Abstract
The involvement of autoantibodies to human lysosome-associated membrane protein-2 (hLAMP-2) in anti–neutrophil cytoplasmic antibody (ANCA)–associated vasculitis is controversial because of the absence of confirmatory data subsequent to the initial reports of their high prevalence in this disease. We characterized three assays for anti-hLAMP-2 antibodies: ELISA and Western blotting assays using unglycosylated recombinant hLAMP-2 expressed in Escherichia coli, and an indirect immunofluorescence assay using stably transfected ldlD cells that expressed glycosylated full-length hLAMP-2 on the plasma membrane. The assays detected autoantibodies to hLAMP-2 in human sera reproducibly and with comparable sensitivity and the assays gave the same results in 80.5% of the test panel of 40 selected positive and negative sera. In untreated patients at presentation, the frequencies of autoantibodies to LAMP-2 were 89%, 91%, and 80%, respectively, among three groups of patients with ANCA-associated vasculitis from Vienna, Austria (n=19); Groningen, the Netherlands (n=50) and Cambridge, United Kingdom (n=53). Prevalence of LAMP-2 autoantibodies was similar in both those with myeloperoxidase-ANCA and proteinase 3-ANCA. Furthermore, we detected LAMP-2 autoantibodies in two ANCA-negative patients. LAMP-2 autoantibodies rapidly became undetectable after the initiation of immunosuppressive treatment and frequently became detectable again during clinical relapse. We conclude that when robust assays are used, circulating autoantibodies to hLAMP-2 can be detected in most European patients with ANCA-associated vasculitis. Large-scale prospective studies are now needed to determine whether they are pathogenic or merely an epiphenomenon.
Pauci-immune focal necrotizing GN (piFNGN) is a severe inflammatory disease that occurs in anti–neutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), such as microscopic polyangiitis (MPA) or granulomatosis with polyangiitis (GPA, formerly Wegener’s granulomatosis).1,2 AAV has a relapsing course and over a quarter of those affected die within 5 years either from uncontrolled disease or complications of treatment,3 which emphasizes the need for more specific immunosuppressive therapy tailored to the underlying pathogenic mechanisms.
Between 85% and 90% of those with piFNGN have antibodies to neutrophil cytoplasmic antigens (ANCA) that recognize either myeloperoxidase (MPO) or proteinase 3 (PR3).1,2 This provides strong evidence for their involvement in pathogenesis, which is supported by in vitro studies4 and experimental models (at least for MPO-ANCA).5–7 Despite this, MPO and PR3 are not normally expressed in kidney although MPO released from infiltrating neutrophils can decorate glomerular endothelium,8 and additional factors are needed for anti-MPO antibodies to cause severe injury in rodent models.6,8,9 The weak correlation between ANCA titers and clinical disease activity suggests that the same is true in humans.10–12 We identified autoantibodies to lysosome-associated membrane protein-2 (LAMP-2) in active piFNGN and proposed that they might contribute to injury because the antigen is expressed in the plasma membrane of glomerular endothelial cells.13,14
Antibodies to hLAMP-2 were originally discovered in 16 of 17 patients with piFNGN by Western blotting in a systematic search for autoantibodies to neutrophil or glomerular membrane proteins.13 We found a similarly high prevalence in a subsequent cohort of 84 patients with active piFNGN.14 Patients’ autoantibodies commonly bind two epitopes, one of which (P41-49) is shared with the bacterial adhesin FimH with which they cross-react. Injection of antibodies to the LAMP-2 extracellular domain induced piFNGN in WKY rats as did immunization with FimH that acted as molecular mimic and provoked synthesis of antibodies to rat LAMP-2. Thus, antibodies to LAMP-2 cause piFNGN in rodents, which raises the issue whether they are similarly pathogenic in humans. Robust assays are required to investigate this further, and development of suitable assays for antibodies to hLAMP-2 has been challenging because of the difficulty in obtaining pure preparations of appropriately glycosylated native or recombinant antigen,15,16 a problem shared with other glycosylated membrane proteins such as the membranous nephropathy antigen, phospholipase A2 receptor.17 Recombinant membrane proteins often need modification to produce soluble substrates for ELISA, and inappropriate glycosylation can affect accessibility of epitopes recognized by patients’ autoantibodies. Only one other group has reported the development of assays for anti-hLAMP-2 antibodies and they have challenged our conclusions.18
In this study, we characterize three assays for antibodies to hLAMP-2 in human sera and show that they give highly concordant results. In applying them to new European cohorts from three different centers, we confirm that antibodies to hLAMP-2 are highly prevalent in patients with piFNGN both at presentation and during clinical relapse. Results of sequential measurements after the start of treatment provide a possible explanation for the disparity between our findings and those of Roth et al.18
Results
Recombinant Escherichia coli Expressed hLAMP-2 for Western Blotting and ELISA
Most patients’ autoantibodies bind epitopes in the protein backbone of the extracellular domain not occluded by glycosylation in native neutrophil and glomerular hLAMP-2.13,14 Consequently, we induced recombinant unglycosylated hLAMP-2 truncated to 342 amino acids of the full extracellular domain as GST fusion protein in E. coli (Figure 1A). After purification on Glutathione-Sepharose, hLAMP-2/GST fusion protein runs as a single band of approximately 65 kD on SDS-PAGE (Figure 1B), whose identity was confirmed by immunoblot with antibodies to hLAMP-2 and GST. It also binds IgG in sera from patients with antibodies to hLAMP-2 but not controls (Figure 1C). Patients’ sera were diluted 1:100 to give the best binding/background ratio (Figure 1D).
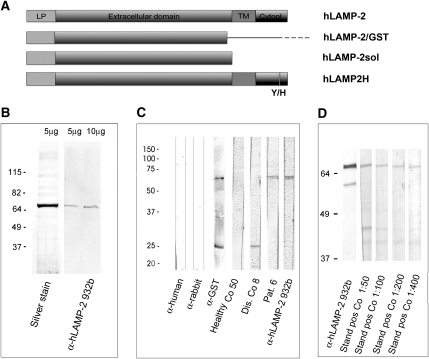
Figure 1.
cDNA constructs, generation, and quality control of recombinant hLAMP-2. (A) Representation of cDNA encoding hLAMP-2A with the 28 amino acid leader peptide (LP), 347 amino acid extracellular domain, 24 amino acid transmembrane domain (TM), and 11 amino acid cytoplasmic domain (Cytopl). The two extracellular domain constructs were utilized to express soluble hLAMP-2 in E. coli (hLAMP-2/GST) and mammalian cells (hLAMP-2sol). Both contain the leader peptide but not the transmembrane domain or cytoplasmic tail. hLAMP-2sol expressed in mammalian cells results in an appropriately glycosylated soluble protein exported into the culture supernatant via the default secretory pathway in mammalian cells. The hLAMP-2 cytoplasmic tail contains the signal that directs its retrieval from the plasma membrane to lysosomes. The critical tyrosine was mutated to a histidine in hLAMP-2H (Y/H), which targets it to the plasma membrane when expressed in ldlD cells. (B) Purified hLAMP-2/GST runs as a single 65-kD band on SDS-PAGE and silver stain. Fractions of high purity were pooled and identity of hLAMP-2 was confirmed with an antibody reactive with hLAMP-2 only. (C) Purified hLAMP-2/GST (10 μg/ml) was separated by SDS-PAGE and transferred onto PVDF before probing with specific antibodies to hLAMP-2 (932b), which bound exclusively to the fusion protein. Antibody to GST (anti-GST) recognized both fusion protein and free GST. A human serum (1:100 dilution) containing anti-hLAMP-2 antibodies (Pat. 6) also bound the fusion protein, whereas serum from a healthy control (Healthy Co 50) did not. Serum from a patient with renal disease (Dis. Co 8) contained antibodies to GST. Secondary antibodies alone were negative (anti-human, anti-rabbit). (D) Sensitivity of the Western blot was assessed from doubling dilutions (1:50 to 1:400) of the standard positive control (Stand pos Co) used for all assays. This also confirmed the optimal binding/background ratio was 1:100.
hLAMP-2/GST was prepared in batches of <10 mg and used within 3 months because large-scale cultures and pre-purification storage of pellets increased degradation and contamination with other proteins (Supplemental Figure 1, A and C) and the recombinant protein degrades rapidly at −20°C and even −80°C after 6 months (Supplemental Figure 1, B and D).
Measuring Antibodies to hLAMP-2 by ELISA
ELISA plates were coated with 5 µg/ml of hLAMP-2/GST for 1 hour, optimal conditions for distinguishing between positive and negative sera (Figure 2A). Coated plates were stable for 4 weeks at 4°C (Figure 2B). When diluted, 1:100 moderately strong positive sera gave an OD of approximately 0.9 compared with a mean OD of 0.27 for normal sera (Figure 2A). Minor degrees of substrate degradation profoundly affected assay performance (Supplemental Figure 1, B and D), necessitating rigorous quality control of hLAMP-2/GST batches by SDS-PAGE and immunoblotting (Supplemental Figure 1C) and testing ELISA plates with standard sera to ensure consistency (Figure 2C). Sera were tested for contamination with FimH-expressing bacteria because these inhibit binding, as does repeated freezing and thawing.
Figure 2.
Development of anti-hLAMP-2 ELISA. (A) Comparison of ELISA plates coated with 0.5 and 5.0 µg/ml of recombinant hLAMP-2/GST fusion protein (FP). Plates coated with 5.0 µg/ml gave much better separation between positive and negative sera. (B) ELISA plates coated with hLAMP-2/GST FP were stored at 4°C and tested after different time points. Serial dilutions of a standard positive control serum gave similar results using plates stored for 1–33 days, but there was a considerable increase in background binding after 41 days. (C) Comparison of ELISA plates coated with two different batches of hLAMP-2/GST FP demonstrates comparable binding of serial dilutions of a standard positive control serum. (D) The standard curve derived from the results of assaying serial dilutions of the standard control serum was used to calculate anti-hLAMP-2 antibody concentrations with 100 U equating to OD of the 1:100 dilution of the positive control.
The ELISA was highly reproducible and our standard positive control gave a mean OD of 0.958±0.159 (coefficient of variation, 16.5%) when assayed 24 times over 6 months using three different hLAMP-2/GST preparations. Anti-hLAMP-2 antibody concentrations were calculated from a standard curve generated with this serum (Figure 2, C and D) with 100 U equating to the OD of the 1:100 dilution. Sera from 80 healthy controls had a mean ± SD of 15.91±6.68 U, giving a 95% confidence limit of the upper limit of normal of 29 units for the ELISA: higher values were scored positive.
Expression of hLAMP-2 in ldlD Cells for Immunofluorescence Assay
Binding of autoantibodies to glycosylated LAMP-2 was tested using an indirect immunofluorescence assay (IIF) assay in which hLAMP-2 was expressed exclusively on the surface of ldlD cells—a Chinese hamster ovary (CHO) cell subline used for studying glycoproteins.19
First, we assessed the complexity of glycosylation of CHO cell expressed hLAMP-2. The expressed extracellular domain had molecular mass of 110 kD similar to native human glomerular hLAMP-2 but less than neutrophil hLAMP-2 (Supplemental Figure 2, A and B). As expected, the soluble extracellular domain was secreted into the supernatant and could not be detected in transfected cells using hLAMP-2–specific antibodies (Supplemental Figure 2, A and D). By contrast, most commercially available anti-hLAMP-2 antibodies cross-reacted with hamster LAMP-2 and stained lysosomes of transfected and untransfected cells, particularly after permeabilization with saponin (Supplemental Figure 2, C and D), negating their use as positive controls for transfection.
We then stably transfected ldlD cells with full-length hLAMP-2 with a tyrosine to histidine mutation in the cytoplasmic motif responsible for retrieval from the plasma membrane (hLAMP-2H). Transfected cells expressed abundant hLAMP-2 on the cell surface, where it was visualized without permeabilization with six different antibodies; however, none was detected in lysosomes with human-specific antibodies (Figure 3, A and B). Test sera containing anti-hLAMP-2 antibodies bound specifically to transfected ldlD cells, whereas control sera did not (Supplemental Figure 3). Sera were routinely diluted to 1:40 but binding of moderately strong positive sera way seen at 1:400 dilution (Figure 3C), indicating equivalent sensitivity to the other two assays.
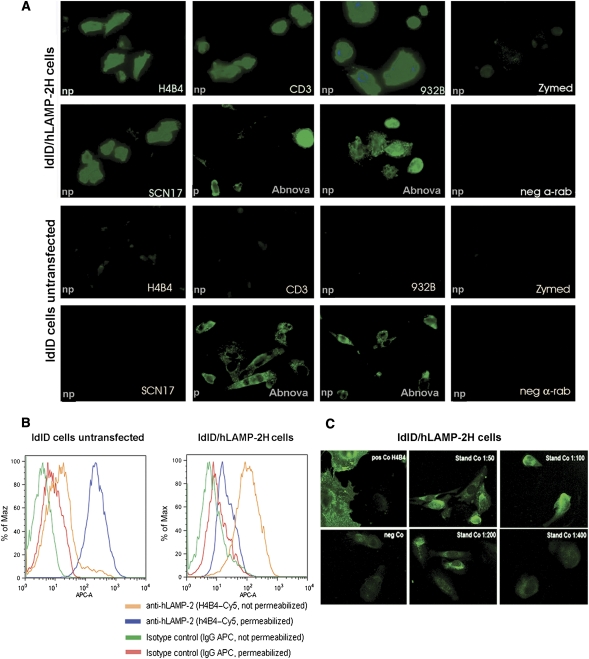
Figure 3.
Indirect immunofluorescence on ldlD cells stably expressing hLAMP-2 on the cell surface (ldlD/hLAMP-2H). (A) ldlD cells were transfected with full-length hLAMP-2 cDNA with a tyrosine to histidine mutation in the cytoplasmic lysosomal retrieval signal. Stable transfectants were sorted by flow cytometry for uniform expression of the transgene and stained with monoclonal (CD3) and polyclonal (932B) antibodies to hLAMP-2 generated by M. Fukuda as well as commercially available monoclonal (H4B4) and polyclonal anti-LAMP-2 antibodies (SCN17 and Abnova). All of the antibodies bound to hLAMP-2 on the surface of nonpermeabilized transfected cells (np). The commercial anti-hLAMP-2 cross-reacted with hamster LAMP-2 in transfected and untransfected cells especially after permeabilization (p). CD3 and 932B were the only antibodies that did not cross-react with hamster LAMP-2. A polyclonal antibody to rat LAMP-2 (Zymed Laboratories) and secondary antibody alone fail to detect either human or hamster LAMP-2. (B) FACS analysis of ldlD/hLAMP-2H cells and control ldlD cells stained with H4B4, a mAb to hLAMP-2 that cross-reacts with hamster LAMP-2. Surface expression of hLAMP-2 is detected only in transfected cells, whereas intracellular staining of native hamster LAMP-2 is apparent after permeabilization with saponin. (C) The sensitivity of the IIF assay for antibodies to hLAMP-2 assessed from doubling dilutions (1:50 to 1:800) of the standard positive control (Stand Co) compared with binding of monoclonal anti-hLAMP-2 antibody (H4B4; pos Co) and negative control serum (neg Co). IgG binding to ldlD/hLAMP-2H cells decreases progressively in titers >1:100 and becomes negative at 1:400, which is 10-fold higher than the standard assay dilution of 1:40.
Comparison of Western, ELISA, and IIF Assays
We compared assay performance using a panel of sera selected for their range of positive and negative results: 16 from AAV (11 active disease; 5 remission), 15 renal disease controls, and 10 healthy controls. Overall, 14 sera (all from the AAV group) were positive in ≥1 assay. Two-way comparisons confirmed the consistency between results of the different assays (Figure 4A) with interassay concordance rates of 83%, 90% and 92% for ELISA versus IIF, ELISA versus Western blot, and IIF versus Western blot, respectively. In all three assays, 33 of the 41 sera gave the same result, yielding an overall concordance rate of 80.5% (Figure 4B). The concordance rates within groups were as follows: 82%, 9 of 11 patients with active AAV; 40%, 2 of 5 patients with AAV in remission; 86% in 13 of 15 controls with renal disease; and 100% in 10 of 10 healthy controls.
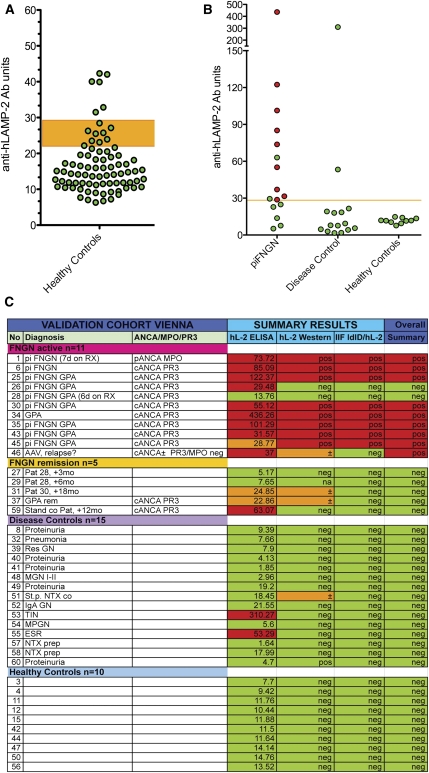
Figure 4.
Evaluation of assays for antibodies to LAMP-2. (A) ELISA results from a panel of sera from 78 healthy controls were used to derive the mean ± SD for this group and the 95% confidence limit of the upper limit of normal for the assay, which was established at 29 U. Six controls had positive ELISA with negative Western blots and IIF assays. (B) ELISA results of the data shown in A. The upper limit of normal in the assay is 29 U. Sera confirmed to have anti-hLAMP-2 antibodies by the other two assays are in red, whereas those in which positive ELISA was not confirmed because ELISA and IIF assays were negative are in green. (C) Measurement of antibodies to hLAMP-2 by ELISA and Western blot using hLAMP-2/GST FP and IIF on ldlD/hLAMP-2H cells was compared using a panel of 41 sera selected to cover a range of positive and negative values. The panel consisted of 16 sera from patients with AAV (11 with active disease and 5 in remission), 15 controls with other renal diseases, and 10 healthy controls. The assays were graded positive (red), low positive (Western and IIF), borderline (ELISA) (orange), or negative (green). The figure illustrates the strong concordance among results from the three assays. Sera were considered to have antibodies to LAMP-2 when ≥2 assays were positive. Abbreviations: TX, renal transplant; MGN, membranous nephropathy; IgA GN, IgA nephropathy; MPGN, membranoproliferative GN; TIN, tubulointerstitial nephritis.
Discrepancies between the assays were most frequent in the inactive piFNGN group, which likely reflects minor differences in assay sensitivity. Notably, the ELISA did not always distinguish low concentrations of specific antibody from high background binding of normal IgG. Consequently, we introduced a borderline range (22–29 U) into the ELISA results. Three of the 41 sera gave false positive ELISA results along with negative results in the other two assays; however, the reasons are unclear because immunoblots excluded binding to GST or contaminating proteins. Consequently, autoantibodies were considered present only when two of the three hLAMP-2 assays were positive.
Prevalence of Anti-hLAMP-2 Antibodies
The three assays were then used to ascertain the prevalence of anti-hLAMP-2 antibodies in three new groups of patients with AAV from Vienna, Austria; Groningen, the Netherlands; and Cambridge, United Kingdom. The Vienna group comprised 19 patients (23 sera: 12 with AAV at presentation, 2 with clinical AAV relapse, and 9 with clinically inactive AAV). The Groningen group comprised 50 patients (50 sera: 35 with untreated AAV at presentation, 9 with AAV at presentation and receiving treatment, and 6 with AAV clinical relapse). The Cambridge group comprised 53 patients (53 sera: 33 with AAV at presentation and 20 with AAV relapse) (Supplemental Tables 1–3). The assays were performed without knowledge of whether sera were from AAV or control groups, or from presentation or follow-up. All assays were performed without knowledge of the other assay results and the data are summarized in Figure 5.
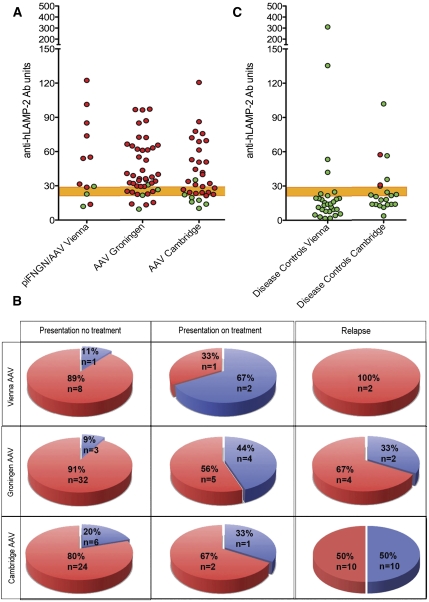
Figure 5.
Antibodies to hLAMP-2 in patients with AAV. (A) ELISA results from patients presenting with active piFNGN/AAV from Vienna (n=14), Groningen (n=44), and Cambridge (n=33). The upper limit of normal is 29 and the shaded area between 22 and 29 indicates borderline. Sera confirmed to have anti-hLAMP-2 antibodies by the other two assays are in red, whereas those without anti-hLAMP-2 antibodies are shown in green. Both ELISA and IIF assays were negative in sera in green with positive ELISA. (B) None of the 30 controls from Vienna with various types of renal disease were judged to have anti-hLAMP-2 antibodies, although 4 patients had isolated positive ELISA with negative Western blots and IIF assays. Two of the 21 SLE patients (9.5%) had anti-hLAMP-2 antibodies and an additional 2 patients had positive ELISA not confirmed by the other two assays. (C) Proportion of patients the Vienna, Groningen, and Cambridge cohorts with active piFNGN/AAV with antibodies to LAMP-2. Results are shown separately for untreated patients at presentation, patients presenting after immunosuppressive treatment had been started, and sera taken during relapse. The overall frequency of antibodies to LAMP-2 at presentation was 82% and was higher in untreated patients than those already on treatment. This difference was significant for the whole group (P=0.0071, Fisher's exact text) and for the Groningen cohort (P=0.0236, Fisher’s exact test).
Of the individuals presenting with piFNGN, 9 of 12 Vienna patients (75%), 37 of 44 Groningen patients (84%), and 26 of 33 Cambridge patients (79%) had positive results (Figure 5). This gives an overall frequency of 81% compared with 93% in our original study,14 which was restricted to patients presenting without prior treatment to avoid confounding effects of immunosuppression. Applying the same restriction to this study further increased the frequency of anti-hLAMP-2 antibodies in 8 of 9 Vienna patients (89%), 32 of 35 Groningen patients (91%), and 24 of 30 Cambridge patients (80%). The difference in frequency of anti-hLAMP-2 antibodies between treated and untreated patients was highly significant (all patients, P=0.0071; Groningen cohort, P=0.0236; Fisher’s exact test).
There was a high degree of agreement between the assays, with the three assays giving identical results in 80%, 67%, and 67% of the patients from Vienna, Groningen, and Cambridge, respectively (Supplemental Tables 1–3). Thus, we can be confident of the high prevalence of anti-hLAMP-2 antibodies in patients presenting with untreated piFNGN in this series (86.5%).
Autoantibodies to hLAMP-2 were equally frequent in MPO- and PR3-ANCA–associated disease and were independent of the clinical diagnosis of GPA or MPA. We also detected the autoantibodies in both ANCA-negative patients presenting with piFNGN (Supplemental Tables 1–3). Other than prior treatment, patients without detectable anti-hLAMP-2 antibodies had no special characteristics. Specifically, they had similar titers of antibodies to MPO or PR3, and equally severe renal involvement and extent of organ involvement. Ten of 11 patients without evidence of renal involvement in the Groningen group had detectable antibodies to hLAMP-2.
We assayed 51 additional participants as disease controls: 30 from Vienna with renal disease and 21 from Cambridge with SLE (Figure 5C). No renal controls had detectable anti-hLAMP-2 antibodies although four had isolated positive or borderline ELISA results. Two of the 21 participants with SLE (9.5%) had anti-hLAMP-2 antibodies and two more had isolated positive ELISA results. Accordingly, the interassay concordance in the ELISA was lower for SLE sera, which emphasizes the need to confirm ELISA results with other assays.
Response of Anti-hLAMP-2 Antibodies to Immunosuppressive Therapy
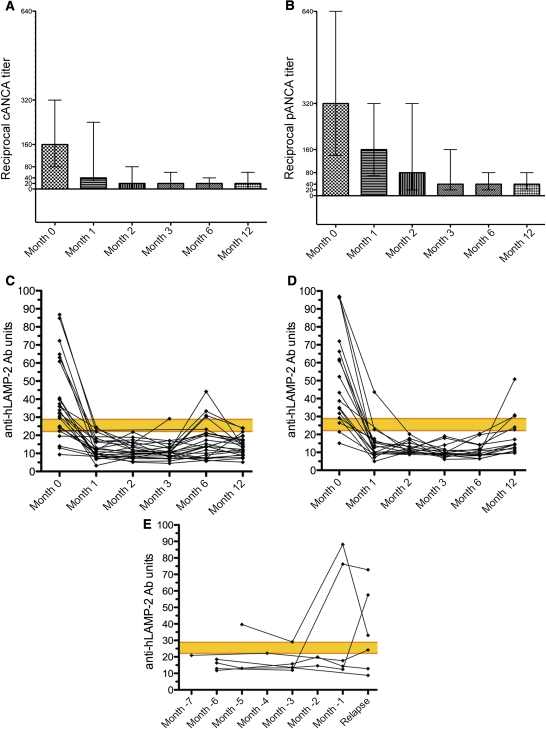
The Groningen patients were managed according to a standard protocol of corticosteroids and cyclophosphamide, together with plasma exchange for those most severely affected; eight patients also received rituximab as part of the Rituximab for ANCA-Associated Vasculitis trial.20 They were followed with clinical and serological assessment at 1, 2, 3, 6, and 12 months after presentation. Once treatment was started, disease activity decreased rapidly accompanied by a decrease in ANCA titers that eventually became negative, at least transiently, in 22 patients and very low titer (1:20) in an additional 9 patients (Figure 6, A and B). However, ANCA titers remained above 1:640 in two patients. Concentrations of anti-hLAMP-2 antibodies fell more rapidly and they became undetectable in 36 of 37 positive patients by 1 month (Figure 6, C and D). Thereafter, antibodies to hLAMP-2 were judged negative in all but one serum throughout the 12-month study—although ELISA increased to just above normal in five patients, the other two assays remained negative. The isolated changes in ELISA were not accompanied by changes in ANCA titers or evidence of relapse.
Figure 6.
ANCA and anti-hLAMP-2 antibody titers in patients with AAV. (A and B) Sequential ANCA titers expressed as median and interquartile range in the 43 patients in the Groningen cohort followed for 12 months. ANCA titers decreased rapidly after the induction of treatment. The patients are separated into those who had PR3-ANCA (n=25; A and C) and MPO-ANCA (n=18; B and D). (C and D) Sequential titers of antibodies to hLAMP-2 measured by ELISA in the 43 patients in the Groningen cohort followed for 12 months. The upper limit of normal is 29 U and the yellow bar indicates the borderline positive. The patients are separated into those who had PR3-ANCA (n=25) and MPO-ANCA (n=18). Anti-hLAMP-2 antibodies became undetectable in <1 month in all but one of the patients. They became undetectable in all 37 positive patients, irrespective of the presence or absence of antibodies to either (C) PR3 or (D) MPO. (E) Sequential titers of antibodies to hLAMP-2 measured by ELISA in six patients from Groningen in the months preceding clinical relapse. The upper limit of normal is 29 U and the yellow bar indicates the borderline positive. Sera from four patients had detectable antibodies to hLAMP-2 before clinical relapse.
Antibodies to hLAMP-2 during Clinical Relapse
Sera were assayed from 28 additional patients with clinical relapses (2 from Vienna, 6 from Groningen, and 20 from Cambridge) (Supplemental Tables 1–3). Seventeen patients had PR3-ANCA, 7 had MPO-ANCA, and 4 were ANCA negative. At relapse, the relevant ANCA was positive in 15 of 24 patients (63%), whereas anti-hLAMP-2 antibodies were detected in 16 of 28 patients (57%) (Figure 5B). Surprisingly, there was no correlation between anti-MPO or PR3 antibodies at relapse and antibodies to hLAMP-2 (hLAMP-2 positive/MPO/PR3 positive, n=10; hLAMP-2 positive/MPO/PR3 negative, n=6; hLAMP-2 negative/MPO/PR3 positive, n=4; and hLAMP-2 negative/MPO/PR3 negative, n=7; P=0.17, Fisher’s exact test). Similarly, there was no correlation between ANCA diagnosis and hLAMP-2 positivity at relapse. Sequential sera taken before relapse from six Groningen patients documented the evolution from negative to positive in four patients and suggested that the appearance of anti-hLAMP-2 antibodies anticipated relapse (Figure 6E), as did ANCA titers.
Discussion
Here we describe three independent assays for antibodies to hLAMP-2 and their application to three new cohorts with AAV. The frequency of anti-hLAMP-2 antibodies at presentation was 80%–90% in these cohorts, confirming our previous findings.13,14 The autoantibodies rapidly become undetectable in response to immunosuppressive therapy but are again frequent at clinical relapse. This emphasizes the need for large prospective studies to analyze relationships between the autoantibodies and disease activity that will require robust, generally applicable assays.
LAMP-2 is a heavily glycosylated membrane protein with cell type and activation-dependent differences in carbohydrate complexity evident from the molecular mass.15,16 Thus, endothelial hLAMP-2 is 110 kD, whereas neutrophil hLAMP-2 is 70–190 kD. Most patients’ autoantibodies bind epitopes in the protein backbone of the extracellular domain that are accessible in native neutrophil and endothelial hLAMP-2 as well as recombinant human antigen expressed in CHO cells.13,14 This determined our selection of the following hLAMP-2 preparations as assay substrates: recombinant human unglycosylated extracellular domain expressed in E. coli for ELISA and Western blotting, and glycosylated full-length hLAMP-2 targeted to the plasma membrane of ldlD cells for IIF.
Recombinant hLAMP-2/GST fusion protein is simple to produce in standard laboratory E. coli that do not express FimH under standard conditions,21 but is susceptible to degradation. Degradation markedly reduces specific binding in the ELISA and the effect is amplified in sera from patients with chronic disease, such as lupus, and after repeated freezing and thawing. Precautions to minimize degradation included truncating hLAMP-2 extracellular domain to 324 amino acids and preparing antigen in small batches. We tested for antibodies that bind glycosylated hLAMP-2 by IIF on ldlD cells stably transfected with full-length hLAMP-2 because the isolated extracellular domain is, like other soluble proteins,22 secreted and not retained within the cell. In contrast, full-length hLAMP-2 traffics to the plasma membrane and is then retrieved to lysosomes. The cytoplasmic retrieval signal was mutated in the full-length construct we used, resulting in exclusive hLAMP-2 cell surface expression. This enabled us to assay antibodies that bind it without cell permeabilization, thus avoiding the potential confusion caused by co-existing autoantibodies that recognize hamster LAMP-2 or intracellular antigens found in autoimmune disease.23,24
The three anti-hLAMP-2 antibody assays gave remarkably similar results, with three-way concordance rates of 80.5% and 74% in the evaluation and test cohorts, respectively. The proportion of positive sera was similar for each assay and we can thus be certain that the prevalence of anti-hLAMP-2 antibodies at presentation is between 80% and 90% in these new AAV cohorts and that the epitopes recognized are not occluded by carbohydrate in mammalian expressed antigen. This replicates our previous reports13,14 but contrasts sharply with that of Roth et al.,18 possibly because of critical differences in the patients studied and assays used.
We have always analyzed sera from patients with carefully defined disease activity, whereas sera in the study by Roth et al. were for the most part not segregated by disease activity. This is important because anti-hLAMP-2 antibodies are confined to those with active disease. Both groups used ELISA and immunoblotting assays but with markedly hLAMP-2 extracellular domain preparations. Roth et al. used complexly glycosylated hLAMP-2 expressed in HEK293 cells,18 whereas we used unglycosylated hLAMP-2 that, in our hands, gives identical results to the less complexly glycosylated ldlD expressed hLAMP-2.14 The ELISA results in the study by Roth et al. are most like our own when direct comparisons can be made. Thus, 7 of 15 (47%) of their presenting patients tested positive compared with 80%–90% in our cohorts. By contrast, results of their other two assays were categorically different by being uniformly negative even in sera that were positive by ELISA, whereas our immunoblotting and IIF assays gave similar results to the ELISA. The difference is highlighted by the four Viennese positive controls provided to validate the Roth ELISA: These were positive in all three Vienna assays and in the Roth ELISA, but were negative by immunoblotting and were not stated for the IIF assay. Possible reasons for these disparities include failure of autoantibodies to bind more complexly glycosylated hLAMP-2 by immunoblotting and use transiently expressed soluble hLAMP-2 extracellular domain that, in our hands, is not retained in the cells.
Anti-hLAMP-2 autoantibodies become undetectable strikingly quickly after starting steroids and immunosuppressive drugs and remained undetectable in the absence of clinical evidence of relapse until the end of the study at 12 months. This explains the significantly lower frequency of anti-hLAMP-2 antibodies in patients presenting after the start of treatment. The ANCA titers decreased more slowly and often remained positive in those without clinical evidence of disease activity. The rapid disappearance of anti-hLAMP-2 antibodies is reminiscent of the fate of anti-MPO antibodies in Churg-Strauss Syndrome.25 This implies a short t1/2 of the autoantibody, which is what we observed in rats injected with antibodies to LAMP-2.14 We attribute this to the well documented rapid internalization of anti-LAMP-2 antibodies after they bind at the cell surface.26,27
In conclusion, results from three new European cohorts confirm the high frequency of antibodies to hLAMP-2 in patients presenting with AAV and show that they rapidly become undetectable once treatment is started but recur during clinical relapses. The critical remaining question is whether they are pathogenic or merely an epiphenomenon.
Concise Methods
Patients and Controls
Sera were studied from three independent groups of patients whose details are summarized in Supplemental Tables 1–3. The groups comprised 19 patients from Vienna, Austria, with piFNGN with or without AAV either at presentation or during follow-up; 50 patients from Groningen, the Netherlands, presenting with AAV and followed prospectively for 12 months, and 6 patients with AAV in relapse in whom sequential serum samples were available leading up to the relapse; 53 patients from Cambridge, United Kingdom with AAV with or without piFNGN either at presentation (33 individuals) or with clinical relapse (20 individuals)). Clinical diagnoses of GPA and MPA were in accordance with the Chapel Hill Consensus criteria.28 Diagnosis of relapse was made on clinical grounds. In addition, we studied a panel of 80 apparently healthy controls aged 21–84 years (mean 48.15 years) from Vienna; 30 controls with various renal diseases from Vienna; and 21 patients presenting with SLE from Cambridge. All sera were assayed without knowledge of whether they were from the AAV or disease control groups, or from presentation or follow-up. The performance of the assays was compared using a test panel of 41 positive and negative sera.
Permission to use patients’ sera for autoantibody testing was granted by the relevant ethics committees of the Medical University of Vienna and the Universities of Groningen and Cambridge.
Antibodies and Reagents
The following primary antibodies were used: monoclonal mouse anti-hLAMP-2, clone H4B4 (Developmental Studies Hybridoma Bank, University of Iowa); polyclonal rabbit (PAB12956; Abnova, Taipei, Taiwan); goat anti-hLAMP-2 (sc-8101; Santa Cruz biotechnology, Santa Cruz, CA); and rabbit anti-rLAMP-2 (Zymed Laboratories, San Francisco, CA). Monoclonal mouse anti-hLAMP-2 (clone CD3) and rabbit anti-hLAMP-2 (932b) were kind gifts from Professor Minoru Fukuda (The Burnham Institute, La Jolla, CA). Secondary antibodies used in IIF were Alexa Fluor 488 conjugated F(ab`)2 fragment of goat anti-mouse IgG (H+L) and goat anti-rabbit IgG (H+L) (Invitrogen, Carlsbad, CA), and FITC conjugated sheep Ig to human IgG (INOVA Diagnostics, San Diego, CA). Peroxidase-conjugated AffiniPure Goat Anti-Human IgG, F(ab') 2 Fragment Specific (Jackson Immuno Research, West Grove, PA) was used in ELISA and alkaline phosphatase conjugated anti-mouse, anti-human, and anti-rabbit IgG (Promega) were used for Western blotting with their respective chromogenic substrates 1,2-phenylendiamine-hydrochloride (Fluka AG, Buchs, Switzerland) and nitro-blue tetrazolium and 5-bromo-4-chloro-3′-indolyphosphate (Pierce, Thermo Fischer Scientific, Rockford, IL).
Standard ANCA and Autoantibody Assays
Serial dilutions of all sera were routinely tested for ANCA, antinuclear antibodies, and anti-glomerular basement membrane antibodies (The Binding Site, Birmingham, UK), as described previously.13,14 One or more ANCA-positive serum from each patient was tested for ANCA specificity using commercially available ELISA systems. For follow-up studies, all sera from individual patients were re-tested by ELISA in the same assay.
Generation of Recombinant and Mutant hLAMP-2
A GST-tagged hLAMP-2 fusion protein (which is more stable that the His-tagged equivalent) corresponding to 342 amino acids of the extracellular domain of hLAMP-2 was amplified from bases −83 to 1026, cloned in frame into pGEX6p1 (pGEX6p1/hl2), and expressed in E. coli. The extracellular domain of hLAMP-2 was amplified from bases −3 to 1119 by 5′ and cloned into pCDNA1 to allow expression of a soluble 373 amino acid protein in mammalian cells (pCDNAI/hl2sol). A construct to express hLAMP-2 on the surface of mammalian cells was generated by mutating tyrosine in position 378 of hLAMP-2 sequence to histidine. The mutated hLAMP-2 cDNA was cloned into pSVK3 (pSVK3/LAMP-2H). All cloning procedures have been described previously.14
Purification of Recombinant hLAMP-2 and Western Blotting
E. coli BL21(DE3)pLysS (CLONTECH laboratories, Palo Alto, CA) were used to avoid toxicity of the pGEX6p1/hl2 construct that had been observed in other host strains. Toxicity and degradation of the fusion protein were also limited by reducing the induction time to a maximum of 2 hours. The cells of 1-L cultures were harvested, kept overnight at −80°C, and solubilized in PBS with 0.05% NaN3 and protease inhibitors (Complete, Roche) from the periplasmic fraction by freezing in liquid N2 followed by sonication. The recombinant protein was purified from cleared supernatant using fresh batches of Glutathione-Sepharose (GE Healthcare Europe, Vienna, Austria). Purity of individual batches of fusion protein was analyzed by SDS-PAGE and silver stain. Only fractions free of contaminating proteins were pooled, dialyzed against PBS, snap frozen, and stored at −80°C for up to 4 months. Production of soluble hLAMP-2 in CHO DG44 cells was induced using methods described previously.14
For Western blot analysis, 10 µg hLAMP-2/GST fusion protein per lane were subjected to SDS-Page, with a subsequent transfer onto nitrocellulose followed by immuno-overlay with patients’ sera in 1:50 and 1:100 dilutions. Size and immunoreactivity of E. coli expressed hLAMP-2 were confirmed with antibodies specific for hLAMP-2 and the GST tag.
ELISA for Antibodies to hLAMP-2
NUNC Maxisorp F ELISA plates were coated with 5 μg/ml of LAMP-2/GST for 1 hour—conditions shown in exploratory experiments to discriminate best between standard positive and negative sera after testing different substrate concentrations (0.2–22 μg/ml) and incubation times (1 hour to overnight). Newly coated ELISA plates were tested to ensure the consistency of binding with standard positive and negative control sera.
A standard positive control from a patient’s plasma exchange bag was used in serial dilutions to generate a standard curve from which anti-hLAMP-2 antibody concentrations were determined. The normal range in the ELISA was established by testing a panel of 80 apparently healthy controls aged 21–84 years (mean 48.15 years) at a dilution of 1:100. Each sample was measured three times and the average of these values was used to derive the mean and SD for this group. All serum samples were analyzed at least twice in triplicate. Sera of healthy participants were used as controls in all experiments.
IIF Assay for Antibodies to hLAMP-2
ldlD/hLAMP-2H cells were sorted by FACS for homogenous expression of hLAMP-2H on the cell surface. A pool of cells were expanded for 2 days and subsequently frozen in aliquots of 106 cells/ml. Each newly thawed batch went through a maximum of five passages before seeding onto eight-well chamber slides to avoid loss of transgene that occurs in around 10% of the cells per passage. ldlD parental or ldlD/hLAMP-2H cells in chamber slides were fixed in freshly prepared 4% paraformaldehyde 24–36 hours after seeding before washing in PBS and incubating for 1 hour at room temperature with patients’ sera diluted 1:40 in PBS. The slides were then incubated with FITC conjugated secondary antibodies. Specific binding of test sera to hLAMP-2 was assessed by comparison with reactivity and staining pattern of antibodies specific for hLAMP-2. The staining pattern and its intensity were scored independently by two observers. Representative photographs of IgG binding to ldlD parental and ldlD/hLAMP-2H cells were taken as a record using a fixed exposure time, based on the average of the optimal exposure time for a standard low binding serum and a standard high binding one.
Statistical Analyses
Median and interquartile ranges were calculated using GraphPad Prism software (GraphPad Software Inc), as were statistical comparisons by Fisher’s exact test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Professor Dontscho Kerajaschki for continual support and for helpful discussions, as well as Régis Dieckmann for helpful discussion.
A.J.R. was supported by an EU Marie Curie Excellence Chair (MACRORIEN), V.H. and T.F. are Marie Curie Fellows in the EU FP7 funded Initial Training Network TranSVIR, and the work in Vienna has been funded by the Vienna Science and Technology Fund (WWTF) through Project LS09-075. Work in Cambridge was funded by the Wellcome Trust, Medical Research Council, and the NIHR Cambridge BioMedical Research Centre. K.G.C.S. is a Lister Prize Fellow. The Cambridge Institute for Medical Research is in receipt of a Wellcome Trust Strategic Award (079895). Work in Groningen was supported by a grant from the Dutch Arthritis Association (06-1-401).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Anti–LAMP-2 Autoantibodies in ANCA-Associated Pauci-Immune Glomerulonephritis,” on pages 378–380.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011090920/-/DCSupplemental.
References
- 1.Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Morgan MD, Harper L, Williams J, Savage C: Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, Höglund P, Jayne D, Luqmani R, Mahr A, Mukhtyar C, Pusey C, Rasmussen N, Stegeman C, Walsh M, Westman K; European Vasculitis Study Group: Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 70: 488–494, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Xiao H, Falk RJ: Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 17: 1235–1242, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeringa P, Little MA: In vivo approaches to investigate ANCA-associated vasculitis: Lessons and limitations. Arthritis Res Ther 13: 204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Geld YM, Hellmark T, Selga D, Heeringa P, Huitema MG, Limburg PC, Kallenberg CG: Rats and mice immunised with chimeric human/mouse proteinase 3 produce autoantibodies to mouse Pr3 and rat granulocytes. Ann Rheum Dis 66: 1679–1682, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P: Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: Role of tumor necrosis factor-alpha. Am J Pathol 167: 47–58, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De’Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD: Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis 25: 380–389, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Schmitt WH, van der Woude FJ: Clinical applications of antineutrophil cytoplasmic antibody testing. Curr Opin Rheumatol 16: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kallenberg CG, Stegeman CA, Bootsma H, Bijl M, Limburg PC: Quantitation of autoantibodies in systemic autoimmune diseases: Clinically useful? Lupus 15: 397–402, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kain R, Matsui M, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D: A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: The lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med 181: 585–597, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D: Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14: 1088–1096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson SR, Roth J, Piller F, Fukuda M: Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem 263: 18911–18919, 1988 [PubMed] [Google Scholar]
- 16.Gough NR, Fambrough DM: Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol 137: 1161–1169, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth AJ, Brown MC, Smith RN, Badhwar AK, Parente O, Chung HC, Bunch DO, McGregor JG, Hogan SL, Hu Y, Yang JJ, Berg EA, Niles J, Jennette JC, Preston GA, Falk RJ: Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis [published online ahead of print]. J Am Soc Nephrol doi:10.1681/ASN.2011030273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remaley AT, Ugorski M, Wu N, Litzky L, Burger SR, Moore JS, Fukuda M, Spitalnik SL: Expression of human glycophorin A in wild type and glycosylation-deficient Chinese hamster ovary cells. Role of N- and O-linked glycosylation in cell surface expression. J Biol Chem 266: 24176–24183, 1991 [PubMed] [Google Scholar]
- 20.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group: Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwan WR, Seifert HS, Duncan JL: Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J Bacteriol 174: 2367–2375, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman JE, Wieland FT: Protein sorting by transport vesicles. Science 272: 227–234, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB: Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349: 1526–1533, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A: Fifty years of anti-ds DNA antibodies: Are we approaching journey’s end? Rheumatology (Oxford) 46: 1052–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Keogh KA, Specks U: Churg-Strauss syndrome: Clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med 115: 284–290, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Vischer UM, Rosnoblet C, Lebrand C, Lindsay M, Parton RG, Kruithof EK, Gruenberg J: The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell 11: 1829–1843, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janvier K, Bonifacino JS: Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell 16: 4231–4242, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CGM, McCluskey RT, Sinico RA, Rees AJ, van Es LA, Waldherr R, Wiik A: Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 37: 187–192, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.