Abstract
The pathogenesis of anti–neutrophil cytoplasmic antibody (ANCA)–associated necrotizing crescentic GN (NCGN) is incompletely understood. Dipeptidyl peptidase I (DPPI) is a cysteine protease required for the activation of neutrophil serine proteases (NSPs) cathepsin G, neutrophil elastase, and proteinase 3, which are enzymes that modulate inflammation. We used a mouse model of anti–myeloperoxidase (MPO) antibody–induced NCGN to determine whether active NSPs contribute to its pathogenesis. MPO-deficient animals immunized with murine MPO, irradiated, and transplanted with wild-type bone marrow developed NCGN. In contrast, transplantation with bone marrow that lacked DPPI or lacked both neutrophil elastase and proteinase 3 protected mice from NCGN induced by anti-MPO antibody. The kidneys of mice reconstituted with DPPI-deficient bone marrow generated significantly less IL-1β than did those of mice reconstituted with wild-type bone marrow; similarly, in vitro, DPPI-deficient monocytes produced significantly less IL-1β in response to anti-MPO antibody than did wild-type monocytes. This reduction in IL-1β was NSP dependent; exogenous addition of PR3 restored IL-β production in DPPI-deficient monocytes. Last, the IL-1 receptor antagonist anakinra protected animals against anti-MPO antibody–induced NCGN (16.7%±6.0% versus 2.4%±1.7% crescents), suggesting that IL-1β is a critical inflammatory mediator in this model. These data suggest that the development of anti-MPO antibody–induced NCGN requires NSP-dependent IL-1β generation and that these processes may provide therapeutic targets for ANCA-mediated diseases in humans.
Anti–neutrophil cytoplasmic autoantibody (ANCA) with specificity to proteinase 3 (PR3) or myeloperoxidase (MPO) is detected in patients with systemic small-vessel vasculitis and necrotizing crescentic GN (NCGN).1,2 The pathogenicity of ANCA for NCGN was established in several animal models.3–7 Extensive in vitro evidence indicates that ANCA binds to target antigens on neutrophils and monocytes and initiates signaling events leading to cell activation.8–10 ANCA-induced respiratory burst and release of proteolytically active granule proteins are thought to be pivotal mediators of the resulting vasculitic damage.
Active serine proteases modulate the inflammatory response by processing cytokines, growth factors, surface receptors, and signaling molecules. PR3, neutrophil elastase (NE), and cathepsin G (CG) are the most abundant neutrophil-derived serine proteases (NSPs) and reside in neutrophil granules and monocyte lysosomes. NSPs participate in intracellular killing of pathogens but also act extracellularly by degrading matrix proteins, generating more or less active chemokines and cytokines, cleaving NF-κB and progranulin, and activating protease-activated receptor 2.11 Most of these effects were shown in vitro; the in vivo pathophysiologic significance remains to be established. Serine protease activation requires proteolytic proform cleavage by the lysosomal cysteine protease dipeptidyl peptidase I (DPPI) also known as cathepsin C.12 Data obtained in DPPI loss-of-function mutation (DPPI−/−) mice further suggest the importance of active serine proteases in inflammatory conditions, such as asthma, arthritis, air pouch inflammation, and abdominal aortic aneurysm.13–15
Cytokines are important inflammatory mediators in ANCA-associated vasculitis. Increased TNFα and IL-1β levels were measured in patients with active disease.16 Priming with cytokines or with anaphylatoxin C5a increased the ANCA response in vitro and in animal models.5,17,18 IL-1β is a key mediator of inflammatory and immune responses, and monocytes are the main source of IL-1β. The significance of this cytokine in ANCA-associated vasculitis is not known. The secretion of IL-1β is a tightly regulated process, requiring proteolytic cleavage of the inactive pro–IL-1β to IL-1β.19 This process is controlled by the inflammasome promoting caspase-1, the classic IL-1β–converting enzyme.20 More recently, several studies have suggested that active NSP may also participate in IL-1β generation;12,21–24 however, a direct link between NSP and IL-1β processing has never been firmly established.
Using specific NSP-deficient mouse strains, we establish that active NSPs, specifically PR3, are essential for anti-MPO antibody–induced NCGN. Our data implicate NSP-dependent IL-1β generation as an important mechanism for NCGN development. We suggest that pharmacologic NSP or IL-1β blockade could provide novel treatment targets in ANCA-associated NCGN in humans.
Results
Absence of DPPI Abrogates Disease in a Mouse Model of Anti-MPO Antibody–Induced NCGN
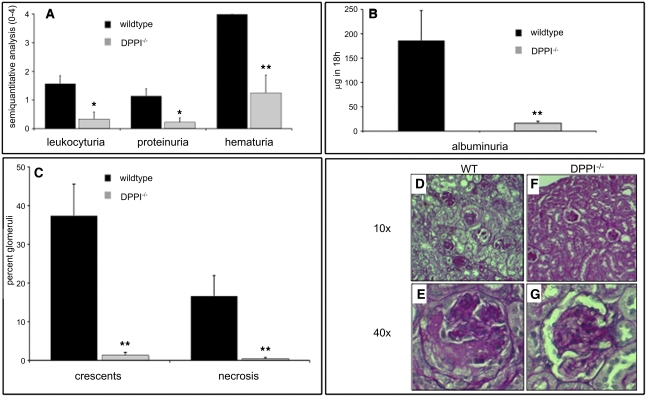
To test the hypothesis that enzymatically active NSPs are necessary for anti-MPO antibody–induced NCGN in vivo, we used a murine model of bone marrow transplantation.4 MPO-deficient animals were immunized with murine MPO, irradiated, and transplanted with bone marrow cells from wild-type (WT) mice or DPPI-deficient (DPPI−/−) animals (n=8 in each group). In mice transplanted with WT bone marrow, we found marked hematuria, leukocyturia, and proteinuria (Figure 1, A and B). All these measures were significantly reduced in mice that received DPPI−/− bone marrow. Eight weeks after transplantation, mice were sacrificed and their kidneys were harvested and analyzed. All eight mice transplanted with WT bone marrow developed focal and segmental crescentic GN (37.4%±8.2% crescents, mean ± SEM). In contrast, only three of eight mice in the group transplanted with DPPI−/− bone marrow showed glomerular crescent formation. Statistically, the disease severity was significantly lower in the DPPI−/− mice (1.3%±0.7% crescents; P<0.001 compared with WT) (Figure 1C). This protective effect in DPPI−/− bone marrow–transplanted animals was not due to different anti-MPO IgG titers because these values did not differ between groups (anti-MPO IgG at week 4: 1.28±0.2 arbitrary units [AU] in WT versus 1.12±0.23 AU in DPPI−/− mice; week 8: 1.0±0.15 AU in WT versus 0.84±0.28 AU in DPPI−/− mice). In addition, immunohistochemistry for IgG, IgA, IgM, and C3 showed minimal deposition with no difference between groups (data not shown).
Figure 1.
Absence of DPPI protects against anti-MPO antibody–induced NCGN. Urine analysis and renal histologic assessment in mice 8 weeks after transplantation with WT or DPPI−/− bone marrow: (A) dipstick analysis, (B) albuminuria by ELISA, and (C) renal tissue analysis. Glomerular crescents and necrosis are expressed as the mean percentage of glomeruli with crescents and necrosis. Typical examples for each transplanted group (n=8 per genotype) are depicted with ×10 magnification in the upper and ×40 magnification in the lower rows in D–G (PAS staining). *P<0.05, **P<0.01.
Protection from Anti-MPO Antibody–Induced NCGN Correlates with Inactivation of NSP
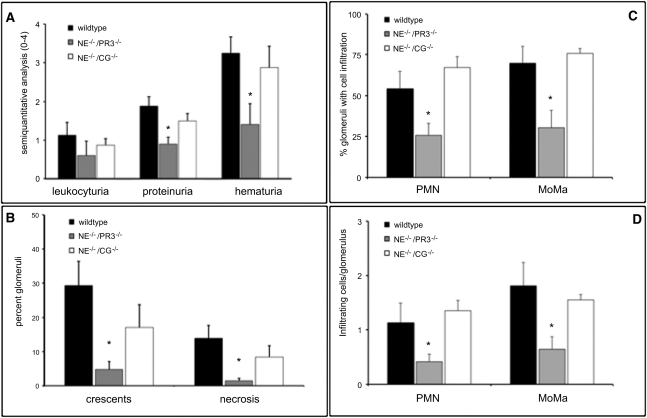
The absence of DPPI may affect several immune effector cell functions that could contribute to the observed phenotype.13,15, 25 To further investigate the mechanism involved in the resistance to NCGN, we transplanted MPO-immunized mice with WT bone marrow (n=8), NE−/−/CG−/− bone marrow (n=8), or NE−/−/PR3−/− bone marrow (n=7) to induce NCGN. We found that mice that received NE−/−/PR3−/− bone marrow displayed less hematuria and proteinuria (Figure 2A) and were protected against anti-MPO antibody–induced NCGN (Figure 2B), whereas reconstitution with bone marrow from NE−/−/CG−/− mice did not significantly protect animals against disease development (Figure 2B). Taken together, these results strongly suggest that the protection against NCGN observed in animals transplanted with DPPI−/− bone marrow is due to inactivation of PR3 or a combination of PR3 and NE, whereas CG probably plays a minor role in this model.
Figure 2.
Absence of NSP protects against anti-MPO antibody–induced NCGN. Urine analysis and renal histologic assessment in mice 8 weeks after transplantation with WT, NE−/−/PR3−/−, or NE−/−/CG−/− bone marrow: (A) dipstick analysis and (B) renal histologic assessment. Glomerular crescents and necrosis are expressed as the mean percentage of glomeruli with crescents and necrosis. (C) Glomerular neutrophil (PMN) and monocyte/macrophage (MoMa) influx are expressed as the percentage of glomeruli with PMN or MoMa infiltration. (D) Absolute number of infiltrating cells per glomerulus. *P<0.05.
Absence of DPPI and NSP Attenuates Glomerular Neutrophil and Macrophage Influx
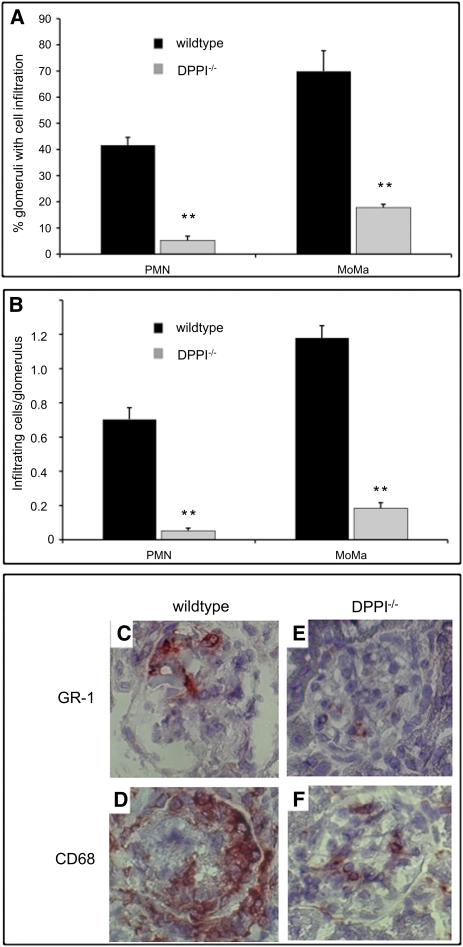
Next, we investigated the glomerular inflammatory cell influx in all animals. Analysis of kidney sections from mice transplanted with WT bone marrow revealed robust glomerular neutrophil and macrophage influx, whereas animals transplanted with DPPI−/− bone marrow showed markedly reduced inflammatory cell infiltrates in the kidneys (Figure 3, A–F). Consistent with the defect seen in mice reconstituted with DPPI−/− bone marrow, mice that received NE−/−/PR3−/− bone marrow also showed a significant decrease in inflammatory cell infiltrates (Figure 2, C and D).
Figure 3.
Absence of DPPI protects against inflammatory cell influx. (A) Percentage of glomeruli with neutrophil (PMN) or monocyte/macrophage (MoMa) infiltration. (B) Absolute number of infiltrating cells per glomerulus. Representative renal cross-sections from mice transplanted with WT (C and D) and DPPI−/− (E and F) bone marrow stained with GR-1 for PMN and CD68 for MoMa. **P<0.01.
Inflammatory Cytokines Are Reduced in the Absence of DPPI and NSP
To gain further insight into the mechanism underlying the renal-protecting effect observed in mice transplanted with DPPI−/− bone marrow, we measured the local production of cytokines in kidneys obtained from mice at 8 weeks after transplantation. In mice transplanted with DPPI−/− bone marrow, we observed a significant reduction of TNFα, MCP1, IL12p70, IL-10, and GM-CSF levels (Table 1). However, the biggest difference, a 15-fold reduction, was detected for IL-1β. In contrast, serum cytokine levels showed no significant difference between study groups (data not shown). Similar to the defect seen in mice transplanted with DPPI−/− bone marrow, we found a significant decrease in IL-1β generation in the kidneys of mice transplanted with NE−/−/PR3−/− bone marrow but not in mice transplanted with NE−/−/CG−/− (1.53±0.42 μg/g in WT mice versus 0.62±0.14 μg/g in NE−/−/PR3−/− mice versus 1.29±0.31 μg/g in NE−/−/CG−/− mice). Because monocytes are a major source of pro–IL-1β, we pursued the hypothesis that NSPs directly control IL-1β release in this cellular compartment.
Table 1.
Renal cytokine levels in MPO-deficient mice transplanted with WT or DPPI−/− bone marrow
| Variable | WT Mice | DPPI−/− Mice |
|---|---|---|
| TNFα (μg/g) | 1.24±0.22a | 0.40±0.11 |
| MCP-1 (μg/g) | 15.9±1.77a | 9.14±2.09 |
| IL-6 (ng/g) | 0.39±0.07 | 0.25±0.12 |
| IFNγ (ng/g) | 0.044±0.002 | 0.13±0.05 |
| IL-12p70 (ng/g) | 0.13±0.03a | 0.023±0.023 |
| IL-10 (ng/g) | 0.42±0.051a | 0.25±0.062 |
| IL-1β (μg/g) | 1.96±0.56a | 0.13±0.032 |
| G-CSF (ng/g) | 0.47±0.06a | 0.26±0.14 |
Data are expressed as the mean ± SEM.
P<0.05.
Anti-MPO Antibody–Induced IL-1β Production in Monocytes Depends on Active NSP
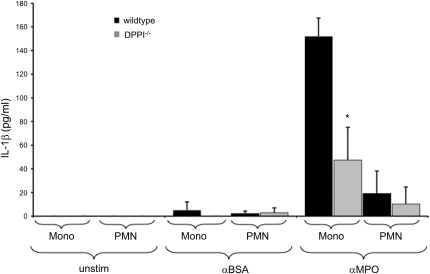
Bone marrow–derived neutrophils and monocytes from WT and DPPI−/− mice were primed with TNFα, stimulated with anti-MPO IgG (purified from MPO-deficient mice immunized with MPO), and evaluated for their ability to secrete IL-1β. Cells stimulated with IgG purified from MPO-deficient mice after immunization with BSA served as controls. We found that anti-MPO antibody induced the release of a substantial amount of IL-1β from monocytes, whereas the release of IL-1β from neutrophils was only modest. The response to anti-MPO IgG was significantly reduced in DPPI-deficient cells (Figure 4).
Figure 4.
Defective IL-1β generation in DPPI−/− monocytes and neutrophils stimulated with anti-MPO IgG. Murine bone marrow neutrophils (PMN) and monocytes (Mono) were primed with TNFα for 30 minutes and subsequently stimulated with anti-BSA IgG (αBSA) and anti-MPO IgG (αMPO) or left unstimulated (unstim). Supernatants were collected after 4 hours and analyzed for IL-1β by ELISA. Anti-MPO IgG stimulation resulted in robust IL-1β release in WT monocytes (Mono) and to a lesser extent in WT neutrophils (PMN), but release was significantly reduced in cells from DPPI−/− mice. Two independent experiments using a total of 24 mice were performed. Each experiment used pooled bone marrow neutrophils and bone marrow monocytes from six WT and six DPPI−/− mice, respectively. *P<0.05.
To confirm that NSPs are directly responsible for the release of IL-1β, monocytes from NSP-deficient mice were stimulated with anti-MPO IgG for induction of IL-1β. Compared with WT mice, anti-MPO IgG–stimulated monocytes from NE−/−/PR3−/− mice displayed significantly reduced IL-1β generation, whereas stimulated monocytes from NE−/−/CG−/− mice showed no reduction (Figure 5A). Finally, we sought to rescue the defect in IL-1β production with exogenous administration of purified human NSP. We found that addition of exogenous human PR3 significantly increased anti-MPO IgG–induced IL-1β generation whereas human NE did not (Figure 5B).
Figure 5.
NSP-dependent anti-MPO IgG–stimulated IL-1β generation. (A) Monocytes from WT, NE−/−/PR3−/−, or NE−/−/CG−/−mice were primed with TNFα for 30 minutes and subsequently stimulated with anti-MPO IgG (αMPO). Supernatants were collected after 4 hours and analyzed for IL-1β by ELISA. NE−/−/PR3−/− mice displayed a significant reduction in anti-MPO IgG–induced IL-1β generation. Four independent experiments using a total of 48 mice were performed. Each experiment used pooled bone marrow monocytes from 4 mice per genotype. *P<0.05. (B) Exogenous addition of human PR3 increases IL-1β generation in anti-MPO IgG–stimulated DPPI−/− monocytes. Human NE or PR3 (2.5 μg/ml each) was added to bone marrow monocytes at the beginning of the assay, and cells were subsequently primed with TNFα for 30 minutes before stimulation with anti-MPO IgG for 4 hours. Supernatants were analyzed by ELISA for IL-1β. Three independent experiments using a total of 24 mice were performed. Each experiment used pooled bone marrow monocytes from four WT and four DPPI−/− mice, respectively. *P<0.05.
These data strongly suggest that anti-MPO IgG stimulation results in a DPPI- (and NSP-) dependent IL-1β release from monocytes and, to a lesser extent, neutrophils.
ANCA from Patients with Vasculitis Promote IL-1β Release by Human Monocytes and Neutrophils in an NSP-Dependent Fashion
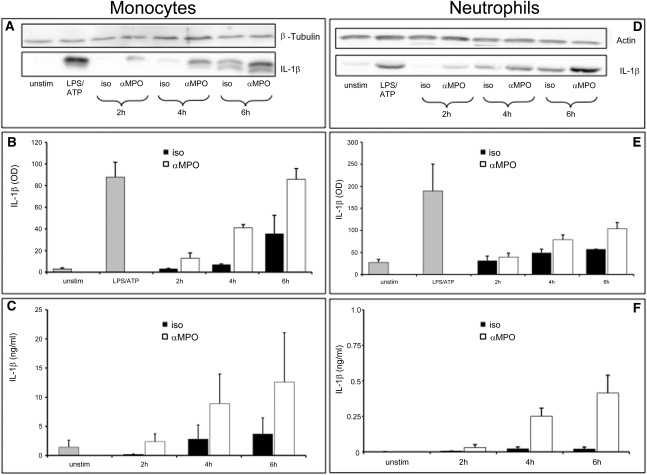
Next, we extended our observations obtained in murine cells and investigated whether TNFα-primed human monocytes and neutrophils produce IL-1β after stimulation with an mAb to MPO. Cleavage of pro–IL-1β into the active 17-kD IL-1β form was analyzed by Western blot analysis in cell lysates. In monocytes, we found a significant, time-dependent generation of active IL-1β with anti–MPO mAb stimulation (Figure 6, A and B). Active IL-1β generation by LPS/ATP stimulation served as a positive control. When we analyzed the cell supernatants for secretion of IL-1β by ELISA, we also observed a significant release of IL-1β in response to anti–MPO mAb stimulation (Figure 6C). Human neutrophils showed a much weaker IL-1β generation in response to anti-MPO mAb stimulation, both by Western blot analysis (Figure 6, D and E) and by ELISA (Figure 6F), approximately a 20-fold reduction compared with monocytes.
Figure 6.
Anti-MPO IgG stimulates IL-1β release from human monocytes and neutrophils. TNFα-primed human monocytes and neutrophils were stimulated with an isotype control antibody (iso) or a mAb to MPO (αMPO) for the indicated time. A (monocytes) and D (neutrophils) show representative Western blot analysis of the active 17-kD IL-1β form. β-tubulin serves as a loading control for protein content. IL-1β mean OD obtained from densitometry of Western blots was calculated from five independent experiments: B = monocytes and E = neutrophils. Secreted IL-1β was also measured by ELISA: C = monocytes and F = neutrophils.
Finally, we compared anti-MPO and anti-PR3 mAbs in parallel and tested whether human ANCA can induce IL-1β generation and secretion by human monocytes. We used three different PR3-ANCA and MPO-ANCA preparations. Generation of active IL-1β was analyzed by Western blot and ELISA. The results indicate that human PR3-ANCA and MPO-ANCA were as efficient as the mAbs to PR3 and MPO in stimulating monocytes to generate and release IL-1β (Figure 7, A–F).
Figure 7.
Human ANCA promotes IL-1β generation in human monocytes. (A) TNFα-primed human monocytes were stimulated with an isotype control antibody (iso) or mAbs to PR3 (αPR3) and MPO (αMPO) for 4 hours. Control cells were left unstimulated (unstim) or stimulated with LPS/ATP. (A) Representative Western blot analysis of the active 17-kD IL-1β secreted form is shown. β-tubulin serves as protein loading control. (B) Mean IL-1β OD (from densitometry) derived from five independent experiments. (C) Secreted IL-1β from anti-PR3– and anti-MPO–stimulated monocytes assayed by ELISA (n=5 independent experiments). (D) TNFα-primed human monocytes were stimulated with IgG isolated from healthy donors (ctrl IgG), PR3-ANCA IgG (PR3 ANCA), or MPO-ANCA IgG (MPO ANCA). A representative Western blot analysis of the active 17-kD IL-1β secreted form is shown. (E) Mean IL-1β OD (from densitometry) derived from five independent experiments. (F) Secreted IL-1β from PR3-ANCA– or MPO-ANCA–stimulated monocytes assayed by ELISA (n=5 independent experiments). *P<0.05, **P<0.01.
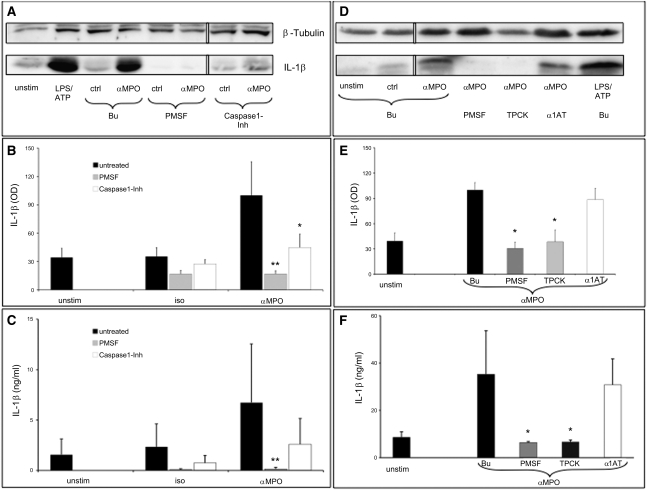
To address whether anti-MPO antibody–induced IL-1β generation in human monocytes is NSP dependent, we preincubated the cells with the serine protease inhibitor phenylmethylsulfonylfluoride (PMSF) or with the specific caspase-1 inhibitor Ac-YVAD-CHO before incubation with the mAb to MPO. By Western blot analysis we observed that IL-1β generation was significantly reduced in cells preincubated with PMSF (Figure 8, A through C). In parallel, preincubation with YVAD-CHO also inhibited IL-1β, albeit to a lesser extent. The data suggest that both NSP- and caspase-1–dependent mechanism were at work. Furthermore, we observed that only the cell-permeable NSP inhibitors PMSF and N-tosyl-L-phenylalanine chloromethyl ketone (TPCK), but not the cell-impermeable inhibitor α1-antitrypsin, prevented IL-1β generation (Figure 8, D–F). Of note, no significant cell toxicity was associated with the inhibitors (data not shown).
Figure 8.
IL-1β release from ANCA-stimulated human monocytes is primarily mediated by NSP. (A) Human monocytes were preincubated for 30 minutes with control buffer (Bu), 1 mM PMSF, or 10 μM Ac-YVAD-CHO (Caspase1-Inh), followed by TNFα priming and stimulation with an isotype control antibody (iso) or a mAb to MPO (αMPO) for 4 hours. Representative Western blot analysis of the active 17-kD IL-1β secreted form is shown. β-tubulin serves as protein loading control. Lanes were run on the same gel but were noncontiguous (white line). (B) Mean IL-1β OD (from densitometry) derived from four independent experiments. (C) Secreted IL-1β from αMPO-stimulated monocytes preincubated with the indicated inhibitors assayed by ELISA (n=4 independent experiments). (D) Monocytes were preincubated with the cell-permeable serine-protease inhibitors PMSF (1 mM) and TPCK (10 μM) or the cell-impermeable inhibitor α1AT (10 μM) before stimulation with αMPO. A representative Western blot analysis of the active 17-kD IL-1β secreted form is shown. Lanes were run on the same gel but were noncontiguous (white line). (E) Mean IL-1β OD (from densitometry) derived from four independent experiments. (F) Secreted IL-1β from αMPO-stimulated monocytes preincubated with the indicated inhibitors assayed by ELISA (n=4 independent experiments). *P<0.05, **P<0.01.
These data, together with the mouse data, support the notion that ANCA-induced IL-1β generation is mediated by active NSP.
IL-1–Receptor Blockade Protects against ANCA-Induced NCGN
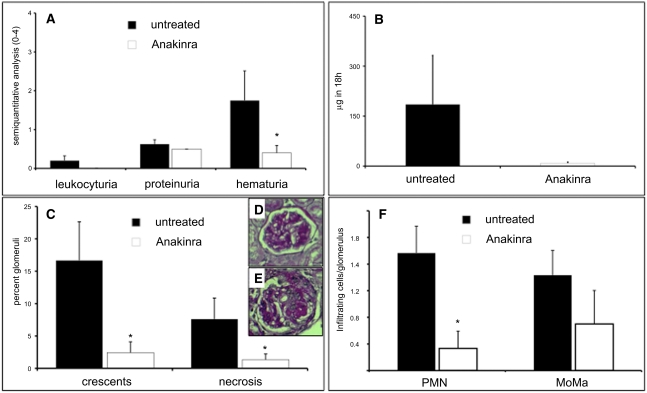
Having established that IL-1β levels in the kidneys of mice with anti-MPO antibody–induced NCGN correlate with disease severity, we wanted to investigate further whether the absence of IL-1β or the interruption of the IL-1β signaling cascade would protect against anti-MPO antibody–induced NCGN. Although the IL-1 receptor–deficient mouse would provide a definitive answer, the use of this loss-of-function model is not optimal for the bone marrow transplantation approach; elegant studies by Timoshanko et al. demonstrated that the IL-1 receptor on renal stromal cells was more important than the receptor on myeloid cells in its ability to induce NCGN.26 As an alternative, we sought to block the inflammatory effects of IL-1β through the well-characterized IL-1–receptor antagonist anakinra.26 NCGN was induced as described above; 4 weeks after bone marrow transplantation, mice were left untreated or received treatment with anakinra daily for 4 weeks (n=5 in each group). Mice in the anakinra-treated group developed significantly less hematuria (mean hematuria, 1.8±0.8 in untreated mice compared with 0.4±0.2 in treated mice, P=0.01), and results showed a trend toward reduction of other urinary abnormalities without reaching statistical significance (Figure 9, A and B). Of note, all the mice in the control group, but only two of five mice in the anakinra-treated group, developed NCGN (mean percentage of crescents, 16.7%±6.0% in untreated mice compared with 2.4%±1.7% in treated mice; P=0.02; Figure 9, C–E). In addition, animals treated with anakinra showed significant reduction in glomerular inflammatory leukocyte influx (Figure 9F). These data strongly suggest that IL-1β is a critical inflammatory mediator in anti-MPO antibody–induced NCGN.
Figure 9.
IL-1–receptor blockade protects against ANCA-induced NCGN. Urine analysis and renal histologic assessment in untreated or anakinra-treated MPO−/− mice 8 weeks after transplantation with WT bone marrow: (A) dipstick analysis, (B) albuminuria by ELISA, and (C) renal tissue analysis. Glomerular crescents and necrosis are expressed as the mean percentage of glomeruli with crescents and necrosis. Representative examples for each treatment group are depicted with ×40 magnification in (D) (untreated) and (E) (anakinra-treated) (PAS staining). (F) Infiltrating glomerular PMN and monocytes/macrophages (MoMa) as absolute number of cells per glomerulus.
Discussion
This study provides genetic evidence that active NSPs, particularly PR3, are intimately involved in anti-MPO antibody–induced NCGN. We showed that DPPI−/− mice (which lack active NSP) and NE−/−/PR3−/− mice were protected from anti-MPO antibody–induced NCGN. The renal protective effect was accompanied by significant decrease in the local (renal) production of inflammatory cytokines, including a strong reduction in IL-1β. Consistent with the in vivo findings, we demonstrate that ANCA-activated monocytes release IL-1β in vitro through a predominantly NSP-dependent process. In addition, specific IL-1–receptor blockade abrogates anti-MPO antibody–induced NCGN, confirming that IL-1β is an important inflammatory mediator in this disease process.
ANCAs directed against PR3 or MPO are found in patients with necrotizing vasculitis and pauci-immune NCGN.1,2 Animal models firmly establish the importance of anti-MPO antibody and neutrophils for the induction of vasculitis and NCGN.3,27,28 Although monocytes also express ANCA antigens,29–31 are activated by ANCA, and are consistently detected in ANCA-induced NCGN, their role is less well understood.31,32 In addition, although ANCA-induced neutrophil and monocyte activation has been extensively characterized in vitro, the effector mechanisms that actually cause the in vivo damage are not yet characterized. It is hypothesized that in a cytokine-rich milieu, ANCAs bind to cell surface membrane–expressed antigens on neutrophils and monocytes, leading to cellular activation and adhesion to the endothelium, the generation of reactive oxygen species, and the release of proteases and other toxic granule proteins, the cumulative effects of which result in small-vessel vasculitis, including NCGN. Here we sought to understand how active NSPs control the in vivo inflammatory response leading to NCGN.
Previous studies have shown that DPPI is required for the processing of NSP proform and that their neutrophils therefore lack catalytically active PR3, NE, and CG.12 However, DPPI activates other serine proteases, including cytotoxic lymphocyte granzymes and mast cell chymase.25,33 To examine the specific contribution of NSP in this model, we turned to the NSP-deficient mice NE−/−/PR3−/− and NE−/−/CG−/−. We found that NE−/−/PR3−/−, but not NE−/−/CG−/−, bone marrow protected mice from NCGN. These findings firmly establish that NSP, catalytically activated by DPPI, plays a critical role in anti-MPO antibody–induced NCGN. The fact that NE−/−/PR3−/− bone marrow protected against NCGN development but NE−/−/CG−/− bone marrow did not suggests that PR3, alone or in combination with NE, probably plays a predominant role in this process.
NSP are traditionally regarded as degradative enzymes that cleave extracellular matrix proteins and promote intracellular and extracellular killing of pathogens.34–37 However, recent evidence suggests that NSPs also modulate inflammation through the processing or release of cytokines, chemokines, growth factors, and receptors.11 Several studies have implicated PR3 (and NE) in the processing of IL-1β.12,21–24 For example, Greten et al. suggest that IL-1β processing and release by neutrophils and monocytes were mediated by intracellular PR3 and NE because a cell-permeable serine protease inhibitor, but not the cell-impermeable α1-antitrypsin, reduced IL-1β secretion.23 IL-1β generation is a tightly regulated process, including transcriptional regulation, regulation of the processing, and release of IL-1β. Processing of Il-1β by proteolytic cleavage of its inactive 31-kD precursor into the bioactive 17-kD fragment depends on an IL-1β–converting enzyme called caspase-1.38 Over the years, alternative IL-1β–converting enzymes other than PR3 and NE have been suggested, including granzyme A,39 mast cell chymase,40 and CG and collagenase.41 However, these conclusions were based on the use of inhibitory compounds that are inevitably associated with specificity concerns or on DPPI gene deletion that has pleiotropic effects on several other serine proteases. Using monocytes derived from DPPI−/− mice, we show for the first time that PR3 modulates IL-1β generation in vitro as addition of exogenous human PR3 rescued the defective IL-1β generation. Recent research showed that monocytic THP-1 (human acute monocytic leukemia cell line) cells internalize PR3.42 This finding, together with our data showing that only the cell-permeable inhibitor TPCK reduced IL-1β generation, implicates intracellular IL-1β processing by PR3. This assumption is also supported by the fact that we observed the generation of the processed IL-1β fragment by Western blot assay.
Moreover, the fact that YVAD-CHO reduced but did not completely abolish IL-1β processing and release from anti-MPO antibody–activated monocytes suggests an additional caspase-1–independent, NSP-dependent alternative mechanism of IL-1β generation.
Although several inflammatory cytokines were significantly elevated in the kidneys of mice with anti-MPO antibody–induced NCGN, we chose IL-1 antagonism as a therapeutic approach for several reasons. In humans, a spectrum of diseases collectively known as cryopyrin-associated periodic syndromes illustrates the highly inflammatory nature of IL-1. These conditions include familial Mediterranean fever, Muckle-Wells syndrome, neonatal-onset multisystem inflammatory disease, familial cold autoinflammatory syndrome, adult-onset Still’s disease, and syndrome of the interleukin-1–receptor antagonist deficiency.43 In our anti-MPO antibody–induced experimental model of NCGN, renal protection was associated with nearly 15-fold reduction in renal levels of IL-1β, the largest reduction measured (although we do not rule out the potential contribution of other inflammatory cytokines). That IL-1 antagonism is currently an established treatment for cryopyrin-associated periodic syndrome, adult-onset Still’s disease, systemic-onset juvenile idiopathic arthritis, and gouty arthritis illustrates the contribution of IL-1 to inflammation and suggests that IL-1–receptor blockade provides an attractive therapeutic option in NCGN.
The observation that IL-1 receptor antagonist treatment abrogated NCGN in the mouse model confirms that IL-1β is an important inflammatory mediator in anti-MPO antibody–induced NCGN and provides a proof-of-concept for this approach. However, additional issues, such as dosage titration and treatment timing, will need to be explored in future studies. In addition, our experimental approach does not distinguish which cells are affected by IL-1β antagonism. Because infiltrating neutrophils and monocytes, as well as resident renal cells, express IL-1 receptors, all these cells are potential IL-1β targets. However, Timoshanko et al. previously showed in an anti–glomerular basement membrane disease model that mice lacking IL-1 receptor in renal stromal cells were protected from NCGN, whereas mice lacking IL-1 receptor on bone marrow–derived cells were not.26 In addition to IL-1β, other investigators have demonstrated a role for TNFα in anti-MPO NCGN.5,28 Results from our studies herein further suggest that anti-MPO antibody–activated monocytes and neutrophils generate IL-1β, which in turn stimulates the release of additional inflammatory cytokines (including TNFα and IL-6), causing local inflammation and renal injury and culminating in NCGN. IL-1β blockade therefore abrogates the cascade of inflammatory cytokines, protecting animals from NCGN development.
In summary, we provide strong evidence that active NSP, specifically PR3 or a combination of PR3 and NE, mediates local cytokine production in anti-MPO antibody–induced NCGN. Their importance was indicated by the dramatic protection against NCGN development. We demonstrate for the first time using a genetic approach that PR3 provides an alternative mechanism of IL-1β processing and release in monocytes. Finally, the observation that specific IL-1–receptor blockade prevents anti-MPO antibody–induced NCGN points to a new therapeutic approach in ANCA-associated vasculitis and GN.
Concise Methods
Materials
Anakinra (Kineret) was obtained from Amgen (Munich, Germany); Borgal, from Intervet (Unterschleissheim, Germany); and the urine dipsticks, from Roche Diagnostics Corp. (Indianapolis, IN). The antibodies to GR-1 (clone RB6-8C5), CD14, and CD68 (clone FA11) were from BD Biosciences (Heidelberg, Germany). FITC-conjugated goat antimouse IgG was from Molecular Probes Invitrogen (Carlsbad, CA); FITC-conjugated goat antimouse C3, IgG, IgM, IgA, and MPO were from ICN/Cappel (Aurora, OH). HBSS, PBS, Roswell Park Memorial Institute medium (RPMI), DMEM, and penicillin and streptomycin were purchased from PAA (Coelbe, Germany), and FCS was from Biochrom (Berlin, Germany). The anti-PR3 mAbs were CLB12.8 (Hiss, Freiburg, Germany) and clone 43-8-3. The mAb to MPO was from Acris (Hiddenhausen, Germany); TNFα, from R&D Systems (Wiesbaden-Nordenstedt, Germany); LPS, from Sigma-Aldrich (Munich, Germany); ATP, from Roche Diagnostics Corp.; and dextran, from GE Healthcare (Amsterdam, the Netherlands). Purified human NE and TPCK were from Calbiochem (Darmstadt, Germany); α1-antitrypsin, from Sigma-Aldrich; and human PR3, from Athens Research & Technology (Athens, GA). The antibody against IL-1β (#2022) was from Cell Signaling (Danvers, MA), and antibodies against actin and tubulin were from Santa-Cruz Biotechnology (Heidelberg, Germany). Endotoxin-free reagents and plastic disposables were used in all experiments.
Mice and Immunization Protocol
C57BL/6J (B6) mice breeding pairs were purchased from Jackson Laboratories (Bar Harbor, ME). DPPI−/− mice were developed and extensively described by C.T.N. Pham.12 DPPI−/− mice were backcrossed to C57BL/6J strain (Jackson Laboratory) for 11 generations, and microsatellite typing of the entire DPPI−/− mouse genome found the genome to be 99.2% congenic with C57BL/6J. NE−/−34 and CG−/−44 mice were backcrossed to C57BL/6J (Jackson Laboratory) for 15 and 10 generations, respectively, before intercrossing to generate double-deficient mice. NE−/−/CG−/− mice are 97.7% congenic with C57BL/6J by microsatellite typing. Mice deficient in NE and PR3 were generated by C.T.N. Pham (Supplemental Figure 1). Mutant mice were backcrossed to C57BL/6J for 9 generations by speed congenics and are 99.2% congenic. Mice lacking MPO (MPO−/− mice) were the sixth-generation progeny of a backcross into B6 mice. MPO−/− mice (8–10 weeks old) were used for immunization. B6 WT mice 9–10 weeks old were used as bone marrow donors. Local authorities approved all animal experiments, which followed American Physiologic Society guidelines for animal care.
The purification of mouse MPO and the immunization of MPO−/− mice were performed as described elsewhere.4 Briefly, murine MPO was purified from WEHI-3 cells by dounce homogenization, Concanavalin A affinity chromatography, ion exchange, and gel filtration chromatography. MPO−/− mice were immunized intraperitoneally with 10 µg of purified murine MPO or BSA, respectively, in complete Freund’s adjuvant and boosted at day 28 with 10 µg of purified murine MPO or BSA in incomplete Freund’s adjuvant. Development of antibodies was monitored by anti-MPO enzyme-linked immunosorbent assay. Total IgG was purified (anti-MPO or anti-BSA murine IgG) from pooled sera of immunized Mpo−/− mice.
Bone Marrow Transplantation in Mice
After immunization the MPO−/− mice were kept at sterile housing conditions with food and water ad libitum (sterile water with trimethoprim and sulfadoxine [Borgal]). After immunization mice were gamma-irradiated with a whole-body dose of 900 rad and reconstituted with bone marrow cells from WT or DPPI−/− mice of the same genetic background. Bone marrow cells were harvested from femurs and tibia, erythrocytes were lysed, and 1.5×107 bone marrow cells were intravenously injected.
Analysis of Kidney Cytokines by Cytometric Bead Arrays
Frozen mouse kidneys were homogenized in 0.5 mL of cold PBS and homogenized with a Tissue Tearor (Biospec, Bartlesville, OK). Samples were spun down at 13,000 revolutions per minute for 30 minutes at 4°C. Supernatants were collected, followed by a second centrifugation of the supernatants. Clear supernatants were collected and stored at −20°C. The concentrations of cytokines were determined by cytometric bead array (Mouse Inflammation Kit, catalog number 552364; Mouse IL-1β Flex Set, catalog number 560232; BD Pharmingen). The analysis was done according to the manufacturer’s instructions. Cytometric analysis was carried out using FACScalibur flow cytometry (Becton Dickinson Immunocytometry Systems, San José, CA). Data were obtained and analyzed by CBA software. IL-1β kidney levels in the second set of experiments using WT, NE−/−/PR3−/−, and NE−/−/CG−/−mice were analyzed by ELISA.
Treatment Protocol
Treatment started 4 weeks after bone marrow transplantation. The control group received food and water ad libitum. The treatment group received anakinra intraperitoneally at a dosage of 100 mg/kg body weight two times daily for up to 4 weeks. Mice were sacrificed thereafter, or earlier if we had to assume the animal would not survive until the next day.
Functional Evaluation of Renal Injury
Mice were placed in metabolic cages 1 day before being sacrificed, and urine was collected for 18 hours overnight. Urine was tested by dipstick for hematuria, leukocyturia, and proteinuria; the extent is expressed as the mean on a scale of 0 (none) to 4 (severe) for leukocyturia and hematuria and 0 to 3 for proteinuria. Albuminuria was quantified by an albumin-specific ELISA (CellTrend, Luckenwalde, Germany).
Histologic Evaluation of Renal Injury
Samples of kidney tissue were collected at the time of sacrifice, fixed in 10% formalin, and embedded in paraffin using routine protocols. Four-micrometer coronal sections of specimens were stained with hematoxylin and eosin and periodic acid–Schiff and evaluated by light microscopy. The extent of glomerular crescents and extent of necrosis were expressed as the mean percentage of glomeruli with crescents and necrosis in each animal. For immunofluorescence microscopy to detect glomerular localization of immune determinants, 4-µm frozen sections were stained with fluoresceinated antibodies. The glomerular IgG deposition was performed by staining using FITC-conjugated goat antimouse IgG. Deposition of mouse complement C3, IgM, IgA, and MPO was visualized with FITC-conjugated goat antimouse C3, IgM, IgA, and MPO. For detection of leukocytes, sections of snap-frozen tissue were stained with rat antibodies to neutrophils and monocytes/macrophages, respectively. Rat antibody binding was detected using peroxidase-labeled secondary rabbit antirat IgG and tertiary goat antirabbit IgG antibodies, followed by 3-amino-9-ethylcarbazole and hydrogen peroxide. Sections were counterstained with hematoxylin.
Preparation of Human Neutrophils, Monocytes, and Human IgG
Neutrophils from healthy donors were isolated from heparinized whole blood as described elsewhere.9 Monocytes were prepared from the PBMC fraction by culture for 1 hour at 37°C in DMEM in cell culture dishes; attached cells were washed twice in PBS and resuspended in RPMI/10% FCS. Cell viability by trypan blue exclusion was found to be greater than 99% in every experiment. Normal IgG and ANCA-IgG were prepared from normal volunteers and patients with active MPO and PR3-ANCA disease using a High-Trap-protein-G column in an Äkta-FPLC system (GE Healthcare).
Preparation of Murine Neutrophils and Monocytes
For the isolation of bone marrow neutrophils and monocytes, mice were sacrificed, femurs and tibias were dissected, and the bone marrow was flushed with ice-cold sterile PBS without calcium and magnesium. Neutrophils and monocytes were further isolated by Ficoll-Hypaque density gradient centrifugation. Contaminating red blood cells in the neutrophil fraction were lysed by incubation with hypotonic saline for 15 seconds, the PBMC fraction was cultured for 1 hour at 37°C in DMEM in cell culture dishes, and attached cells were washed twice in PBS and resuspended in RPMI/10% FCS. The cell viability was greater than 99% according to trypan blue exclusion.
Western Blot and ELISA Analysis of IL-1β
IL-1β processing was analyzed by Western blot using whole-cell extracts, and IL-1β secretion was analyzed by supernatant analysis by ELISA. Neutrophils and monocytes were primed in RPMI/10% FCS for 30 minutes with 2 ng of TNFα per mL and subsequently stimulated by monoclonal antibodies to PR3 or MPO, or human IgG preparation for 4 hours at 37°C. Afterward, samples were centrifuged and the supernatant was stored at −80°C for later analysis by ELISA. The cell pellets were lysed, incubated for 5 minutes at 95°C in loading buffer (250 mM Tris-HCl, pH of 6.8, with 4% SDS, 20% glycerol, 0.01% bromphenol blue, and 10% β-mercaptoethanol). Thirty micrograms of protein of monocyte-lysates and 150 μg of neutrophil-lysates were loaded, electrophoresed on a 15% sodium dodecyl sulfate–polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was blocked and incubated overnight with the indicated primary antibodies, followed by a horseradish peroxidase–labeled secondary antibody. Densitometry was performed with the Chemicapt 12.8 software using a Chemi-smart 5000 scanner (Vilber Lourmat, Germany). IL-1β secretion was analyzed in the supernatants by IL-1β–specific ELISA according to the manufacturer’s guidelines (R&D Systems).
Exogenous Reconstitution with Purified NSPs
When indicated, human NE and human PR3 were added to isolated murine monocytes at the beginning of the assays at a final concentration of 2.5 µg/ml.
Statistical Analyses
Results are given as means ± SEM. Comparisons were made using ANOVA with post hoc analysis. Differences were considered significant at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Sylvia Krueger, Gisela Philipp, Baerbel Kuhlmann, and Antonina Akk for excellent technical assistance.
We would like to acknowledge the Rheumatic Disease Core Center’s Speed Congenics Laboratory at Washington University for microsatellite genotyping of DPPI−/−, NE−/−/CG−/−, and NE−/−/PR3−/− mice.
This work was supported in parts by DFG grants (SCHR 771/5-1, SCHR 771/6-1, and KE 576/7-1), Experimental and Clinical Research Center grants, and National Institutes of Health Grant AI049261.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2010080892/-/DCSupplemental.
References
- 1.van der Woude FJ: Anticytoplasmic antibodies in Wegener’s granulomatosis. Lancet 2: 48, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber A, Xiao H, Falk RJ, Jennette JC: Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol 17: 3355–3364, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JWC, Jennette JC, Heeringa P: Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: Role of tumor necrosis factor-alpha. Am J Pathol 167: 47–58, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruth A-J, Kitching AR, Kwan RYQ, Odobasic D, Ooi JDK, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD: Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 106: 2050–2058, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Ben-Smith A, Dove SK, Martin A, Wakelam MJ, Savage CO: Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: comparison with conventional Fcgamma receptor ligation. Blood 98: 1448–1455, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Kettritz R, Jennette JC, Falk RJ: Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol 8: 386–394, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne H-J, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pham CTN: Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol 40: 1317–1333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adkison AM, Raptis SZ, Kelley DG, Pham CTN: Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 109: 363–371, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, Pham CTN: Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA 104: 2855–2860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Pham CTN: Dipeptidyl peptidase I regulates the development of collagen-induced arthritis. Arthritis Rheum 52: 2553–2558, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Akk AM, Simmons PM, Chan HW, Agapov E, Holtzman MJ, Grayson MH, Pham CTN: Dipeptidyl peptidase I-dependent neutrophil recruitment modulates the inflammatory response to Sendai virus infection. J Immunol 180: 3535–3542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noronha IL, Krüger C, Andrassy K, Ritz E, Waldherr R: In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int 43: 682–692, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Falk RJ, Terrell RS, Charles LA, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 87: 4115–4119, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R: C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. : A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Mayor A, Tschopp J: The inflammasomes: guardians of the body. Annu Rev Immunol 27: 229–265, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M: Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum 60: 3642–3650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J: Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA 96: 6261–6266, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greten FR, Arkan MC, Bollrath J, Hsu L-C, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M: NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130: 918–931, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joosten LAB, Netea MG, Fantuzzi G, Koenders MI, Helsen MMA, Sparrer H, Pham CT, van der Meer JWM, Dinarello CA, van den Berg WB: Inflammatory arthritis in caspase 1 gene-deficient mice: Contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum 60: 3651–3662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham CT, Ley TJ: Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA 96: 8627–8632, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG: Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am J Pathol 164: 1967–1977, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Timmeren MM, van der Veen BS, Stegeman CA, Petersen AH, Hellmark T, Collin M, Heeringa P: IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J Am Soc Nephrol 21: 1103–1114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little MA, Bhangal G, Smyth CL, Nakada MT, Cook HT, Nourshargh S, Pusey CD: Therapeutic effect of anti-TNF-alpha antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J Am Soc Nephrol 17: 160–169, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ohlsson S, Hellmark T, Pieters K, Sturfelt G, Wieslander J, Segelmark M: Increased monocyte transcription of the proteinase 3 gene in small vessel vasculitis. Clin Exp Immunol 141: 174–182, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowack R, Schwalbe K, Flores-Suárez LF, Yard B, van der Woude FJ: Upregulation of CD14 and CD18 on monocytes in vitro by antineutrophil cytoplasmic autoantibodies. J Am Soc Nephrol 11: 1639–1646, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Charles LA, Falk RJ, Jennette JC: Reactivity of antineutrophil cytoplasmic autoantibodies with mononuclear phagocytes. J Leukoc Biol 51: 65–68, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Weidner S, Neupert W, Goppelt-Struebe M, Rupprecht HD: Antineutrophil cytoplasmic antibodies induce human monocytes to produce oxygen radicals in vitro. Arthritis Rheum 44: 1698–1706, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH: Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem 276: 18551–18556, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD: Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 4: 615–618, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Belaaouaj A, Kim KS, Shapiro SD: Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 289: 1185–1188, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW: Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416: 291–297, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Campbell EJ, Campbell MA, Owen CA: Bioactive proteinase 3 on the cell surface of human neutrophils: Quantification, catalytic activity, and susceptibility to inhibition. J Immunol 165: 3366–3374, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA: Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267: 2000–2003, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Irmler M, Hertig S, MacDonald HR, Sadoul R, Becherer JD, Proudfoot A, Solari R, Tschopp J: Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med 181: 1917–1922, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS: Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med 174: 821–825, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR: Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem 265: 6318–6322, 1990 [PubMed] [Google Scholar]
- 42.Källquist L, Rosén H, Nordenfelt P, Calafat J, Janssen H, Persson A-M, Hansson M, Olsson I: Neutrophil elastase and proteinase 3 trafficking routes in myelomonocytic cells. Exp Cell Res 316: 3182–3196, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Goldbach-Mansky R, Kastner DL: Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol 124: 1141–1149, quiz 1150–1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ: Normal neutrophil function in cathepsin G-deficient mice. Blood 94: 4282–4293, 1999 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.