Abstract
Rodent models exhibit only the earliest features of human diabetic nephropathy, which limits our ability to investigate new therapies. Hypertension is a prerequisite for advanced diabetic nephropathy in humans, so its rarity in typical rodent models may partly explain their resistance to nephropathy. Here, we used the Cyp1a1mRen2 rat, in which the murine renin-2 gene is incorporated under the Cytochrome P4501a1 promoter. In this transgenic strain, administration of low-dose dietary indole-3-carbinol induces moderate hypertension. In the absence of hypertension, streptozotocin-induced diabetes resulted in a 14-fold increase in albuminuria but only mild changes in histology and gene expression despite 28 weeks of marked hyperglycemia. In the presence of induced hypertension, hyperglycemia resulted in a 500-fold increase in albuminuria, marked glomerulosclerosis and tubulointerstitial fibrosis, and induction of many of the same pathways that are upregulated in the tubulointerstitium in human diabetic nephropathy. In conclusion, although induction of diabetes alone in rodents has limited utility to model human diabetic nephropathy, renin-dependent hypertension and hyperglycemia synergize to recapitulate many of the clinical, histological, and gene expression changes observed in humans.
Diabetic nephropathy (DN) is the single largest cause of end stage renal failure in the Western world.1 Although the development of novel therapeutic strategies for DN remains a research priority, we are constrained by the fact that current rodent models replicate only the earliest stages of human DN.2 One potential explanation for the resistance of rodents to DN is that they tend not to develop hypertension, which is critical for progressive DN in humans. Abnormalities in BP, such as loss of nocturnal dipping, occur early in the course of human DN3 and rigorous BP control is at least as effective as glycemic control in retarding disease progression.4,5 Indeed, in patients with diabetes and co-existing unilateral renal artery stenosis there may be no evidence of nephropathy in the kidney downstream of the stenosis, despite severe nephropathy in the contralateral kidney, suggesting that transmission of systemic hypertension to the diabetic glomerulus is a prerequisite for the development of advanced nephropathy.6,7
Despite the crucial role of hypertension in the pathogenesis of DN, there is a paucity of data regarding how high BP and hyperglycemia interact at a molecular level to promote nephropathy. Whereas it is difficult to dissect the relative contribution of hypertension and diabetes in humans, rodent studies may be informative as hyperglycemia and high BP synergize to promote nephropathy in a number of hypertensive rodent models.8–10
The renin-dependent hypertensive (mRen-2)27 rat has been extensively use to model DN9; however, it is limited by the development of malignant phase hypertension.11,12 To determine how hyperglycemia and hypertension interact at a molecular level, we used the Cyp1a1mRen2 rat, which harbors the murine Ren2 cDNA under the control of the cytochrome P4501a1 promoter,13 such that hypertension may be induced by dietary supplementation with indole-3-carbinol (I-3-C). Unlike the constitutive (mRen-2)27 rat, hypertension can be induced after the onset of diabetes to mimic the natural history of human DN and the I-3-C dose may be titrated to avoid malignant phase hypertension.
Cyp1a1mRen2 rats were allocated into four groups: controls (n=6), streptozotocin-induced diabetes (DM; n=6), I-3-C–induced hypertension (HTN; n=7), and combined hypertension and diabetes (DN+HTN; n=8). During the subsequent 28 weeks, blood sugar levels were 20–30 mM in both diabetic groups with no significant difference between the DM and DM+HTN animals (Figure 1A). Dietary I-3-C induced an equivalent increase in tail-cuff BP in both hypertensive groups compared with their nonhypertensive counterparts (Figure 1B). The tail-cuff readings were consistent with those obtained by arterial cannulation under terminal anaesthesia (mean arterial pressure of 127±2.3, 136±2.8, 181±6.4, and 169±8.7 mmHg in controls, DM, HTN, and DM+HTN animals, respectively).
Figure 1.
Diabetes and hypertension synergise to promote albuminuria. (A) Mean (±SD) early morning nonfasting blood sugar level, (B) mean (±SD) tail-cuff systolic BP, and (C) median (interquartile range) albumin/creatinine ratio in the four groups of rats over the 28-week course of the experiment. ***P<0.001 versus control; #P<0.05, ##P<0.01, and ###P<0.001 versus diabetic alone; $P<0.05 and $$P<0.01 versus HTN alone.
DM animals exhibited a modest increase in albuminuria, with a 14-fold higher median albumin/creatinine ratio than that of controls at 28 weeks, equivalent to microalbuminuric levels in humans (Figure 1C). Hypertension and diabetes synergized to promote albuminuria, such that by 28 weeks the median albumin/creatinine ratio in the DM+HTN group was 500-fold higher than controls and significantly greater than that in either the DM or HTN groups.
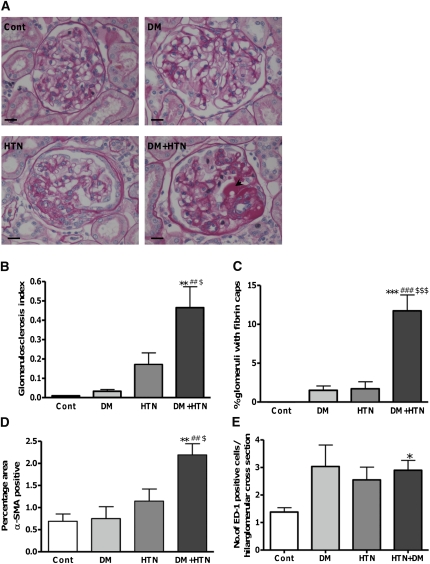
There was very mild histological injury in the DM group; however, induction of hypertension alone promoted FSGS and a nonsignificant increase in the glomerulosclerosis index (GSI; Figure 2, A and B). Concurrent diabetes and hypertension significantly increased the GSI compared with all other groups (Figure 2B) and resulted in the development of intraglomerular fibrin caps, which were rarely observed with either DM or HTN alone but are typical of human DN (Figure 2, A and C). Importantly, there was no histological evidence of malignant phase hypertension, such as onion-skinning of the renal arterioles in either hypertensive group. Only the DM+HTN rats had a significant increase in mesangial cell activation as indicated by α-smooth muscle actin (α-SMA) staining (Figure 2D). There was an increase in glomerular macrophage infiltration in all of the intervention groups, which reached significance in the DM+HTN animals (Figure 2E). Few glomerular lymphocytes were observed with no significant differences between the groups.
Figure 2.
Diabetes and hypertension together promote glomerulosclerosis. (A) Representative images of the glomeruli from kidneys from each group. Arrowhead indicates example of a fibrin cap. Bars represent 25 μM. (B) Mean (±SEM) glomerulosclerotic index (GSI) for each group. (C) Mean (±SEM) percentage of glomeruli from each group that exhibit fibrin caps. (D) Mean (±SEM) percentage α-SMA positive area in the glomeruli for each group. (E) Mean (±SEM) number of macrophages (ED-1 positive cells per glomerular hilar cross-sectional area). *P<0.05, **P<0.01, and ***P<0.001 versus control; ##P<0.01 and ###P<0.001 versus DM; $P<0.05 and $$$P<0.001 versus HTN.
Tubulointerstitial fibrosis (TIF) and inflammation are key components in the pathogenesis of DN; indeed, the severity of TIF more accurately predicts prognosis than the glomerular findings.14 The absence of overt TIF in rodent models of DN compromises their ability to effectively model human DN. Indeed, even in the endothelial nitric oxide synthase knockout mouse, which develops moderate hypertension and significant glomerular pathology and is arguably the most convincing model of DN to date, there is scant evidence of TIF.15,16 As anticipated, there was no evidence of TIF after induction of diabetes alone; however, overt TIF developed in the DM+HTN animals as indicated by a significant increase in collagen deposition (Figure 3, A and C) and myofibroblast activation (Figure 3, B and D). The innate immune system plays a major role in the pathogenesis of DN,17 and although this was not activated by diabetes alone, marked macrophage infiltration was observed in the tubulointerstitium of both hypertensive groups (Figure 3E). The role of the adaptive immune system in DN is less well characterized; however, tubulointerstitial T cell and B cell infiltration is observed in human DN18 and T cells may be pathogenic in rodent DN.19 There was an increase in tubulointerstitial T lymphocytes in the hypertensive animals, which was not evident with diabetes alone (Figure 3F). In addition, focal B cell aggregates were observed solely in the DM+HTN group, often adjacent to blood vessels (Figure 3G). Lymphocyte recruitment may be mediated by the increase in chemokines and chemokine receptors observed predominantly in the DM+HTN group (Supplemental Table 1).
Figure 3.
Diabetes and hypertension together promote tubulointerstitial inflammation and fibrosis. Representative images of interstitial (A) picrosirius red and (B) α-SMA staining in kidneys from each group. Bars represent 50 μM. Mean (±SEM) percentage of tubulointerstitial area in each group staining with (C) picrosirius red and (D) α-SMA. Mean (±SEM) number of (E) macrophages (ED-1+ve) or (F) T cells (CD-3+ve) per 200-power tubulointerstitial field in each group. (G) B cells (CD45RA+ve) were found solely in the DM+HTN animals in (i) aggregates and (ii) adjacent to blood vessels, in particular. Bars represent 25 μM. ***P<0.001 versus control; #P<0.05, ##P<0.05, and ###P<0.001 versus DM; $P<0.05 and $$P<0.01 versus HTN.
To determine the molecular signature of the interaction between hypertension and hyperglycemia we performed microarray analysis on whole kidney tissue (n=4 per group). Remarkably, despite prolonged severe hyperglycemia in the DM group, only 8 and 15 genes were significantly upregulated and downregulated (corrected P<0.01), respectively, versus controls. Indeed, the vast majority of genes were dysregulated only by concurrent diabetes and hypertension (Figure 4). The top 60 upregulated and downregulated genes in DM+HTN animals compared with controls are given in Supplemental Tables 2 and 3. Analysis using the Database for Annotation, Visualization and Integrated Discovery (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Frederick, MD) identified pathways that contain a significant over-representation of genes that were upregulated (Figure 4B) or downregulated (Figure 4C) in the DM+HTN group. Examples of pathways and networks identified by GeneGo Metacore pathway analysis and Network Building software from the upregulated genes in the DM+HTN rats include the classical complement pathway (Supplemental Figure 1), the antigen presenting cell-mediated regulation of the cell cycle (Supplemental Figure 2) and an extracellular matrix gene network (Supplemental Figure 3).
Figure 4.
Patterns of renal gene expression changes with diabetes and hypertension. (A) Venn diagrams demonstrating number of genes upregulated or downregulated >1.5-fold in the DM, HTN, or DM+HTN groups compared with control animals at a corrected P<0.01. Kegg pathways that contain an over-representation of genes that are (B) upregulated and (C) downregulated in DM+HTN rats compared with controls.
It is, however, worth noting that because the microarray was performed on terminal tissue samples, many of the changes in gene expression will be secondary to the presence of an inflammatory cell infiltrate or to modification of the intrinsic cells due to anchorage to a scarred extracellular matrix, rather than reflect the primary causal pathways of hyperglycemic and hypertensive damage.
Because the glomeruli comprise a small proportion of the total renal mass, whole-kidney microarray predominantly reflects gene expression in the tubulointerstitium. Hence, to determine whether the pattern of gene expression observed in our study reflects the molecular pathophysiology of human DN, we compared the upregulated genes from each group with those preferentially expressed in the tubulointerstitium of patients with DN.20 Remarkably, none of the genes that were upregulated in human DN were induced by diabetes alone, whereas 21% and 42% were significantly upregulated in the HTN and DM+HTN groups, respectively (Supplemental Table 4). Conversely, 43% and 39% of the top 60 upregulated and downregulated genes in the DM+HTN rats were similarly dysregulated in the tubulointerstitium in human DN (Supplemental Tables 2 and 3; http://www.nephromine.org). The inability of hyperglycemia alone to activate many of the pathways that promote DN in humans suggests that a “second hit” such as hypertension is required and this is readily illustrated by examining the expression of individual genes.
Havcr1, which encodes the tubular injury marker kidney injury molecule-1, is upregulated early in the course of human DN.21 Although it was highly expressed in the DM+HTN animals (Supplemental Table 2 and Supplemental Figure 4), the lack of induction by diabetes alone implies minimal tubular injury despite 28 weeks of marked hyperglycemia. The Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway plays a key role in human DN and the absence of TIF in standard rodent models of DN may reflect a failure to activate this pathway.22 In keeping with this theory, multiple JAK-STAT pathway genes, such as JAK1, JAK2, and STAT1, were induced by the combination of diabetes and hypertension, but not by diabetes alone (Supplemental Figure 5).
Gene expression analysis from conventional rodent models of DN may not be reliably informative regarding the pathogenesis of human DN. For example, the increase in vascular endothelial growth factor-A (VEGF-A) expression observed in rodent models of incipient DN23 implicated VEGF-A inhibition as an attractive therapeutic strategy. However, in human DN, VEGF-A expression is reduced20 and VEGF-A antagonists may be detrimental and promote proteinuria.24 Likewise in this study, whereas hyperglycemia alone tended to increase VEGF-A expression, the combination of hypertension and hyperglycemia reduced VEGF-A expression (Supplemental Figure 6), suggesting that superimposing hypertension on diabetes more closely reflects the pathophysiology of human DN.
One limitation of this study is that it cannot determine whether the development of nephropathy is due to hypertension or activation of the renin-angiotensin-aldosterone-system per se. In addition, whereas the DM+HTN rats mimicked many of the hallmarks of human DN, some cardinal features were absent such as arteriolar hyalinosis and a significant (>50%) decline in renal function. There was, however, a trend toward a reduction in mean (±SD) inulin clearance in both hypertensive groups (1.9±0.6 ml/min [n=6] and 2.6±0.6 ml/min [n=8] in the HTN and HTN+DM groups, respectively) compared with the control and DM animals (3.2±1.4 ml/min [n=6] and 3.4±0.9 ml/min, [n=5], respectively). At the level of BP used in this study, hypertension seems to be at least as important as hyperglycemia in mediating renal injury; this may also be the case in human DN, as emphasized by the case reports of patients with diabetes and unilateral renal artery stenosis in which the failure of transmission of systemic hypertension to the kidney prevents development of nephropathy.6,7
In conclusion, this study reaffirms that induction of diabetes alone in rodents is of limited utility in modelling human DN. However, superimposing moderate renin-dependent hypertension results in many of the clinical, histological, and molecular features of human DN and is a relevant model in which to test novel therapies and dissect the pathogenesis of the disease.
Concise Methods
Animal Studies
Cyp1a1mRen2 rats were generated as described.13 Diabetes was induced by a single intravenous injection of 20 mg/kg streptozotocin and blood sugars were maintained in the 20–30 mM range by serial subcutaneous insulin implants (Research Pack, Linshin, Canada). Two weeks after onset of diabetes, hypertension was induced by dietary supplementation of 0.125% by mass I-3-C (Sigma, UK). Tail-cuff BP measurements were performed twice weekly in conscious, trained animals and 24-hour urine collections were obtained every 4 weeks. After 28 weeks, animals were anesthetized (Inactin; 120 mg/kg intraperitoneally) and inulin clearance was determined as described.25 All procedures were preformed under a UK Home Office license.
Albumin and Creatinine Measurements
Albumin and creatinine measurements were performed on a Cobas Fara centrifugal analyzer (Roche Diagnostics Ltd, Welwyn Garden City, UK) using a commercial immunoturbidimetric assay (Microalbumin Kit, Olympus Diagnostics Ltd, Watford, UK) and a creatininase-based enzymatic method26 (Alpha Laboratories Ltd, Eastleigh, UK), respectively.
Immunohistochemistry
Staining was performed on 4-μM methacarn-fixed, paraffin-embedded sections using standard protocols with the following primary antibodies: mouse anti-rat α-SMA (1:500; Sigma-Aldrich, Dorset, UK), mouse anti-rat ED-1 (macrophage marker, 1:100; AbD Serotec, Kidlington, UK), rabbit anti-rat CD3 (T cell marker, 1:1000; Abcam, Cambridge, UK), anti-rat CD45RA (B cell marker, 1:500; AbD Serotec), goat anti-rat kidney injury molecule-1 (1:100; R&D Systems, Abingdon, UK), or a species-specific isotype. Sections were also stained for periodic acid–Schiff and picrosirius red using standard protocols. The glomerulosclerosis index for each animal was determined from all glomeruli in one kidney cross-section (mean 180 glomeruli/animal) as described previously.27 The mean number of ED-1 and CD3+ve cells were obtained from 60 hilar glomerular cross-sections per animal and twenty 200-power tubulointerstitial fields per animal. The mean glomerular and tubulointerstitial area staining for α-SMA or picrosirius red was calculated from 30 hilar glomerular cross-sections per animal or 20 interstitial fields using Adobe Photoshop software.
Microarray Analysis
RNA was extracted using the Nucleospin RNA II kit (Macherey-Nagel, Duren, Germany) from snap-frozen, homogenized whole kidney tissue from four representative animals from each group. RNA was processed using standard Affymetrix protocols, including one round of cDNA amplification, and was hybridized to the Affymetrix Rat Genome 230 2.0 GeneChip. Data were extracted by using GeneChip Operating Software software, and Celestia files were used for further data processing in Bioconductor,28 normalized by Robust Multiarray Average in the Affy module,29 and analyzed with the Limma30 and Rank Products packages.31 Gene ontology32 and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Frederick, MD).33 Metacore pathway analysis software (GeneGo, St. Joseph, MI) was used to identify functional links in the differentially expressed genes and networks were built using Metacore's default network building algorithm. Microarray data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress; accession no. E-MEXP-3165). The relative expression levels of selected genes from the microarray were validated by real-time PCR using inventoried Taqman gene expression assays and the 2-ΔΔCt method per the manufacturer’s instructions (Applied Biosystems, Cheshire, UK).
Statistical Analyses
Data are presented as mean ± SD and median (interquartile range) where the data are normal or skewed, respectively. The groups were compared by one-way ANOVA (after log-transformation for nonparametric data where necessary).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Forbes Howie, Dr. Linda Mullins, Dr. Chris Kenyon, Louise Evans, Robert Menzies, and the staff of the animal facility for useful discussions and technical support.
B.R.C. was supported by a Medical Research Council Clinician Scientist Fellowship. D.R.D. and J.R.M. are supported by the University of Edinburgh BHF Centre for Research Excellence.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011060577/-/DCSupplemental.
References
- 1.US Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 2.Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium: Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D: Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347: 797–805, 2002 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 5.Mogensen CE: Combined high blood pressure and glucose in type 2 diabetes: Double jeopardy. British trial shows clear effects of treatment, especially blood pressure reduction. BMJ 317: 693–694, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkman J, Rifkin H: Unilateral nodular diabetic glomerulosclerosis (Kimmelstiel-Wilson): Report of a case. Metabolism 22: 715–722, 1973 [DOI] [PubMed] [Google Scholar]
- 7.Béroniade VC, Lefebvre R, Falardeau P: Unilateral nodular diabetic glomerulosclerosis: Recurrence of an experiment of nature. Am J Nephrol 7: 55–59, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Cooper ME, Allen TJ, Macmillan P, Bach L, Jerums G, Doyle AE: Genetic hypertension accelerates nephropathy in the streptozotocin diabetic rat. Am J Hypertens 1: 5–10, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL: A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR). Kidney Int 54: 343–352, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Janssen U, Riley SG, Vassiliadou A, Floege J, Phillips AO: Hypertension superimposed on type II diabetes in Goto Kakizaki rats induces progressive nephropathy. Kidney Int 63: 2162–2170, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Mullins JJ, Peters J, Ganten D: Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 344: 541–544, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Hartner A, Cordasic N, Klanke B, Wittmann M, Veelken R, Hilgers KF: Renal injury in streptozotocin-diabetic Ren2-transgenic rats is mainly dependent on hypertension, not on diabetes. Am J Physiol Renal Physiol 292: F820–F827, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ: Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem 276: 36727–36733, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert RE, Cooper ME: The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int 56: 1627–1637, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC: Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T: Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473–1484, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesch GH: Macrophages and diabetic nephropathy. Semin Nephrol 30: 290–301, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Cohen CD, Lindenmeyer MT, Eichinger F, Hahn A, Seifert M, Moll AG, Schmid H, Kiss E, Gröne E, Gröne HJ, Kretzler M, Werner T, Nelson PJ: Improved elucidation of biological processes linked to diabetic nephropathy by single probe-based microarray data analysis. PLoS ONE 3: e2937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim AK, Ma FY, Nikolic-Paterson DJ, Kitching AR, Thomas MC, Tesch GH: Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia 53: 1772–1782, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlöndorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV: Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 79: 464–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE: Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 48: 2229–2239, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Bellamy CO, Bailey MA, Mullins LJ, Dunbar DR, Kenyon CJ, Brooker G, Kantachuvesiri S, Maratou K, Ashek A, Clark AF, Fleming S, Mullins JJ: Angiotensin-converting enzyme is a modifier of hypertensive end organ damage. J Biol Chem 284: 15564–15572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J: Plasma creatinine determination in mice and rats: An enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Iturbe B, Quiroz Y, Shahkarami A, Li Z, Vaziri ND: Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int 68: 1041–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J: Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautier L, Cope L, Bolstad BM, Irizarry RA: Affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Smyth GK: Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article 3, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Breitling R, Armengaud P, Amtmann A, Herzyk P: Rank products: A simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573: 83–92, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium: Gene ontology: Tool for the unification of biology. Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.