Abstract
Impaired kidney function is a risk factor for upper gastrointestinal (GI) bleeding, an event associated with poor outcomes. The burden of upper GI bleeding and its effect on patients with ESRD are not well described. Using data from the US Renal Data System, we quantified the rates of occurrence of and associated 30-day mortality from acute, nonvariceal upper GI bleeding in patients undergoing dialysis; we used medical claims and previously validated algorithms where available. Overall, 948,345 patients contributed 2,296,323 patient-years for study. The occurrence rates for upper GI bleeding were 57 and 328 episodes per 1000 person-years according to stringent and lenient definitions of acute, nonvariceal upper GI bleeding, respectively. Unadjusted occurrence rates remained flat (stringent) or increased (lenient) from 1997 to 2008; after adjustment for sociodemographic characteristics and comorbid conditions, however, we found a significant decline for both definitions (linear approximation, 2.7% and 1.5% per year, respectively; P<0.001). In more recent years, patients had higher hematocrit levels before upper GI bleeding episodes and were more likely to receive blood transfusions during an episode. Overall 30-day mortality was 11.8%, which declined significantly over time (relative declines of 2.3% or 2.8% per year for the stringent and lenient definitions, respectively). In summary, despite declining trends worldwide, crude rates of acute, nonvariceal upper GI bleeding among patients undergoing dialysis have not decreased in the past 10 years. Although 30-day mortality related to upper GI bleeding declined, perhaps reflecting improvements in medical care, the burden on the ESRD population remains substantial.
More than 600,000 patients in the United States have advanced CKD and require maintenance dialysis or kidney transplantation. Most of these patients are covered through Medicare’s ESRD program, which spends more than $27 billion annually for the care of these patients.1 Previous studies have indicated that patients requiring dialysis are particularly prone to developing upper gastrointestinal (GI) bleeding, with subsequent poor outcomes.2–4 In the general (non-ESRD) population, the incidence of, and mortality associated with, acute nonvariceal upper GI bleeding has been declining over time.5–11 It is unknown, however, whether the ESRD population has experienced similar changes in rates or complications of this bleeding event and its outcomes. One may posit that patients undergoing hemodialysis have unique risks for upper GI bleeding because of repeated exposure to anticoagulants. Residual uremia might also render patients resistant to the benefits of certain therapeutics. Thus, the beneficial trends toward lower incidence and better outcomes of upper GI bleeding observed in the general population may not have materialized in the ESRD population.
We used the national registry of ESRD patients in the United States to study the occurrence of acute nonvariceal upper GI bleeding and its outcomes in patients undergoing dialysis. We tested the null hypotheses that the occurrence of, and mortality from, upper GI hemorrhage over the past decade did not change among patients in the United States who are undergoing dialysis.
Results
From 1998 to 2007, 948,345 unique patients were represented in our study, contributing 2,296,323 person-years of observation. Characteristics of the study population in calendar years 1998 and 2007 are shown in Table 1. During the decade of observation, mean age, the proportion of men, median dialysis vintage, and prevalence of certain comorbid conditions increased.
Table 1.
Patient characteristics in 1998 and 2007
| Characteristic | 1998 | 2007 | ||||
|---|---|---|---|---|---|---|
| Total | No Acute Nonvariceal Upper GI Bleeding | Acute Nonvariceal Upper GI Bleeding | Total | No Acute Nonvariceal Upper GI Bleeding | Acute Nonvariceal Upper GI Bleeding | |
| No. of patients (%) | 250,474 | 240,219 (95.9) | 10,255 (4.1) | 341,890 | 329,797 (96.5) | 12,093 (3.5) |
| Age (yr) | 61.2±15.5 | 61.2±15.5 | 62.9±14.8 | 62.4±15.2 | 62.3±15.2 | 63.0±14.8 |
| Male | 130,479 (52.1) | 125,193 (52.1) | 5286 (51.5) | 187,187 (54.8) | 180,693 (54.8) | 6494 (53.7) |
| BMI (kg/m2) | 25.8±6.7 (n=157,290) | 25.9±6.7 (n=151,464) | 25.0±6.3 (n=5826) | 28.6±7.6 (n=338,880) | 28.6±7.6 (n=327,394) | 27.9±7.2 (n=11,486) |
| Race | ||||||
| white | 144,077 (57.5) | 138,325 (57.6) | 5752 (56.1) | 195,924 (57.3) | 189,304 (57.4) | 6620 (54.7) |
| black | 93,034 (37.1) | 89,067 (37.1) | 3967 (38.7) | 125,020 (36.6) | 120,200 (36.4) | 4820 (39.9) |
| Asian | 7795 (3.1) | 7483 (3.1) | 312 (3.0) | 13,589 (4) | 13,165 (4.0) | 424 (3.5) |
| Native American | 3638 (1.5) | 3505 (1.5) | 133 (1.3) | 5128 (1.5) | 4979 (1.5) | 149 (1.2) |
| other | 1806 (0.7) | 1719 (0.7) | 87 (0.8) | 2126 (0.6) | 2053 (0.6) | 73 (0.6) |
| missing | 124 (0) | 120 (0.0) | 4 (0.0) | 103 (0) | 96 (0.0) | 7 (0.1) |
| Medicaid eligibility | ||||||
| yes | 127,533 (50.9) | 122,018 (50.8) | 5515 (53.8) | 180,189 (52.7) | 173,122 (52.5) | 7067 (58.4) |
| missing | 45,522 (18.2) | 43,688 (18.2) | 1834 (17.9) | 4132 (1.2) | 4006 (1.2) | 126 (1.0) |
| Dialysis modality | ||||||
| hemodialysis | 218,818 (87.4) | 209,844 (87.4) | 8974 (87.5) | 314,241 (91.9) | 303,045 (91.9) | 11,196 (92.6) |
| peritoneal dialysis | 27,866 (11.1) | 26,724 (11.1) | 1142 (11.1) | 23,694 (6.9) | 22,911 (6.9) | 783 (6.5) |
| missing | 3790 (1.5) | 3651 (1.5) | 139 (1.4) | 3955 (1.2) | 3841 (1.2) | 114 (0.9) |
| Dialysis vintage (yr)a | 1.7 (0.2-4.0) | 1.7 (0.2-4.0) | 1.8 (0.4-4.1) | 2.0 (0.4-4.6) | 2.0 (0.4-4.6) | 2.3 (0.6-4.9) |
| Primary kidney disease | ||||||
| GN | 33,081 (13.2) | 31,929 (13.3) | 1152 (11.2) | 34,183 (10) | 33,171 (10.1) | 1012 (8.4) |
| hypertensive | 74,239 (29.6) | 71,137 (29.6) | 3102 (30.2) | 99,502 (29.1) | 96,035 (29.1) | 3467 (28.7) |
| diabetes | 99,523 (39.7) | 95,100 (39.6) | 4423 (43.1) | 150,228 (43.9) | 144,442 (43.8) | 5786 (47.8) |
| other | 34,707 (13.9) | 33,500 (13.9) | 1207 (11.8) | 45,130 (13.2) | 43,746 (13.3) | 1384 (11.4) |
| missing | 8924 (3.6) | 8553 (3.6) | 371 (3.6) | 12,847 (3.8) | 12,403 (3.8) | 444 (3.7) |
| History of comorbid conditions | ||||||
| diabetes | 129,184 (51.6) | 123,171 (51.3) | 6013 (58.6) | 217,936 (63.7) | 209,270 (63.5) | 8666 (71.7) |
| hypertension | 190,776 (76.2) | 182,439 (75.9) | 8337 (81.3) | 322,096 (94.2) | 310,501 (94.1) | 11,595 (95.9) |
| coronary artery disease | 75,512 (30.1) | 71,792 (29.9) | 3720 (36.3) | 13,1204 (38.4) | 125,609 (38.1) | 5595 (46.3) |
| cerebral vascular disease | 37,303 (14.9) | 35,274 (14.7) | 2029 (19.8) | 67,394 (19.7) | 64,242 (19.5) | 3152 (26.1) |
| heart failure | 109,336 (43.7) | 103,992 (43.3) | 5344 (52.1) | 190,269 (55.7) | 182,531 (55.3) | 7738 (64.0) |
| arrhythmia | 60,101 (24) | 57,000 (23.7) | 3101 (30.2) | 116,267 (34) | 111,135 (33.7) | 5132 (42.4) |
| valvular heart disease | 37,355 (14.9) | 35,244 (14.7) | 2111 (20.6) | 94,080 (27.5) | 89,793 (27.2) | 4287 (35.5) |
| peripheral vascular disease | 67,734 (27) | 64,199 (26.7) | 3535 (34.5) | 127,878 (37.4) | 122,326 (37.1) | 5552 (45.9) |
| COPD | 40,170 (16) | 37,990 (15.8) | 2180 (21.3) | 88,831 (26) | 84,853 (25.7) | 3978 (32.9) |
| cancer | 23,302 (9.3) | 22,181 (9.2) | 1121 (10.9) | 43,407 (12.7) | 41,695 (12.6) | 1712 (14.2) |
| chronic liver disease | 52,032 (20.8) | 49,545 (20.6) | 2487 (24.3) | 39,663 (11.6) | 37,685 (11.4) | 1978 (16.4) |
| past kidney transplant | 13,386 (5.3) | 12,924 (5.4) | 462 (4.5) | 18,955 (5.5) | 18,365 (5.6) | 590 (4.9) |
| acute nonvariceal upper GI bleeding | 1969 (0.8) | 426 (0.2) | 1543 (15.0) | 6701 (2) | 3746 (1.1) | 2955 (24.4) |
| Inability to ambulate | ||||||
| yes | 6215 (2.5) | 5893 (2.5) | 322 (3.1) | 13,501 (3.9) | 12,983 (3.9) | 518 (4.3) |
| missing | 73,999 (29.5) | 70,771 (29.5) | 3228 (31.5) | 10,006 (2.9) | 9627 (2.9) | 379 (3.1) |
| Inability to transfer | ||||||
| yes | 2039 (0.8) | 1920 (0.8) | 119 (1.2) | 5607 (1.6) | 5372 (1.6) | 235 (1.9) |
| missing | 74,000 (29.5) | 70,772 (29.5) | 3228 (31.5) | 10,007 (2.9) | 9628 (2.9) | 379 (3.1) |
| Alcohol dependence | ||||||
| yes | 2648 (1.1) | 2497 (1.0) | 151 (1.5) | 4399 (1.3) | 4154 (1.3) | 245 (2.0) |
| missing | 74,000 (29.5) | 70,772 (29.5) | 3228 (31.5) | 10,007 (2.9) | 9628 (2.9) | 379 (3.1) |
| Drug dependence | ||||||
| yes | 1654 (0.7) | 1583 (0.7) | 71 (0.7) | 4022 (1.2) | 3817 (1.2) | 205 (1.7) |
| missing | 74,000 (29.5) | 70,772 (29.5) | 3228 (31.5) | 10,007 (2.9) | 9628 (2.9) | 379 (3.1) |
| Tobacco use | ||||||
| yes | 9552 (3.8) | 9139 (3.8) | 413 (4.0) | 19,324 (5.7) | 18,508 (5.6) | 816 (6.7) |
| missing | 74,000 (29.5) | 70,772 (29.5) | 3228 (31.5) | 10,006 (2.9) | 9627 (2.9) | 379 (3.1) |
Values expressed with a plus/minus sign are the mean ± SD. Unless other wise noted, other values are number of patients (percentage). COPD, chronic obstructive pulmonary disease.
Data are the median (interquartile range).
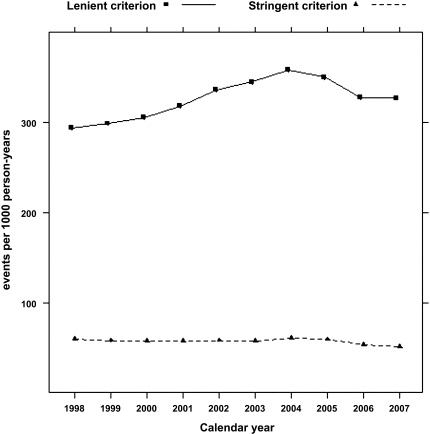
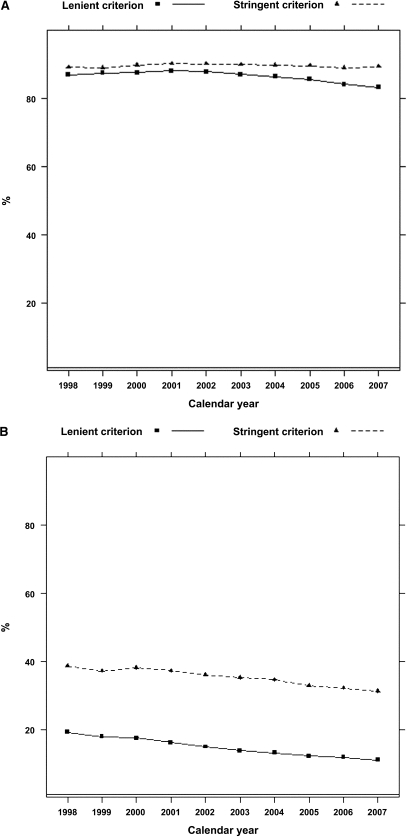
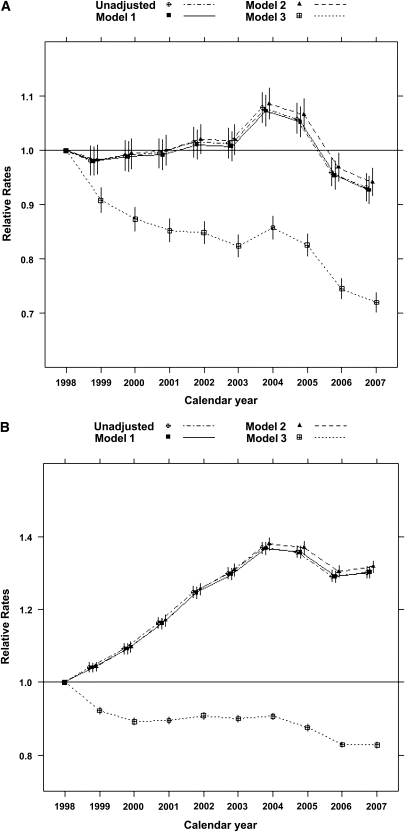
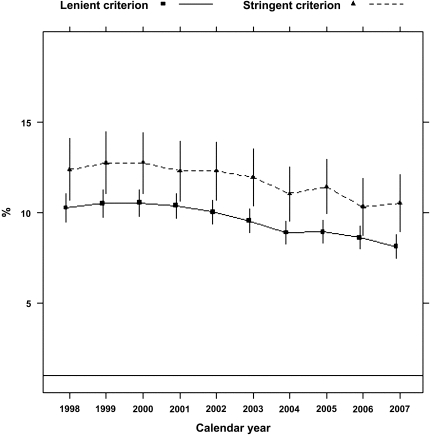
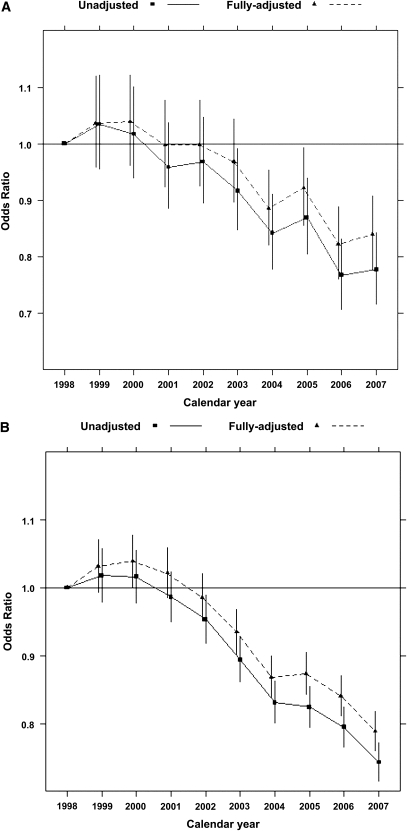
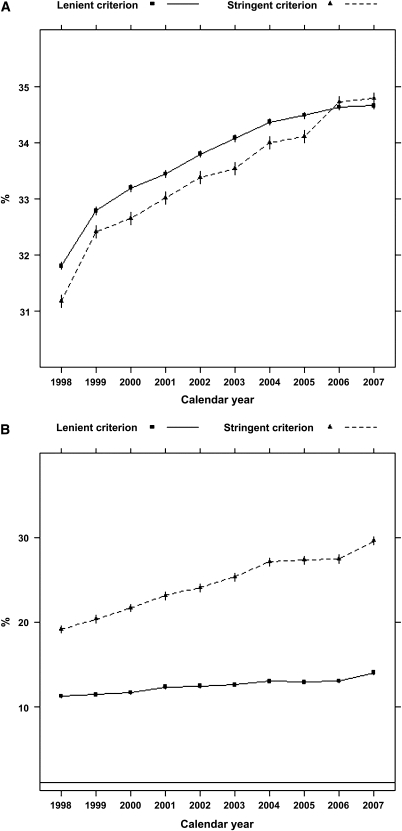
According to the stringent algorithm for the ascertainment of acute nonvariceal upper GI bleeding, a total of 101,561 patients experienced at least one upper GI bleeding event during the observed period, with a total of 131,022 episodes (57 per 1000 person-years). According to the more lenient algorithm, 380,343 patients experienced 753,508 events (328 per 1000 person-years). Trends in occurrence rates over time are shown in Figure 1. The proportion of hospitalized episodes remained unchanged: approximately 85%–90% according to either definition (Figure 2). The proportion of bleeding episodes related to peptic ulcer disease declined from 38.7% to 31.2% for the stringent definition of acute nonvariceal upper GI bleeding and from 19.3% to 11.1% for the lenient definition (Figure 2). In unadjusted analyses, the occurrence rate of acute nonvariceal upper GI bleeding using the stringent criteria was essentially unchanged from 1998 to 2007 (decline per year: 0.2%; 95% confidence interval [CI], 0%–0.4%; P=0.12; Table 2 and Figure 3). However, after adjustment for age, sex, race, dialysis vintage, modality, previous kidney transplantation, history of acute nonvariceal upper GI bleeding, Medicaid coverage, and comorbid conditions, the occurrence of acute nonvariceal upper GI bleeding declined by 2.7% per year (95% CI, 2.5%–2.9%; P<0.001). According to the lenient criteria, the adjusted rate of bleeding declined by 1.5% (95% CI, 1.4%–1.6%) per year. Overall 30-day mortality rates after bleeding were 11.8% (stringent criterion) and 9.6% (lenient criterion), respectively. Mortality was slightly higher for first episodes: 12.5% and 10.0% for stringent and lenient criterion episodes, respectively. Mortality declined from 12.3% in 1998 to 10.5% in 2007 according to the stringent criterion and from 10.2% to 8.1% according to the lenient criterion (Figure 4). Formal tests for trend showed that the adjusted 30-day mortality odds from acute nonvariceal upper GI bleeding decreased by 3.3% per year (relative change; 95% CI, 2.8%–3.8%) for the stringent criterion and by 3.6% (95% CI, 3.4%–3.8%) for the lenient criterion, respectively (Table 2 and Figure 5).
Figure 1.
Lower and upper bounds of the occurrence rate of acute nonvariceal upper GI bleeding. Note: 95% confidence intervals not shown (essentially superimposed with rate estimates).
Figure 2.
Trends in the proportions of hospitalized and peptic ulcer disease–related acute nonvariceal upper GI bleeding episodes. (A) Proportion of hospitalized events. (B) Proportion of peptic ulcer disease–related events. Note: 95% confidence intervals not shown (essentially superimposed with rate estimates).
Table 2.
Annual changes in the occurrence of and 30-day mortality after acute nonvariceal upper GI bleeding
| Criterion for Acute Nonvariceal Upper GI Bleeding | Change for an Increment of 1 yr (%) (95% CI) | P Value |
|---|---|---|
| Stringent | ||
| occurrence rate | ||
| unadjusted | −0.17 (−0.39 to 0.04) | 0.12 |
| adjusteda | −2.73 (−2.92 to −2.53) | <0.001 |
| mortality odds | ||
| unadjusted | −2.28 (−2.72 to −1.83) | <0.001 |
| adjusteda | −3.28 (−3.77 to −2.79) | <0.001 |
| Lenient | ||
| occurrence rate | ||
| unadjusted | 3.16 (3.05 to 3.26) | <0.001 |
| adjustedb | −1.48 (−1.56 to −1.39) | <0.001 |
| mortality odds | ||
| unadjusted | −2.75 (−2.96 to −2.54) | <0.001 |
| adjustedb | −3.57 (−3.80 to −3.35) | <0.001 |
CI, confidence interval.
Adjusted for age, sex, race, Medicaid coverage, dialysis vintage, modality, history of kidney transplantation, prior acute nonvariceal upper GI bleeding, all comorbid conditions, alcohol dependence, tobacco use, drug dependence, ability to transfer, ability to ambulate, and baseline BMI.
Adjusted for age, sex, race, Medicaid coverage, dialysis vintage, modality, peptic ulcer disease related, hospitalized, history of renal transplantation, prior acute nonvariceal upper GI bleeding, all comorbid conditions, alcohol dependence, tobacco use, drug dependence, ability to transfer, ability to ambulate, and baseline BMI.
Figure 3.
Relative occurrence rates of acute nonvariceal upper GI bleeding (1998–2007). (A) Stringent criterion. (B) Lenient criterion. Note: The occurrence rates were 60 (stringent) and 294 (lenient) per 1000 person-years in 1998, which served as the referent for the estimated annual rate ratios and corresponding 95% confidence intervals. Model 1: adjusted for age, sex, and race. Model 2: also adjusted for Medicaid coverage, dialysis vintage, and modality. Model 3: also adjusted for history of kidney transplantation, history of acute nonvariceal upper GI bleeding and all comorbid conditions, alcohol dependence, tobacco use, drug dependence, ability to transfer, ability to ambulate, and baseline BMI.
Figure 4.
Crude 30-day mortality after acute nonvariceal upper GI bleeding, 1998–2007.
Figure 5.
Trends in the relative odds ratios and corresponding 95% confidence intervals for 30-day mortality after acute nonvariceal upper GI bleeding (1998–2007). (A) Stringent criterion. (B) Lenient criterion. Note: Fully adjusted model includes age, sex, race, dialysis vintage, modality, Medicaid coverage, peptic ulcer disease related, hospitalized and receiving transfusion or not, history of renal transplantation, history of acute nonvariceal upper GI bleeding, all comorbid conditions, alcohol dependence, tobacco use, drug dependence, ability to transfer, ability to ambulate, and baseline BMI.
To explore correlates of declining mortality trends, we studied patterns of hematocrit values before acute nonvariceal upper GI bleeding events as well as use of blood transfusions during these episodes. Mean hematocrit and the proportion of patients receiving blood transfusions increased significantly over time (Figure 6).
Figure 6.
Trends in the most recent recorded hematocrit level and the proportion of patients receiving a transfusion during episodes of acute nonvariceal upper GI bleeding. (A) Hematocrit. (B) Proportion of patients receiving a transfusion. Note: 95% confidence intervals not shown (essentially superimposed with rate estimates).
Discussion
From a comprehensive database detailing the experience of patients in the United States undergoing dialysis over a decade, we found occurrence rates of acute nonvariceal upper GI bleeding almost two orders of magnitude higher than in the general population, where rates of 50–150 events per 100,000 person-years had recently been reported.7–11 In contrast to the general population, in which occurrence of acute nonvariceal upper GI bleeding and associated mortality have declined over the past decade, we observed an increase in occurrence rates after adjustment for demographic factors and comorbid conditions. Of note, 30-day mortality declined during the same period, consistent with reductions in non-ESRD populations.6,7,10
Relatively little is known about the occurrence and outcomes of upper GI bleeding in patients undergoing dialysis. In an analysis of the US Renal Data System (USRDS) Dialysis Morbidity and Mortality Study, Wasse and colleagues reported a rate of 23 upper GI bleeding events per 1000 person-years, less than half of our conservative estimate.12 Of note, that study was oversampled for patients undergoing peritoneal dialysis and included person-time spent after kidney transplantation. Patients undergoing peritoneal dialysis and kidney transplant recipients had a lower risk for upper GI bleeding than patients undergoing hemodialysis.12 Differences in reported rates must be considered in light of different approaches to ascertaining and studying upper GI bleeding.
For example, some studies included only bleeding episodes that were deemed to be related to peptic ulcer disease. In the general population, only 40%–60% of all upper GI bleeding episodes are due to peptic ulcer disease; 10%–20% are attributed to variceal bleeding in the general population.7–10 The proportions of peptic ulcer disease–related and variceal bleeding episodes were reported to be approximately 60% and 10%, respectively, in a cross-sectional survey of 727 patients with CKD.13 Because we were interested in the overall effect of acute nonvariceal upper GI bleeding in patients undergoing dialysis, we used a more extensive selection of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM), diagnosis codes to identify all episodes of acute nonvariceal upper GI bleeding, rather than including only those related to peptic ulcer disease. In our cohort, 35.2% of acute nonvariceal upper GI bleeding episodes appeared related to peptic ulcer disease.
Most previous published studies only considered the first episode or hospitalized cases with primary discharge diagnosis indicating upper GI bleeding. However, upper GI bleeding episodes are acute events that can be fully resolved and later recur. Previous studies have indicated that patients undergoing dialysis are at particular risk for recurrence of upper GI bleeding.2,4 Although upper GI bleeding frequently complicates the course of patients already hospitalized, the management of episodes that begin in the ambulatory setting does not necessarily mandate admission. Almost 40% of upper GI bleeding episodes in Medicare patients were managed in the outpatient setting,14 and 20% of these episodes recurred.15 In our cohort, 28.3% of episodes were recurrent and approximately 10% episodes were managed in the outpatient setting. We would have missed many events had we not meticulously investigated outpatient claims and identified recurrent episodes.
Such high rates of upper GI bleeding might also be explained by the special characteristics of the dialysis population itself. The dialysis population is enriched by individuals exhibiting several well-known risk factors for acute nonvariceal upper GI bleeding: older age, anticoagulant use, platelet dysfunction, cardiovascular disease, and disability.12 Further, patients undergoing dialysis are exposed to heightened medical surveillance because of their regular interactions with health care providers and routine laboratory surveillance, where management of anemia has been an important focus of care related to quality metrics and reimbursement.16 In response to the ESRD Prospective Payment System (the “bundle”), instituted in January 2011, it is likely that vigilance for GI bleeding will remain because of the 8% higher reimbursement for dialysis services in patients with a diagnosis of GI bleeding.
Although upper GI bleeding has declined over the past decades worldwide,7–11 we found consistently high occurrence rates of acute nonvariceal upper GI bleeding in the dialysis population from 1998 to 2007. The most probable explanation for the lack of decline in occurrence rates is the changing character of the dialysis population.
Advanced age, male sex, and certain medications (such as antiplatelet or anticoagulant agents) are well known risk factors for acute nonvariceal upper GI bleeding.17–19 A subtle but important change in the dialysis population is the shift away from peritoneal dialysis and toward hemodialysis; the latter obligates regular, intermittent use of anticoagulants. Only after adjustment for multiple comorbid conditions that increase the risk for acute nonvariceal upper GI bleeding were the adjusted rates found to be declining, albeit slightly (Figure 2).
The prevalence rates of several comorbid conditions, including diabetes, hypertension, heart failure, arrhythmia, valvular heart disease, peripheral vascular disease, chronic obstructive pulmonary disease, and previous upper GI bleeding, all increased over time (Table 1). It is important to note, however, that more aggressive coding practices may have contributed to these apparent increases,20 a type of information bias. Thus, adjustment for these comorbid conditions may have actually led to overestimation of any true reductions in the occurrence of acute nonvariceal upper GI bleeding over time.
The decline of upper GI bleeding in the general population is mainly due to a decrease in peptic ulcer disease–related bleeding, especially in younger populations.7,8 In the past decades, there have been many advances in prevention and treatment of peptic ulcer disease, such as introduction of histamine-2–receptor antagonists, proton-pump inhibitors, and eradication regimens for Helicobacter pylori. Whether these treatments are as effective in patients with ESRD as in the general population for acute nonvariceal upper GI bleeding related or unrelated to peptic ulcer disease is unknown.21–25
Mortality associated with upper GI bleeding ranged between 5% and 15% in the general population, depending on the specific population studied, specific definition of upper GI bleeding, and definitions of mortality (in-hospital versus all settings).6–11,14 The 30-day mortality rate in our report was within the same range, although the mortality rate after outpatient episodes of acute nonvariceal upper GI bleeding was much lower than that among hospitalized patients (7.3% versus 13.6%), again similar to the Medicare population.14 Earlier studies reported no change in upper GI bleeding–related mortality rate over time, probably because of co-occurring trends in age and comorbid conditions.8,9,11 However, as we saw in patients undergoing dialysis, recent reports demonstrated an improvement in outcomes even though patients with upper GI bleeding became more vulnerable over time in terms of age and comorbidity.6,7,10 The declining trends of acute nonvariceal upper GI bleeding–related 30-day mortality rates were demonstrated in bleeding episodes related and unrelated to peptic ulcer disease.
Despite an increasingly sicker dialysis population, the 30-day mortality rate for acute nonvariceal upper GI bleeding declined by 2%–3% per year, which may reflect success of one or more therapeutic strategies or improvements in the general care for this population. At least two obvious changes occurred during past decade: (1) the evolution of endoscopic hemostatic techniques and (2) a trend toward higher hemoglobin concentrations. Combined endoscopic modalities for hemostasis improved bleeding outcomes.26 Patients undergoing dialysis might particularly benefit from these advancements because they are viewed as a high-risk group for surgery. With effective nonsurgical therapies, these patients may have better chances to survive even massive acute nonvariceal upper GI bleeding without the need for operative intervention. Similarly, more aggressive treatment with erythropoiesis-stimulating agents and intravenous iron and, subsequently, higher hemoglobin targets have contributed to an increased “hematocrit reserve,” which might also have translated into the observed better outcomes (i.e., providing a larger “margin of error” in the face of acute nonvariceal upper GI bleeding); more liberal use of transfusion and increasing use of proton-pump inhibitors may have contributed to the decline in case fatality. Finally, it is possible that more subtle cases of acute nonvariceal upper GI bleeding may have been noticed and coded in more recent years (also known as “code creep”), although the stable proportion of hospitalized episodes (approximately 90%) during the study period argues against this possibility.
To our knowledge, this study is the most comprehensive analysis of acute nonvariceal upper GI bleeding in the dialysis population to date. We applied both stringent and lenient algorithms to approximate the upper and lower ranges of occurrence rates for acute nonvariceal upper GI bleeding, and we considered outpatient, inpatient, and recurrent episodes. We ascertained multiple covariates in addition to basic demographic data and primary outcomes for adjustment, including comorbid conditions and transfusion events.
Despite these strengths, our study also has some limitations, mostly from use of administrative billing claims for our research. The accuracy of the coding for our primary outcome is the most prominent concern. The algorithm we used to identify acute nonvariceal upper GI bleeding events came from a validated list of ICD-9 diagnosis codes proposed by Cooper and colleagues27 and modified by Targowinik and Nabalamba.11 The former authors reported good sensitivity and positive predictive values (85%–95%) of diagnosis coding for source of hemorrhage and upper endoscopy among 882 patients with upper GI hemorrhage. The latter group modified the algorithm by adding nonspecific GI bleeding diagnostic codes and procedure codes to exclude variceal bleeding, lower GI bleeding, or chronic GI bleeding in an attempt to increase sensitivity and positive predictive value.11,28 Because the latter codes did not cover most non–ulcer-related GI bleeding, we augmented the algorithm by adding several lesion-specific diagnostic codes, as detailed in the methods section. For the stringent algorithm, 100% of patients had endoscopy within 3 days of the event date, which again indicates high reliability of this algorithm; for the lenient definition, 40% of patients had endoscopy within 3 days of an assumed event date.
Another inherent limitation from administrative claims is the absence of other clinically relevant data, including most laboratory results, biometric values, endoscopic findings, or detailed medications, all unavailable. Finally, there were some missing data for certain variables that we imputed using standard methods,29,30 which assume that missingness occurred at random.
Finally, the primary goal of this study was to describe secular trends in the occurrence and outcomes of acute nonvariceal upper GI bleeding, which guided us in the specific set-up of our cohort. We did not focus on studying risk factors of acute nonvariceal upper GI bleeding because this would have required a different cohort assembly (inception cohort) and analytical approach.
In summary, we found an exceptionally high occurrence rate of acute nonvariceal upper GI bleeding among dialysis patients in the United States, at least an order of magnitude higher than in the general population. In contrast to trends in the general population toward lower rates of these events, occurrence rates among patients undergoing dialysis did not change materially between 1998 and 2007. Although 30-day mortality of acute nonvariceal upper GI bleeding declined from 12.3% to 10.5% in the recent decade, further investigation is needed to identify care pathways that may reduce the burden of this bleeding in the dialysis population.
Concise Methods
Data Source
We used the USRDS database for this study, with data available from 1996 through 2008. We obtained information on the outcome of interest, acute nonvariceal upper GI bleeding, as well as presence of several comorbid conditions, from Medicare claims files. We obtained demographic data and selected clinical information from USRDS Standard Analysis Files, including the Medical Evidence Report (CMS-2728). To use clinician-assigned codes as often as possible, we used ICD-9-CM codes; we excluded from consideration diagnostic codes from Part B clinical laboratory, diagnostic imaging, and durable medical equipment claims.31
Study Population
We included all patients eligible for and covered by Medicare who underwent maintenance dialysis at some point between January 1, 1998, and December 31, 2007. Patients younger than age 18 years or whose kidney function recovered were excluded. To ensure complete ascertainment of events of interest, we restricted all analyses to periods during which a patient was covered by Medicare (Part A and B) as primary payer of medical expenses. Patients older than age 65 years were eligible for Medicare benefits on the basis of their age. For patients younger than age 65, primary Medicare coverage became active 36 months after the start of dialysis.
Study Outcomes
We modified the methods proposed by Targownik and Nabalamba11 to define acute nonvariceal upper GI bleeding events using two criteria: a specific or stringent criterion and a more inclusive or lenient criterion. According to the stringent criterion we defined acute nonvariceal upper GI bleeding events as those corresponding to a group of ICD-9 diagnostic codes, which specify the identified cause of the bleeding (e.g., the ICD-9 codes for gastroesophageal laceration-hemorrhage [530.7] or for gastric ulcer with hemorrhage [531.0]). According to the lenient criterion, we defined acute nonvariceal upper GI bleeding events by also including incidents with corresponding diagnosis codes that did not specify the lesions responsible for the bleeding but coincided with bleeding-related medical procedure codes. In addition, for both definitions we included several important lesion-specific events not considered by Targownik and Nabalamba11 (e.g., 537.83 for angiodysplasia of stomach and duodenum with hemorrhage; 537.84 for Dieulafoy lesion [hemorrhagic] of stomach and duodenum). This modification was based on a systematic review of all potentially relevant ICD-9 codes and consultation with an experienced gastroenterologist (T.-C.L.). The stringent algorithms and codes used are listed in Supplemental Material. We excluded all claims with diagnosis codes of “variceal bleeding” on the bleeding date in our algorithm.
Given trends toward outpatient management of a variety of health conditions, including GI disorders over the past decade,14 we used outpatient claims information when ascertaining acute nonvariceal upper GI bleeding events. Such data are important to investigate recurrent events of interest, which may have been less severe than a first event and would be missed if we had considered only events requiring hospitalization. On the one hand, care must be taken in defining an event from outpatient claims only because a single diagnostic code might not reliably represent a true event. On the other hand, a single true event could be represented by multiple reimbursement claims that spanned several days. The former error would reduce specificity of event ascertainment, and the latter would lead to overestimation of true event rates. To reduce these potential errors, we defined a single acute nonvariceal upper GI bleeding event from outpatient claims according to the presence of two outpatient claims within a 7-day time period. Alternatively, one outpatient claim was considered sufficient if it was accompanied by a claim for esophagogastroduodenoscopy performed on the same date.14 Further, if the onset dates of two events were within a 30-day period, these two events were counted as a single episode to avoid overestimating the occurrence rate of acute nonvariceal upper GI bleeding.14
The second outcome of interest, which applies only to patients who experienced an acute nonvariceal upper GI bleeding event, is short-term (30-day) mortality after such an episode, defined as death within 30 days of the diagnosis date for acute nonvariceal upper GI bleeding regardless of discharge status.6,14
Patient Characteristics
From the USRDS, we ascertained basic demographic variables, such as age, sex, race, and primary kidney disease causing ESRD. Dialysis vintage, dialysis modality, transplantation status, and Medicaid eligibility as a representation of lower socioeconomic status were defined as time-dependent variables. We systematically surveyed ICD-9-CM diagnostic and procedure codes and referred to previous studies to derive a comprehensive table of codes for relevant comorbid conditions (Supplemental Material).32–35 The initial status of comorbidity was based on information from the Medical Evidence Report forms and then updated according to claims data. Corresponding codes had to appear at least twice in outpatient claims or be present in any inpatient claims to be counted.31 For all comorbid conditions and histories of gastrointestinal bleeding, we searched claims back to January 1, 1996 (i.e., 2 years before the start date of the observation period). We matched the date of GI bleeding events to institutional claims in order to obtain the most recently recorded hematocrit value. Transfusion events were identified via ICD-9 procedure codes (99.03, 99.04) and Healthcare Common Procedure Coding System codes (P9010, P9011, P9016, P9021, P9022, P9038, P9039, P9040, and 36430) from claims data.36
Statistical Analyses
Modeling
To address whether the occurrence of acute nonvariceal upper GI bleeding events changed over time, we used Poisson regression to model the number of such episodes each year with adjustment for the length of time observed via an offset term. Logistic regression models were used to evaluate changes in 30-day mortality after acute nonvariceal upper GI bleeding events over time. For each outcome of interest, we fitted three models that adjusted for different sets of covariates. In model 1, we adjusted for age, sex, and race. Model 2 included covariates in model 1 and also adjusted for Medicaid coverage, dialysis vintage, and modality. Model 3 (the full model) included covariates in model 2 and also adjusted for history of kidney transplantation, prior acute nonvariceal upper GI bleeding, all comorbid conditions (Supplemental Material), alcohol dependence, tobacco use, drug dependence, inability to transfer, inability to ambulate, and baseline Quetélet (body mass) index (BMI) for analysis of acute nonvariceal upper GI bleeding events. For mortality analyses, two additional event-related variables were included: whether the episode was related to peptic ulcer disease and whether it involved hospitalization. The observation of a single patient may have been interrupted by ineligible payor histories (with Medicare Part A and B not being the primary payor), dialysis modality switches, transplantation, or calendar years. This means that a patient can have more than one observation per year and several observations between years. Because each patient may contribute more than one outcome, we expect correlation among observations within subject. The generalized estimating equations method with an exchangeable working correlation structure was applied to adjust for the expected dependence of observations.37 Validity of results obtained with this method, however, relies on an assumption that the data are missing completely at random.38
Handling of Missing Data
Some of the key covariates were missing for a proportion of the patients; missingness ranged from less than 1% (race) to 16% (BMI). Examination of the patterns of missingness revealed that the data were not missing completely at random. We therefore used multiple imputation techniques29,30 to handle the missing data. More specifically, standard multiple imputation techniques were used to yield 10 imputed data sets to which we subsequently fit the generalized estimating equation Poisson regression (or generalized estimating equation logistic regression depending on the outcome), and applied the combination rules described by Roderick and Little39 to provide parameter estimates that address our questions of interest. Multiple imputation relies on the assumption that the data are missing at random or that missingness is related to observed variables only. This assumption is reasonable in our context but would be violated if missingness were related to the missing values themselves (e.g., if we were more likely to be missing BMI for those with higher or lower BMI values). One challenge posed by implementing the imputation related to the dependence of observations. Although methods exist for imputation in the presence of correlated data, it can be computationally demanding to format a data set with a large number of time-varying covariates, as in our case. We therefore assumed independence of observations when performing multiple imputation, where the imputation model included all variables used in the scientific model of interest. All analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). This work was approved by the Institutional Review Board of Stanford University School of Medicine.
Disclosures
W.C.W. served as a scientific advisor or consultant to Affymax, Amgen, Astellas, Vifor-Fresenius, GlaxoSmithKline, and Sandoz. He has received unrestricted research support from Fibrogen. G.M.C. serves on the Board of Directors of Satellite Healthcare, Inc., and on the Scientific Advisory Board of DaVita Clinical Research and has received research support and served as an advisor to Amgen. No other author has reported any disclosures.
Supplementary Material
Acknowledgments
We thank the staff of the USRDS (Chronic Disease Research Group) for their kind assistance.
We gratefully acknowledge the generous sponsorship of the Far Eastern Memorial Hospital, New Taipei City, Taiwan, which supported J.-Y.Y.’s visiting scholarship at Stanford University.
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. This work was conducted under a data use agreement between W.C.W. and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript was reviewed by NIDDK and approved for submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011070658/-/DCSupplemental.
References
- 1.United States Renal Data System: USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN: Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc 71: 44–49, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Chiu PW, Ng EK: Predicting poor outcome from acute upper gastrointestinal hemorrhage. Gastroenterol Clin North Am 38: 215–230, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT: Long-term peptic ulcer rebleeding risk estimation in patients undergoing haemodialysis: A 10-year nationwide cohort study. Gut 60: 1038–1042, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Chiu PW, Sung JJ: Acute nonvariceal upper gastrointestinal bleeding. Curr Opin Gastroenterol 26: 425–428, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Crooks C, Card T, West J: Reductions in 28-day mortality following hospital admission for upper-gastrointestinal hemorrhage. Gastroenterology 141: 62–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loperfido S, Baldo V, Piovesana E, Bellina L, Rossi K, Groppo M, Caroli A, Dal Bò N, Monica F, Fabris L, Salvat HH, Bassi N, Okolicsanyi L: Changing trends in acute upper-GI bleeding: A population-based study. Gastrointest Endosc 70: 212–224, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Theocharis GJ, Thomopoulos KC, Sakellaropoulos G, Katsakoulis E, Nikolopoulou V: Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol 42: 128–133, 2008 [DOI] [PubMed] [Google Scholar]
- 9.van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Tytgat GN: Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 98: 1494–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Di Fiore F, Lecleire S, Merle V, Hervé S, Duhamel C, Dupas JL, Vandewalle A, Bental A, Gouerou H, Le Page M, Amouretti M, Czernichow P, Lerebours E: Changes in characteristics and outcome of acute upper gastrointestinal haemorrhage: A comparison of epidemiology and practices between 1996 and 2000 in a multicentre French study. Eur J Gastroenterol Hepatol 17: 641–647, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Targownik LE, Nabalamba A: Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993-2003. Clin Gastroenterol Hepatol 4: 1459–1466, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Wasse H, Gillen DL, Ball AM, Kestenbaum BR, Seliger SL, Sherrard D, Stehman-Breen CO: Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 64: 1455–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Cotsonis G, Wilcox CM: Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol 91: 2329–2332, 1996 [PubMed] [Google Scholar]
- 14.Cooper GS, Kou TD, Wong RC: Outpatient management of nonvariceal upper gastrointestinal hemorrhage: Unexpected mortality in Medicare beneficiaries. Gastroenterology 136: 108–114, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Terdiman JP, Ostroff JW: Risk of persistent or recurrent and intractable upper gastrointestinal bleeding in the era of therapeutic endoscopy. Am J Gastroenterol 92: 1805–1811, 1997 [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services: Dialysis Facility Compare. Available at: http://www.cms.gov/DialysisFacilityCompare/. Accessed June 30, 2011
- 17.Shafi MA, Fleischer DE: Risk factors of acute ulcer bleeding. Hepatogastroenterology 46: 727–731, 1999 [PubMed] [Google Scholar]
- 18.Lanas A, Bajador E, Serrano P, Fuentes J, Carreño S, Guardia J, Sanz M, Montoro M, Sáinz R: Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med 343: 834–839, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S: Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet 348: 1413–1416, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Wiener RS, Schwartz LM, Woloshin S: Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch Intern Med 171: 831–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagiannis S, Goulas S, Kosmadakis G, Galanis P, Arvanitis D, Boletis J, Georgiou E, Mavrogiannis C: Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data). World J Gastroenterol 12: 5182–5185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khedmat H, Ahmadzad-Asl M, Amini M, Lessan-Pezeshki M, Einollahi B, Pourfarziani V, Naseri MH, Davoudi F: Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc 39: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Al-Mueilo SH: Gastroduodenal lesions and Helicobacter pylori infection in hemodialysis patients. Saudi Med J 25: 1010–1014, 2004 [PubMed] [Google Scholar]
- 24.Závada J, Sulková S, Lukás M: [Gastrointestinal hemorrhage and endoscopic findings in patients with chronic kidney failure]. Vnitr Lek 48: 1011–1016, 2002 [PubMed] [Google Scholar]
- 25.Abou-Saif A, Lewis JH: Gastrointestinal and hepatic disorders in end-stage renal disease and renal transplant recipients. Adv Ren Replace Ther 7: 220–230, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Laine L, McQuaid KR: Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol 7: 33–47, quiz 1–2, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Cooper GS, Chak A, Lloyd LE, Yurchick PJ, Harper DL, Rosenthal GE: The accuracy of diagnosis and procedural codes for patients with upper GI hemorrhage. Gastrointest Endosc 51: 423–426, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Abraham NS, Cohen DC, Rivers B, Richardson P: Validation of administrative data used for the diagnosis of upper gastrointestinal events following nonsteroidal anti-inflammatory drug prescription. Aliment Pharmacol Ther 24: 299–306, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, J. Wiley & Sons, 1987 [Google Scholar]
- 30.Rubin DB: Multiple imputation after 18+ years (with discussion). J Am Stat Assoc 91: 473–489, 1996 [Google Scholar]
- 31.Klabunde CN, Potosky AL, Legler JM, Warren JL: Development of a comorbidity index using physician claims data. J Clin Epidemiol 53: 1258–1267, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S: The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 22: 349–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano PS, Roos LL, Jollis JG: Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol 46: 1075–1079, discussion 1081–1090, 1993 [DOI] [PubMed] [Google Scholar]
- 34.D’Hoore W, Bouckaert A, Tilquin C: Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol 49: 1429–1433, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim HN, Ishani A, Foley RN, Guo H, Liu J, Collins AJ: Temporal trends in red blood transfusion among US dialysis patients, 1992-2005. Am J Kidney Dis 52: 1115–1121, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Stürmer T, Glynn RJ, Kliebsch U, Brenner H: Analytic strategies for recurrent events in epidemiologic studies: Background and application to hospitalization risk in the elderly. J Clin Epidemiol 53: 57–64, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Rubin DB: Inference and missing data. Biometrika 63: 581–592, 1976 [Google Scholar]
- 39.Roderick JA, Little DBR: Statistical Analysis with Missing Data, Hoboken, New Jersey, John Wiley & Sons. Inc., 2002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.