Abstract
Nephron loss in a diseased kidney invokes adaptations in the remaining nephrons. Whether and how these adaptations condition the response of the kidney to injury is not known. We examined the susceptibility of the kidney after subtotal (5/6th) nephrectomy (STN) to ischemic injury in rats. GFR in STN kidneys did not significantly change after ischemia reperfusion (IR), whereas GFR fell by 70% after IR in unilateral nephrectomy controls. In micropuncture experiments, single-nephron GFR responses mirrored the whole-kidney responses: in STN, single-nephron GFR decreased by 7% after IR compared with 28% in controls. Furthermore, we found that tubuloglomerular feedback, a mechanism that links proximal tubular injury to a fall in GFR, was inoperative in STN but was normal in controls. Restoration of normal feedback in STN attenuated the functional resistance to IR. In addition to the functional resilience, the morphology of the kidney was better preserved in STN. In STN kidneys, the S3 segment of the proximal tubule, normally injured after ischemia, constitutively expressed hypoxia-inducible factor-1α (HIF-1α), which is cytoprotective in ischemia. Inducing HIF before IR improved GFR in control animals, and inhibiting the HIF target heme-oxygenase-1 before IR reduced GFR in STN animals. Taken together, these data suggest that fewer functioning nephrons in a diseased kidney do not increase the susceptibility to injury, but rather, hemodynamic and molecular adaptations in the remnant nephrons precondition them against ischemic injury.
Clear evidence to support the intrinsic susceptibility of the diseased kidney to injury is lacking.1 The physiologic and biochemical consequences of kidney disease are primarily influenced by the rapidity and extent of nephron obliteration, largely independent of the specific etiology. Hence, the functional capacity of a diseased kidney is determined by the flexibility of the surviving nephrons to adapt with the progression of disease. That the surviving nephrons do so with remarkable competence is evident from the uniformity of glomerulotubular balance, hyperfiltration, and tubular transport adaptations until the late stages of disease. This striking functional and regulatory capacity in the residual nephrons despite the extensive parenchymal damage was presented as the intact nephron hypothesis nearly four decades ago.2,3
In animal studies, using the 5/6th nephrectomy or subtotal nephrectomy (STN) model of renal mass reduction, several hemodynamic, metabolic, and molecular responses have been identified in response to the nephron loss. Many of these adaptations are thought to be maladaptive that may make the kidney vulnerable to injury. For instance, the increased intraglomerular pressure eventually leads to glomerulosclerosis,4 whereas the increased sodium transport-related oxygen consumption caused by compensatory hyperfiltration imposes a metabolic stress on the tubules.5,6 However, adaptations such as increased single-nephron blood flow and increased solute excretion per nephron can make the kidney more resilient. We have recently observed a lack of normal tubuloglomerular feedback responses in the STN kidney.7 Tubuloglomerular feedback activation caused by decreased reabsorption by the injured proximal tubule is implicated in the pathogenesis of various forms of AKI. Therefore, the aberrant tubuloglomerular feedback activity observed in STN could influence the susceptibility to filtration failure in AKI.
The questions of import to us are whether the kidney in the aftermath of nephron loss is more susceptible to injury when exposed to an insult or whether hemodynamic and molecular adaptations condition the kidney to withstand the insult. With these objectives in mind, we undertook to examine the hemodynamic (at the whole-kidney and single-nephron levels) and cellular responses of the kidney after STN to an ischemic insult. These experiments are the first detailed analyses at a single-nephron level that specifically assess the role of tubuloglomerular feedback activation in ischemic injury in STN. In conjunction with the hemodynamic analyses, we performed some preliminary investigations into the role of hypoxia-inducible factor-1α (HIF-1α) in the cellular response of the kidney to ischemia.
Results
Set 1: Micropuncture Experiments (Distal Single-Nephron GFR)
Systemic Data
Micropuncture experiments were performed in rats 1 week after STN or unilateral nephrectomy (UN) surgery, which served as the control. Some rats were subjected to ischemia reperfusion (IR), designated as UN+IR and STN+IR, and underwent micropuncture 1 day after IR. Systemic characteristics during micropuncture experiments are presented in Table 1. Both STN and STN+IR animals were hypertensive relative to UN and UN+IR, but the mean arterial pressure (MAP) was not significantly different pre- and postischemia in each group. There were no other significant intergroup differences.
Table 1.
Systemic data for distal micropuncture experiments: Set 1
| Groups | Body weight (g) | MAP (mmHg) | Hematocrit | Urine Flow Rates (µl/min) |
|---|---|---|---|---|
| UN (n=5) | 361±4 | 119±2 | 46±0.6 | 15±2 |
| UN+IR (n=4) | 320±8 | 112±3 | 45±1 | 13±2 |
| STN (n=6) | 315±24 | 136±6a | 48±1 | 20±5 |
| STN+IR (n=4) | 321±8 | 131±6b | 44±0.6 | 13±3 |
P=0.04 versus UN.
P=0.04 versus UN+IR.
Whole-Kidney and Single-Nephron Responses
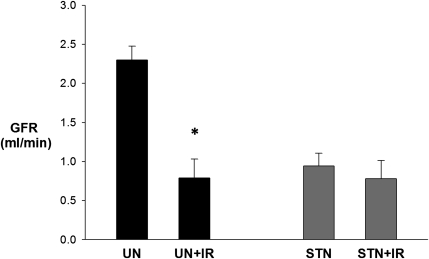
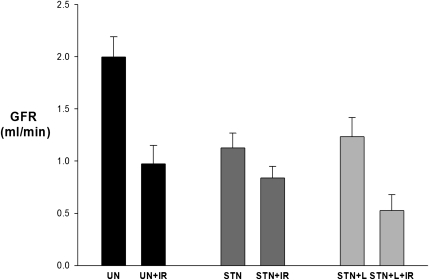
Whole-kidney GFR was determined by inulin clearances during micropuncture experiments. GFR in STN rats was less than one-half the GFR in UN (P<0.001), reflecting the difference in nephron number (Figure 1). IR reduced the GFR by 70% in UN+IR (P<0.001 versus UN). Surprisingly, STN rats, despite fewer functioning nephrons and lower baseline GFR than UN, were resistant to IR with no significant change in GFR (P=0.30).
Figure 1.
Functional resistance of STN to IR: whole kidney response. Whole-kidney GFR measured by 3-H inulin clearance during micropuncture experiments. The effect of IR was significant (P<0.001) as was the interaction between STN and IR (P=0.004). The effect of IR was significant in UN versus UN+IR (*P<0.001). At baseline, STN rats had a significantly lower GFR than UN (P<0.001). STN versus STN+IR (P=0.30).
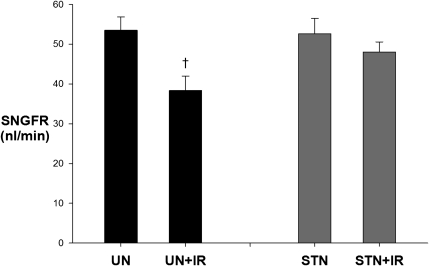
In addition to whole-kidney response to IR, ambient single-nephron GFR (SNGFR) was measured by randomly sampling nephrons in different quadrants of available kidney surface for free-flow early distal collections. SNGFR responses mirrored the whole-kidney responses (Figure 2). SNGFR dropped by 15 nl/min (28%) in UN+IR compared with UN (P=0.004), whereas in STN, the decrease was only 4 nl/min (7%) after IR (P=0.40).
Figure 2.
Functional resistance of STN to IR: single nephron response. The effect of IR was significant (P=0.03), but the interaction between STN and IR was not significant (P=0.10). The effect of IR on SNGFR was significant (†P=0.004) between UN and UN+IR groups but not between STN and STN+IR (P=0.40).
Set 2: Micropuncture Experiments (Tubuloglomerular Feedback Responses)
In a separate group of animals (UN, UN+IR, STN, and STN+IR), SNGFR was measured two times from the late proximal tubules with and without tubuloglomerular feedback activation by perfusion of Henle’s loop in 142 nephrons (total of 284 collections) to assess the responsiveness of tubuloglomerular feedback by closed-loop analysis. The timing of STN, UN, and IR was similar to the micropuncture experiments described above. The systemic characteristics for these groups are shown in Table 2.
Table 2.
Systemic data for tubuloglomerular feedback micropuncture experiments: Set 2
| Groups | Body weight (g) | MAP (mmHg) | Hematocrit | Urine Flow Rates (µl/min) |
|---|---|---|---|---|
| UN (n=8) | 339±12 | 120±4 | 48±1 | 24±6 |
| UN+IR (n=7) | 344±13 | 122±3 | 45±1 | 45±14 |
| STN (n=9) | 310±11 | 168±4a | 52±1b | 22±2 |
| STN+IR (n=9) | 289±7c | 176±4d | 46±1 | 32±6 |
| STN+L (n=4) | 357±4 | 104±6 | 46±1 | 20±4 |
| STN+L+IR (n=4) | 325±8 | 96±6 | 42±1 | 15±5 |
P<0.001 versus STN+L and UN.
P=0.005 versus STN+L and UN.
P=0.004 versus UN+IR.
P<0.001 versus STN+L+IR and UN+IR.
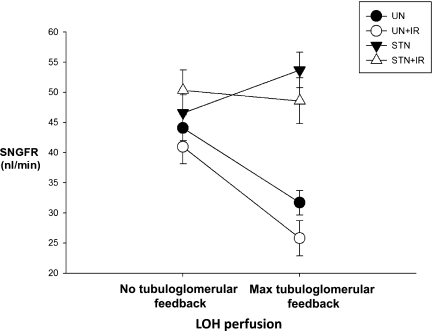
Typical feedback responses were observed in both UN groups, with the decrease in SNGFR of 12–15 nl/min with tubuloglomerular feedback activation. IR did not alter the responsiveness or range of tubuloglomerular feedback in UN. Activating tubuloglomerular feedback in STN had the anomalous effect of a paradoxical increase in SNGFR of 7 nl/min. In STN+IR, no discernible feedback response was observed (Figure 3). The change in SNGFR with feedback activation in the STN groups was significantly different pre- (P<0.001) and post-IR (P=0.002) from the UN groups. Thus, mean feedback responses were normal in UN and UN+IR, paradoxical in STN pre-IR, and not evident in STN post-IR.
Figure 3.
Aberrant tubuloglomerular feedback responses in STN before and after IR. No tubuloglomerular feedback activation at 0-nl/min loop of Henle perfusion. Maximal tubuloglomerular feedback activation was 60 nl/min. Normal tubuloglomerular feedback responses were observed in UN and UN+IR, but aberrant TGF responses were observed in STN and STN+IR. The effect of IR was significant (P<0.001). The change in SNGFR with feedback activation in the STN groups was significantly different pre- (P<0.001) and post-IR (P=0.002) from the UN groups.
Response to Angiotensin AT-1 Receptor Blockade
Our hypothesis that IR decreases GFR to a lesser degree in STN kidneys because of the absence of normal tubuloglomerular feedback was testable if tubuloglomerular feedback could be selectively normalized in STN. We have previously discovered that blocking angiotensin receptors with losartan in STN completely corrected the aberrant tubuloglomerular feedback response.7 Using an identical protocol, we administered losartan to STN rats and performed micropuncture experiments to confirm the preservation of a normal feedback response pre- and post-IR and test whether this administration would increase the sensitivity to IR. These groups are designated as STN+L and STN+L+IR.
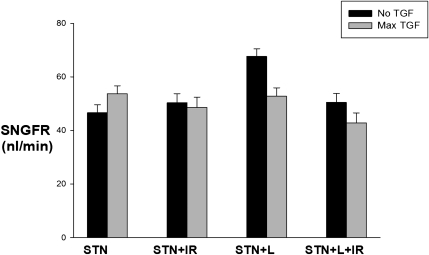
Systemic data are shown in Table 2. Micropuncture experiments to examine the range and responsiveness of tubuloglomerular feedback as described above were performed in 64 additional nephrons (total of 128 collections) in STN+L and STN+L+IR rats (Figure 4). With losartan treatment, normal feedback responses were observed in STN, with the decrease in SNGFR of 15 nl/min with tubuloglomerular feedback activation. In STN+IR rats treated with losartan, a feedback response of 8 nl/min was observed. The change in SNGFR with feedback activation was significantly different between untreated and losartan-treated STN (P<0.001).
Figure 4.
Preservation of normal tubuloglomerular feedback responses in STN with angiotensin blockade. Normal feedback responses observed in STN with angiotensin blockade. The effect of IR was significant (P<0.001). The change in SNGFR with feedback activation was significantly different between untreated and losartan-treated STN (P<0.001).
Whole-Kidney Responses
Whole-kidney GFR in STN was, again, resistant to IR, corroborating the result of the earlier micropuncture studies (Figure 5). Losartan markedly sensitized STN to the effect of IR. The differences in GFR pre- and postischemia were significant in the UN (P=0.002) and losartan-treated STN groups (P=0.02) but not the STN group (P=0.10). The percent reduction in GFR in the STN+L after IR (58%) approximated the percent reduction observed in UN (55%). Therefore, with preservation of normal tubuloglomerular feedback responses in STN with losartan treatment, the functional resilience of STN against IR was lowered.
Figure 5.
Preserving normal tubuloglomerular feedback responses lowered functional resistance of STN against IR. Whole-kidney GFR measured by 3-H inulin clearance. The effect of IR was significant (P<0.001). The effect of IR was significant in UN+IR (P<0.002 versus UN) and STN+L+IR (P=0.02 versus STN+L).
Histology
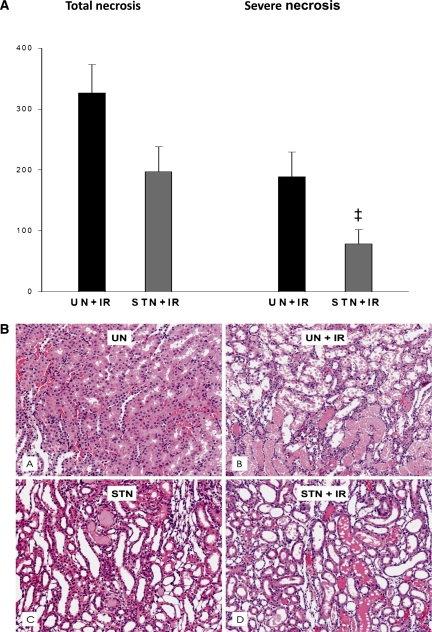
Morphologic examination of kidney tissue was performed to examine whether, in addition to the functional resistance to ischemic injury, the STN kidney shows evidence of cellular resistance. Tubular hypertrophy was observed in both STN and UN. Some mild glomerulosclerosis in a few nephrons was observed in STN. By a semiquantitative method to evaluate tubular necrosis described in Concise Methods, the composite scores for average tubular necrosis (mild, moderate, and severe) after IR were lower in STN compared with UN (197±41 versus 326±47, P=0.05) (Figure 6). The degree of severe tubular necrosis was significantly less in STN (78±23 versus 189±40, P=0.03). A modest degree of variability was observed in the injury among animals consistent with the relatively short duration of ischemia. Figure 6B represents the more severe injury observed in some animals after IR. Tubular necrosis was observed mainly in the outer stripe of medulla in the S3 segment of proximal tubule in both STN+IR and UN+IR. In the UN+IR groups, areas of medullary thick ascending limb necrosis were also observed.
Figure 6.
Cellular resistance of STN to IR. (A) The mean histology scores for severe necrosis were lower in STN+IR (‡P=0.03) as were the total score for necrosis (P=0.05). (B) Representive photomicrographs of histological appearance of kidneys before and after IR in STN and UN groups by hematoxylin & eosin staining (A, UN; B, UN+IR; C, STN; D, STNIR). B and D represent the more severe injury observed in some animals mainly in the outer stripe of medulla in the S3 segment of proximal tubule in both STN+IR and UN+IR. In the UN+IR groups, areas of medullary thick ascending limb necrosis were also observed. IR injury resulted in increased medullary congestion, hemorrhage, and proteinaceous casts that obliterated tubular lumen.
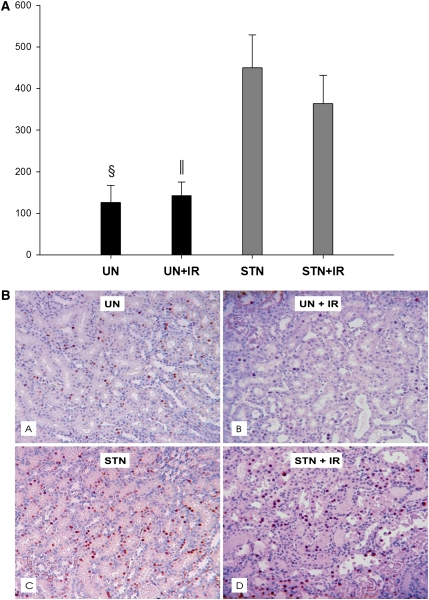
In addition to tubular hypertrophy observed in both STN and UN, we examined 5-bromodeoxyuridine (BrdU) incorporation in kidney tissue as a measure of cellular hyperplasia. Although a few proliferating cells were observed in UN and UN+IR, a robust proliferative response was observed in STN and STN+IR (Figure 7) both in the cortex and medulla.
Figure 7.
Cellular hyperplasia in STN. (A) Using 5-bromodeoxyuridine (BrdU) incorporation in kidney tissue as a measure of cellular hyperplasia, BRDU positive cells were counted in all groups. The mean BrdU-positive cells were significantly higher in STN versus UN (§P=0.02) and STN+IR versus UN+IR (‖P=0.03). IR had no effect in either group. (B) Representative photomicrographs of kidneys before and after IR in STN and UN groups by BRDU staining showing cellular proliferation in all groups (A, UN; B, UN+IR; C, STN; D, STN+IR). Few proliferating cells were observed in tubular epithelium in UN and UN+IR, but a more robust response was observed in STN and STN+IR.
HIF Pathway
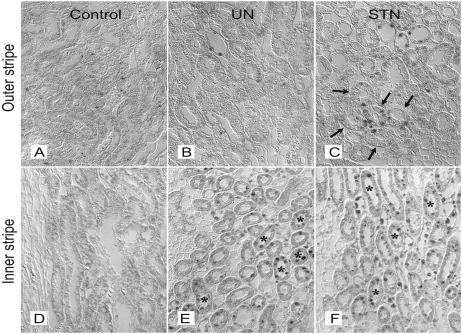
High oxygen consumption in the STN kidney5,6 has been proposed to contribute to a hypoxic environment in the kidney. We examined a potential consequence of this high oxygen consumption by testing for expression and cellular localization of HIF-1α in the STN kidney (Figure 8). By immunohistochemistry, at 1 week, clusters of HIF-1α–positive cells in the S3 segment of the proximal tubule were observed in STN but not in UN. Tubular cells in medullary thick ascending limbs (mTALs) were also positive for HIF-1α in STN and UN, with a modestly stronger signal in STN.
Figure 8.
Inherent HIF expression in STN. Representative photomicrographs showing HIF1α expression by immunohistochemical staining in the outer and inner stripes of medulla in control (A and D), UN (B and E), and STN (C and F) kidneys. Clusters of HIF-1α–positive cells observed in the S3 segment of proximal tubule in STN kidneys and in the medullary thick ascending limbs in UN and STN kidneys. Arrows point to S3 segment of proximal tubule. *mTAL.
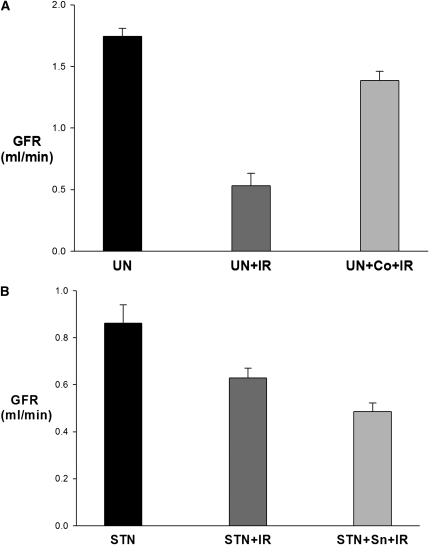
To further substantiate the role of HIF in this response, we performed clearance experiments in UN rats treated with cobalt chloride (CoCl2) to induce HIF expression before IR.8 The systemic characteristics are shown in Table 3. After IR, whole-kidney GFR was significantly improved in CoCl2-treated UN compared with untreated UN (0.5±0.1 versus 1.4±0.07, P<0.001) (Figure 9A).
Table 3.
Systemic data for clearance experiments
| Groups | Body weight (g) | MAP (mmHg) | Hematocrit | Urine Flow Rates (µl/min) |
|---|---|---|---|---|
| UN (n=6) | 325±16 | 122±6 | 45±1 | 29±5 |
| UN+IR (n=6) | 318±13 | 111±5 | 40±1 | 21±3 |
| UN+Co+IR (n=4) | 243±14a | 120±2 | 50±1a | 28±6 |
| STN (n=6) | 280±14 | 149±5 | 49±2 | 17±3 |
| STN+IR (n=7) | 275±8 | 157±5 | 43±2 | 20±4 |
| STN+Sn+IR (n=4) | 250±7 | 138±4 | 45±1 | 13±8 |
P<0.01 versus UN and UN+IR.
Figure 9.
Manipulation of HIF pathway prior to IR. (A) Response to HIF induction. Whole-kidney GFR by inulin clearances significantly improved in CoCl2-treated compared with untreated UN (P<0.001). (B) Response to HO-1 blockade. Inhibition of HO-1 with tin-protoporphyrin (STN+Sn+IR) led to a modest but significant reduction in GFR post-IR compared with untreated STN (P=0.04).
Next, we attempted to examine the role of a specific pathway downstream to HIF activation in this response. Among the many HIF target proteins, heme-oxygenase-1 (HO-1) has been shown to have a significant protective effect against ischemic and nephrotoxic insults.8–13 Therefore, we manipulated HO-1 activity by administering tin-protoporphyrin, a highly effective inhibitor of HO-1,10,14–16 to STN rats before IR. Inhibition of HO-1 led to a modest but significant reduction in GFR post-IR compared with untreated STN (0.4±0.03 versus 0.6±0.04, P=0.04) (Figure 9B). This finding suggests that HO-1 is one of the pathways downstream to HIF that plays a role in preconditioning the STN kidney.
Discussion
We examined the hemodynamic and cellular response of the kidney after severe nephron mass reduction to IR and obtained some significant results. First, the remnant kidney, despite of a 5/6th reduction in the functioning mass, shows a striking resistance to ischemia by largely maintaining the whole-kidney GFR and SNGFR. Second, in addition to this functional response, the morphology of the renal tissue in STN was better preserved. Third, typical tubuloglomerular feedback responses are absent in STN before and after ischemia, and restoration of tubuloglomerular feedback in STN sensitizes it to IR. Fourth, in STN, the S3 segment of the proximal tubule, normally injured after ischemia reperfusion, constitutively expresses HIF-1α, which has known cytoprotective actions. Finally, induction of HIF-1α protects against IR, and this effect may partly be mediated by HO-1. We discuss these results and interpretations in the following paragraphs.
Lately, investigative effort surrounding AKI has focused on mechanisms of tubular injury, but it is equally imperative to explain the mechanisms that translate tubular injury to a fall in GFR. Tubuloglomerular feedback is one such mechanism that lowers SNGFR when activated by a decrease in proximal reabsorption (because of tubular injury) in AKI. The work by Mason et al.17 found a significant role of tubuloglomerular feedback in ischemic and nephrotoxic AKI. Even with severe ischemia, tubular obstruction was observed early, but a day after IR, marked preglomerular vasoconstriction, presumably caused by tubuloglomerular feedback activation, was prominent.18 Peterson et al.19 also observed a direct role for tubuloglomerular feedback, where intratubular administration of uranyl nitrate immediately reduced SNGFR. With no systemic exposure to the nephrotoxin, only a local tubuloglomerular feedback mechanism could explain the immediate decline in SNGFR, and with tubuloglomerular feedback inhibition, SNGFR was preserved.
Tubular injury can also decrease GFR directly by tubular obstruction with epithelial debris, which raises tubular pressure and lowers net filtration pressure. Decreased clearance can also occur with transepithelial backleak of solutes across damaged tubular epithelium. Neither tubular obstruction nor backleak has been found to contribute to the pathogenesis of ischemic AKI with ischemia duration of less than 1 hour.20,21 Hence, these mechanisms cannot be invoked to explain the decrease in filtration in UN or the resilience observed in STN.
Unilateral nephrectomy controls for nephron mass reduction per se and the consequent compensatory growth and hyperfiltration achieved by increasing nephron plasma flow observed in STN. However, the more severe nephron reduction in STN may invoke or suppress other mechanisms to sustain hyperfiltration. Because tubuloglomerular feedback curbs any increase in SNGFR, the stronger stimulus posed by STN eliminates tubuloglomerular feedback as a brake on hyperfiltration. It is likely that the mechanism suppressed in the course of preserving overall GFR after STN also confers resistance against IR.
We have previously reported that typical tubuloglomerular feedback responses are absent in STN.7 We confirmed this finding in the current study and also showed normal tubuloglomerular feedback responses in UN. Furthermore, we have shown that preserving normal tubuloglomerular feedback in STN with losartan diminished the functional resistance of STN. Although we cannot rule out the impact of other hemodynamic and cellular effects of Ang II blockade, these data further support the role of tubuloglomerular feedback in the functional response after IR. Based on this finding and the above discussion, we believe that the lack of tubuloglomerular feedback-mediated decline in GFR typically observed after ischemic injury preserves renal function in STN after IR. Tubuloglomerular feedback activity may differ at various time points after renal mass reduction, and this activity may affect the response to ischemia. Blantz et al.22 have studied tubuloglomerular feedback in UN at 2–4 and 12 hours after surgery and have observed tubuloglomerular feedback adaptation by 12 hours but no suppression of tubuloglomerular feedback activity or sensitivity at either time point. Data on tubuloglomerular feedback at other time points in STN are not available.
There may be deleterious consequences for the kidney if after an insult GFR is preserved while tubular function and reabsorptive capacity remain impaired. Increasing transport activity after IR or improving SNGFR without alleviating tubular damage further exacerbates tubular injury.23,24 Therefore, we evaluated and found that preservation of GFR in STN was accompanied with better morphology. In addition, numerous proliferating cells were observed in STN. Although compensatory growth after nephron mass reduction is typically thought to be hypertrophic, we and others have observed significant hyperplasia in STN.25–28 Interestingly, proliferating cells are more resistant to ischemic injury.29 These findings cannot be explained on the basis of the hemodynamic alterations discussed above, and therefore, we investigated molecular adaptations in STN that may precondition the cellular response to IR.
There may be several molecular pathways involved in the cellular response of STN to IR. We focused on HIF; it is upstream to several of these pathways, and activation of HIF-1α in normal kidneys before ischemia or nephrotoxins has gained much traction as a preconditioning measure.8,9,30–35 HIF-1α is a transcriptional complex that regulates the cellular hypoxia response by its actions on various target genes. Moreover, increased renal oxygen consumption in STN is thought to contribute to intrarenal hypoxia and stimulate HIF expression. HIF activation also seems to retard the progression of disease in STN by halting proteinuria, preventing podocyte injury, and preserving peritubular capillary networks.36,37 Tubular hypoxia and upregulation of HIF-1α in early STN has been reported before.38 Our study confirms HIF-1α activation at early stages of STN and also shows its cellular localization, which has not been reported before.
The novel and striking feature of the inherent HIF-1α expression in STN is its presence in the S3 segment of the proximal tubule and the mTAL, the two most susceptible sites to ischemic injury. Even in conditions of experimental hypoxia, HIF-1α expression was seen mainly in the S1 and S2 segments, and practically none of the expression was in the mTALs.39 However, without altering the level of HIF expression, its direct role is difficult to confirm. Additional induction of HIF in STN would not be helpful, and therefore, we investigated the response to HIF induction in UN before IR and observed significant improvements in GFR with this treatment.
Several HIF-1α target proteins influence renal hemodynamics and cellular metabolism.9 To further investigate the specific downstream pathway of protection, we examined HO-1, a HIF target protein with known beneficial effects against ischemic and nephrotoxic insults.8–13 Inhibition of HO-1 in STN lowered its resistance against IR, suggesting that HO-1 may be one of the major downstream pathways to HIF in this preconditioning response. In addition to the known cytoprotective effects of HO-1, recently, HO-1 induction has been shown to inhibit tubuloglomerular feedback activity.40 Whether the protective effects of HO-1 observed in this model are primarily hemodynamic or cellular in nature remain to be examined.
A limitation of our study is that these findings may not translate to other models of kidney injury. However, there is extensive prior literature that suggests that preconditioning by a preceding injury of a similar or dissimilar nature confers protection against subsequent insults in the kidney.29 These studies include preconditioning by a prior ischemic insult, ureteral obstruction, nephrotoxic antiserum, gentamicin exposure, pretreatment with sodium arsenite, repeat exposure to uranium and/or single exposure after nephritis, repeat exposure to glycerol, and protection against mercuric chloride in animals with prior glycerol nephrotoxicity and vice versa.41–52
Another limitation is that the STN model does not capture etiological or phenotypical features of kidney disease, but it does recapitulate the consequences of nephron reduction common to nearly all forms of kidney disease. Similar findings have been observed in different strains of rats and at different time points after STN.50,53 Importantly, the work by Vercauteren et al.53 examined STN at 10 weeks, when severe glomerulosclerosis and tubular fibrosis were well-established, and even with 60-minute ischemia, this work found lesser degrees of functional and histologic impairment in STN animals compared with controls, showing the persistent resilience of STN even at later stages.
In summary, with nephron loss in a kidney, the compensatory adaptations that brace the surviving nephrons also confer a functional and structural resistance against ischemic injury. In STN, two rather independent factors seem to be operative—there is less tubular injury, presumably because of prior HIF activation, and the mechanism that connects tubular injury to filtration failure, to wit, tubuloglomerular feedback is inoperative. These hemodynamic and molecular preconditioning pathways have the potential to be artificially manipulated as preventive and therapeutic measures in other models of kidney injury.
Concise Methods
All experimentation was conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Effects of ischemia on STN and UN animals were studied in male Wistar rats (Harlan). All rats received free access to tap water and standard rat chow. Chronic angiotension I receptor blockade was achieved by adding losartan (200 mg/L) to the drinking water.7 Placebo controls were given a water bottle with no losartan. For HIF induction, CoCl2 was administered at 30 mg/kg two times a day subcutaneously for 2 days, with the last dose 6 hours before IR.8 Pharmacologic inhibition of HO-1 was achieved by administering tin-protoporphyrin (1.5 mg/100 g body weight) subcutaneously daily for 3 days to STN before ischemia.10,14–16
Micropuncture or clearance experiments were conducted 7–8 days after STN or UN surgery. IR groups underwent an ischemic insult 1 week after creation of STN or UN. In these groups, micropuncture or clearance experiments were performed 1 day after the ischemic insult. Kidneys were harvested for histologic or immunohistochemical examination at the same time points as micropuncture or clearance experiments.
Unilateral Nephrectomy
This procedure was performed with sterile technique on a temperature-controlled surgical table and under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally). A small right flank incision (1.5 cm long) was made. The muscle was clamped for 1 minute before cutting to prevent bleeding. The adrenal gland was carefully separated from the right kidney. The exposed right kidney was ligated with 4-0 silk suture tied tightly around the renal artery and vein. The right kidney was then cut from the vasculature and removed. The adrenal gland and attached vascular tissues were returned to the retroperitoneum. The fascia was closed with silk suture, and the skin was closed with steel wound clips.
Subtotal Nephrectomy
Right unilateral nephrectomy was performed as described above. A left flank incision was then made, and the left kidney was maneuvered to expose the renal artery. Two branches of left renal artery were ligated with 4-0 silk suture. The kidney was replaced back into the body, and the incision was closed as above. Rats were kept warm with a heating pad until ambulatory and administered a dose of buprenorphine analgesic.
Ischemia Reperfusion
This procedure is performed with sterile technique on a temperature-controlled surgical table to maintain body temperature at 37°C and under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally). A small left flank incision (1.5 cm long) was made. The muscle was clamped for 1 minute before cutting to prevent bleeding. The left kidney was exposed, and using an arterial clamp, the left renal pedicle was clamped for 30 minutes. Ischemia was visually confirmed by the change in color of the kidney. After the ischemic period, the clamp was removed, and the abdomen was closed. The animal was kept warm on the heating pad until ambulatory.
Clearance Experiments
Under inactin anesthesia (100 mg/kg body weight intraperitoneally), a tracheostomy tube (PE250 tubing) was placed, and the left internal jugular (PE50), left femoral artery (PE50), and urinary bladder (PE50) were cannulated. Body temperature was maintained at 37°C. At the end of the surgical preparation, rats underwent a 60-minute equilibration period, during which time 3H-Inulin in NaCl-NaHCO3 at a rate of 1 ml/h and NaCl-NaHCO3 solution at 1.4 ml/h were infused. MAP was monitored by connecting the femoral artery catheter to a transducer and a desktop computer loaded with the WinDaq software (DATAQ Instruments, Akron, OH). Blood samples for hematocrit and 3H-Inulin were obtained from the femoral artery catheter at the beginning and end of each urine collection.
Renal Micropuncture
Animals were surgically prepared according to previously established protocols54 and the surgical preparation for clearance experiments described above. In addition, the left kidney was exposed by flank incision and immobilized in a Lucite cup. The left ureter was cannulated (PE50). Tubular localization was carried out with a micropipette (3- to 5-m tip) containing Ringer saline stained lightly with FD&C green #3 (artifical food coloring). For ambient SNGFR measurements, free-flow collections of tubular fluid were made from early distal tubules after localization and assayed for volume of 3H-inulin content.
Tubular microperfusion was carried out using artificial tubular fluid delivered through a 7- to 9-m-tip pipette connected to a nanoliter perfusion pump (University of Tuebingen). For orthograde perfusion of Henle’s loop from the late proximal tubule, artificial tubular fluid contained 130 mM NaCl, 10 mM NaHCO3, 4 mM KCl, 2 mM CaCl2, 45 mM mg-percent urea, and 0.1% FD&C green, pH 7.4. Late proximal nephrons were localized, and an obstructing wax block was inserted immediately upstream from the most downstream accessible segment. Timed collections of tubular fluid were made upstream from the wax block to measure SNGFR. For tubuloglomerular feedback experiments, a microperfusion pipette containing artificial tubular fluid was inserted downstream from the wax block to perfuse the loop of Henle. Controlled perfusion of the loop of Henle was performed to activate tubuloglomerular feedback and thereby cause SNGFR to change. Collections were made from each nephron during minimal tubuloglomerular feedback activation (loop of Henle microperfusion at 0 nl/min) and maximal tubuloglomerular feedback activation (loop of Henle microperfusion at 50–60 nl/min). Several nephrons were studied in each animal, and the two perfusions per nephron were done in alternating order. Nephrons were vented upstream from the wax block before each collection to prevent pressure from building up in the proximal tubule. Two minutes were allowed for equilibration before each collection, and each collection was taken for 3 minutes or more. Tubular fluid samples were assayed for volume by transferring to a constant-bore glass capillary and then counting for radioactivity to determine SNGFR at both extremes of tubuloglomerular feedback activation.
Histology
Kidneys were harvested from all four groups of rats by perfusion fixation 1 week after STN or UN and 1 day after IR in both groups and evaluated in a blinded manner by a pathologist. Kidneys were perfused with cold PBS, fixed with 4% paraformaldehyde in situ by cannulation of the abdominal aorta, and then suspended in 4% formaldehyde in PBS for 24 hours. Four equally spaced 10× images were made from a paraffin-embedded 3-µm section of kidney tissue obtained after perfusion fixation from each rat. Tubular necrosis was labeled as mild when tubular epithelium was mostly intact but cells were flattened and the lumen contained a few sloughed epithelial cells or small amounts of cellular debris. It was labeled moderate when tubular epithelium was partially intact or nearly intact but with severely flattened cells and the lumen partially filled with sloughed epithelial cells and cellular debris. Tubules were labeled as severely necrotic when tubular epithelium was completely or nearly completely necrotic, such that the basement membrane was denuded and the lumen was filled with sloughed cells and/or cellular debris. Tubules with mild, moderate, or severe necrosis were counted. The number of tubules with moderate necrosis was weighed by two, and the number of severe ones was weighed by three. A sum of these values per section was calculated, and the average of the four images for each rat was considered as the final score.
Cellular Proliferation by BrdU Incorporation
Rats were given BrdU daily (50 mg/kg intraperitoneally; Sigma, St. Louis, MO) starting on day 3 after STN or UN until day 7 and continued until 1 day after IR in the groups subjected to IR. Kidneys were perfused-fixed as described above; 5-μm slices were cut from paraffin-embedded tissue. The sections were stained with biotinylated mouse anti-BrdU antibody and related reagents provided from a commercial kit (Zymed Laboratories Catalog #93-3944; Invitrogen) according to the manufacturer’s instructions. Ten 40× fields were examined in each animal, and BrdU-positive cell numbers in each slide were counted in both cortex and medulla of each section. The means for each group were calculated and compared.
HIF-1α Immunohistochemistry
Immunohistochemical stainings were performed on 3-µm paraffin sections using a rabbit anti-human HIF-1α antibody (1:20,000; Cayman Chemical, Ann Arbor, MI) as previously reported.39 Detection of bound antibodies is performed by using biotinylated secondary anti-mouse antibodies and a catalyzed signal amplification system (Dako, Hamburg, Germany) based on the streptavidin-biotin-peroxidase reaction, according to the instructions provided by the manufacturer. Antigen retrieval was performed for 90 s in preheated Dako target retrieval solution using a pressure cooker. All incubations are performed in a humidified chamber. Between incubations, specimens are washed two to four times in buffer (50 mM Tris⋅HCl, 300 mM NaCl, 0.1% Tween-20, pH 7.6).
Statistical Analyses
Data were analyzed by one- or two-way ANOVA as suitable, which was done with commercial software (Sigmaplot) with appropriate post hoc tests. Unless stated otherwise, results are presented as group mean ± SEM.
Disclosures
None.
Acknowledgments
This work was supported by Grants NIH-K08-DK084305 (to P.S.), R01-DK28602 (to R.C.B.), and P30-DK 079337 and Vererans Affairs Research Service Merit Awards (R.C.B. and S.C.T.).
Some of the results have been presented as abstracts at the American Society of Nephrology Renal Week, San Diego, CA, 2009, and Denver, CO, 2010.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Singh P, Rifkin DE, Blantz RC: Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol 5: 1690–1695, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Bricker NS: On the meaning of the intact nephron hypothesis. Am J Med 46: 1–11, 1969 [DOI] [PubMed] [Google Scholar]
- 3.Bricker NS, Morrin PA, Kime SW, Jr: The pathologic physiology of chronic Bright’s disease. An exposition of the “intact nephron hypothesis.” Am J Med 28: 77–98, 1960 [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Meyer TW, Rennke HG, Brenner BM: Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 76: 612–619, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng A, Tang T, Singh P, Wang C, Satriano J, Thomson SC, Blantz RC: Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int 75: 197–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath KA, Croatt AJ, Hostetter TH: Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol 258: F1354–F1362, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Singh P, Deng A, Blantz RC, Thomson SC: Unexpected effect of angiotensin AT1 receptor blockade on tubuloglomerular feedback in early subtotal nephrectomy. Am J Physiol Renal Physiol 296: F1158–F1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Maxwell P: HIF-1: An oxygen response system with special relevance to the kidney. J Am Soc Nephrol 14: 2712–2722, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME: Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salom MG, Cerón SN, Rodriguez F, Lopez B, Hernández I, Martínez JG, Losa AM, Fenoy FJ: Heme oxygenase-1 induction improves ischemic renal failure: Role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293: H3542–H3549, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA: Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int 48: 1298–1307, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A: Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A, Nick HS: Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA: Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol 169: 21–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwar YS: Heme oxygenase-1 in renal injury: Conclusions of studies in humans and animal models. Kidney Int 59: 378–379, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Mason J, Takabatake T, Olbricht C, Thurau K: The early phase of experimental acute renal failure. III. Tubologlomerular feedback. Pflugers Arch 373: 69–76, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Arendshorst WJ, Finn WF, Gottschalk CW: Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res 37: 558–568, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Peterson OW, Gabbai FB, Myers RR, Mizisin AP, Blantz RC: A single nephron model of acute tubular injury: Role of tubuloglomerular feedback. Kidney Int 36: 1037–1044, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Mason J, Olbricht C, Takabatake T, Thurau K: The early phase of experimental acute renal failure. I. Intratubular pressure and obstruction. Pflugers Arch 370: 155–163, 1977 [DOI] [PubMed] [Google Scholar]
- 21.Olbricht C, Mason J, Takabatake T, Hohlbrugger G, Thurau K: The early phase of experimental acute renal failure. II. Tubular leakage and the reliability of glomerular markers. Pflugers Arch 372: 251–258, 1977 [DOI] [PubMed] [Google Scholar]
- 22.Blantz RC, Peterson OW, Thomson SC: Tubuloglomerular feedback responses to acute contralateral nephrectomy. Am J Physiol 260: F749–F756, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Bird JE, Milhoan K, Wilson CB, Young SG, Mundy CA, Parthasarathy S, Blantz RC: Ischemic acute renal failure and antioxidant therapy in the rat. The relation between glomerular and tubular dysfunction. J Clin Invest 81: 1630–1638, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brezis M, Rosen S, Silva P, Epstein FH: Transport activity modifies thick ascending limb damage in the isolated perfused kidney. Kidney Int 25: 65–72, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC: Renal protection in chronic kidney disease: Hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol 299: F1365–F1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miskell CA, Simpson DP: Hyperplasia precedes increased glomerular filtration rate in rat remnant kidney. Kidney Int 37: 758–766, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Mulroney SE, Pesce C: Early hyperplastic renal growth after uninephrectomy in adult female rats. Endocrinology 141: 932–937, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A: Tweak induces proliferation in renal tubular epithelium: A role in uninephrectomy induced renal hyperplasia. J Cell Mol Med 13: 3329–3342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonventre JV: Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Nangaku M, Eckardt KU: Hypoxia and the HIF system in kidney disease. J Mol Med (Berl) 85: 1325–1330, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, Arend M, Klaus SJ, Heyman SN: Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant 23: 3472–3478, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Nangaku M: The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens 19: 43–50, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Câmpean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C: HIF activation protects from acute kidney injury. J Am Soc Nephrol 19: 486–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, Joo KW, Han JS, Na KY: Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant 25: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M: Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M: Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15: 1277–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Ren Y, D’Ambrosio MA, Wang H, Liu R, Garvin JL, Carretero OA: Heme oxygenase metabolites inhibit tubuloglomerular feedback (TGF). Am J Physiol Renal Physiol 295: F1207–F1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell JA: Subcutaneous fat necrosis, haemolysis without siderosis, and renal tubular atrophy following repeated glycerol injections. J Pathol Bacteriol 76: 473–481, 1958 [DOI] [PubMed] [Google Scholar]
- 42.Elliott WC, Houghton DC, Gilbert DN, Baines-Hunter J, Bennett WM: Gentamicin nephrotoxicity. I. Degree and permanence of acquired insensitivity. J Lab Clin Med 100: 501–512, 1982 [PubMed] [Google Scholar]
- 43.Hayes JM, Boonshaft B, Maher JF, O’Connell JM, Schreiner GE: Resistance to glycerol induced hemoglobinuric acute renal failure. Nephron 7: 155–164, 1970 [DOI] [PubMed] [Google Scholar]
- 44.Macnider WD: A pathological and physiological study of the naturally nephropathic kidney of the dog, rendered acutely nephropathic by uranium or by an anesthetic: Part II. J Med Res 34: 199–230, 1916 [PMC free article] [PubMed] [Google Scholar]
- 45.Macnider WD: The functional and pathological response of the kidney in dogs subjected to a second subcutaneous injection of uranium nitrate. J Exp Med 49: 411–433, 1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oken DE, Mende CW, Taraba I, Flamenbaum W: Resistance to acute renal failure afforded by prior renal failure: Examination of the role of renal renin content. Nephron 15: 131–142, 1975 [DOI] [PubMed] [Google Scholar]
- 47.Park KM, Chen A, Bonventre JV: Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV: Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem 277: 2040–2049, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Yang CW, Kim BS, Kim J, Ahn HJ, Park JH, Jin DC, Kim YS, Bang BK: Preconditioning with sodium arsenite inhibits apoptotic cell death in rat kidney with ischemia/reperfusion or cyclosporine-induced Injuries. The possible role of heat-shock protein 70 as a mediator of ischemic tolerance. Exp Nephrol 9: 284–294, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Zager RA, Baltes LA: Progressive renal insufficiency induces increasing protection against ischemic acute renal failure. J Lab Clin Med 103: 511–523, 1984 [PubMed] [Google Scholar]
- 51.Zager RA, Baltes LA, Sharma HM, Jurkowitz MS: Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689–700, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Zager RA, Iwata M, Burkhart KM, Schimpf BA: Post-ischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int 45: 1760–1768, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Vercauteren SR, Ysebaert DK, De Greef KE, Eyskens EJ, De Broe ME: Chronic reduction in renal mass in the rat attenuates ischemia/reperfusion injury and does not impair tubular regeneration. J Am Soc Nephrol 10: 2551–2561, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V: Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]