Abstract
Signaling through both angiotensin AT1 receptors (AT1R) and dopamine D1 receptors (D1R) modulates renal sodium excretion and arterial BP. AT1R and D1R form heterodimers, but whether treatment with AT1R antagonists functionally modifies D1R via allosterism is unknown. In this study, the AT1R antagonist losartan strengthened the interaction between AT1R and D1R and increased expression of D1R on the plasma membrane in vitro. In rat proximal tubule cells that express endogenous AT1R and D1R, losartan increased cAMP generation. Losartan increased cAMP in HEK 293a cells transfected with both AT1R and D1R, but it did not increase cAMP in cells transfected with either receptor alone, suggesting that losartan induces D1R activation. Furthermore, losartan did not increase cAMP in HEK 293a cells expressing AT1R and mutant S397/S398A D1R, which disrupts the physical interaction between AT1R and D1R. In vivo, administration of a D1R antagonist significantly attenuated the antihypertensive effect of losartan in rats with renal hypertension. Taken together, these data imply that losartan might exert its antihypertensive effect both by inhibiting AT1R signaling and by enhancing D1R signaling.
Sodium excretion and renal vascular tonus are bi-directionally regulated by dopamine, acting on dopamine D1 receptors (D1R) and angiotensin II, acting on angiotensin AT1 receptors (AT1R).1–3 Several lines of evidence suggest that AT1R and D1R form a heteromeric signaling complex.4–6 We recently reported that activation of either AT1R or D1R might cause internalization and/or interruption of the signaling capacity of the other receptor.6 These observations imply that AT1R and D1R may allosterically modulate each other. Several recent studies have shown that the G-protein coupled receptor (GPCR) often forms heteromers and it has been suggested that allosteric modification within such heteromers will fine-tune receptor signal strength and offer novel opportunities for therapeutic approaches.7–10 Because AT1R antagonists are far more commonly used therapeutically than AT1R agonists, we decided to test whether losartan, an AT1R antagonist,11 might influence AT1R and D1R interaction and modulate D1R signaling. Losartan has been widely used to lower arterial BP and to prevent or attenuate the progression of renal disease.12–15 Notably, the losartan binding site in AT1R does not overlap with the angiotensin binding site.16,17
This study was performed on rat renal proximal tubule cells (RPTCs) in primary culture and on HEK 293a (HEK) cells, a cell line derived from human embryonic kidney. The endogenous expressions of D1R and AT1R are relatively high in RPTCs1,18 and low in HEK cells. Therefore, HEK cells were used for expression of fluorescently tagged receptors and mutant receptors. We performed a series of biochemical studies, which indicated that losartan strengthens the interaction between AT1R and D1R and that losartan increases the availability of D1R inserted in the plasma membrane. The latter observation was confirmed in a study of live cells expressing fluorescently tagged D1R. Because D1R is positively and AT1R is negatively coupled to adenylyl cyclase,19,20 the effect of losartan-bound AT1R on D1R signaling was evaluated by determination of cAMP generation. We previously showed that the amino acid residues S397 and S398 in the C-terminus tail of D1R are of critical importance for AT1R and D1R interaction.6 To test the effect of AT1R and D1R interaction for effects of losartan on plasma membrane expression of D1R and on cAMP generation, wild-type AT1R and D1R S397A/S398A were expressed in HEK cells. Collectively, the results from these studies indicated that losartan strengthens the interaction between AT1R and D1R and exerts an allosteric stimulating effect on D1R signaling. Because the in vitro studies suggested that losartan-bound AT1R has a positive effect on D1R signaling, we hypothesized that the therapeutic effects of this drug might also be mediated via D1R activation. To test this hypothesis, we performed an in vivo study, in which rats with experimental hypertension of primarily renal origin21 were treated for 10 days with either losartan alone or with losartan and a D1 receptor antagonist.
Results
Interaction in the AT1R and D1R Complex Is Strengthened by Losartan
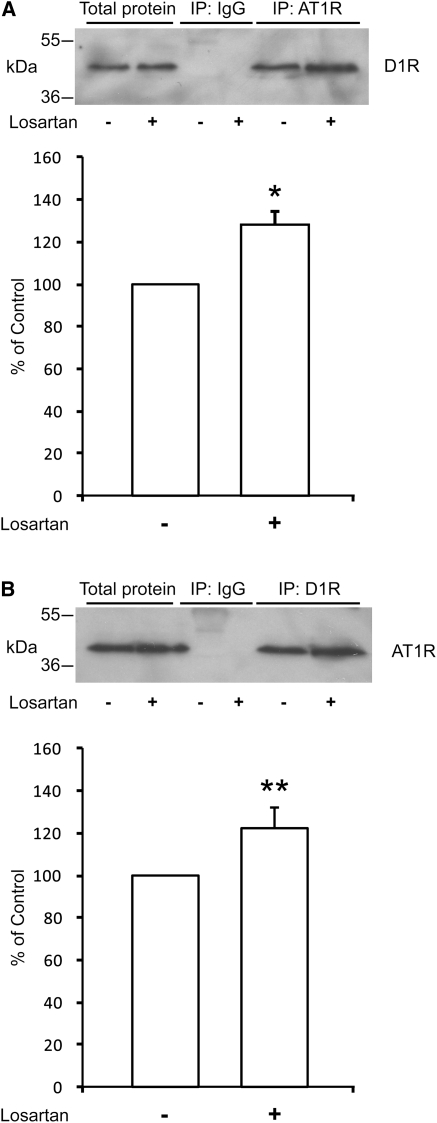
To test the role of losartan for the interaction between AT1R and D1R, we first performed co-immunoprecipitation studies, using the outer 250-µm layer of renal cortical slices from 40-day-old rats. The slices were incubated with losartan 10−5 M or vehicle for 15 minutes. Lysates from these slices were immunoprecipitated with either a D1R antibody or AT1R antibody and prepared for Western blot. We found, in line with what we previously reported,6 that D1R is co-immunoprecipitated with AT1R and vice versa. Exposure to losartan significantly increased the strength of interaction between the receptors (Figure 1, A and B). In addition, we also used HEK cells transfected with a V5-tagged AT1R and a Venus-tagged D1R for treatment with losartan 10−5 M or vehicle for 15 minutes followed by immunoprecipitation with GFP or V5 antibody and detection by V5 or GFP antibody, respectively. The strengthened interaction between D1R and AT1R in the losartan-exposed HEK cells can be seen as a stronger signal intensity (Supplemental Figure 1).
Figure 1.
Losartan increases the strength of interaction between D1R and AT1R. Effect of losartan (10−5 M, 15 minutes) on co-immunoprecipitation from renal cortical slices using (A) AT1R antibody and blot for D1R (n=4; 28% increase; *P<0.05 versus control) and (B) using D1R antibody and blot for AT1R (n=5; 22% increase; **P<0.01 versus control). Densitometric quantification of bands was done for the respective blots. The density of vehicle-treated controls was set to 100%. Statistical analysis was performed using the Mann–Whitney U test. Values are mean ± SEM.
Losartan Increases Abundance of D1R in the Plasma Membrane
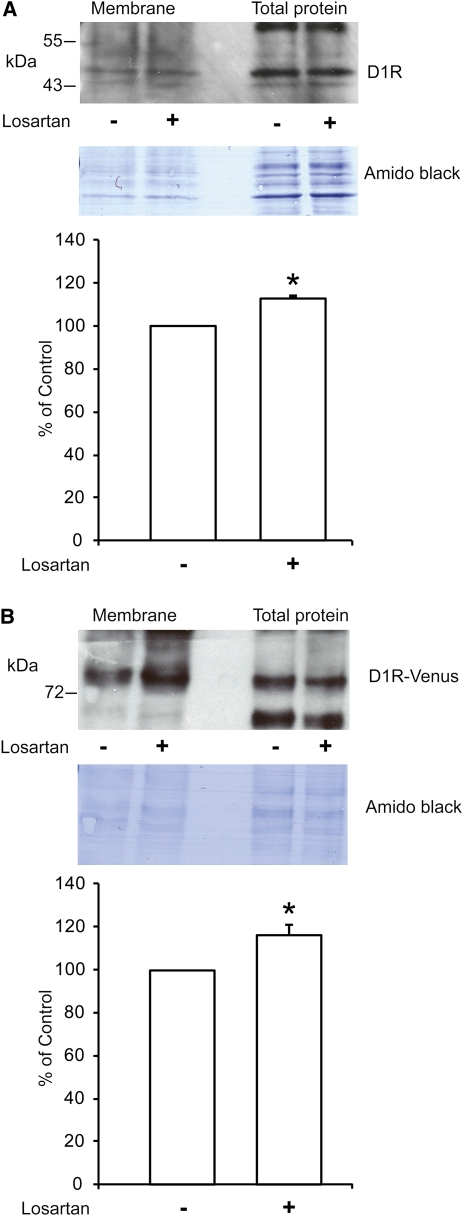
We tested whether losartan-bound AT1R would have an effect on the subcellular distribution of D1R. We first performed cell-surface biotinylation studies. We found that losartan significantly increased the abundance of biotinylated D1 receptors in the plasma membrane of RPTCs (Figure 2A). A similar increase in the abundance of D1R in the plasma membrane was observed in HEK cells double transfected with D1R and AT1R (Figure 2B).
Figure 2.
Losartan increases D1R abundance in the plasma membrane in RPTCs and HEK cells. Plasma membrane expression of D1R was studied using the biotinylation technique. Losartan (10−5 M, 20 minutes) increased membrane biotinylated D1R in (A) RPTCs (n=4) by 13% (*P<0.05 versus control) and (B) HEK cells transfected with AT1R and D1R (n=4) by 17% (*P<0.05 versus control). Densitometric quantification of bands was done for the respective blots. The density of vehicle-treated controls was set to 100%. Statistical analysis was performed using the Mann–Whitney U test. Values are mean ± SEM. In A and B, the top shows a typical image of the Western blot for D1R of biotinylated membrane and total protein, the middle is the corresponding filter stained with amido black for loading control, and the bottom is the graph for the quantification.
Losartan Increases Plasma Membrane Abundance of D1R in a Time-Dependent Manner
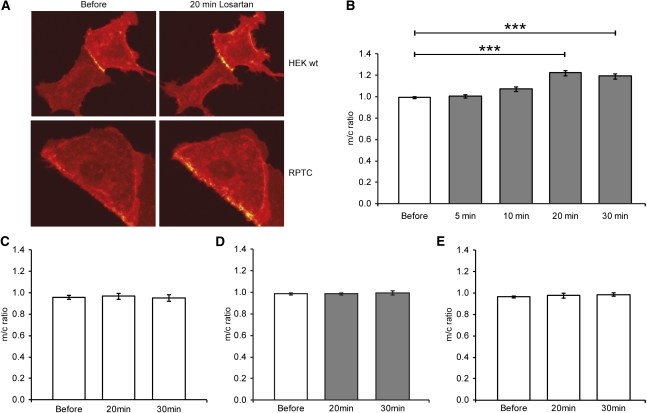
We studied the time dependence of the effect of losartan on the subcellular distribution of D1R using live cell imaging. RPTCs or HEK cells were first transfected with wild-type D1R and AT1R. Images were collected before exposure and after 5, 10, 20, or 30 minutes of exposure to losartan. We found that the effect of losartan is time dependent, with a maximal increase of D1R at the plasma membrane after 20 minutes exposure to losartan (Figure 3, A and C). Vehicle treatment had no effect on D1R localization (Figure 3B). To examine whether this effect is dependent on a physical interaction between AT1R and D1R, we used a mutant form of D1R (S397A/S398A), which does not interact with AT1R as efficiently as wild-type D1R.6 HEK cells were transfected with wild-type AT1R and mutant S397A/S398A D1R, losartan had no effect on the expression of mutant D1R (Figure 3D).
Figure 3.
Losartan increases D1R expression in the plasma membrane in a time-dependent manner. (A) Typical images showing D1R localization in HEK cells and RPTCs transfected with D1R and AT1R before and 20 minutes after losartan (10−5 M) exposure. (B) Membrane/cytosol ratio for HEK cells transfected with AT1R and wild-type D1R treated with vehicle (white bars) or losartan (10−5 M, gray bars). The effect of losartan is time dependent (n=16; ***P<0.001) for 20 and 30 minutes versus before (ANOVA, t test). (C) Vehicle has no effect on wild-type D1R localization. (D) Losartan (10−5 M, gray bars) treatment for 20 or 30 minutes has no effect on membrane/cytosol ratio for HEK cells transfected with AT1R and mutant S397A/S398. (E) Vehicle has no effect on mutant S397A/S398A D1R localization. All values are mean ± SEM.
Losartan-Bound AT1R Acts as an Allosteric Modifier of D1R
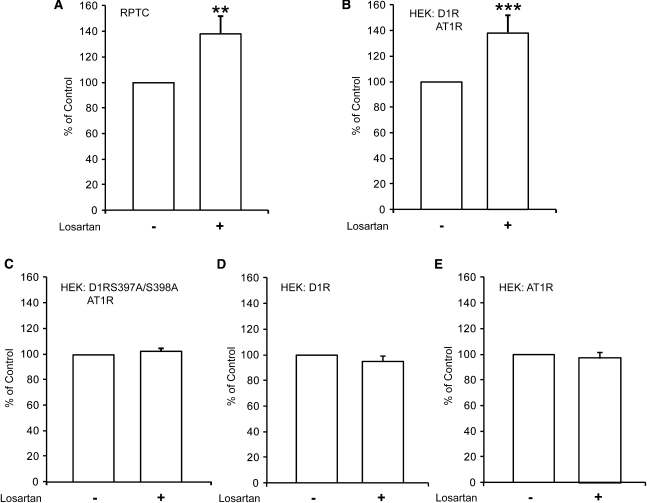
We tested whether losartan-bound AT1R might have an allosteric effect on D1R, resulting in D1R activation. D1R signaling is positively and AT1R is negatively coupled to adenylyl cyclase and generation of cAMP. We first recorded the effect of losartan on cAMP generation in RPTCs, which express relatively high levels of endogenous AT1R and D1R. We found that losartan significantly increased cAMP in RPTCs (Figure 4A). We then recorded the effect of losartan on cAMP generation in HEK cells, double transfected with AT1R and D1R. Losartan significantly increased cAMP (Figure 4B). In contrast, losartan did not have an effect on cAMP levels in cells transfected with AT1R or D1R alone (Figure 4, D and E). To examine whether the cAMP-generating effect of losartan required physical interaction between AT1R and D1R, cells were transfected with wild-type AT1R and mutant S397A/S398A D1R. Losartan did not have any effect on cAMP production in HEK cells transfected with AT1R and the D1R mutant (Figure 4C). To confirm the functionality of the mutant D1R, transfection of HEK cells with mutant D1R alone was performed. Treatment with the dopamine D1R agonist SKF38393 resulted in a significant increase of cAMP (Supplemental Figure 2A). The exposure to a D1R agonist, SKF38393 (10−5 M, 5 minutes) did not further increase the level of cAMP in cells treated with losartan (10−5 M, 20 minutes) in HEK cells transfected with AT1R and wild-type D1R (Supplemental Figure 2B).
Figure 4.
Losartan acts as an allosteric modifier of D1R. (A) Losartan (10−5 M, 20 minutes) significantly increases the amount of cAMP in RPTCs compared with control, vehicle-treated cells (n=7; **P< 0.01 versus control). (B) Losartan (10−5 M, 20 minutes) significantly increases the amount of cAMP in HEK cells transfected with AT1R and wild-type D1R (n=10; ***P< 0.001 versus control). (C) Losartan does not affect the cAMP production in HEK cells transfected with AT1R and mutant S397A/S398A D1R (n=9). Losartan does not affect the cAMP production in HEK cells transfected with (D) only D1R (n=7) or (E) only AT1R (n=7). For each experiment, the cAMP level of control, vehicle-treated cells, was set to 100%. Statistical analysis was performed using the Mann–Whitney U test. Values are mean ± SEM.
Losartan Enhances D1R Effects In Vivo
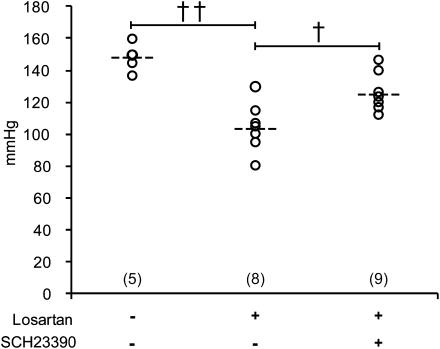
To study whether the antihypertensive effect of losartan might also involve D1R activation, we used a modification of the Goldblatt-2 kidney hypertensive rat model, in which hypertension is generated by performing a surgical procedure that leads to aortic coarctation proximal to the renal artery.21 This procedure resulted in an increased pressure proximal to the coarctation (carotid 148±4 mmHg; n=5) in rats studied 18 days after the surgical procedure and treated with only vehicle. In rats treated with losartan 8–18 days after the surgical procedure, the pressure proximal to the coarctation was 103±5 mmHg (n=8). Addition of a D1R antagonist significantly attenuated the antihypertensive effect of losartan. In rats treated with losartan 8–18 days after surgery and with losartan and the D1R antagonist SCH 23390 15–18 days after surgery, the pressure proximal to the coarctation was 125±4 mmHg (n=9) (Figure 5). Rats that were only given SCH23390 15–18 days after surgery had a pressure of 141±3 mmHg (n=5), which was not significantly different from the pressure of the control rats. Treatment had little effect on the pressure distal to the coarctation.
Figure 5.
The antihypertensive effect of losartan is attenuated by a D1R antagonist. Mean arterial BP (dashed line) in hypertensive rats receiving vehicle (control, n=5), losartan (n=8), and losartan and SCH 23390 (n=9). ††P<0.01 for losartan versus vehicle-treated rats and †P<0.05 for losartan and SCH 23390 versus losartan-treated rats. Open rings show arterial BP in all individuals in each treatment group. Statistical analysis was performed using ANOVA and the Newman–Keuls multiple comparison test.
Discussion
Summary of Findings
The kidney plays a central role for BP regulation by its control of sodium balance. Angiotensin, acting on AT1 receptors, and dopamine, acting on the D1 family of receptors, exert opposite effects on sodium excretion.2 Recent studies showed that AT1R and D1R form a dimer, in which they act as a unit of opposites.6 This study confirms the concept that AT1R and D1R form a heteromeric signaling complex and identifies losartan, an AT1R antagonist and a widely used antihypertensive drug, as an allosteric modifier of this complex. The finding that an AT1R antagonist can activate D1R signaling and that this effect is dependent on AT1R and D1R interaction is novel and has potential pharmacologic implications.
AT1R and D1R: A Heterodimer that Can Be Modified by AT1R Ligands
The results from the co-immunoprecipitation studies are compatible with the notion that D1R and AT1R form a heterodimer. This is in line with previously reported data.6 Interestingly, agonist and antagonist binding to AT1R have opposite effects on the AT1R-D1R heteromeric complex. Results from a previous study indicated that the strength of interaction between AT1R and D1R is decreased by angiotensin.6 In contrast, results from this study indicate that the strength of AT1R and D1R interaction was increased by the AT1R antagonist losartan.
Losartan Occupied AT1R Increases Plasma Membrane Availability of D1R
Losartan caused a small but significant increase in the plasma membrane expression of D1R. In a previous study, we identified two amino acids in the C-terminus tail of D1R, S397 and S398, to be of critical importance for D1R and AT1R interaction. Here, we used transfected HEK cells to test whether AT1R might increase the surface expression of mutant D1R that lacked the motif of importance for the interaction with AT1R. Because this was not the case, we conclude that the effect of losartan-bound AT1R on D1R plasma membrane expression can be attributed to the function of the AT1R/D1R heterodimer.
The losartan-triggered increase in plasma membrane expression of D1R was confirmed in the live cell studies. This methodological approach allowed us to perform a time course recording, which showed that the increase in D1R plasma membrane expression was not immediate but seemed to reach a plateau after 20–30 minutes. Assuming that losartan would activate a signal that recruits D1R to the plasma membrane, we expected a more instant effect. The relatively slow accumulative increase in D1R plasma membrane expression is more compatible with a reduced rate of D1R internalization. The finding that losartan failed to increase plasma membrane expression of the mutant D1R, which does not form a heterodimer with AT1R, supports the notion that losartan-bound AT1R attenuates the internalization and recycling of D1R rather than enhances D1R recruitment.
Allosteric Modulation of D1R by Losartan-Bound AT1R
We previously reported that in rat renal cells, AT1R activation by angiotensin uncouples D1R from its signaling pathway and activation of D1R uncouples AT1R signaling.6 Because D1R is positively and AT1R is negatively coupled to adenylyl cyclase, we used the generation of cAMP as an index of D1R activation. Losartan increased cAMP in cells expressing both AT1R and wild-type D1R, but did not do so in cells expressing AT1R and the mutant D1R lacking the motif to interact with AT1R, nor in cells expressing AT1R alone.
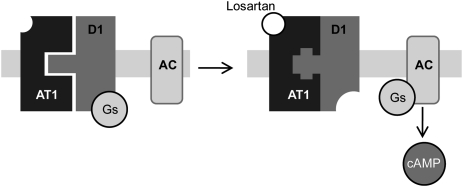
Taken together, these results indicate that losartan, by binding to AT1R, exerts an allosteric effect on its D1R protomer, resulting in activation of D1R signaling. This concept is illustrated in Figure 6. Allosteric modulation within a heterodimer, in which a structural modification in one protomer will affect the structure and function of the other protomer, has been intensively studied in the past decade and is now considered to be an important indirect mechanism for control of receptor function. Allosteric modulation among heterodimeric GPCRs, whereby agonist actions at one member of a heterodimer influence functional coupling of the other protomer, has been demonstrated for many GPCRs, including the D1, D2, and D3 dopamine receptors. However, to the best of our knowledge, the action of an antagonist on one member of a heterodimer has not previously been reported to influence the functional coupling of the other protomer.
Figure 6.
Model of the allosteric effects of losartan-bound AT1R on its protomer D1R. As previously shown, AT1R and D1R form a dimer that is disrupted by agonist binding to either AT1R or D1R. In contrast, antagonist binding to AT1R strengthens the interaction between the protomers. Binding of the antagonist losartan to the AT1R should result in an allosteric modification of the receptor. Results from this study indicate that this allosteric modification is transferred to D1R and that it will trigger a coupling of D1R to its signaling pathway.
Is There a Tissue-Specific Role of AT1R–D1R Interaction?
It is generally believed that, although circulating angiotensin levels exert direct effects on vascular resistance, the circulating levels of dopamine are too low to play a physiologic or pathophysiologic role. The situation is different in the renal epithelium-like cells, in which the availability of locally produced dopamine is high and might be physiologically controlled.22 Thus, interaction between AT1R and D1R has been most extensively studied in these cell types. This study was performed on renal tubular cells that play a pivotal role for regulation of salt balance, and indirectly, for BP regulation. Will the results from this study be applicable for other tissues? AT1R plays a role for BP regulation at all levels. Activation of AT1R in the brain (limbic, hypothalamic, and brainstem) increases arterial BP and plays a role for central pressure control.23,24 Much attention has been given to the effects of D1R activation on mood, behavior, and motor control, but there is little or no information available on the role of D1R in the central nervous system for cardiovascular function.25–27 There is currently little or no information on whether AT1R and D1R may be co-expressed in the brain. AT1R and D1R are both expressed in the heart and vessels,28,29 but little is known about whether AT1R and D1R are co-localized in these tissues. It is noteworthy that the interaction between AT1R and D1R was similar in the primitive HEK cell line and RPTCs, suggesting that AT1R and D1R allosterism does not require the presence of additional cell specific proteins. Thus, it is important to examine the role of AT1R-D1R in other cells of importance for cardiovascular function and BP regulation, such as the cardiomyocyte.
Medical Implications
The receptor heteromer concept is now widely accepted and it was recently postulated that more knowledge about the functional significance of receptor heterodimers will take drug development to a new level of specificity and efficacy.10 To test the concept of allosteric interaction between losartan-bound AT1R and dopamine D1 receptors in an in vivo model, we compared the antihypertensive effects of losartan alone with co-treatment of losartan and a D1R antagonist in rats with experimental hypertension. We found that addition of a D1R antagonist significantly attenuated the antihypertensive effect of losartan. These results provide proof of principle of a functionally important allosteric interaction between AT1R and D1R and imply that attempts should be made to develop low-dose combination therapy of AT1R antagonists and D1R agonists in the treatment of hypertension.
Concise Methods
Complete details of methods for all experiments are described in the Supplemental Material.
Animals, Tissues, and Cells
All animals used were male Sprague-Dawley rats aged 4–8 weeks. Studies performed in Sweden were approved by the Stockholm North Ethical Evaluation Board for Animal Research. Studies performed in Argentina were approved by the Comité Institucional Para el Cuidado y Uso de Animales de Laboratorio of the Faculty of Medicine, University of Buenos Aires. Primary cultures of RPTCs were prepared as described previously.30 HEK 293a cells, human embryonic kidney cell line QBI293A, were obtained from Qbiogene Inc.
Immunoprecipitation
Renal cortical slices were incubated for 15 minutes at 20°C with losartan (10−5 M) or vehicle. Slices were lysed and precleared with Sepharose-G. Lysates were incubated with precipitating antibody against D1R or AT1R, followed by a second incubation with Sepharose-G. Samples were washed, eluted with Laemmli sample buffer, and resolved by Western blot. Densitometric quantification of films was done, correction for total protein was made, and control values were set to 100%.
Cell-Surface Biotinylation
Biotinylation was performed using RPTCs or HEK cells. Cells were treated in presence of vehicle or losartan (10−5 M, 20 minutes). After treatment cells were incubated with EZ linked Sulfo-NHS-SS Biotin. Cells were rinsed, homogenized, and centrifuged, and the resulting pellets were resuspended and protein amounts were adjusted. Biotinylated proteins were captured with streptavidin-agarose beads, washed, and eluted with Laemmli sample buffer, and were resolved by Western blot. Densitometric quantification of films was done, assessment of equal total protein loaded was made, and control values were set to 100%.
Transfection and Constructs
Cloning of D1R expressing constructs was described previously.31 Cloning and constructs of AT1a receptor are described in the Supplemental Material. Transfection of RPTCs was performed using Lipofectamine 2000 and transfection of HEK cells was performed using Exgene 500, all according to the manufacturer’s suggestion. Cells were cultured an additional 24 hours after transfection before experiments.
cAMP Assay
Two hours before treatment, RPTCs or HEK cells were exposed to a serum-free medium. Cells were then incubated with losartan (10−5 M) or vehicle for 20 minutes at 37°C. Cells were washed and lysed and protein concentration was measured. cAMP was determined using the Direct Cyclic AMP Enzyme Immunoassay Kit (Assay Designs). Results from each experiment were normalized to the control (vehicle) value that was set to 100%.
Live Cell Imaging
Complete details of confocal microscopy equipment used for experiments are described in the Supplemental Material. Images were collected before and 5, 10, 20, or 30 minutes after treatment. The membrane/cytosol ratio was calculated by dividing the average intensity of an area in the region of the plasma membrane by the average intensity of an identical area of the cytosolic region immediately subjacent to the chosen region of the plasma membrane.
BP Recording in Rats with Experimental Hypertension
Seven-week-old rats were subjected to a surgical procedure to perform aortic coarctation proximal to the renal artery, which resulted in hypertension.21 One week after surgery, rats were randomly divided into control (n=5), SCH23390 only (n=5), losartan (n=8), and losartan and SCH23390 (n=9) groups. The losartan treatment groups received losartan (20 mg/kg per day) in the drinking water. One week later, the rats receiving SCH23390 were treated with SCH 23390 (1 mg/kg body wt per day), administered subcutaneously, divided in 2 doses per day for 4 days. Control rats and rats treated with losartan only received vehicle. After 4 days, rats were anesthetized and catheters were inserted into the carotid and right femoral arteries for recording of mean arterial pressure above and below the aortic constriction.
Statistical Analyses
Statistical analyses of data obtained from immunoprecipitation, biotinylation, and cAMP studies were performed using the Mann–Whitney U test. Statistical analyses of results from live cell imaging studies were performed using repeated-measures ANOVA and multiple t test comparisons. Statistical analysis of data from the in vivo study was performed using ANOVA and Newman–Keuls multiple comparison test. P values <0.05, <0.01, or <0.001 were used as the levels of significance.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by the Torsten and Ragnar Söderberg Foundations, the Swedish Heart Lung foundation, the Swedish Research Council, and Familjen Erling-Perssons Stiftelse.
This study was presented at the Experimental Biology meeting in Washington, DC, April 9–13, 2011.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011040344/-/DCSupplemental.
References
- 1.Aperia A, Bertorello A, Seri I: Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol 252: F39–F45, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Jose PA: Dopamine receptors: Important antihypertensive counterbalance against hypertensive factors. Hypertension 57: 11–17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aperia A, Holtbäck U, Syrén ML, Svensson LB, Fryckstedt J, Greengard P: Activation/deactivation of renal Na+,K(+)-ATPase: A final common pathway for regulation of natriuresis. FASEB J 8: 436–439, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Zeng C, Wang Z, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA: Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension 46: 799–805, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Gildea JJ: Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens 18: 28–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A: Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 295: F1110–F1116, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Martin B, Brenneman R, Luttrell LM, Maudsley S: Allosteric modulators of G protein-coupled receptors: Future therapeutics for complex physiological disorders. J Pharmacol Exp Ther 331: 340–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan BA, Devi LA: G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399: 697–700, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith NJ, Milligan G: Allostery at G protein-coupled receptor homo- and heteromers: Uncharted pharmacological landscapes. Pharmacol Rev 62: 701–725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R: Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5: 131–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu F, Mao C, Liu Y, Wu L, Xu Z, Zhang L: Losartan chemistry and its effects via AT1 mechanisms in the kidney. Curr Med Chem 16: 3701–3715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruilope LM: Angiotensin receptor blockers: RAAS blockade and renoprotection. Curr Med Res Opin 24: 1285–1293, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Smith DH: Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs 68: 1207–1225, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Aulakh GK, Sodhi RK, Singh M: An update on non-peptide angiotensin receptor antagonists and related RAAS modulators. Life Sci 81: 615–639, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Elliott WJ, Calhoun DA, DeLucca PT, Gazdick LP, Kerns DE, Zeldin RK: Losartan versus valsartan in the treatment of patients with mild to moderate essential hypertension: Data from a multicenter, randomized, double-blind, 12-week trial. Clin Ther 23: 1166–1179, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Bhuiyan MA, Ishiguro M, Hossain M, Nakamura T, Ozaki M, Miura S, Nagatomo T: Binding sites of valsartan, candesartan and losartan with angiotensin II receptor 1 subtype by molecular modeling. Life Sci 85: 136–140, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Oliveira L, Costa-Neto CM, Nakaie CR, Schreier S, Shimuta SI, Paiva AC: The angiotensin II AT1 receptor structure-activity correlations in the light of rhodopsin structure. Physiol Rev 87: 565–592, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS: Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol 273: F170–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG: Dopamine receptors: From structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 20.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T: International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 21.Eklöf AC, Aperia A: Renal hypertension following aortic constriction is abolished by angiotensin II converting enzyme inhibitor, but not by low-salt diet. Acta Physiol Scand 139: 435–440, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Eklöf AC, Holtbäck U, Sundelöf M, Chen S, Aperia A: Inhibition of COMT induces dopamine-dependent natriuresis and inhibition of proximal tubular Na+,K+-ATPase. Kidney Int 52: 742–747, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Buckley JP, Bickerton RK, Halliday RP, Kato H: Central effects of peptides on the cardiovascular system. Ann N Y Acad Sci 104: 299–311, 1963 [DOI] [PubMed] [Google Scholar]
- 24.Rettig R, Ganten D, Lang RE, Unger T: The renin-angiotensin system in the central control of blood pressure. Eur Heart J 8[Suppl B]: 129–132, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG: Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X: Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23: 9395–9402, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuvonen PJ, Westermann E: On the cardiovascular action of dopamine in rats. Naunyn Schmiedebergs Arch Pharmacol 280: 363–371, 1973 [DOI] [PubMed] [Google Scholar]
- 28.Carey RM: Angiotensin type-1 receptor blockade increases ACE 2 expression in the heart. Hypertension 43: 943–944, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ozono R, O’Connell DP, Vaughan C, Botkin SJ, Walk SF, Felder RA, Carey RM: Expression of the subtype 1A dopamine receptor in the rat heart. Hypertension 27: 693–703, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Li J, Zelenin S, Aperia A, Aizman O: Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J Am Soc Nephrol 17: 1848–1857, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Scott L, Zelenin S, Malmersjö S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A: Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci USA 103: 762–767, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.