to vasoactive drugs therefore has an indirect effect on tumor blood flow. That is, vasoconstrictors produce constriction of normal vessels, which “pushes” blood into the tumor and increases tumor blood flow. In contrast, vasodilators dilate normal vessels and divert blood flow from the tumor vessel bed. This difference in the reactive response between tumor and normal vessels should provide a more tumor-specific blood flow modulation strategy to enhance tumor RF ablation treatment.
In this study, we sought to determine whether the intravenous injection of a vasoconstrictor—phenylephrine or a vasodilator—hydralazine could be used to modulate blood flow in a subcutaneous rat tumor model. We also attempted to determine which agent could best enhance the size of tumor RF ablation. CT perfusion was used to quantify changes of blood flow in tumor and normal tissue.
MATERIALS AND METHODS
Drugs
(R)-(−)-phenylephrine hydrochloride (PE) was purchased from Sigma–Aldrich, Inc. (St. Louis, MO), and diluted to 0.001% in distilled water. Hydralazine hydrochloride (HYZ) powder was purchased from MP Bio-medicals, Inc. (Solon, OH), and dissolved with distilled water to a concentration of 0.5%. The drug solution was filtered with a 0.22-µm filter (MILLEX®GP, Carrigtwohill, Co. Cork, Ireland) before injection.
Animals and Tumor Model
A well-established rat tumor model, DHD/K12/TRb adenocarcinoma in the subcutaneous tissue of BDIX rats, was used in this study. The animal experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. For all experiments, procedures were carried out under general gas anesthesia, which has 1% isoflurane with an O2 flow rate of 1 L/min (EZ150 Isoflurane Vaporizer, EZ Anesthesia™). The DHD/K12/TRb rat colorectal carcinoma cell line, originating from a 1,2-dimethylhydrazine-induced colon adenocarcinoma in BDIX rats, was kindly donated by the laboratory of Dr. W.G. Pitt, Brigham Young University (original source was the European Collection of Cell Cultures), and stored in liquid nitrogen. The cells were cultured in RPMI-1640 with 10% fetal bovine serum and passaged weekly. Six-week-old male BDIX rats (body weight 150–180 g, purchased from Charles River Laboratories Inc., Wilmington, MA) were used for inoculation. Tumors were inoculated subcutaneously with a bilateral injection of 0.05 mL DHD/K12/TRb cell suspension (2 × 106 cells/mL) on the upper back using a 26-gauge hypodermic needle. Tumor size was measured weekly using calipers. Tumors were grown for 5–6 weeks. The maximum longitudinal diameter (a) and the maximum transverse diameter (b, perpendicular to maximum axis) were measured with calipers before treatment. Tumor volume (V) was calculated with the following formula: V = 0.5 × ab2.
Overall Experimental Design
The study included two experiments. Experiment 1 was performed to explore the effects of vasoactive drugs on tumor blood flow. Twenty-four rats with 48 tumors were used in this experiment. The blood flow of tumors in response to PE (10 µg/kg, 24 tumors) or HYZ (5 mg/kg, 24 tumors) was evaluated by functional CT. In experiment 2, the influence of PE or HYZ on tumor coagulation size induced by RF ablation was explored. An additional 17 rats were used in this experiment. Seventeen tumors (1 per rat) with tumor size 1.0–1.4 cm were randomly divided into 3 groups: control (n = 6), PE (n = 6) and HYZ (n = 5) groups. In the control group, tumors were treated with RF ablation alone (80 °C ± 2, 2 min). Tumors in the PE or HYZ groups were treated with i.v. PE or HYZ followed by RF ablation (80 °C ± 2, 2 min) 1 or 5 min after PE (10 µg/kg) or HYZ (5 mg/kg) administration, respectively. The determination of delay times for PE and HYZ was based on the effect-time curves reported in previous studies.34,43 Coagulation sizes were measured 24 h after treatment.
Radiofrequency Ablation
A 500 kHz RF generator (Radionics, Cool-tip RF system, Radionics Inc., Burlington, MA) and a custom-designed 21-gauge monopolar electrode needle were used for RF ablation. The hair on the back surrounding the tumor and abdomen was shaved before treatment. A standard grounding pad was placed on the rat’s abdomen with ultrasound gel to ensure proper contact. The tumor area was sterilized with betadine. A small incision was made with a scalpel on the skin surrounding the tumor to avoid burning, and the electrode was inserted into the tumor center. During ablation, the temperature was manually adjusted to 80 °C ± 2 for 2 min. The power was approximately 2–3 W. After ablation, the incision was closed with sutures.
CT Perfusion
CT perfusion was performed with a multi-slice CT scanner (SOMATOM Sensation Open, Siemens Medical System, Erlangen, Germany). The rat was laid prone on the CT table and the rat position was adjusted so that the centers of both tumors were parallel to the scan plane. An initial planar scan was carried out to determine the optimal position for the perfusion scan. Then, the perfusion scan was performed with a 0.5 mL bolus of contrast (Optiray® 240, Tyco Healthcare, Mallinckrodt Inc., St. Louis, MO) administrated through a 24-g catheter (EXELint® Safelet Cath, Exelint Inc., Los Angeles, CA) in the tail vein. The perfusion protocol, which imaged 12 consecutive slices every 0.5 s for 40 s, was executed using the following parameters: axial scan, 80 kV; 150 mAs; rotation time, 0.5 s; detector width, 1.2 mm; reconstruction width, 2.4 mm; field of view, 60 mm; matrix, 512 × 512. Thus, each perfusion scan obtained 960 images. One minute after PE (10 µg/kg) or 5 min after HYZ (5 mg/kg) i.v. administration (time delays differed because of drug activity), the perfusion scan was repeated as above. The interval time between baseline and post-drug perfusion scan was 10 min to allow for contrast agent clearance. The animal was void of movement to ensure the consistence of the scan positions during the pre and post perfusion scan.
Image Analysis
Image analysis was performed by one observer (H.W.) who has 5 years of experience in CT diagnosis and 3 years of experience in CT image post processing. All pre and post perfusion studies were analyzed on the Wizard workstation using commercial compartmental analysis-based perfusion software (Body Perfusion CT, Siemens Medical Solutions) and the tumor perfusion algorithm. Tumor specific parameters, blood flow (measured in mL/100 mL/min, based on the maximum slope method), permeability (measured in mL/100 mL/min, based on Patlak analysis) and relative blood volume (measured in mL/100 mL, based on Patlak analysis) were used. A single 2.4-mm slice that best depicted both of the bilateral tumors was chosen from the 12 perfusion slices. The analyzed slices of pre and post perfusion of each animal were consistent.
CT images of the chosen slice were loaded into the software. The image stack was examined and images with severe motion artifacts were removed before perfusion calculation. A rectangular reference region of interest (ROI) which contained the tumors was drawn, and 2D and 3D motion correction were performed automatically. The arterial input curve of contrast medium concentration was obtained from an ROI (50–70 pixels) in the descending aorta or common carotid artery. A processing threshold of −20 to 200 Hu was selected. The time-attenuation curves (TDC) of artery and mean tissue were generated by the software and showed in an arterial input function & optimization dialog box. The times of arterial shift and Patlak start for Patlak model, and the start time, rise time, peak time for maximum slope model were adjusted whenever necessary based on the TDCs of artery and mean tissue. A grayscale maximum intensity projection (MIP) image and 3 pseudocolor perfusion maps (blood flow, permeability and relative blood volume) were generated corresponding to each voxel.
Three ROIs, entire tumor, tumor rim and normal surrounding muscle, were then drawn freehand on the MIP image to measure perfusion parameters (Fig. 1). The tumor rim was defined as a contrast enhancing peripheral zone. Care was taken to exclude the surrounding vessels and fat when drawing the tumor boundary. The mean values for the three tumor perfusion parameters of each ROI for each tumor were then noted by the observer. The perfusion analysis of each animal was reanalyzed 3 weeks later to assess intra-observer agreement and minimize recall bias.
FIGURE 1.
Representative CT perfusion images demonstrating the manual segmentations of the regions of interest. ROI1, tumor rim of left tumor; ROI2, entire tumor of right tumor; ROI3, normal muscle.
TTC Stain and Measurement
The treated rats were euthanized with carbon dioxide inhalation 24 h after treatment. Tumor tissue slices about 2 mm in thickness were cut perpendicular to the ablation needle track and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Fisher Scientific, Fairlawn, NJ) for 30 min at room temperature to assess mitochondrial enzyme activity.16 Viable tissue with intact mitochondrial activity is stained pink, while ablated tumor tissue with irreversible cellular damage is white. The stained tissue slice was photographed with a digital camera (Nikon Coolpix 5400, Japan). The area of the coagulation region was measured using ImageJ 1.36b software (National Institutes of Health, available at http://rsb.info.nih.gov/ij/download.html). The coagulation region was manually segmented and measured by 2 independent, blinded observers (H.W. and A.A.E.).
Data and Statistical Analysis
Unless otherwise noted, all data, including the perfusion parameters, tumor size, and coagulation size, are presented as mean ± standard deviation (SD). Intraobserver agreement was assessed using two separate analyses. First, correlation coefficients were calculated for each of the three perfusion parameters (blood flow, permeability, and relative blood volume). Second, the mean differences, SD, and 95% limits of agreement for each of the three perfusion parameters were calculated using the Bland-Altman analysis.3,14 A paired t-test was used to compare the perfusion parameters before and after vasoactive drugs injection. The tumor size (maximum diameter and volume) and coagulation area were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test using Minitab 15.1. A p value of less than 0.05 was considered statistically significant.
RESULTS
Intraobserver Agreement of CT Perfusion Analysis
Table 1 shows the results of linear regression and Bland-Altman analysis between the paired values of perfusion parameters (blood flow, permeability and relative blood volume) in 3 ROIs and pooled data of the dual perfusion analyses. Satisfactory correlations were noted between the parameters in first and second perfusion analysis (r2 = 0.66–0.92, p < 0.001). The best intraobserver agreement was noted in blood volume of the entire tumor [mean of difference, 0.02%; 95% CI (−12.1 to 1.25)%]. A somewhat reduced intraobserver agreement was noted in blood flow in muscle [mean of difference, 0.03 mL/100 mL/min; 95% CI (−4.03 to 4.09) mL/100 mL/min]. The correlation and Bland-Altman analysis of the pooled data are shown in Fig. 2.
TABLE 1.
Results of linear regression and Bland-Altman analysis of the intra-observer agreement.
| Blood flow (mL/100 mL/min) | Permeability (mL/100 mL/min) | Blood volume (%) | |
|---|---|---|---|
| Entire tumor (n = 96) | |||
| Slope | 0.87 | 0.87 | 0.91 |
| r2 | 0.82 | 0.72 | 0.82 |
| Mean | 27.52 | 16.01 | 5.10 |
| Mean of difference | −0.17 | −0.05 | 0.02 |
| 95% CI | −11.20 to 10.86 | −4.68 to 4.58 | −1.21 to 1.25 |
| Tumor rim (n = 96) | |||
| Slope | 0.87 | 0.81 | 0.88 |
| r2 | 0.80 | 0.66 | 0.80 |
| Mean | 35.37 | 18.60 | 5.10 |
| Mean of difference | 0.09 | −0.15 | −0.01 |
| 95% CI | −12.34 to 12.52 | −5.68 to 5.38 | −1.38 to 1.36 |
| Muscle (n = 96) | |||
| Slope | 0.78 | 0.77 | 0.76 |
| r2 | 0.68 | 0.73 | 0.81 |
| Mean | 3.18 | 7.86 | 1.48 |
| Mean of difference | 0.03 | 0.00 | 0.05 |
| 95% CI | −4.03 to 4.09 | −3.43 to 3.43 | −0.75 to 0.85 |
| Total (n = 288) | |||
| Slope | 0.95 | 0.93 | 0.96 |
| r2 | 0.92 | 0.86 | 0.92 |
| Mean | 22.02 | 14.15 | 3.69 |
| Mean of difference | −0.02 | −0.07 | 0.02 |
| 95% CI | −9.86 to 9.82 | −4.66 to 4.52 | −1.14 to 1.18 |
FIGURE 2.
(a–c) Scatter plots showing correlation between the pooled data in dual measurements of perfusion parameters, blood flow (a), permeability (b), and relative blood volume (c). There is good agreement between duplicate measurements (a–c solid line 5 linear regression line). (d–f) Bland-Altman plots of blood flow (d), permeability (e), and relative blood volume (f) show a relationship between differences and means of the duplicate perfusion measurements. The difference between each pair is plotted against the average value of the same pair (d–f, solid lines 5 mean value of differences, dotted lines 5 mean value of differences ± 1.96 SDs).
Effects of Vasoactive Drugs on Tumor Perfusion
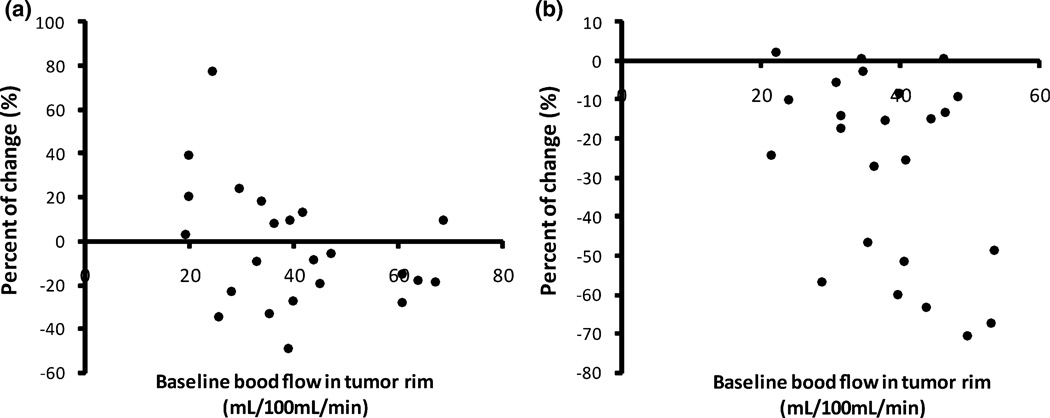
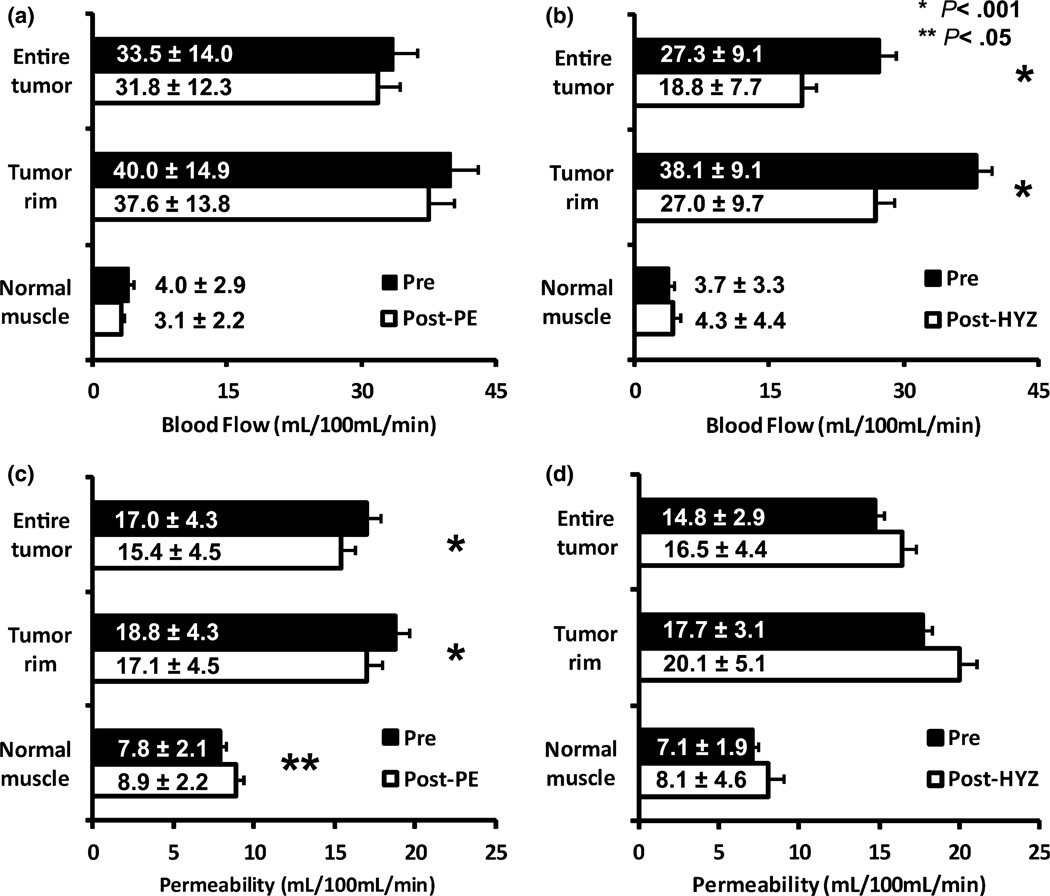
Figures 3a and 3b show the percentage change of tumor rim blood flow in each tumor induced by PE and HYZ. A wide variability in the effect of PE on tumor blood flow (−49.3 to 77.5%) was noted. Among 24 tumors, 13 showed a blood flow decrease and 11 showed a blood flow increase in response to PE. The tumor blood flow changed more consistently (−70.7 to 2.4%) in response to HYZ. It decreased in 21 of 24 tumors, and slightly increased in 3 tumors. Figures 4a and 4b show the mean blood flow changes induced by PE and HYZ. HYZ significantly decreased entire tumor blood flow from 27.3 mL/100 mL/min ± 9.1 to 18.8 mL/100 mL/min ± 7.7 (p < 0.001) and tumor rim blood flow from 38.1 mL/100 mL/min ± 9.1 to 27.0 mL/100 mL/min ± 9.7 (p < 0.001), corresponding to a decrease of 31.1 and 29.1% respectively. PE produced a 5.1% decrease in entire tumor blood flow (33.5 mL/100 mL/min ± 14.0 vs. 31.8 mL/100 mL/min ± 12.3, p = 0.30) and a 6.0% decrease in tumor rim blood flow (40.0 mL/100 mL/min ± 14.9 vs. 37.6 mL/100 mL/min ± 13.8, p = 0.22). PE induced a 22.1% decrease and HYZ induced a 14.5% increase in normal muscle blood flow, but the differences were not statistically significant.
FIGURE 3.
Percent change of tumor rim blood flow of each tumor induced by PE (a) or HYZ (b).
FIGURE 4.
Changes in blood flow (a, b) and permeability (c, d) in entire tumor, tumor rim and surrounding normal muscle in response to PE (a, c) or HYZ (b, d). * denotes significant differences (p < .001) in pre vs. post measurements. ** denotes significant differences (p < .05). Error bars are standard error of mean.
Figures 4c and 4d show the permeability changes induced by PE and HYZ. PE significantly decreased entire tumor permeability from 17.0 mL/100 mL/min ± 4.3 to 15.4 mL/100 mL/min ± 4.5 (p < 0.001) and tumor rim permeability from 18.8 mL/100 mL/min ± 4.3 to 17.7 mL/100 mL/min ± 4.5 (p < 0.001), increased normal muscle permeability from 7.8 mL/100 mL/min ± 2.1 to 8.9 mL/100 mL/min ± 2.2 (p = 0.045). HYZ increased the permeability in entire tumor, tumor rim and normal muscle. However, the changes were not statistically significant. No significant correlation was noted between the change rates of blood flow in 3 ROIs of each tumor and those of permeability (data not shown).
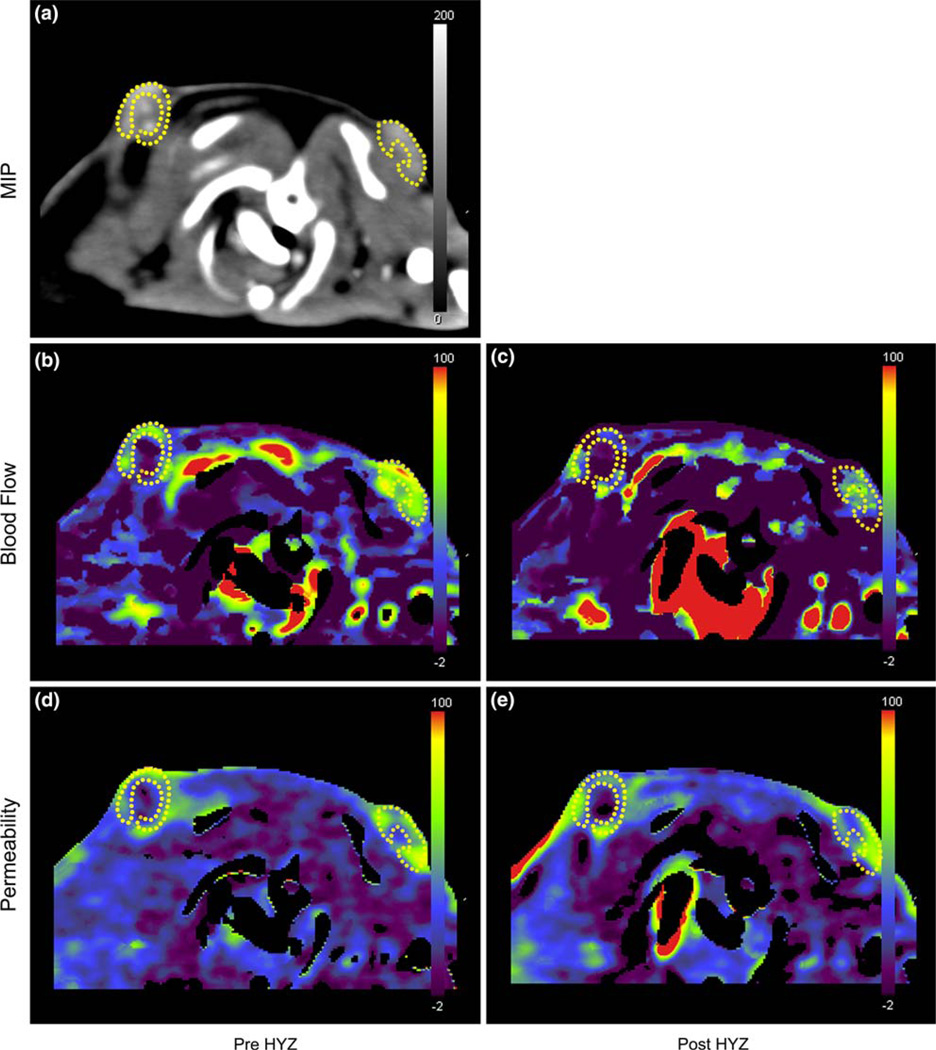
No significant changes of blood volume (including entire tumor, tumor rim and normal muscle) were induced by PE or HYZ (values not shown). Figures 5 and 6 show representative perfusion images of rats with 2 shoulder subcutaneous tumors before and after PE (Fig. 5) or HYZ (Fig. 6) injection. Tumor rim shows a higher perfusion than tumor center where the perfusion is near zero. PE decreased tumor blood flow and permeability slightly, while HYZ decreased tumor blood flow and increased tumor permeability.
FIGURE 5.
Representative perfusion images of a rat with 2 shoulder tumors (dotted lines: tumor rim) before and after phenylephrine (PE) injection. (a) MIP image of perfusion scan images. After PE administration, tumor blood flow decreased slightly (b, pre; c, post) and tumor permeability decreased (d, pre; e, post).
FIGURE 6.
Representative perfusion images of a rat with 2 shoulder tumors (dotted lines, tumor rim) before and after hydralazine (HYZ) injection. (a) MIP image of perfusion scan images. After HYZ administration, tumor blood flow decreased markedly (b, pre; c, post) and tumor permeability increased slightly (d, pre; e, post).
Effects of Vasoactive Drugs on RF Ablation Size
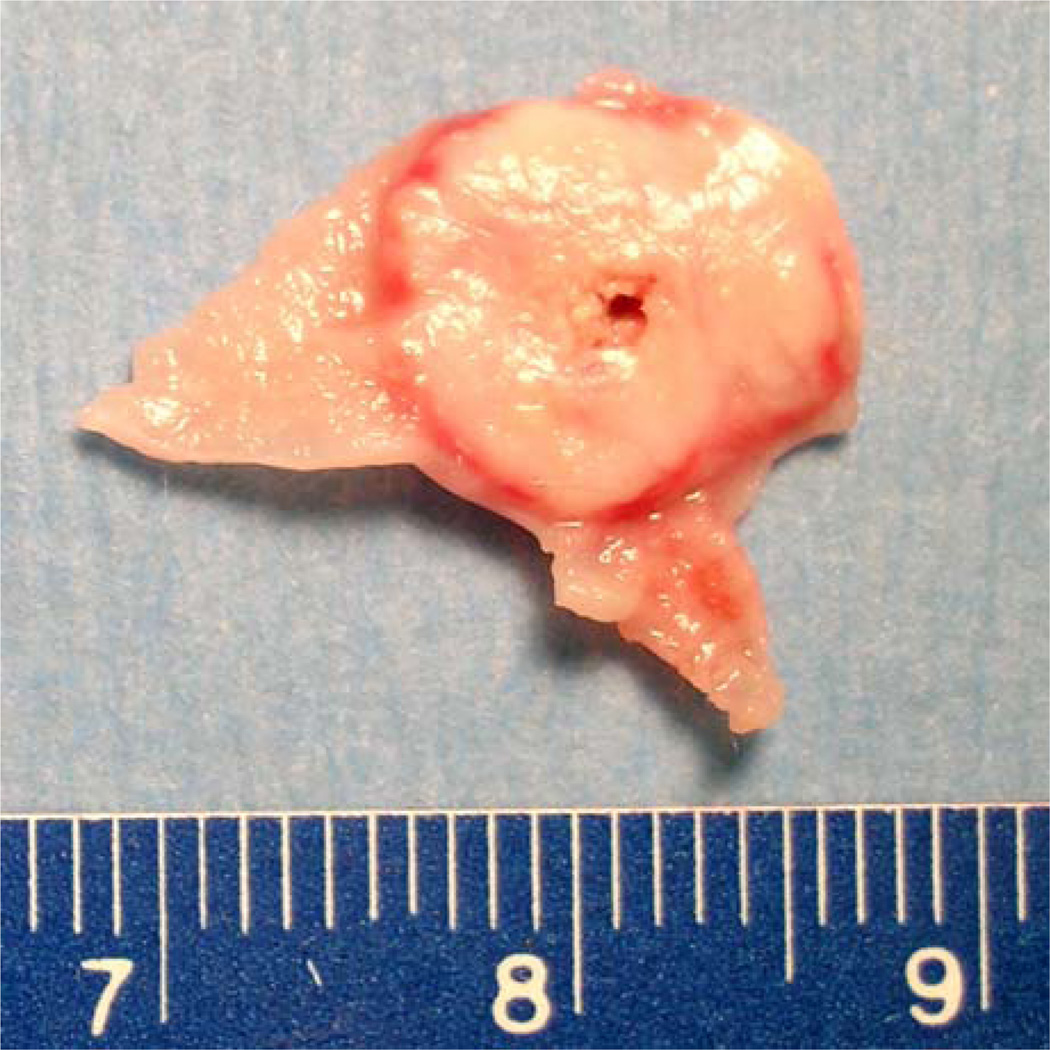
Table 2 reports the pre-treatment tumor size and coagulation area in control, PE and HYZ groups. No significant difference was noted in either maximum tumor diameter or tumor volume among three groups before treatment. RF ablation alone produced 0.29 cm2 ± 0.13 in area of coagulation. Injection of PE before RF ablation resulted in similar size of coagulation (0.30 cm2 ± 0.18 in area). Greater coagulation was obtained by using the combination of HYZ injection followed by RF ablation (0.59 cm2 ± 0.24 in area). ANOVA analysis demonstrated a significant difference in the coagulation area among the three groups (p = 0.028). A subsequent Dunnett’s multiple comparison test demonstrated that the coagulation area in the HYZ group was significantly greater than that in the control and PE group. No difference in coagulation area between control and PE groups was noted. Figure 7 shows a representative gross tumor specimen stained with TTC after treatment with a combination of HYZ injection and RF ablation.
TABLE 2.
Comparison of initial tumor size and coagulation size after ablation in the three treatment groups.
| Control (n = 6) | PE (n = 6) | HYZ (n = 5) | p | |
|---|---|---|---|---|
| Maximum diameter of tumor before treatment (cm) | 1.19 ± 0.09 | 1.17 ± 0.12 | 1.30 ± 0.14 | 0.169 |
| Tumor volume before treatment (cm3) | 0.47 ± 0.27 | 0.43 ± 0.17 | 0.62 ± 0.40 | 0.532 |
| Coagulation area following RF ablation (cm2) | 0.29 ± 0.13 | 0.30 ± 0.18 | 0.59 ± 0.24 | 0.028 |
FIGURE 7.
Gross tumor specimen stained with triphenyltetrazolium chloride (TTC) after treatment with a combination of HYZ i.v. injection followed by RF ablation. The section was cut in the transverse plane perpendicular to the electrode track. The pink peripheral region represents residual viable tumor, whereas the white region represents the zone of ablation-induced coagulation.
DISCUSSION
Many techniques have been used for studying the modulation of tumor blood flow to enhance the effects of chemotherapy, radiotherapy and thermotherapy in the last four decades. These techniques include the radioactive microsphere technique,27 isotope (e.g. 85Kr, 133Xe) clearance,11,31 uptake of radioactive tracer,39 and the thermal clearance method.4 The invasive nature of these tests prevents them from being used clinically on a regular basis. In contrast, CT perfusion enables noninvasive quantification of tissue perfusion parameters such as blood flow and permeability. These measurements have previously been shown to correlate well with gold standard measurements of the same parameters. 36 In addition, this technique has been shown to be superior to conventional imaging in early detection and differentiation of ischemic lesions and occult tumors because it enables the quantification of slight changes in tissue perfusion.8,12 Because it is noninvasive, fast and convenient to perform, CT perfusion has become an important method to explore tumor vascularity, monitor tumor response to antiangiogenic therapies35 and other treatments.28
CT perfusion measurements are intrinsically variable because of a combination of internal and external factors. The accuracy and reproducibility of functional CT perfusion measurements have been assessed by various investigators.14,21,40 A high degree of inter-observer agreement was reported by these authors (r2 = 0.81–0.94). Our study assessed the intra-observer agreement, from which a high correlation was noted between dual measurements of each parameter in every ROI (r2 = 0.66–0.82). The intra-observer agreements of permeability and blood volume were greater than those of blood flow. This may be attributed to the method of perfusion parameter calculation. In this study, blood flow was calculated with the maximum slope method, using only limited CT data in initial enhancement phase. Arbitrary deletion of motion images may influence the measurement of blood flow. On the contrary, permeability and blood volume were calculated with the Patlak method, which used more CT data in a relatively long washout phase. Deleted motion images may thus have less influence on the result. We also noted that the agreements of the perfusion parameters in entire tumor and tumor rim were greater than that in muscle. This might be attributed to the fact that perfusion in normal muscle is much lower than that in tumor, with more noise present in the muscle.
The use of vasoactive drugs to modulate tumor blood flow to enhance efficacy of various treatments has been attempted by many investigators. Among these agents, the vasodilator, HYZ, is one of the most widely studied. HYZ was one of the first available oral antihypertensive drugs and is currently primarily utilized to treat pregnancy-associated hypertension.2 Although the exact mechanism of action of this drug is not fully understood, it may lower blood pressure by exerting a peripheral vasodilating effect through a direct relaxation of vascular smooth muscle. In early publications HYZ has been shown to reduce blood flow in many tumor models in both rodents and dogs.7,44 The effects are dose-dependent and can last for several hours. Recently, Nielsen et al.33 confirmed reduction of blood flow in mammary tumors noninvasively using single voxel 1H localized spectroscopy. In the current study, we found that HYZ consistently decreased entire tumor blood flow about 31.1% and increased normal muscle blood flow about 14.5%. This is in agreement with previous reports. The counter-intuitive response of tumor and normal tissue to HYZ, which was in line with our hypothesis, can be explained by the dilation of normal blood vessels causing them to “steal” the blood flow from the tumor vessels due to their unresponsiveness upon exposure to the vasodilator.
Alpha adrenergic agonists, such as noradrenaline, 20,31 isoprenaline,31 PE,6 have been used as vasoconstrictors to modulate tumor blood flow since 1960s. PE is a powerful postsynaptic alpha-1 receptor stimulant with little effect on the beta receptors of the heart. It produces vasoconstriction that lasts longer than that of epinephrine and ephedrine and can be administrated orally. The changes in tumor blood flow induced by these agents appear to be disparate and are affected by many factors such as the tumor model, organ, administration route etc. Mattson et al.30 reported that intravenous infusion of noradrenaline significantly reduced the blood flow in transplanted muscle tumor and normal muscle. Chan et al.6 reported that PE decreased the blood flow in “hard tumors”, where the vessels had a certain amount of vascular smooth muscle, but had no effect on “soft tumor”, where the vessels lacked smooth muscle. Ackerman et al.1 reported that in a liver tumor model, capillary blood flow increased briefly but significantly with intraportal administration of epinephrine, norepinephrine and PE. In contrast, a reduction or no change in tumor blood flow induced by epinephrine, norepinephrine and ethylphenylephrine or PE, was noted by Li et al.29 also in a liver model. The disparity of the effect of adrenergic agonists on tumor blood flow was interpreted as heterogeneity of tumor vessel maturation 9 and the reaction of recruited, pre-existing normal vasculature with well-defined intimal, medial and adventitial structures.25,38 In the current study, a non-significant 5.1% decrease in tumor blood flow and a non-significant change (−22.1%) in blood flow to normal muscle was observed. This did not support our hypothesis, which expected an increase in tumor blood flow and decrease in normal muscle blood flow. We speculate that heterogeneity of tumor vessel maturation in this tumor model may be responsible for the variable results, further supporting the notion that tumor blood vessels react multivariably to α-adrenergic agonists.
A significant decrease or non-significant increase of permeability in tumor and normal muscle induced by PE or HYZ was also detected in this study. The study of the effect of vasoactive drugs on the permeability of tumor or normal tissue has not been widespread. One study reported that PE decreased the permeability in burned skin.5 This effect was thought to be secondary to the vasoconstrictive effect of PE. If this mechanism is valid, there should be a positive correlation between the percent change of blood flow and permeability. Our data did not support this hypothesis. Further study is needed to verify the PE effect on vascular permeability.
Our results imply that there is a strong relationship between tumor blood flow and the coagulation size induced by RF ablation. For example, PE changed tumor blood flow slightly and thus had no effect on RF ablation. In contrast, HYZ decreased tumor blood flow over 30% and correspondingly increased the coagulation size. The same relationship was reported by other investigators using other drugs, such as arsenic trioxide,23,26 halothane,17 or other techniques, such as tumor feeding artery occlusion18 and embolization 45 to decrease the tumor blood flow.
The results of this study suggest that a vasodilator is an appropriate agent to enhance tumor RF ablation efficacy. However, it is important to consider that different organs respond differently to certain vasodilators. For example, Hasegawa et al.22 reported that in a rat tumor model an intra-arterial injection of HYZ significantly reduced the blood flow in most normal abdominal organs, whereas it markedly increased the blood flow in muscle. No change in blood flow in the liver tumor was noted, but marked decreases in blood flow of other abdominal tumors and subcutaneous tumors were noted after HYZ injection. This indicates that the decrease in blood flow in tumors and most normal tissues was caused mainly by diversion of blood to muscle. However, HYZ could markedly decrease the systemic blood pressure, which may limit its clinical application. In a final application, an organ or tissue specific vasomodulator would be an ideal agent for this approach.
Our study has several limitations. First, only one vasoconstrictor, PE, was tested. Because the results were contrary to the proposed hypothesis, additional agents should be examined. Second, although the results demonstrated that HYZ could enhance RF ablation, optimal efficacy may not have been achieved, since only a single dose of the agent was studied. A dose–response study could be beneficial for finding an optimal dose, one that would induce significant blood flow changes with minor effects on systemic blood pressure. Third, while this study has verified our hypothesis that a vasodilator can divert the blood from tumor to surrounding normal tissue, it is only the initial step of a broader project that includes proper agent selection, optimization of the treatment protocol and examination of the effects in a clinically pertinent model. Ongoing experiments are examining the vasomodulation approach in kidney and liver tumor models, which may be superior to the subcutaneous model used in the current study.
In conclusion, functional CT is a useful tool to monitor and explore blood flow changes induced by vasoactive drugs and was used in the current study to find a practical vasoactive agent to enhance RF ablation damage in a tumor model. Our results suggest that HYZ can decrease tumor blood flow and enhance the coagulation size induced by RF ablation. The proposed vasomodulation technique may be a simple, cost effective and noninvasive approach to enhance RF efficacy. Although the agents and protocols utilized in this study are clinically relevant, more validation experiments in animal models with hepatic or renal tumors are needed before this approach can be translated to clinical use.
ACKNOWLEDGMENT
This work was supported by NIH (5P30 043703-17S3).
REFERENCES
- 1.Ackerman NB, Jacobs R, Bloom ND, Poon TT. Increased capillary flow in intrahepatic tumors due to alpha-adrenergic effects of catecholamines. Cancer. 1988;61:1550–1554. doi: 10.1002/1097-0142(19880415)61:8<1550::aid-cncr2820610811>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Arce C, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Candelaria M, Duennas-Gonzalez A. Hydralazine target: from blood vessels to the epigenome. J. Transl. Med. 2006;4:10. doi: 10.1186/1479-5876-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet. Gynecol. 2003;22:85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 4.Carter LP, Erspamer R, Bro WJ. Cortical blood flow: thermal diffusion vs isotope clearance. Stroke. 1981;12:513–518. doi: 10.1161/01.str.12.4.513. [DOI] [PubMed] [Google Scholar]
- 5.Cassuto J, Tarnow P, Yregard L, Lindblom L, Rantfors J. Adrenoceptor subtypes in the control of burn-induced plasma extravasation. Burns. 2005;31:123–129. doi: 10.1016/j.burns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Chan RC, Babbs CF, Vetter RJ, Lamar CH. Abnormal response of tumor vasculature to vasoactive drugs. J. Natl. Cancer Inst. 1984;72:145–150. doi: 10.1093/jnci/72.1.145. [DOI] [PubMed] [Google Scholar]
- 7.Chaplin DJ, Acker BD, Horsman MR. Reduction of tumour blood flow by vasoactive drugs: a role in cancer therapy. Biomed. Biochim. Acta. 1989;48:S264–S268. [PubMed] [Google Scholar]
- 8.Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clement O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556–561. doi: 10.1148/radiology.218.2.r01fe10556. [DOI] [PubMed] [Google Scholar]
- 9.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 10.Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radio-frequency hyperthermia with a ‘cooled-tip needle’. A preliminary clinical experience. Eur. J. Ultrasound. 1999;9:145–153. doi: 10.1016/s0929-8266(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 11.Fraser IS, Brown BW, Mattner PE. Endometrial blood flow in anaesthetized sheep as measured with krypton-85 clearance and microsphere techniques. Q. J. Exp. Physiol. Cogn. Med. Sci. 1980;65:19–26. doi: 10.1113/expphysiol.1980.sp002488. [DOI] [PubMed] [Google Scholar]
- 12.Gaggl A, Penka B, Schultes G, Karcher H. Assessment of perfusion of facial microvascular transplants and early detection of ischemia by perfusion-CT scan. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;94:425–431. doi: 10.1067/moe.2002.127586. [DOI] [PubMed] [Google Scholar]
- 13.Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology. 2004;233:729–739. doi: 10.1148/radiol.2333032052. [DOI] [PubMed] [Google Scholar]
- 14.Goh V, Halligan S, Hugill JA, Bassett P, Bartram CI. Quantitative assessment of colorectal cancer perfusion using MDCT: inter- and intraobserver agreement. AJR Am. J. Roentgenol. 2005;185:225–231. doi: 10.2214/ajr.185.1.01850225. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad. Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Torchillin VP, Halpern EF, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222:797–804. doi: 10.1148/radiol.2223010861. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998;209:761–767. doi: 10.1148/radiology.209.3.9844671. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 19.Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002;8:33–39. doi: 10.1097/00130404-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Gullino PM, Grantham FH. Studies on the exchange of fluids between host and tumor. II. The blood flow of hepatomas and other tumors in rats and mice. J. Natl. Cancer Inst. 1961;27:1465–1491. [PubMed] [Google Scholar]
- 21.Hakime A, Peddi H, Hines-Peralta AU, Wilcox CJ, Kruskal J, Lin S, de Baere T, Raptopoulos VD, Goldberg SN. CT perfusion for determination of pharmacologically mediated blood flow changes in an animal tumor model. Radiology. 2007;243:712–719. doi: 10.1148/radiol.2433052048. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa T, Song CW. Effect of hydralazine on the blood flow in tumors and normal tissues in rats. Int. J. Radiat. Oncol. Biol. Phys. 1991;20:1001–1007. doi: 10.1016/0360-3016(91)90197-c. [DOI] [PubMed] [Google Scholar]
- 23.Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology. 2006;240:82–89. doi: 10.1148/radiol.2401050788. [DOI] [PubMed] [Google Scholar]
- 24.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, Iwakiri H, Uto H, Kato J, Ido A, Hayashi K, Tsubouchi H. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J. Gastroenterol. 2003;38:977–981. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 25.Hori K, Zhang QH, Saito S, Tanda S, Li HC, Suzuki M. Microvascular mechanisms of change in tumor blood flow due to angiotensin II, epinephrine, methoxamine: a functional morphometric study. Cancer Res. 1993;53:5528–5534. [PubMed] [Google Scholar]
- 26.Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, Goldberg SN. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J. Vasc. Interv. Radiol. 2004;15:269–274. doi: 10.1097/01.rvi.0000109396.74740.c4. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson O, Widmark A, Grankvist K, Damber JE, Henriksson R. Effects of clonidine-induced hypotension and dopamine-induced hypertension on blood flows in prostatic adenocarcinoma (Dunning R3327): and normal tissues. Prostate. 1992;20:225–232. doi: 10.1002/pros.2990200307. [DOI] [PubMed] [Google Scholar]
- 28.Kan Z, Kobayashi S, Phongkitkarun S, Charnsangavej C. Functional CT quantification of tumor perfusion after transhepatic arterial embolization in a rat model. Radiology. 2005;237:144–150. doi: 10.1148/radiol.2371040526. [DOI] [PubMed] [Google Scholar]
- 29.Li YL, Li HC, Zhang LH. The effects of angiotensin II and other vasoactive agents on the blood flow in Yoshida ascites hepatoma. Zhonghua Yi Xue Za Zhi. 1993;73:23–25. 61. [PubMed] [Google Scholar]
- 30.Mattson J, Appelgren L, Karlsson L, Peterson HI. Influence of vasoactive drugs and ischaemia on intra-tumour blood flow distribution. Eur. J. Cancer. 1978;14:761–764. doi: 10.1016/0014-2964(78)90006-3. [DOI] [PubMed] [Google Scholar]
- 31.Mattsson J, Lilja J, Peterson HI. Influence of vasoactive drugs on local tumor blood flow. Eur. J. Cancer Clin. Oncol. 1982;18:677–684. doi: 10.1016/0277-5379(82)90214-0. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen DT, Laursen HB, Rokkjaer M, Astrup LB. Radiofrequency ablation of malignant liver tumors. Ugeskr. Laeger. 2002;164:4642–4645. [PubMed] [Google Scholar]
- 34.Ohyama N, Yamaguchi S. Effects of phenylephrine and prazosin on axial movement of the rat incisor and arterial blood pressure. Jpn. J. Pharmacol. 1999;80:271–274. doi: 10.1254/jjp.80.271. [DOI] [PubMed] [Google Scholar]
- 35.Pollard RE, Broumas AR, Wisner ER, Vekich SV, Ferrara KW. Quantitative contrast enhanced ultrasound and CT assessment of tumor response to antiangiogenic therapy in rats. Ultrasound Med. Biol. 2007;33:235–245. doi: 10.1016/j.ultrasmedbio.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 36.Pollard RE, Garcia TC, Stieger SM, Ferrara KW, Sadlowski AR, Wisner ER. Quantitative evaluation of perfusion and permeability of peripheral tumors using contrast-enhanced computed tomography. Invest. Radiol. 2004;39:340–349. doi: 10.1097/01rli.0000124456.82985.35. [DOI] [PubMed] [Google Scholar]
- 37.Rossi S, Di Stasi M, Buscarini E, Cavanna L, Quaretti P, Squassante E, Garbagnati F, Buscarini L. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J. Sci. Am. 1995;1:73. [PubMed] [Google Scholar]
- 38.Ruddock MW, Burns DM, McKeown SR, Murphy L, Walsh IK, Keane PF, Hirst DG. Contractile properties of human renal cell carcinoma recruited arteries and their response to nicotinamide. Radiother. Oncol. 2000;54:179–184. doi: 10.1016/s0167-8140(99)00163-2. [DOI] [PubMed] [Google Scholar]
- 39.Rust TC, Kadrmas DJ. Rapid dual-tracer PTSM+ATSM PET imaging of tumour blood flow and hypoxia: a simulation study. Phys. Med. Biol. 2006;51:61–75. doi: 10.1088/0031-9155/51/1/005. [DOI] [PubMed] [Google Scholar]
- 40.Sanelli PC, Nicola G, Tsiouris AJ, Ougorets C, Knight I, Frommer S, Veronelli B, Zimmerman RD. Reproducibility of postprocessing of quantitative CT perfusion maps. AJR Am. J. Roentgenol. 2007;188:213–218. doi: 10.2214/ajr.05.2188. [DOI] [PubMed] [Google Scholar]
- 41.Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ. Differential expression of markers for endothelial cells, pericytes, basal lamina in the microvasculature of tumors and granulation tissue. Am. J. Pathol. 1991;138:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- 42.Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur. J. Ultrasound. 2001;13:149–158. doi: 10.1016/s0929-8266(01)00127-6. [DOI] [PubMed] [Google Scholar]
- 43.Tozer GM, Maxwell RJ, Griffiths JR, Pham P. Modification of the 31P magnetic resonance spectra of a rat tumour using vasodilators and its relationship to hypotension. Br. J. Cancer. 1990;62:553–560. doi: 10.1038/bjc.1990.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voorhees DW, 3rd, Babbs CF. Hydralazine-enhanced selective heating of transmissible venereal tumor implants in dogs. Eur. J. Cancer Clin. Oncol. 1982;18:1027–1033. doi: 10.1016/0277-5379(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 45.Yamakado K, Nakatsuka A, Kobayashi S, Akeboshi M, Takaki H, Kariya Z, Kinbara H, Arima K, Yanagawa M, Hori Y, Kato H, Sugimura Y, Takeda K. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc. Intervent. Radiol. 2006;29:389–394. doi: 10.1007/s00270-004-0090-9. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki T, Kimura T, Kurokawa F, Aoyama K, Ishikawa T, Tajima K, Yokoyama Y, Takami T, Omori K, Kawaguchi K, Tsuchiya M, Terai S, Sakaida I, Okita K. Percutaneous radiofrequency ablation with cooled electrodes combined with hepatic arterial balloon occlusion in hepatocellular carcinoma. J. Gastroenterol. 2005;40:171–178. doi: 10.1007/s00535-004-1516-5. [DOI] [PubMed] [Google Scholar]